Abstract
Fluoride concentration in groundwater sources used as major drinking water source in rural area of block Nawa (Nagaur District), Rajasthan was examined and the toxic effects by intake of excess fluoride on rural habitants were studied. In block 13, habitations (30%) were found to have fluoride concentration more than 1.5 mg/l (viz. maximum desirable limit of Indian drinking water standards IS 10500, 1999). In five habitations (11%), fluoride concentration in groundwater is at toxic level (viz. above 3.0 mg/l). The maximum fluoride concentration in the block is 5.91 mg/l from Sirsi village. As per the desirable and maximum permissible limit for fluoride in drinking water, determined by World Health Organization or by Bureau of Indian Standards, the groundwater of about 13 habitations of the studied sites is unfit for drinking purposes. Due to the higher fluoride level in drinking water, several cases of dental and skeletal fluorosis have appeared at alarming rate in this region. There is an instant need to take ameliorative steps in this region to prevent the population from fluorosis. Groundwater sources of block Nawa can be used for drinking after an effective treatment in absence of other safe source. The evaluation of various defluoridation methods on the basis of social and economical structure of India reveals that the clay pot chip, activated alumina adsorption, and Nalgonda techniques are the most promising.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The groundwater contains variety of substances in different concentration either in suspension or in solution (Day 1987; Hussain 2001). Fluoride is an essential microelement for human health. Smaller quantities (<1.0 mg/l) in drinking water are usually considered good to have a beneficial effect on the rate of occurrence of dental carries, particularly among children (WHO 1997; Hussain et al. 2004, 2010). On the other hand, due to its strong electronegativity, fluoride is attracted by positively charged calcium ions in teeth and bones. Excessive intake results in pathological changes in teeth and bones, such as mottling of teeth or dental fluorosis followed by skeletal fluorosis (Shusheela 1993; Hussain et al. 2002; USEPA 1995; USPHS 1991; WHO 1984).
Fluorosis is a considerable health problem worldwide, which is afflicting millions of people in many areas of the world, for example East Africa (Nanyaro et al. 1984; Gaciri and Davies 1993; Gizaw 1996), Turkey (Oruc 2003), India (Hussain 2001, 2005; Subba and John 2003; Gupta et al. 2005), southeastern Korea (Kim and Jeong 2005), and northern China (Wang and Reardon 2001).
The permissible limit of fluoride in drinking water is 1.0 mg/l as per WHO (1997). Per day fluoride intake is directly related with the daily water intake. Therefore, in year 1962, USPHS set a range of allowable concentrations for fluoride in drinking water according to the climatic conditions (especially temperature).
Rajasthan is the largest state in India having 342,239 km2 area with relatively low population density, i.e., 165 persons per square kilometer. According to physiographic divisions, the north and western part of the state is under the Great Plain of north India, while south and middle as well as eastern part is classified under the Peninsular Plateau. In the state, Thar Desert occupies about 61% of the total area. Groundwater is a major source for drinking and domestic and irrigation purpose (Census 2001). In groundwater, the natural concentration of fluoride depends on the geological, chemical, and physical characteristics of the aquifer, porosity, and acidity of the soil and rocks, temperature, and the depth of source. It has been observed that low calcium and high bicarbonate alkalinity favor high fluoride content in groundwater. Figure 1 depicts the general mechanism of formation of fluoride.
Many workers (Hussain et al. 2000, 2003, 2005; Gupta et al. 1983; Choubisa et al. 1995) have reported the level of fluoride concentration in different districts of Rajasthan; however, in Nagaur, a centrally located district, no studies have been undertaken yet in the study area with regard to fluoride and fluorosis problem. Therefore, the objective of this study was to investigate the quality of drinking water (underground water) with special reference to the concentration of fluoride in some rural habitations of Central Rajasthan, India.
Material and methods
Groundwater samples of 44 habitations located in Nawa block of Nagaur District were collected in pre-cleaned polythene bottles following standard sampling techniques. The fluoride concentration in water was determined electrochemically, using fluoride ion-selective electrode (APHA 1991). This method is applicable to the measurement of fluoride in drinking water in the concentration range of 0.01–1,000 mg/l. The electrode used was an Orion fluoride electrode, coupled to an Orion electrometer. Standards fluoride solutions (0.1–10 mg/l) were prepared from a stock solution (100 mg/l) of sodium fluoride. As per experimental requirement, 1 ml of total ionic strength adjusting buffer grade III (TISAB III) was added in 10 ml of sample. The ion meter was calibrated for a slop of −59.2 ± 2. The composition of TISAB solution was 385.4 g ammonium acetate, 17.3 g of cyclohexylene diamine tetraacetic acid, and 234 ml of concentrate hydrochloric acid per liter. All the experiments were carried out in triplicate and the results were found reproducible with ±2% error. A general observation was also conducted with respect to the incidence of dental and skeleton fluorosis.
Study area
Nagaur District is located at latitude 26°25′ to 27°40′ N and longitude 73°18′ to 75°15′ E. Its average elevation is about 300 m, ranging below 250 m in the south and 640 m in the north. There are 1,396 habitations in the district. The main lithological units include gneisses, schists, granites, quartzites, phyllites, and limestones belonging to the Bhilwara and Delhi Supergroup of rocks of Archaean and Proterozoic ages, respectively. Although groundwater occurs mainly under water table condition in all the formations, the quaternary alluvium forms good aquifers in Nagaur District. In hard rock terrain, the occurrence and movement of groundwater are controlled by secondary porosity such as fractures, fissures, joints, foliation, etc.
Results and discussion
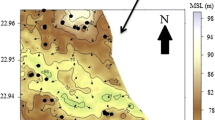
Fluoride concentration in groundwater of 44 habitations of Nawa block was examined. The village census code wise concentration of fluoride is shown in Fig. 2a–e. All the habitations were categorized according to following concentration range (Table 1):
-
Category I
Fluoride concentration below 1.0 mg/l
-
Category II
Fluoride concentration between 1.0 and 1.5 mg/l
-
Category III
Fluoride concentration between 1.5 and 3.0 mg/l
-
Category IV
Fluoride concentration between 3.0 and 5.0 mg/l, and
-
Category V
Fluoride concentration above 5.0 mg/l.
The distribution of fluoride in the groundwater of Nawa block is shown in Fig. 2a–e. Fluoride concentration ranges from 0.3 to 5.9 mg/l. The minimum concentration was recorded for Rajliya village while maximum concentration was recorded from Sirsi village (5.9 mg/l). Data on the concentration of fluoride in different samples of Nawa block indicate that maximum habitations have fluoride concentration between 0.4 and 1.5 mg/l (Fig. 3). The present investigation reveals that 19 habitations (43%) fall in category I (Fig. 2a) in which fluoride concentration is below 1.0 mg/l, a maximum desirable limit of standards for drinking water recommended by Bureau of Indian Standard (BIS) in IS 10500 (1991). There is no possibility of fluorosis in these habitations because this concentration of fluoride is beneficial for calcification of dental enamel especially for children below 10 years of age. Once fluoride is incorporated into teeth, it reduces the solubility of the enamel under acidic conditions and thereby provides protection against dental carries.
Out of 44 habitations of Nawa block, 13 habitations (30%) have fluoride concentration between 1.0 and 1.5 mg/l and fall in category II (Fig. 2b). The maximum permissible limit of fluoride in standard for drinking water is 1.5 mg/l (IS 10500; BIS 1991). In 32% population of these habitations, fluoride intake through drinking water is more than 4 mg/day in an individual. Therefore, an incidence of first and second degree dental fluorosis is possible in local residents of these habitations (Fig. 4).
About 17% of population of 7 habitations (16%) consumes water with fluoride concentration between 1.5 and 3.0 mg/l, which is above the maximum permissible limit as recommended by BIS (Fig. 2c). Therefore, dental fluorosis is common in these habitations. At this concentration, teeth lose their shiny appearance and chalky black, gray, or white patches develop known as mottled enamel (Dean 1942). In some cases, the pre-stage of skeletal fluorosis may occur after 45 years of age (Ziauddin 1974). In four habitations (9%), fluoride concentration in groundwater is above 3.0 mg/l and below 5.0 mg/l, and this fall in category IV (Fig. 2d). The intake of fluoride per day by an adult in these habitations is very high. About 6% population of these habitations may have all degree of dental fluorosis (mild, moderately, moderately severe, and severe fluorosis) including skeletal fluorosis after 30 years of age. However, the probability of second stage skeletal fluorosis age may be common after the age of 45 (Olsson 1979). In the entire survey, Sirsi was the only village that falls in category V (Fig. 2e), which contributes 1% population of Nawa block (Fig. 4). In this village, fluoride concentration is above 5.0 mg/l, which may result in all types of fluorosis among inhabitants. In the second clinical stage, the affected persons may have pain in bones, which causes further calcification in ligaments. It has been reported that such persons may suffer from stiffness in joints. At this concentration, the vertebrae partially fuse together crippling the patient which is known as “crippling skeletal fluorosis”. A detailed survey of health hazards particularly fluorosis-induced symptoms and empirical data on affected population are required in Nawa.
Control and prevention of fluoride and fluorosis
The lack of resources and low-cost efficient technology acceptable to the affected populations restrict the development of an effective fluoride and fluorosis control and prevention program in developing countries. Precipitation and adsorption are most preferred methods for the defluoridation. Precipitation process is based on the addition of chemicals and removal of insoluble compounds as precipitates. A comparative account of various common defluoridation methods has been summarized in Table 2; many of these methods are discussed in detail by Heidweiller (1990) and references within. Nalgonda technique of community defluoridation is the most common available method in India, which is based on precipitation process. Although it is a very efficient and cost-effective technique, the main limitations of this technique are daily additions of chemicals, large amount of sludge production, and least effective with water having high TDS and high hardness (Apparao et al. 1990). Furthermore, it converts a large portion of ionic fluoride (67–87%) into soluble aluminum complex and practically removes only a small portion of fluoride in the form of precipitate (18–33%). The risk of secondary contamination by metal ions such as aluminum and the search for simple, low-cost methods has sustained to use local materials as adsorbent (Table 2). This material includes the use of plant residue such as coconut shell carbon (Arulanantham et al. 1992), activated alumina (Kumar 1995), chemically activated carbon (Muthukumaran et al. 1995), bone media and clay (Heidweiller 1990), fishbone charcoal (Killedar and Bhargava 1993), natural zeolites (Shrivastava and Deshmukh 1994), burnt clay (Karthikeyan et al. 1999), and other low-cost adsorbents. Although the low-cost defluoridation methods have received increasing attention, these are not yet technically feasible and culturally acceptable. Although based on the field test, economical evaluation and religious consideration clay pot chip, activated alumina adsorption, and Nalgonda methods seem more promising for defluoridation in India.
Provisions of alternative water sources such as the delivery of water from low fluoride sources have also been considered for fluorosis prevention strategies. A major problem in the delivery of water from low fluoride sources and defluoridated water is the scarcity of piped distribution systems in rural communities. Construction of piped networks for the purpose of distributing alternative water supplies initially may cost more than the defluoridation but it is economically feasible during the operation and maintenance phases in the absence of treatment systems. Furthermore, it is suggested that the sources of municipal water supply must be established in a region where adequate levels of fluoride have been observed (Ravindra and Garg 2006). One more way to avoid excessive fluoride intake is rainwater harvesting. The dilution of high fluoride water with rainwater will make small amount of rainwater to last long.
Community participation has been identified as an important factor in the success and sustainability of community water programs. Motivating local people to participate in community-based fluorosis prevention efforts has been difficult due to a combination of the chronic nature of fluorosis, lack of awareness of its progressive course and irreversible pathology, and the general deficiency and failure of fluorosis control programs.
References
APHA. (1991). Standard methods for the examination of water and wastewater (17th ed.). Washington: American Public Health Association Washington, DC.
Apparao, B. V., Meenakshi, S., & Karthikayan, G. (1990). Nalgonada technique of defluoridation of water. Indian Journal of Environmental Protection, 10, 292–298.
Arulanantham, A. J., Ram, K. T., & Balasubramaniam, N. (1992). Studies on fluoride removal by coconut shell carbon. Indian Journal of Environmental Health, 13, 531–536.
Bureau of Indian Standards (BIS). (1991). Specification for drinking water IS 10500, New Delhi (2–4).
Census, (2001). District Nagaur, Rajasthan, Government of Rajasthan
Choubisa, S. L., Sompura, K., Choubisa, D. K., Pandya, H., Bhatt, S. K., Sharma, O. P., et al. (1995). Fluoride content in domestic water sources of Dungarpur district of Rajasthan. Indian Journal of Environmental Health, 37(3), 154–160.
Day, A. K. (1987). Environmental chemistry (2nd ed.). New Delhi: Wiley.
Dean, H. T. (1942). The investigation of physiological effects by the epidemiological method. American Association for the Advancement of Science, 19, 23–33.
Gaciri, S. J., & Davies, T. C. (1993). The occurrence and geochemistry of fluoride in some natural waters of Kenya. Journal of Hydrology, 143, 395–412.
Gizaw, B. (1996). The origin of high bicarbonate and fluoride concentrations in waters of the main Ethiopian Rift Valley. Journal of African Earth Sciences, 22, 391–402.
Gupta, B. L., Kothari, K. S., & Gupta, S. C. (1983). Quality of groundwaters in southeast Rajasthan. Transactions Isdt & Ucds, 8(1), 52–57.
Gupta, S. K., Deshpande, R. D., Agarwal, M., & Raval, B. R. (2005). Origin of high fluoride in groundwater in the North Gujarat-Cambay region, India. Hydrogeology Journal, 13, 596–605.
Heidweiller, V. M. L. (1990). Fluoride removal methods. In J. E. Frencken (Ed.), Proc. symposium on endemic fluorosis in developing countries: causes, effects and possible solutions (chapter 6) (pp. 51–85). Leiden: NIPG–TNO.
Hussain, J. (2001). Studies on the impact of industrial and domestic waste on groundwater quality, Ph.D. Thesis, MDS University Ajmer Rajasthan, India.
Hussain, J., Sharma, K. C., Ojha, K. G., & Hussain, I. (2000). Fluoride distribution in ground waters of Sirohi district in Rajasthan. Indian Journal of Environment and Ecoplanning, 3(3), 661–664.
Hussain, I., Hussain, J., Sharma, K. C., & Ojha, K. G. (2002). Fluoride in drinking water and health hazards: some observations of fluoride distribution in Rajasthan. Environmental scenario of 21st centaury (pp. 355–374). New Delhi: APH.
Hussain, J., Sharma, K. C., & Hussain, I. (2003). Fluoride distribution in groundwater of Raipur Tehsil in Bhilwara District. International Journal of “Bioscience Reporter”, 1(3), 580–587.
Hussain, J., Sharma, K. C., & Hussain, I. (2004). Fluoride in drinking water and its ill affect on human health: a review. Journal of Tissue Research, 4(2), 263–273.
Hussain, J., Sharma, K. C., & Hussain, I. (2005a). Fluoride contamination in groundwater sources of Hurda Tehsil of Bhilwara, Rajasthan. Pollution Research, 24(2), 431–434.
Hussain, J., Sharma, K. C., & Hussain, I. (2005b). Fluoride distribution in groundwater of Banera Tehsil in Bhilwara District, Rajasthan. Asian Journal of Chemistry, 17(1), 457–461.
Hussain, J., Hussain, I., & Sharma, K. C. (2010). Fluoride and health hazards: community perception in a fluorotic area of Central Rajasthan (India): an arid environment. Environmental Monitoring and Assessment, 162, 1–14.
Karthikeyan, G., Andal, M. N., & Sundar, S. G. (1999). Defluoridation property of burnt clay. Journal of Indian Water Works Association, 31, 291.
Killedar, D. J., & Bhargava, D. S. (1993). Effect of stirring rate and temperature on fluoride removal by fishbone charcoal. Indian Journal of Environmental Health, 35, 81–87.
Kim, K., & Jeong, Y. G. (2005). Factors influencing natural occurrence of fluoride-rich ground waters: a case study in the southeastern part of the Korean Peninsula. Chemosphere, 58, 1399–1408.
Kumar, S. (1995). Studies on desorption of fluoride from activated alumina. Indian Journal of Environmental Protection, 12, 50–53.
Muthukumaran, V., Balasubramaniam, N., & Ramkrishna, T. V. (1995). Removal of fluoride by chemically activated carbon. Indian Journal of Environmental Protection, 12, 514–517.
Nanyaro, J. T., Aswathanarayana, U., Mungere, J. S., & Lahermo, P. (1984). A geochemical model for the abnormal fluoride concentrations in waters in parts of northern Tanzania. Journal of African Earth Sciences, 2, 129–140.
Olsson, B. (1979). Dental finding in high fluoride areas. Epidemiology, 7, 51–56.
Oruc, N. (2003). Problems of high fluoride waters in Turkey (hydrogeology and health aspects). The short course on medical geology-health and environment. Canberra, Australia
Ravindra, K., & Garg, V. K. (2006). Distribution of fluoride in groundwater and its suitability assessment for drinking purpose. International Journal of Environmental Health Research, 16, 163–166.
Shrivastava, P. K., & Deshmukh, A. (1994). Defluoridation of water with natural zeolite. Journal of the Institute of Public Health Engineers (India), 14, 11–14.
Shusheela, A. K. (1993). Prevention and control of fluorosis in India. Rajeev Gandhi National Drinking Water Mission. Ministry of Rural Development, New Delhi Health, 1, 20–22.
Subba, R. N., & John, D. D. (2003). Fluoride incidence in groundwater in an area of peninsular India. Environmental Geology, 45, 243–251.
USEPA. (1995). Drinking water criteria document on fluoride office of drinking water (pp. TR- 832–TR- 51985). Washington: USEPA.
USPHS, (1991). US Public Health Service, PHS review of fluoride: benefits and risk: report of ad hoc sub-committee on fluoride. Committee to co-ordinate environments health and related programs
USPHS (United State Public Health Service). (1962). Drinking water standard-1962. Washington: USPHS, Publication 956, USGPO.
Wang, Y., & Reardon, E. J. (2001). Activation and regeneration of a soil sorbent for defluoridation of drinking water. Applied Geochemistry, 6, 531–539.
WHO, (1984). Fluorine and fluoride. Environmental health criteria, Vol. 36, Geneva.
WHO. (1997). Guideline for drinking water quality health criteria and other supporting information (2nd ed., Vol. 2). Geneva: World Health Organization.
Ziauddin, M. (1974). Endemic fluorosis in parts of Dharwar and Raichur District, Karnataka state. Proceeding of the Symposium on Fluorosis, Hyderabad Paper, 10, 85.
Acknowledgment
The authors are thankful to Dr. Manoj Sharma, Junior Chemist, Public Health Engineering Department, Nagour and Mrs. Nishy, P. Scientist E-II, NISCAIR, Council of Scientific and Industrial Research (CSIR), New Delhi, for helping and for necessary guidance.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hussain, I., Arif, M. & Hussain, J. Fluoride contamination in drinking water in rural habitations of Central Rajasthan, India. Environ Monit Assess 184, 5151–5158 (2012). https://doi.org/10.1007/s10661-011-2329-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-011-2329-7