Abstract
Anomalous high fluoride concentration up to 7.59 mg/dm3 is found in groundwater from “La Victoria” area. This water is used to supply drinking water to Hermosillo City, Sonora. Geochemistry of groundwater, relationship between physicochemical parameters, hydrogeology and geologic setting were correlated to define the origin and the geochemical mechanisms of groundwater fluorine enrichment. High fluoride concentration is associated with high bicarbonates, pH and temperature, and it decreases toward the west and south of the area. Fluoride is in negative correlation to calcium concentration. Sodium sulphate facies of regional deep water flow are related to high fluoride concentration. High electric resistivity rocks associated with granites from the Sierra Bachoco basement might be the deep source of fluoride. Outcropping of Sierra Bachoco in the west causes upward regional flow. Groundwater of longer residence time can be pumped there. The anomalous area is restricted to “La Victoria” because calcareous paleozoic rocks outcrop to the south.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoride is an indispensable element for the maintenance of dental health. Fluoride concentrations up to 1.5 mg/dm3 are beneficial for reducing cavities in children during the calcification period (Trilles 1970; Srinivasa 1997). Fluoride concentrations above 1.5 mg/dm3 may lead to dental mottling (fluorosis), characterized initially by opaque white patches on teeth. In advanced stages of dental fluorosis, teeth display brown to black staining, followed by pitting of teeth surfaces during the tooth calcification stage from fetal to 12 years of age (Apambire et al. 1997). The frequency of fluorosis, far from disappearing as a public health problem, tends to increase around the world. The most completely effective action to prevent fluorosis is to reduce the permissible levels of fluoride concentration in drinking water. Commonly, fluoride reduction is achieved by dilution of high water fluoride concentration with waters of lower fluoride content until an acceptable concentration is reached. Since this technique is not possible in the study area, other alternates should be considered.
Fluoride groundwater contamination in Nayagarh, India, happens because of the deep mixing of hot spring water with normal groundwater. Hot spring can be thought of as a result of prolonged interaction with granitic rocks of the area. There is evidence of deep/seated fractures/fissures as channel ways for hot water (Kundu et al. 2001).
Extensive research of fluoride groundwater levels have been carried out in India. The most important factors that influence the fluoride concentration in water are the fluoride availability in the hydrogeological system, presence of fractures/fissures in volcanic rocks that act like channels of thermal water, and water residence time in volcanic rock (Gaciri 1993).
In Mexico, water is an indispensable but scarce and unequally distributed resource. It has been affected by a growing exploitation, often in an uncontrolled way, even with the possibility to decrease the groundwater levels. Extension and geographic diversity of Mexico cause a restrictive and irregular distribution of water. In San Luis Potosi, Mexico, a comparison of the water temperatures for 1962 with those of 1972, showed an abnormal increase of >5°C in 60% of wells with high fluoride content (Carrillo-Rivera et al. 2002). Fluoride concentrations of 1.5 to 4.0 mg/L were related to thermal flow in volcanic rocks and regional flow systems (Gallegos et al. 2004).
The northern half of Mexico including the Sonora state, suffers a constant deficit of rainfall (INEGI 1992). The city of Hermosillo, the state capital, (Fig. 1) has an increase in water demand because the population is increasing by 3.13% annually. The population went up to 711,512 in 2005 (http://www.hermosillo.gob.mx, Municipal Government), which makes it the most populated city in the State. The volume of groundwater extracted from 15 wells is around 26.2 Mm3/year (Mm3 = 1 × 106 m3). Fluoride concentration in groundwater varies from 0.24 to 7.82 mg/dm3 (Quintanar 1992).
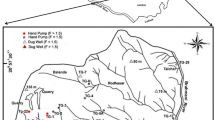
Location of study area. Geological map showing the principal rock types and the normal NW-SE fault system and lateral displacements faults over Sonora and San Miguel rivers. Geologic map was taken from Peña and others (1998)
The groundwater supply to Hermosillo has fluoride concentrations over 1.5 mg/L, which exceeds the Mexican Official Norm (NOM-127-SSAI-1994). Water primarily comes from wells located in “La Victoria” area in the San Miguel river valley. The main aquifer is formed by recent alluvial deposits with granulometric sizes varying from boulders and gravels to limes and clays, it is a free aquifer with a permeability coefficient from 1 × 10−4 to 7 × 10−2 m2/s (INEGI 1992). The total dissolved solids (TDS) content varies from 200 to 900 mg/dm3.
There is little current research about the fluoride content in the water in “La Victoria” area. The objective of the present work is to examine the origin of the high fluoride level in the groundwater for human consumption in Hermosillo city.
The study area
The aquifer with a high fluoride concentration in water which supplies the city of Hermosillo is located in the “La Victoria” region, (Fig. 1) within the boundaries 29° to 29°15′ of north latitude and 110°58′ to 111°00′ of west longitude.
The northwest portion of the Sonora state includes the physiographic province called Llanura Sonorense, with a very dry-hot to very dry-very hot climate. The mean annual and maximum summer temperatures are 22° and 48°C, respectively. The average annual rainfall is 300 mm (INEGI 1992). The water supply of Hermosillo city comes mainly from two sources: (1) superficial water supply is obtained from Sonora and San Miguel rivers, which is stored behind Abelardo L. Rodríguez and Rodolfo Felix Valdez dams located at the west margin of Western Sierra Madre Mountain, and (2) groundwater obtained from a mesh of wells located to the north and northeast of the city.
Geological setting
Granitic rocks in Sonora State have been grouped in “Laramide Batholith of Sonora” with ages varying from 40 up to 90 Ma. These rocks form a belt coarsely parallel to the Pacific Ocean coast that includes batholith bodies formed by multiple intrusions.
The western-center part of Sonora state is characterized by Paleozoic, Mesozoic and Tertiary rocks, its stratigraphic log are incomplete but rocks represent their described ages (Rodriguez-Castañeda 1981). The geologic setting is complex. Igneous, sedimentary and metamorphic rocks with ages that vary from the Precambrian to the Quaternary (Peña et al. 1998) all outcrop in the area. Four main tectonic events occurred in the region. The fourth during Tertiary age was the most extensive. It produced a series of horsts and grabens with N-S orientation (Rodríguez-Castañeda 1981) (Fig. 1). One of these grabens is located in the study area.
The central part of this graben is covered by Tertiary and Quaternary materials forming alluvial valleys limited by normal faults (Rodríguez-Castañeda 1981). In the study area, the horst to the west is formed by Batamote, Las Bateas and Bachoco mountains with a granite, granodiorite and quartz monzonite composition. On the east, the horst is represented by a volcanic system with normal faulting of N-S orientation bounding both sides.
The Sonora and San Miguel rivers flow throughout two lateral displacement faults that cut, in the NE direction, the east part of the tectonic depression. San Miguel river flows to the south in the same orientation of Bachoco normal fault after Sanjon river joints to San Miguel river, in the central portion of the alluvial valley. These change direction to N-S before discharge into the Abelardo L. Rodríguez dam reservoir.
The granitic intrusive is part of the west boundary of the alluvial valley. Granitic rocks that out cropping to the east of Bachoco Range were found in lithological cuttings of San Pedro wells at 150 m of depth. It rises west toward Bachoco Range. A low permeability aquifer is present and no deep wells are found. Anomalous fluoride content has been registered in some wells. On the other hand, rock samples from wells located in the left margin of the San Miguel river basin show sedimentary material derived from river alluvial deposits and intercalated layers of fine to coarse sand with clay lenses (Espinoza 1998). The granitic basement has not been found after drilling 300 m of depth. The Baucarite formation composed by conglomerate continental sediments is present in terraces. This aquifer, of high hydraulic transmissivity, shows an elevated fluoride content and high abnormal temperature (>30°C), that points out deep geothermal activity. Basalt, andesite and ignimbrites interspersed in sand and gravel units on the esatern part of the study area, i.e the bloquera well shows cut basaltic lava at 120 m depth.
A resistivity profile (A-A′) was obtained by GyE (2000) using lithology columns of wells and transient electromagnetic soundings interpretation (Fig. 2). This section is oriented from southwest to northeast, from the granitic outcrop in the Bachoco range to the left margin of Sonora river (Fig. 3). The shallowest layer (U1), with resistivity of 10–16 ohm-m and 10–29 m of thickness, almost disappears towards the Bachoco mountain range. A resistive body (229–569 ohm-m) is identified in the western part of the profile. This unit can be considered as a Bachoco range basement deepening to the east to more than 500 m (U5).
Resistivity transversal profile A-A′. For location see Fig. 3. Altitude is in meters above sea level
A depression of continental conglomeratic sediments from the Baucarit formation (U2-U4) is present in the central part of the profile with low to medium resistivities (5–54 ohm-m). This depression extends from the surface about 500 m deep and is the unit where the “La Victoria” wells are located (Fig. 3).
Geochemistry of fluoride
The fluoride salts dissolved in water originate from minerals present in rocks through which the groundwater flows. Water discharge passing through granite rocks shows an average fluoride content of 810 mg/dm3 (Ramamohana et al. 1993), higher than water moving through metamorphic and sedimentary rocks (Lisa 1994). Fluoride in mineral form is only found as fluorite (CaF2), most of the time as an accessory mineral of granite rocks (Kundu et al. 2001). While fluoride solubility in natural-origin waters has been studied (Ellis and Mahon 1977) the quantification of fluoride enrichment process in natural waters has not been sufficiently studied, especially in groundwaters (Kundu et al. 2001). Nevertheless, Saxena and Ahmed (2001, 2003) points out that high fluoride concentrations in water are related to rocks containing fluoride like granites, granite gneisses, quartzite and pegmatite. Through weathering of the primary minerals in rocks, fluoride is released into the soil and groundwater, i.e. leaching of fluoride-containing minerals may yield fluoride in solution (Saxena and Ahmed 2003). Concentration of fluoride in rock, or ionic species, long residence time of rock water interaction and Ca++ and HCO −3 contents of groundwater are important factors in determining the degree of fluoride dissolution.
Fluoride occurs in almost all natural waters from trace concentrations to as high as 15,000 mg/dm3 in mine water from Kola Peninsula (Kraynov et al. 1969). Its concentration in natural waters depends on several factors such as temperature, pH, presence or absence of ion complexes or precipitation of ions and colloids, solubility of fluorine-bearing minerals, anion exchange capacity of aquifer materials (OH− for F−), the size and type of geological formations through which the water flows and the time water is in contact with a particular formation (Apambire et al. 1997). Principally, controls are governed by climate, host rock composition and hydrogeology. Areas of semiarid climate, crystalline rocks and alkaline soils are mainly affected (Sujatha 2003). The amount of fluoride dissolved in waters of low ionic strength is around 8–10 mg/L. However, the concentrations of Ca++, Na+ and OH−, as well as certain ionic complexes such as Fe3+, Al3+, B3+, Si4+, Mg++ and H+, may modify fluoride concentration.
A strong negative correlation between Ca++ and F− in groundwater that contains Ca++ in excess takes into account the common ion effect. The majority of groundwaters are greatly undersaturated with respect to fluorite (CaF2). Since moderate hydrothermal alteration in water increases the solubility of fluoride more than calcium, it is common to observe high concentrations of fluoride in warmer waters.
On the other hand, sodium may exhibit a positive correlation with fluoride. High concentrations of Na+ will increase the solubility of fluorite in waters (Apambire et al. 1997).
Hydrogeological settings
The hydrogeological system is formed of three hydrologic units. The first one called the Upper Aquifer, is a free type aquifer formed by recent alluvial deposits. The second, called the Intermediate Aquifer is semiconfined, and is composed of alluvial terraces. The deepest aquifer, at 150 m depth, is constituted of clastic rocks of tertiary age, composed by eroded rocks that surrounding the sedimentary basin. These sediments have several consolidation degrees depending on their clay content. The most important recharge of the Upper Aquifer comes from the irrigation water by seepage, and in less volume, from superficial streams. The Intermediate Aquifer is recharged by the Upper Aquifer from the top and horizontal flow from the basin borders. The deepest aquifer is recharged through regional flow from the north and northeast flanks (Valenzuela and Coronado 1999). In the alluvial valley formed by the Zanjon and San Miguel rivers between Pesqueira town and “La Victoria” town, more than 200 wells are located. About 60% are shallow dug wells (locally called norias) up to 30 m depth. The others are deep drilled wells from 250 to 300 m depth and with discharge rates up to 100 l/s. Some of these deep wells present a high fluoride content and abnormally high temperature of water. They are used to supply potable water to the Hermosillo city. The deep wells with a high fluoride concentration are located between the left margin of San Miguel river and the right margin of Sonora river. Faulting associated with the river bed allows underground water circulation and heating, with subsequent enrichment in fluoride.
Several tectonic structures buried under sedimentary deposits were detected based on the geologic and geophysic records and geologic photo interpretation (Espinoza 1998). Some were detected in the right margin of` San Miguel river fault in the granite basement, at 112 m depth and then rises to the west. On the west side, an aquifer of low production and low transmissivity (1 × 10−4 m2/s) is present, while on the east, the aquifer has a higher (7 × 10−2 m2/s), but high fluoride concentration.
Low Cl− concentration could be an evidence of a fast flow. High concentration of Li+ and F− could be associated with flow fractured through rocks and it can be considered as evidence of the length and depth of flow circulation. Concentrations of Na+, Ca++ and SO =4 seem to be determined by the reactions between rocks and water (Carrillo-Rivera et al. 1996, 2002). Ascendant regional flows are due to faults and fractures within lithological units (Gallegos et al. 2004).
Methodology
Water samples were collected from 15 wells during 1995–2003, each one in two polyethylene bottles, one of them was acidified with 35% nitric acid for cation analysis, whereas the other was used for the determination of dissolved anions. PH, temperature and electric conductivity (EC) were measured in situ. All wells sampled were located between the left margin of San Miguel river and the right margin of Sonora river (Fig. 3). Chlorine was determined using ion-sensitive electrodes. SPADNS was the analytic method utilized to determine fluoride concentration in water. This method consists of reacting water fluoride content with a mixture zirconium (ZrOCl28H2O) acid solution and a stabilizer called SPADNS [sodium 2-(parasulfophenylazo)-1,8-dihydroxy-3,6-naphthalene disulfonate], the absorbance of this mixture is directly related to water fluorine content. The procedure consists of mixing prepared fluoride standards in the range of 0–1.40 mgF/dm3 with acid-zirconyl-SPADNS reagent; read absorbance. Plot a curve of the milligrams fluoride-absorbance relationship. Similarly, add reagent to water well samples; mix well and read absorbance. Absorbance readings from water samples are compared with standard fluoride-absorbance curves to obtain the fluoride concentration (APHA et al. 1992). Sulphate was determined by means of a spectrophotometer, bicarbonate by titration and metal concentrations by atomic absorption spectrophotometer.
Geochemical analysis was done to obtain the average concentration of the analyzed parameters in groundwater from 2 to 6 samples. Related variations of physicochemical parameters were obtained by plotting them against fluoride. Classification of water type was done by means of the Piper’s diagram.
Isoconcentration lines of majority ions in water samples were related to fluoride concentration variations in order to identify their spatial variations.
Results
The average concentration of the analyzed parameters is presented in Table 1. Groundwater fluoride concentration oscillates between 0.53 and 7.59 mg/dm3. The water pH varies between 7.24 and 9.15 bicarbonate concentration between 74.8 and 617 mg/dm3, and calcium concentration oscillated between 7 to 232 mg/dm3 (Table 1).
Groundwater chemical analysis from shallow dug wells and deep wells showed a salinity stratification since the salt content decreases as depth increases. This is associated with infiltration of greatly mineralized surface water from percolation of irrigation water. In those deep wells located in the area of volcanic rock, a temporary increase in the fluoride concentration was detected (Fig. 4). Water from Tronconal well increased their annual average concentration of fluoride more intensely from 3.55 to 7.59 mg/dm3, during 1994 to 2003. Likewise, Bloquera water well increased its fluoride concentration 0.4 mg/dm3 per year for the period of 1995 to 2003. Victoria-14 well showed a rise of fluoride concentration from 2.86 to 5.68 mg/dm3 in the same period.
Relationships among temperature, pH, calcium and bicarbonates in water samples with fluoride are displayed (Fig. 5). Temperature exhibits a positive correlation with fluoride. Anomalous high temperature and fluoride content in water was found in Tronconal y Bloquera wells with values: 38.67°C, 7.59 mg/dm3 and 38.8°C, 7.33 mg/dm3, respectively. Equally, a positive correlation between anomalous high fluoride concentration and pH is observed. Calcium concentration shows a negative correlation with fluoride (Fig. 5b). Higher values of calcium were found in R-1 with 232 mg/dm3 of calcium and only 0.99 mg/dm3 of fluoride. Inversely, Bloquera with 7.33 mg/dm3 of fluoride has 14.49 mg/dm3 of calcium. Relationship between the fluoride and the bicarbonates concentration is similar to those of calcium.
Water samples were classified according to Piper diagram (Fig. 6). Tronconal, Cruz-7, Bloquera, R. Silva-1 and R. Silva 2, Victoria-3, Victoria-11, Victoria-12, Victoria-14, Granjas-1 and San Pedro, were classified as sodium-sulphate facies. Victoria-15 and Cordova wells correspond to sodium bicarbonate facies and Granjas II and R1 correspond to calcium-bicarbonate facies. Finally, R2, R3, MS-6, MS-7, Victoria-1, Victoria-2 and R11 wells were classified as calcium-sodium bicarbonate facies.
Isoconcentration lines were plotted to show the spatial relationship (Fig. 7). Carbonates plus bicarbonates concentration increases in parallel along the San Miguel river main stream and to the west and south of “La Victoria”. Maximum concentration is found in San Miguel and Sonora rivers’ junction. This increment pattern is not modified in the fluoride anomalous high areas of concentration. The highest bicarbonates value is present in R-1 well (617.3 mg/dm3) and the lowest in Bloquera well (74.83 mg/dm3) (Fig. 7a).
The area of highest groundwater temperatures coincide with the highest anomalous fluoride area, “La Victoria”. The highest temperature is achieved by Bloquera well. R-1 well located in the reservoir has the lowest temperature (17°C). Groundwater temperature diminish when it brings closer to Miguel and Sonora rivers’ junction. (Fig. 7b). Groundwater fluoride concentration contrary to calcium and bicarbonates, increases toward the northeast. The highest concentration value is the Tronconal well (7.59 mg/dm3), while the lowest value is in 6 Mesa del Seri well (0.53 mg/dm3) located where the Sonora river discharges to the reservoir Abelardo L Rodríguez (Fig. 7a, c, e). Groundwater pH values present the same spatial behavior as fluoride concentration, ranging from alkaline waters to the northeast of the study area (Bloquera and Tronconal wells) to neutral waters with pH near 7.0 at the San Miguel and Sonora rivers’ junction (Fig. 7d, e). Calcium concentrations diminish toward northeast of the study area. “La Victoria” area coincides with the lowest calcium concentration and the highest fluoride groundwater content (Fig. 7c, e).
Discussion
Waters showing concentration of fluoride higher than 1.5 mg/dm3 are located over granitic rocks with medium electric resistivity (25 to 54 ohm-m) from 100 m and conglomerate surface sediments (Fig. 2). This coincides with the values pointed out by Saxena and Ahmed (2003) which relates high concentration of fluoride in water with rocks that contain fluorite, like granite, quartzite, pegmatite and granodiorite.
Water fluoride concentration increases as depth increases up to values higher than 1.5 mg/dm3. This value is the maximum accepted by the Official Mexican Norm (NOM-127-SSAI-1994) for evaluation of potable water.
High fluoride concentration in water has been related to temperature in volcanic rocks and regional flows (Gallegos et al. 2004). Positive correlation is presented between high groundwater temperature and fluoride content, with temperatures up to 38°C in wells with high fluoride concentration: (7 mg/dm3).
Water with higher fluoride content comes from the deep aquifer. Faults must allow contact with warmer bedrocks of granitic composition below 200 m. Thermal alteration of granite could have caused fluorite dissolution and increases in groundwater, coincident with result pointed out by Carrillo-Rivera et al. (2002). These regional and anomalous thermal flows ascend to approach the to resistive granitic rocks of Sierra Bachoco. The presence of high electric resistivity granitic rocks underlying conglomerate strata of Baucarit formation was identified by electromagnetic transitory soundings (section A-A´ Fig. 2) (GyE 2000).
Carbonate rocks have not been identified in the area. Paleozoic calcareous rocks crop out in the south of the area (Hermosillo City); they seem not to extend beneath “La Victoria” area. Wells located in the reservoir (R-1, R-2 and R-3) show high calcium and bicarbonate concentration and low fluoride. The absence of calcareous rocks in “La Victoria” area is corroborated by the decrease of calcium and bicarbonate concentration to the northeast direction, (Fig. 7a, c).
Temporary increase of groundwater fluoride concentration observed in Bloquera, Victoria 14, and Tronconal wells must be related with longer residence of time water the from deeper aquifer.
Positive correlation among pH, temperature and fluoride concentration appear in Fig. 5a, d, in agreement with previous studies (Apambire et al. 1997), particularly in the Bloquera and Tronconal wells.
Conclusions
The origin of groundwater fluoride is related to deep regional flows, heating processes and fluorite dissolutions in granitic rocks that appear to the west of the study area.
Anomalous high fluoride content in groundwater from the “La Victoria” area can be explained by upward flows through the crystalline rocks with high electrical resistivity that form the Sierra Bachoco basement and through normal faulting that bounds the study area aquifer to the west.
Paleozoic calcareous rocks crop out from the south of the study area, in Hermosillo City, to ‘La Victoria’ area. The absence of calcareous rocks in the study area is corroborated by the decrease of calcium and bicarbonate concentration in the northeast.
Wells located in the reservoir near the calcareous outcrop show high calcium and bicarbonate concentration and low fluoride due to calcium solubility coefficient and common ion effect.
Thus, temporary increase of groundwater fluoride concentration observed in several wells must be related to a longer residence time of water from a deeper aquifer.
References
APHA-AWWA - WPCF (1992) Métodos Normalizados para el análisis de agua potable y residual. Ediciones Días Santos S.A. Madrid. Cap. 3 y 4
Apambire WB, Boyle DR, Michel FA (1997) Geochemistry, genesis, and health implications of fluoriferous groundwater in the upper regions of Chana. Environ Geol 33(1):13–24
Carrillo-Rivera JJ, Cardona A, Moss D (1996) Importance of the vertical component of ground water flow: a hydrogeochemical approach in the valley of San Luis Potosi, México. J Hydrol 185:23–44
Carrillo-Rivera JJ, Cardona A, Edmundo WM (2002) Use of abstraction regime and knowledge of hidrogeological conditions to control high-fluoride concentration in abstracted groundwater: San Luis Potosi basin. Méx J Hydrol 261:24–47
Ellis AJ, Mahon WAJ (1977) Chemistry and geothermal systems. Academic Press, Belton
Espinoza VA (1998) Investigación y Análisis Piezométrico del acuífero a Victoria y Mesa del Seri. Ciudad de Hermosillo, Sonora. Handwritings
Gaciri SJ, Davies TC (1993) The occurrence and geochemistry of fluoride in some natural waters. J Hydrol Amst 143:395–412
Gallegos G, Medellin MP y Passo EMS (2004) Extracción de Agua Subterránea a Partir de Sistemas de Flujo Regional en San Luis Potosí: Fluoruro y Geología del Subsuelo. XXXIII Congreso IAH, 7° Congreso ALHSUD. Zacatecas México
GyE (2000) Geofísica y Exploraciones, S.A. Estudio de Actualización Geohidrologica del Acuífero del Valle del Río Zanjón, Municipio de Carbo, San Miguel Horcaditas y Hermosillo, Sonora
INEGI (1992) Instituto Nacional de Estadística e Informática. Estudio Hidrológico del Estado de Sonora. Government of the State of Sonora
Kraynov SR, Merkov AN, Petrova NG Baturinskaya IV, Zharikova VM (1969) Highly alkaline (pH 12) fluosilicate waters in the deeper zone of the Lovozero Massif. Geochem Int 6:635–640
Kundu N, Panigrahi MK, Tripathy S, Munshi S, Powell MA, Hart BR (2001) Geochemical appraisal of fluoride contamination of groundwater in the Nayagarh District of Orissa, India. Environ Geol. 41:451–460. DOI 10.1007/s002540100414
Lisa SB (1994) Factors influencing fluoride concentration in Norwegian lakes. Water Air Soil Pollut 77(1–2):151–167
Peña LJL, Zamora TE, Nuñez Othon A, Orantes CV (1998) Informe de la carta geológico-minera y geoquímica hermosillo clave H12-8, escala 1:250,000, Estado de Sonora, Consejo de Recursos Minerales, Subdirección de Infraestructura Geológico-Minera, Gerencia de Geología y geoquímica. Oficina Regional Hermosillo
Quintanar VAI (1992) Niveles de flúor en el agua potable consumo humano y su relación con la salud dental del estado de Sonora, México. Masters Degree Thesis. Centro de Investigación en Alimentación y Desarrollo A. C. Hermosillo, Son
Ramamohana RNV, Rao N, Prakash RSK, Schuiling RD (1993) Fluorine distribution in water Nalgonda District, Andhra Pradesh. Environ Geol 21:84–89
Rodríguez-Castañeda JL (1981) Notas sobre geología del área de Hermosillo, Sonora. Instituto de Geología. Revista vol. 5 num, 1 UNAM
Saxena VK, Ahmed S (2001) Dissolution of fluoride in groundwater: a water rock interaction study. Environmental Geology. National Geophysical Research Institute. DOI 10.1007/s002540100290
Saxena VK, Ahmed S (2003) Inferring the chemical parameters for the Dissolution of fluoride in groundwater. Environ Geol 43:731–736. DOI 10.1007/s00254-002-0672-2
Srinivasa RN (1997) The occurrence behaviors of fluoride in the groundwater of the Lower Vamsadhara River Basin India. Hidrol Sci-J Sci Hidrol 42(6):877–891
Sujatha D (2003) Fluoride levels in the groundwater of the south-eastern part of Ranga Reddy district, Andhra Pradesh, India. Environ Geol 44:587–591. DOI 10.1007/s00254-003-0795-0
Trilles AR (1970) El Problema Sanitario de las aguas destinadas a la bebida humana con contenido elevado de arsénico, vanadio y flúor. Facultad de Ingeniería. University of Buenos Aires, Argentina
Valenzuela SH, Coronado CCA (1999) Estudio hidrogeológico comprendido entre las presas Abelardo L. Rodríguez y Rodolfo Félix Valdez. Tesis profesional. Universidad de Sonora
Acknowledgments
The authors would like to thank Ing. Rafael García Flores and Marco Antonio Alvarado from the Water of Hermosillo for allowing access to information files and well sites in the study area to the personnel of the Chemical Laboratory of the University of Sonora, and Centro de Estudios Superiores del Estado de Sonora. This investigation was partially supported by SEP-PROMEP program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valenzuela-Vásquez, L., Ramírez-Hernández, J., Reyes-López, J. et al. The origin of fluoride in groundwater supply to Hermosillo City, Sonora, México. Environ Geol 51, 17–27 (2006). https://doi.org/10.1007/s00254-006-0300-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-006-0300-7