Abstract
The present study focuses on the hydrogeochemical composition of groundwater in Chhatarpur area with special focus on nitrate and fluoride contamination, considering the fact that groundwater is the only major source of drinking water here. Carbonate and silicate mineral weathering followed by ground water–surface water interactions, ion exchange and anthropogenic activities are mainly responsible for high concentrations of cations and anions in the groundwater in the region. The average concentration of nitrate and fluoride found in 27 samples is 1.08 and 61.4 mg/L, respectively. Nitrate enrichment mainly occurs in areas occupied with intense fertilizer practice in agricultural fields. Since the area is not dominated by industrialization, the possibility of anthropogenic input of fluoride is almost negligible, thus the enrichment of fluoride in groundwater is only possible due to rock–water interaction. The highly alkaline conditions, which favor the fluorite dissolution, are the main process responsible for high concentration of fluoride.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The groundwater resources are under huge risk due to speedy population growth, industrialization, intensive agriculture, urbanization and changing land use pattern, which in turn is endangering the water resources in terms of quality and quantity. The variation in the groundwater quality in an area is a function of physical and chemical parameters. These parameters are greatly influenced by geological formations and anthropogenic activities (Subramani et al. 2005). Groundwater chemical signature mainly depends on the interaction pattern with aquifer minerals or by internal mixing among different groundwater along flow paths in the subsurface (Domenico 1972; Datta et al. 1996; Belkhiri et al. 2011). The groundwater quality is also strongly affected by change in land use/land cover pattern (Basnyat et al. 1999; Roth et al. 1996; Osborne and Wiley 1988). Therefore, assessment of groundwater quality is needed to ensure its safer use (Vijith and Satheesh 2007). The pesticides and fertilizers used in agriculture to increase the production causes degradation of water quality (Carpenter et al. 1998; Matson et al. 1997; Kolpin 1997; Griffith 2001).
Groundwater contamination is a global problem that has severe impacts on human health as well as on ecosystem and economy (Addiscott et al. 1991). This problem is mainly because of the ineffective environmental conservation policies as well as areas with intensive industrialization, use of fertilizers, mining, etc. To minimize the impacts of groundwater pollution, regular monitoring of groundwater quality is needed. Dissolved nitrogen in the form of nitrate (NO3) is the most common contaminant of groundwater. The source of nitrate in groundwater originates from nonpoint sources such as leaching of chemical fertilizers, animal manure and sewage discharges. Nitrate enrichment (more than 45 mg/L) in drinking water is an environmental health concern because it increases the risks of methemoglobinemia in infants. It is found that 21 out of 29 states of India have localized nitrate pollution in groundwater (CGWB 2010).
High concentration of fluoride (more than 1.5 mg/L) in groundwater or surface water is also a case of great concern because it causes fluorosis (Alagumuthu and Rajan 2010). The main sources of fluoride in groundwater are rocks containing fluoride minerals such as fluorspar, cryolite, muscovite, biotite, fluorite and fluorapatite are the most common. (Handa 1975; Wenzel and Blum 1992; Msonda et al. 2007). Mica is also one of the potential sources of fluoride where it is found as fluorapatite (Singh et al. 2011). The content of fluoride in groundwater can be credited to the weathering/leaching of fluoride containing minerals (Singh et al. 2011) as well as pH, temperature and concentration of calcium and bicarbonate ions in water (Alagumuthu and Rajan 2010), where human interference like mining and industrial activities are almost negligible. Thus, the areas where such rock types are dominant probability of fluoride concentration can be questionable (Sarma and Rao 1997; Nordstrom et al. 1989; Banks et al. 1995; Gizaw 1996; Frengstad et al. 2001). In the case of India, there are several locations in the States of Andhra Pradesh, Gujarat, Karnataka, Madhya Pradesh, Rajasthan, Chhattisgarh, Haryana, Orissa, Punjab, Haryana, Uttar Pradesh West Bengal, Bihar, Delhi, Jharkhand, Maharashtra, and Assam where the fluoride in groundwater exceeds 1.5 mg/l (CGWB 2010).
The main objective in the study is to assess groundwater quality of Chhatarpur District of Madhya Pradesh, India, to determine the factors affecting groundwater quality as well as identification of the main geochemical processes controlling the groundwater quality.
Study area
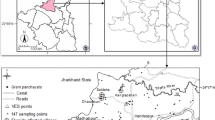
The study area is located on the central portion of Bundelkhand plateau in Madhya Pradesh state of India. It is bordered by Uttar Pradesh state and lies between 24°06′–25°20′ latitudes and 79°59′–80°26′ longitudes (Fig. 1). The climate of study area is semi-arid type, although this is highly variable depending on the monsoon. The area has mean annual maximum and minimum temperatures of 32.7 and 18.1 °C, respectively. The annual rainfall in the area is about 1,060 mm, with the highest precipitation (about 90 % of the total amount) occurring between June and September.
Geology of the area
The geology plays an important role in the groundwater quality determination as different geological units have different weathering rates and process (Duan et al. 2002). Figure 2 shows the geology of the study area. Geologically, the area is covered by Bundelkhand granite (older than 2.6 billion years), Bijawar group (2.6–2.4 billion years), Vindhyan supergroup (1.4–0.9 billions years), and alluvium (Auden 1933). Bundelkhand granites are frequently associated with pegmatites and quartz reefs (Ahmad 1984). Most of the part of the study area is covered by Bundelkhand granite in northern and north central part. Bundelkhand granites are overlain by the Bijawar group of rocks (Bhattacharya and Bhattacharya 1976). The alluvium is present in north eastern part of the area. The Bijawar group of the area consists of terrigenous sequence of basal carbonates and shales with green schists or pillow basalts, chloritic shales, ferruginous quartzites and banded iron formations. The Vindhyan basin is a very large intra-cratonic sedimentary basin, which contains a sequence of sandstone–shale–limestone (Rao et al. 2005). Vindhyan super group have been broadly divided into four major groups, namely the Semri (lower), Kaimur, Rewa and Bhander (upper) based on lithological similarities and into Lower and Upper Vindhyans on the basis of major tectonic (major unconformity) evidences (Mallet 1869). The upper Kaimur group rocks of the Vindhyan supergroup are represented by sandstones all over the Vindhyan basin. The sandstones are red to greyish-white, medium to fine grained, compact and highly jointed. Lower Vindhyan formations are represented by Tirohan limestone, Baghain sandstone; diamond-bearing conglomerate of Kaimur group is present in Panna District (Bhattacharya and Bhattacharya 1976; Avtar et al. 2011).
Land use/land cover of the area
The land use/land cover map was generated using unsupervised classification of Landsat TM data of February, 2009. The study of land use/land cover is important to analyze the spatial–temporal characteristics of land use/land cover change and the spatio-temporal variability of groundwater quality (Jinfeng and Quanjun 2009). The area was classified into 10 classes, viz. dense forests, current fallow land/agriculture area, land without scrap, degraded forest, land with scrub, fallow land, bare exposed rock, water body, built-up area and water logged area. Most of the area is covered by forests fallowed by agricultural area (Fig. 3).
Materials and methodology
A total of 27 water samples were collected in April 2008 in polypropylene bottles (Tarsons) from shallow hand pumps, dug-wells and tube wells. Table 4 in the appendix shows the location of the sampling points. At the time of sampling, bottles were thoroughly rinsed 2–3 times with sample water to be taken. The non-conservative and physical parameters (pH, temperature, electrical conductivity) were measured in situ with their respective probes. Bicarbonate (HCO3 −) was analyzed using titration method with sulfuric acid. Other anions (Cl−, SO4 2−, NO3 − and F−) have been analyzed by ion chromatography and major cations (Na+, Ca2+, Mg2+ and K+) were analyzed by atomic absorption spectrometer (AAS). In order to discern regional trends and to identify chemical processes, it is necessary to make distinction between water types and the classification of samples into different groups (Stuyfzand 1999). Presentation of geochemical data in the form of graphical charts such as the Piper diagram is helpful to recognize and differentiate various water facies (Piper 1944). In this study the Piper diagram is drawn using the AqQA software (RockWare).
In order to understand the origin of groundwater mineralization and the processes responsible for chloride, fluoride and nitrate enrichment in groundwater in the study area, saturation index (SI) was calculated using PHREEQC code (Parkhurst and Appelo 1999). SIs are used to evaluate the degree of equilibrium between water and respective mineral. Changes in saturation state are useful to distinguish different stages of hydrochemical evolution and help to identify which geochemical reactions are important in controlling water chemistry (Coetsiers and Walraevens 2006; Langmuir 1997). The SI of a mineral can be obtained using following equation (Garrels and Christ 1965).
where SI is the saturation index, K IAP is the ion activity product of particular solid phase and K SP is the solubility product of the phase. Based on the value of the SI, the saturation states are recognized as saturation (equilibrium; SI = 0), unsaturation (dissolution; SI < 0) and oversaturation (precipitation; SI > 0). In other words, SI < 0 which specifies case of undersaturation could reflect the character of water from a formation with insufficient amount of the mineral for solution or short residence time and SI > 0 specifies that the groundwater is oversaturated with respect to the particular mineral and therefore incapable of dissolving more of the mineral. Such an index value reflects groundwater discharging from an aquifer containing ample amount of the mineral with sufficient resident time to reach equilibrium.
The matrix of hydrogeochemical data obtained was subjected to multivariate analytical techniques. These techniques help to simplify and organize large data sets in order to make useful generalizations. The data set was subjected to correlation analysis using Spearman’s rank coefficient which is based on the ranking of the data and not their absolute value [Statistical Package for Social Sciences (SPSS) software package (Version 20)] (Kumar et al. 2010).
Results and discussion
Hydrochemical characteristics
A statistical summary of the analytical results (average, minimum, maximum, standard deviation and range) for each water-quality characteristic analyzed is shown in Table 1. Slightly alkaline nature of water with high EC are the combined effect of the high concentration of dissolved solids in the groundwater which can be attributed to high reactivity of the soil, local variation in soil type, multiple aquifer system and agricultural activities in the area. Among anions the trend was HCO3 − > Cl− > NO3 − > SO4 2− > F−, while for cations the trend was Ca2+ > Na+ > Mg2+ > K+. The average meq/L ratio trend of Ca2++Mg2+/Na++K+ is 3.1 indicates the dominance of carbonate rock weathering in the Chhatarpur area. Relation between Tz+ (here Tz+ defined as the sum of all major cations, i.e. Ca2+, Na+, Mg2+ and K+) versus (Ca2+ + Mg2+) and (Na+ + K+) is shown in Fig. 4. The (Ca2++Mg2+) versus Tz+ plot for the groundwater yields a linear trend (with R 2 = 0.96 and calculated p value = 0.000014) indicating that Ca2+ + Mg2+ accounts for most of the cations. In contrast, the trend between Na+ + K+ and Tz+ is poor (with R 2 = 0.27 and calculated p value = 0.104). These results infer that the contribution of cations via alumino-silicate weathering is low in comparison to carbonate weathering.
Contaminations for chemical species
The statistical analysis for the comparison of the values of different chemical species in the groundwater samples from the standard given by WHO and ISO for desirable limit and permissible limit has been given in Table 2. The concentrations of cations, such as Na+, K+, Ca2+ and Mg2+ all were within the maximum permissible limits for drinking, except for a few samples in the case of magnesium. However, for some chemical species there is a great difference between the percentage of samples which were found beyond permissible limit (PL) and the percentage of samples which was found beyond desirable limits (DL) (Table 2). For major anions like HCO3 − and Cl−, concentrations lie within permissible limits except sporadic enrichment of Cl−. Generally, high HCO3 − concentration is indicative of carbonate weathering and decomposition of organic matter; however, higher concentrations of Cl− can be attributed to the input from sewage effluents in the village areas. For minor anions likes NO3 − and F− concentration are of main concern from the health perspective as 48 and 20 % of the water samples had high concentrations of nitrate and fluoride, respectively, compared to WHO permissible limit. With an average concentration of 61.4 mg/L for NO3 −, groundwater samples clearly show an indication for the influence of fertilizer use in surrounding agricultural areas as well as microbial mineralization in the groundwater. Usually the ammonia within nitrogen fertilizers is transformed into nitrate by the process of nitrification, and the possible reaction responsible here is shown:
Furthermore, the higher concentration of nitrate may also be a result of the presence of E. coli, Staphylococcus aureus, Proteus vulgaris, Salmonella typhi, and Pseudomonas aeruginosa in the fecal matter, as reported by Saha and Kumar (2006). This seems reasonable as there is a severe lack of a proper sanitation system in the district. Further, animal waste sources are also a significant contributor of nitrate to groundwater, especially within mixed land use. Some part of the study area is occupied by industrial and urban land use; therefore, some nitrate leaching from landfill sites and industrial effluents cannot be neglected (Kundu and Mandal 2009; Kumar et al. 2010).
Fluoride is one of the main trace elements in groundwater and generally occurs as a natural constituent. Its pollution and health aspect was also reported earlier by Chaurasia et al. (2007). Weathering of bedrock containing fluoride minerals (like biotite, muscovite) is generally responsible for high concentration of fluoride in groundwater. Generally in acidic medium, i.e. groundwater with low pH, fluoride remains adsorbed in clay due to its low solubility. However, in alkaline medium, i.e. groundwater with high pH, the hydroxyl (OH−) group replaces the exchangeable fluoride from minerals like biotite/muscovite and results in its enrichment. The possible reaction responsible for muscovite is shown below (Singh et al. 2011):
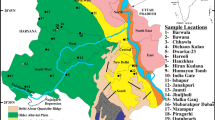
The distribution pattern of NO3 − and F− is shown in Figs. 5 and 6, respectively. When we compare these distribution patterns from the land use–land cover pattern of the study area in Fig. 3, we found that nitrate enrichment corresponds well to the area dominated by agricultural practices; however, fluoride enrichment mainly corresponds to the land without scrub and with very sparse amount of forest/agriculture.
The major ions of water analyses are depicted in the Piper Trilinear equivalence diagram (Fig. 7). Based on the geochemical facies and their percentage, water samples can be classified into five groups, viz. (1) Ca-HCO3 type (40 %), (2) Mg-HCO3 type (37 %), (3) Ca–Cl type (15 %), (4) Mg–Cl type (4 %), and (5) Na-HCO3 type (4 %). Focusing on the Piper diagram of cations, a clear migration tendency from bicarbonate pole to chloride pole is evident, which may indicate water quality shift from pristine environment to the system with much anthropogenic activities.
A correlation matrix of the physiochemical parameters for the water samples is shown in Table 3. The correlation matrix (with statistical significance level at the 0.05) showed good to moderate positive correlation of pH with EC, Ca2+ and HCO3 −. A good to moderate correlation was observed between the following pairs: Ca2+–Mg2+, Ca2+–HCO3 −, Ca2+–SO4 2−, Ca2+–F−, Mg2+–HCO −,3 Na+–K+, Na+–NO3 −, K+–NO3 −, Cl−–SO4 2−, Cl−–NO3 − and SO4 2−–NO3 −. Also there is an interesting negative correlation found between HCO3 −–NO3 −. The pairwise relationships between physiochemical parameters show that water quality is mainly controlled by carbonate weathering. There is presence of dolomite as well, which acts as a common source for both calcium and magnesium. The presence of minerals like fluorspar, apatite and fluorapatite are also indicated by a good correlation between calcium and fluoride. Anthropogenic sources, especially excess use of fertilizers, may control the geochemistry at point scales and can be shown by high correlation between chloride, sulphate and nitrate. Negative correlation between bicarbonate and nitrate is a strong indicator of microbial denitrification process (Chkirbene et al. 2009).
Origin of groundwater mineralization in Chhatarpur area
Speciation modeling was performed using program PHREEQC (Parkhurst and Appelo 1999) to check the possibility of solubility control for fluorite and other species. Most samples are undersaturated with respect to fluorite, gypsum, dolomite, calcite, aragonite and anhydrite except at some of the locations. On the other hand, groundwater is strongly undersaturated with respect to halite.
The positive values of the calculated SI with respect to calcite, dolomite at few sampling points suggest their oversaturation in respect to those minerals which mainly has a geological origin derived from the abundant carbonate weathering points in the plain (Fig. 8). Thus, calcite as well as dolomite might act as the dual origin of Ca2+ and HCO3 − in these samples. This explanation for calcite it well described by Appelo and Postma (2005), as shown below through following equation:
Furthermore, gypsum and anhydrite show same saturation pattern to that of calcite and dolomite suggesting that calcium is also being supplied through gypsum and anhydrite. Saturation of fluorite minerals at some points suggests contact of alkaline water (supported by high pH value in the study area) with fluoride-bearing minerals. Basically, fluorite dissolution might be catalyzed by bicarbonates produced through hydrolysis of alumino-silicate minerals which is well supported by localized granitic or sandstone dominant aquifers in this study area. The possible reaction responsible here is shown as following equations:
So using saturation index, it is concluded that weathering, dissolution and hydrolysis are the main processes determining water quality at spatial scale.
Conclusion
The groundwater quality of the study area has a primary problem of nitrate and fluoride contamination. The groundwater chemistry is mainly controlled by natural conditions of rock–water interaction, i.e., geogenic processes along with the intense agricultural activities at some point. The alkaline nature of groundwater triggers replacement of the exchangeable fluoride from minerals like biotite/muscovite and results in its enrichment. Another possible mechanism is that granitic rocks, which contain abundant fluoride-bearing minerals, upon weathering release it to the dissolved phase in groundwater. The groundwater mineralization in the study area is not a homogenous process, but it is related to different sources and dynamics with space variation. The results of this study make clear that deep groundwater monitoring with numerical simulation for future water quality is important for understanding contaminant migration.
References
Addiscott TM, Whitmore AP, Powlson DS (1991) Farming, fertilizers and the nitrate problem. CAB International, Wallingford, UK, p 170
Ahmad SM (1984) Hydrogeological investigations for augmenting water supply in drought affected areas of Banda District, Uttar Pradesh. Central Ground Water Board Report, Lucknow
Alagumuthu G, Rajan M (2010) Chemometric studies of water quality parameters of Sankarankovil block of Tirunelveli, Tamilnadu. J Environ Biol 31(5):581–586
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. Balkema, Rotterdam, p 649
Auden JB (1933) Vindhayans of Son valley. Memoir Geological Survey of India, Vol. XII, Part II, Lucknow
Avtar R, Singh CK, Singh G, Verma RL, Mukherjee S, Sawada H (2011) Landslide susceptibility zonation study using remote sensing and GIS technology in the Ken–Betwa River link area, India. Bull Eng Geol Environ 70(4):595–606
Banks D, Reimann C, Røyset O, Skarphagen H, Sæther OM (1995) Natural concentrations of major and trace elements in some Norwegian bedrock groundwaters. Appl Geochem 10:1–16
Basnyat P, Teeter L, Flynn K, Lockaby B (1999) Relationships between landscape characteristics and nonpoint source pollution inputs to coastal estuaries. Environ Manag 23(4):539–549
Belkhiri L, Mouni L, Tiri A (2011) Water-rock interaction and geochemistry of groundwater from the Ain Azel aquifer, Algeria. Environ Geochem Health. doi:10.1007/s10653-011-9376-4
Bhattacharya DS, Bhattacharyya TK (1976) Geological and geophysical investigations of a basaltic layer in Archaean terrain of eastern India. Bull Geol Soc Am 81:3073–3078
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8(3):559–568
CGWB (Central Ground Water Board), Ministry of water resources, Government of India (2010) Ground water quality in shallow aquifers of India
Chaurasia OP, Kumari C, Ankita S (2007) Genotoxic effect of ground water salts rich in fluoride. Cytologia 72(2):141–144
Chkirbene A, Tsujimura M, Charef A, Tanaka T (2009) Hydro-geochemical evolution of groundwater in an alluvial aquifer: case of Kurokawa aquifer, Tochigi Prefecture, Japan. Desalination 246:485–495
Coetsiers M, Walraevens K (2006) Chemical characterization of the Neogene Aquifer, Belgium. Hydrogeol J 14:1556–1568
Datta PS, Bhattacharya SK, Tyagi SK (1996) 18O studies on recharge of phreatic aquifers and groundwater flow-paths of mixing in the Delhi area. J Hydrol 176:25–36
Domenico PA (1972) Concepts and models in groundwater hydrology. McGraw-Hill, New York
Duan L, Hao J, Xie S, Zhou Z, Ye X (2002) Determining weathering rates of soils in China. Geoderma 110(3–4):205–225
Frengstad B, Banks D, Siewers U (2001) The chemistry of Norwegian groundwaters: IV. The dependence of element concentrations in crystalline bedrock groundwaters. Sci Total Environ 277:101–117
Garrels RM, Christ CL (1965) Solutions, minerals and equilibria. Harper and Row, New York, p 458
Gizaw B (1996) The origin of high bicarbonate and fluoride concentration in waters of the main Ethiopian rift valley, East African rift system. J Afr Earth Sci 22:391–402
Griffith AJ (2001) Geographic techniques and recent applications of remote sensing to landscape-water quality studies. Water Air Soil Pollut 138:181–197
Handa BK (1975) Geochemistry and genesis of fluoride containing groundwater in India. Ground Water 13:275–281
Jinfeng Y, Quanjun G (2009) Impact of land use change on groundwater quality in Oasis. In: IEEE, Information Engineering and Computer Science-2009, pp 1–4
Kolpin D (1997) Agricultural chemicals in groundwater of the midwestern United States: relations to land use. J Environ Qual 26:1025–1037
Kumar P, Kumar M, Ramanathan AL, Tsujimura M (2010) Tracing the factors responsible for arsenic enrichment in groundwater of the middle Gangetic Plain, India: a source identification perspective. Environ Geochem Health 32:129–146
Kundu MC, Mandal BP (2009) Nitrate enrichment in groundwater from long-term intensive agriculture: its mechanistic pathways and prediction through modeling. Environ Sci Technol 1, 43, 15:5837–5843
Langmuir D (1997) Aqueous environmental geochemistry. Prentice Hall, Engelwood Cliffs
Mallet FR (1869) Vindhayans of central provinces, Memoir Geological Survey of India, vol II, part I
Matson P, Parton J, Power A, Swift M (1997) Agricultural intensification and ecosystem properties. Science 277:504–509
Msonda KWM, Masamba WRL, Fabiano E (2007) A study of fluoride groundwater occurrence in Nathenje, Lilongwe, Malawi. Phys Chem Earth 32:1178–1184
Nordstrom DK, Ball JW, Donahoe RJ, Whittemore D (1989) Groundwater chemistry and water–rock interaction at Stripa. Geochem Cosmochim Acta 53:1727–1740
Osborne LL, Wiley MJ (1988) Empirical relationship between land use-cover and stream water quality an agricultural watershed. J Environ Manag 26:9–27
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC: a computer program for speciation, reaction-path, 1-D transport, and inverse geochemical calculations, US Geological Survey Water-Resources Investigations Report, 99-4259
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analysis. Am Geophys Union Trans 25:914–923
Rao GVSP, Rao JM, Prasad NPR, Venkateswarlu M, Rao BS, Prakash SR (2005) Tectonics and correlation of upper Kaimur group sandstones by their paleomagnetic study. J Indian Geophys Union 9(2):83–95
Roth NE, Allan JD, Ericson DE (1996) Landscape influences on stream biotic integrity assessed at multiple spatial scales. Landsc Ecol 11(3):141–156
Saha LC, Kumar S (2006) Comparative quality of potable waters at Bhagalpur, India. Acta Hydrochim Hydrobiol 18(4):459–467
Sarma DRR, Rao SLN (1997) Fluoride concentrations in ground waters of Visakhapatnam, India. Bull Environ Contam Toxicol 58:241–247
Singh CK, Kumari R, Singh RP, Shashtri S, Kamal V, Mukherjee S (2011) Geochemical modeling of high fluoride concentration in groundwater of Pokhran Area of Rajasthan, India. Bull Environ Contam Toxicol 86:152–158
Stuyfzand PJ (1999) Patterns in groundwater chemistry resulting from groundwater flow. Hydrogeol J 7(1):15–27
Subramani T, Elango L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environ Geol 47:1099–1110
Vijith H, Satheesh R (2007) Geographical Information System based assessment of spatiotemporal characteristics of groundwater quality of upland sub-watersheds of Meenachil River, parts of Western Ghats, Kottayam District, Kerala, India. Environ Geol 53:1–9
Wenzel W, Blum WEH (1992) Fluoride speciation and mobility in fluoride contaminated soil and minerals. Soil Sci 153:357–364
WHO (1993) Guidelines for drinking water quality: recommendation, vols 1–2. World Health Organization, Geneva
Acknowledgments
R. Avtar is highly thankful to the Council for Scientific and Industrial Research (CSIR), New Delhi, for the financial support, School of Environmental Sciences, Jawaharlal Nehru University, New Delhi, for laboratory support. R. Avtar and P. Kumar are also thankful to the Japanese Government (Monbukagakusho-MEXT) Scholarship to pursue doctoral research in Japan.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Avtar, R., Kumar, P., Surjan, A. et al. Geochemical processes regulating groundwater chemistry with special reference to nitrate and fluoride enrichment in Chhatarpur area, Madhya Pradesh, India. Environ Earth Sci 70, 1699–1708 (2013). https://doi.org/10.1007/s12665-013-2257-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2257-7