Abstract
In order to achieve a better understanding of the nature of the factors influencing ground water composition as well as to specify them quantitatively, multivariate statistical analysis (factor analysis) were performed on the hydrochemical data of this area. R-mode factor analysis was carried out on the geochemical results of the 79-groundwater samples and the factor scores were transferred to areal maps. Fundamental chemical parameters of the groundwater have been compounded together for characterizing and interpreting a few empirical hydrogeochemical factors controlling the chemical nature of water. R-mode factor analysis reveals that the groundwater chemistry of the study area reflects the influence of anthropogenic activities, silicate weathering reactions, precipitation, dissolution and subsequent percolation into the groundwater. The data have been put into few major factors and the seasonal variation in the chemistry of water has been clearly brought out by these factors. Factor scores were transferred to contour diagrams and the factor score analysis has been used successfully to delineate the stations under study with various factors and the seasonal effect on the sample stations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater contamination in urban environment is a major issue and is complicated by the large number of potential sources of contamination, and the numerous, distinct contaminants that can be found in a city. Studies on the Geochemical processes that control groundwater chemical composition may lead to improved understanding of hydrochemical systems in such areas. Such studies contribute to effective management and utilization of the groundwater resources. Exploitation of groundwater has increased greatly, particularly for agricultural purpose. Groundwater quality is as important as the quantity. Poor quality of water adversely affects the plant growth and human health. In order to understand the factors controlling the geochemistry of groundwater in the study area, multivariate statistical technique was performed.
The characterization and interpretation of various groundwater parameters is often a complex problem, factor analysis offers a powerful means of identifying the similarities among the variables present in the chemical budget of water. To identify the likely factors that cause the variations in hydrochemical compositions, multivariate statistical methods of analysing hydrochemical data such as principal component factor analysis can be a very useful tool. Such analysis are especially useful because it brings out the relative significance of the combinations of chemical variables that can be evaluated and the subsequent interpretation is simplified since these are statistical tools which reduce and categorize complex sets of data into groups with similar characteristics. The conventional techniques of histograms and trilinear techniques (Dalton and Upchurch 1978) such as Stiff and Piper plots which consider only the major and minor ions with equal emphasis to interpret the group of variables to evaluate the chemical nature of groundwater has several limitations. In order to overcome these limitations of these conventional methods, factor analytical technique has been used to understand a number of geochemical processes by several professionals (Dawdy and Feth 1967; Hitchon and others 1971; Ashley and Lloyd 1978; Lawrence and Upchurch 1976, 1983; Seyhan and others 1985; Usunoff and Guzman 1989; Razack and Dazy 1990; Subbarao and others 1996; Jayakumar and Siraz 1997; Olmez et al. 1994; Bakac 2000).
The statistical analysis of the basic geochemical data of the groundwater using R-mode factor analysis is a widely accepted technique for characterizing and interpreting a few empirical hydrochemical factors controlling the chemical budget of water. Reeder and others (1972) identified the likely weathering processes controlling the chemical composition of surface waters of the Mackenzie River drainage basin in Canada. Ashley and Lloyd (1978) used the factor analysis to evaluate the hydrogeochemical process in the Santiago basin of Chile and the Derbyshire Dome of England. Lawrance and Upchurch (1983) also used the technique to delineate the zones of natural recharge to groundwater in the Floridan aquifer. Ruiz and others (1990) mentioned that the basic purpose of such an analysis is to study the hydrogeochemistry of aquifers by simplifying the numerous and complex groundwater data into a set of factors, few in number which can explain a large amount of the variance of the analytical data and also indicates the source of origin of various ions present in the water. Briz-kishore and Murali (1992) have delineated the areas prone to salinity hazard in Chitravati watershed of India. Similarly Syed Munaf and others (2005) used the multivariate factor analytical technique to assess the water quality and source of contamination in a irrigation project at Al-Fadhli, Saudi Arabia.
Many studies combining the effects of multiple water quality variables evaluating the water quality and the extent and nature of contamination have been undertaken (Shuxia et al. 2003). Chemical composition in groundwater is determined by a number of factors, such as precipitation, infiltration, groundwater flow patterns and characteristics of soil type of the aquifer.
Factor score studies reflect the station-wise variation of the geochemical factors controlling the water chemistry. Scores showing negative values denote areas essentially unaffected by the said factors; most affected areas are denoted by extreme positive score and a near zero score may be considered as areas affected to an average degree to unaffected (Dalton and Upchurch 1978; Gupta and Subramanian 1994). The results of factor scores of groundwater in the study area are represented as contour diagrams.
The R-mode factor analysis is used in the present work, eigenvalues and eigenvectors of the correlation matrix are extracted after applying the varimax rotation and the least important among them are then discarded (Davis 2002). In general, factors showing Eigenvalues greater than 1 are taken up for interpretation of the results and in the present work also, the factor extraction has been done with a minimum acceptable eigenvalue greater than 1 (Harman 1960).
Study area
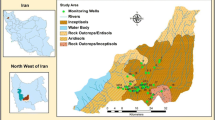
The Neyveli Mine-industrial complex is located in Cuddalore District, Tamil Nadu, India. The study area lies on 11°25′N to 11°40N and 79°25′E to 79°40′E (Fig. 1a). Neyveli is located 200 km south of Chennai and 40 km east of Bay of Bengal lies on the immediate hinderland of coastal zone. The area has a tropical climate with the highest and lowest temperatures recorded in May and January respectively. The precipitation of this study area mainly depends upon North East monsoon, which is cyclonic in nature and attributed to the development of low pressure in the Indian Ocean and Bay of Bengal. This area receives about an annual rainfall of 1,162 mm. The study area includes two very large (Mines I and II) and one small (Mine IA) opencast lignite mines, associated industries (two pit-head thermal power plants, a urea plant, and a briquetting and carbonization plant) that are operated by Neyveli Lignite Corporation Ltd. (NLC), and an independent power plant.
Geology of the area
The study area (Fig. 1a, b) is underlain by the Tertiary Cuddalore Formation and by recent alluvium. The Cuddalore sandstones cover mostly the northern and western areas, while the alluvium covers mostly the eastern and southeastern areas of the Neyveli lignite region. The Tertiary and Mesozoic sequence form a E-SSW belt along the east coast of Tamil Nadu, and uncomformably rest over the Archaean rocks to the west. Few outliers of the sedimentary rocks over the Archaean basement have been noticed. The borehole data of Subramani (1970) indicate a thick sequence of sandstones, sands, clays and gravel down to a depth of 300 m in and around the mine cut. The clays, which separate the granular beds below the lignite disappear exposing the sands to direct recharge. The lignite occurs in the Cuddalore Formation at depths ranging from 45 to 120 m below ground level (bgl).
Table showing the geological evolution of the area.
Recent | Soils, Alluvium, Blown sands, Laterite and kankar | |
|---|---|---|
Tertiary | Upper Miocene(Cuddalore) | Argilaceous Sandstones, Pebbles bearing sand stones, Grits, Sands, Clays with pebles and lignite beds. |
Probable Unconformity | ||
Eocene | Black clays or shales, Grey coloured Sandstones. Calcareous Sandstones and shales and siliceous limestones with fossils | |
Unconformity | ||
Mesozoic | Cretaceous (Ariyalur) | Shell limestones, Silious limestones, Marls etc |
Unconformity | ||
Archean | Gneissic Complex | Granites, Gneisses, Charnakites with dolerite, pegmatites and quartz veins |
Hydro geology
The area gently slopes towards southeast and east, and is not drained by any major river except for a small ephemeral stream (the Paravannar River) flowing to the east. This carries mine water and industrial effluents instead of natural water, and discharges into the Walaza and Perumal Ponds east of the lignite mines. The hydrogeology of the Neyveli Groundwater basin is extremely complex, consisting of a series of productive, confined aquifers below the lignite seam in both Mine I and II areas, while a semi-confined aquifer lies above the seam and occurs only in the Mine II area. In the Cuddalore sandstones, groundwater occurs in unconfined, semi-confined, and confined conditions; in the alluvium, it occurs in unconfined condition. In the study area, both Tertiary Cuddalore Formation and the Recent alluvium form a potential aquifer system.
Methodology
Groundwater samples were collected during May 2006 and December 2006 representing pre- and post-monsoon seasons to evaluate the seasonal variations in chemical compositions. The water samples were collected from wells at a depth of 10–20 m (shown as + in Figs. 2, 3). The samples were collected in new 1-l HDPE bottles pre-washed with dilute hydrochloric acid and rinsed three to four times with the water sample before filling it to capacity and then labelled accordingly. The samples were stored at a temperature below 4°C prior to analysis in the laboratory. For collection, preservation and analysis of the samples, the standard methods (Rainwater and Thatcher 1960; Brown and others 1970; AWWA 1971; Hem 1985; APHA 1995) were followed. EC and pH of water samples were measured in the field immediately after the collection of the samples using pH and SEC meters. Before each measurement, the pH meter was calibrated with reference buffer solution of pH = 4. Na+ and K+ were measured by using a flame photometer (Model: Systronics Flame Photometer 128). Total dissolved solids (TDS) were measured by evaporation and calculation methods. Ca2+ and Mg2+ were determined titrimetrically using standard EDTA. Chloride was estimated by AgNO3 titration. Sulphate was analysed using the turbidimetric method (Clesceri et al. 1998). Nitrate, nitrite, phosphate, fluoride were analysed using UV-Visible spectrophotometer (Rowell 1994). Standard solutions for the above analysis were prepared from the respective salts of Analytical Reagent grade. Trace metals were determined by Graphite Furnace Atomic Absorption Spectrophotometer (Perkin–Elmer AAnalyst 700). Multi element Perkin–Elmer standard solutions were used for the estimation of trace metals.
Discussion
The analytical results of the chemical analysis showing minimum, maximum and mean of the geochemical data are presented in Table 1 for both premonsoon and post monsoon.
Correlation studies
A strong correlation between two variables is exhibited by a high correlation coefficient (near +1 or −1) and its value around zero means no relationship between them. In other words, it can be said that the parameters showing correlation coefficient >0.7 are considered to be strongly correlated whereas value between 0.5 and 0.7 shows moderate correlation.
In this study, the correlation between various elements has been presented in Table 2. The correlation matrix shows high levels of positive and negative correlations among different elements. The chemical budget of ions in the groundwater depends primarily on Rock-water interactions and anthropogenic activities. Na+ and Mg2+ are strongly correlated with HCO −3 . Ca2+ also have positive correlation with HCO −3 in both the seasons. Moreover the correlation of Mg2+ with SO 2−4 in both the pre and post monsoon periods are significantly high but since neither was found to be correlated with NO −3 , the source of these ions are not likely from the anthropogenic activities viz., agricultural inputs such as fertilizers but may be derived from the lithological sources.
Na+ is strongly correlated with Cl− in both the seasons. During premonsoon, the variation of Na+ with Cl− is related to nitrate but in post monsoon, the variation is unrelated to nitrate reflecting that some contribution from the anthropogenic activities is expected in premonsoon and the dissolution of dispersed halite would have contributed to the water chemistry during post monsoon. For both the post-monsoon and pre-monsoon seasons, some pairs of species show moderate to strong correlation (r > 0.6), e.g. Cl−–SO 2−4 , Mg2+−SO 2−4 and Na+−SO 2−4 . Similarly, during both the seasons, the major exchangeable ions Na+–Ca2+ and Na+–Mg2+ correlate positively. It can therefore be postulated that the concurrent increase/decrease in the composition of ions in these waters is predominantly due to the result of dissolution/precipitation reaction and concentration effects.
Moreover, in groundwater three different sets of strong relationships exist between major cations and anions (Douglas and Leo 1977). They are:
-
1.
The highly competitive relationship between ions having same charge but a different valence number e.g. Ca2+ and Na+
-
2.
The affinity between ions having different charges but the same valence number e.g. Na+ and Cl−.
-
3.
The non-competitive relationship between ions having the same charge and same valence number e.g. Ca2+ and Mg2+.
On the above basis and from the results of the correlation coefficient matrix (Table 2), the correlation of ions in groundwater samples in premonsoon is as follows:
-
1.
Highly competitive ion relationship: Ca2+ with Na+; Mg2+ with Na+ have high positive correlation. SO 2−4 with Cl−, HCO −3 ; Ca2+ with K+ have low positive correlation.
-
2.
Affinity ion relationship: Na+ with Cl−, NO −3 , HCO −3 ; Ca2+ with SO 2−4 ; Mg2+ with SO 2−4 have significant correlation. K+ with HCO −3 , F− has low positive correlation.
-
3.
Non-Competitive ion relationship: Ca2+ with Mg2+; HCO −3 with Cl−; Cl−with NO −3 have significant correlation. Na+ with K+; HCO −3 with NO −3 ; NO −3 with NO − 2 have low positive correlation. F− with NO − 3 has negative correlation.
The correlation of ions in groundwater samples in Post monsoon is as follows:
-
1.
Highly competitive ion relationship: Ca2+ with Na+; Mg2+ with Na+; HCO −3 with SO 2−4 ; SO 2−4 with Cl− have positive correlation. Ca2+ with K+; Mg2+ with K+; SO 2− 4 with NO −3 , NO −2 have low positive correlation.
-
2.
Affinity ion relationship: Ca2+ with SO 2−4 ; Mg2+ with SO 2−4 ; Na+ with Cl−, NO −3 , HCO −3 have significant correlation. Na+ with NO −2 ; K+ with HCO −3 , F− have low positive correlation. Negative correlation exists between Na+ with F−; K+ with NO2−.
-
3.
Non-Competitive ion relationship: Ca2+ with Mg2+; HCO −3 with Cl−; Cl− with NO −3 ; shows positive correlation. Na+ with K+; HCO3− with NO −3 , NO −2 ; F− with NO −3 , NO −2 ; shows negative correlation.
Factor analysis
Factor analysis is used to reduce the complex data to an easily interpretable form (Davis 2002). In this work, R-mode factor analysis is chosen which provides several positive features that allows interpretation of the data set more scientifically. In order to evaluate the variables belonging to a specific chemical process and also to find out the dominance and contribution of the major elements in terms of the total data set, Factor loadings and Eigen values (Table 3) are being examined.
The first six factors which accounts for about 71% of variance during premonsoon and first seven factors accounting for 79% of variance during post monsoon with eigen values >1 have been extracted from the principal factor matrix after varimax rotation.
Factor 1 of the premonsoon which explains 28% of the total variance has high loadings in the ions Mg2+, HCO −3 , SO 2−4 , Na+ and Ca2+. The percentage abundance of the above variables indicates that their source of origin may be expected from (1) Anthropogenic sources such as residential water softeners or agricultural activities (Vengosh and Keren 1996; Wayland et al. 2003) and (2) the dissolution of silicate minerals. Moreover, the above variables are positively correlated with pH reflecting that alkalinity increases dissolution of these ions and acidity decreases the dissolution of ions.
Factor 1 of the post monsoon which explains 27% of the total variation has high loading in the ions Ca2+, Mg2+, Na+, HCO3−, SO 2−4 and Cl-. It is observed that there is an increase in Ca2+ and Cl− loadings compared to premonsoon. Since the effect of monsoon is obviously seen, this factor may be termed as precipitation and silicate weathering factor. The areal distribution of the factor scores during premonsoon is shown in Fig. 2a. A small zone in the east shows high scores suggesting the transport of contaminants through dissolution and percolation. The wells in most parts on the Northern direction shows negative factor scores and are apparently unaffected. The wells in the southwest are moderately affected. The natural recharge process would have induced bicarbonate to the groundwater as the atmosphere surrounding the thermal plant area is rich in CO2. The dissolution of the CO2 in the adjacent aquifers due to precipitation would have increased the concentration of these ions in the water bodies. The areal distribution of post monsoon (Fig. 3a) also follows the same pattern as far the northern parts are concerned. A small portion in the East is found to be affected as in the case of premonsoon but the wells located in the south are found to be moderately affected. The seasonal effect is visible in this region.
Factor 2 of premonsoon, which accounts for 16% of the total variance has high loading in the variables Ca2+, Na+, Cl−, and NO −3 . Nitrate has no significant lithologic source in the study area and it must be associated with the anthropogenic activities. During post monsoon, Factor 2, which explains 14% of variance has no significant loading on the major ions and nutrients but it has high loadings on Fe, Mn, Pb and Cr. In the case of factor 2, the seasonal effect is very significant and dilution predominates over leaching in the case of major ions and nutrients except heavy metals. The areal distribution map of premonsoon (Fig. 2b) with regard to factor 2 shows that the wells located in the southern parts are not affected as far as the high loading ions of factor 2. A small portion in the mid zone shows high value of this factor reflecting that the wells fall under this area are found to be affected. The wells in the Northwest direction show some high factor scores reflecting that the groundwater in this area is affected to some extent. The post monsoon areal distribution map (Fig. 3b) shows that the Northwest and Southwest parts are affected with regard to the heavy metal pollution. Northeast and the middle parts are also found to have high factor scores reflecting that the wells in these areas are also affected.
Factor 3 of premonsoon accounting for about 8% of the total variance is explicitly a heavy metal factor showing high loadings on the metals Fe, Mn, Cr and Pb. During post monsoon, factor 3, which explains 7% of the total variance, has high loadings in the ions NO −2 , Cr and Pb. The contour diagrams of premonsoon factor scores (Fig. 2c) shows that the wells in the Southwest parts are found to be highly affected with regard to the heavy metals. The wells in the south and southeast parts shows slightly elevated concentration of these metals. But in the case of post monsoon areal distribution map (Fig. 3c), it is observed that majority of the wells shows negative factor scores indicating that the groundwater in these area is not affected much with regard to the variables viz., NO −2 , Cr and Pb. But some of the wells located in the Northeast and southern parts shows some significant values indicating that these wells are affected with respect to these variables.
Factor 4 is found to have high loadings in fluoride for both the seasons especially in the post monsoon. In addition to Carbonates and fluorides, Cu shows some high loadings in premonsoon and Nickel shows predominance among other metals in post monsoon. During premonsoon, pH is negatively correlated with fluorides indicating that an increase in pH decreases the dissolution of F− ions. The concentration of F− in an area is mainly caused by the leaching of fluoride-bearing minerals (apatite, hornblende, mica, etc.) of the country rocks, and also from the influence of clay minerals, intensive irrigation, high rate of evapotranspiration and long residence time of the waters in the aquifers (Ramamohana Rao and others 1993; Wodeyar and Srinivasan 1996; Subba Rao and others 1998a, b). The lithology of the study area does not support the increased concentration of fluoride but the influence of clay minerals, intensive irrigation, high rate of evapotranspiration and long residence time of the waters in the aquifers would have contributed to the high fluoride loadings. The premonsoon areal distribution map of factor 4 (Fig. 2d) shows that most of the wells falls in the region of unaffected to slightly affected. A small region in the southeast and in the eastern part shows some significant factor scores suggesting that the wells lying in this part contains higher concentration of fluoride and copper ions. The contour maps of post monsoon (Fig. 3d) suggests that the wells in the northwest, southwest and the eastern parts falls in the region of unaffected to slightly affected. A small portion in the upper part of the study area lying in the north is found to have high positive scores.
Factor 5 of premonsoon, which accounts for 6.5% of the total variance is explicitly a nitrite factor. Nitrite has no significant lithologic source in the study area and it must be associated with the anthropogenic activities. The areal distribution map of factor scores (Fig. 2e) shows that majority of the wells are found to be unaffected with respect to this factor. The wells in the southwest, northwest and some parts in the east are found to exhibit slight positive factor scores. A small portion in the north shows highly significant scores suggesting that the wells in this area are affected. During post monsoon, Factor 5, which explains 6% of variance has high loading on CO 2−3 and it does not show any significance in the case of trace metals. Falling in the same line as that of premonsoon, the factor score contour maps (Fig. 3e) of post monsoon suggests that many of the wells are found to be unaffected with regard to factor 5. Some of the wells in the east and southeast parts are found to exhibit highly significant factor scores.
Factor 6 of premonsoon which accounts for only 5% of the total variance may be called as a phosphate factor. Apart from high loading on phosphate, it does not have any significant loadings on other major ions and trace metals. The high loadings on phosphate may be attributed to the anthropogenic activities. Factor 6 areal distribution map of factor scores (Fig. 2f) shows that about 70% of the wells are found to be unaffected with respect to this factor. Some wells in the north, east and southeast parts are found to be slightly affected. A few wells in the north, southeast and in the eastern parts show high positive factor scores. Factor 6 of post monsoon, which accounts for 5.6% of the total variance is also a phosphate factor but there is a slight decrease in the loadings on phosphate comparing to the premonsoon. Loadings on other variables follows as that of the premonsoon loadings. The factor score contour maps (Fig. 3f) of post monsoon shows that wells falling in the northwest and southwest parts are found to be not affected with regard to this factor. Wells in the northeast, southeast and central parts falls in the region of unaffected to slightly affected. A small portion in the north and eastern parts show highly significant factor scores.
Factor 7 of post monsoon accounting for 5% of the total variance does not have any significance except it shows some high loading on Copper. The areal distribution map (Fig. 3g) reveals that majority of the wells in the north, northwest and in the eastern parts falls in the slightly affected region. A few wells in the northeastern and in mid east exhibits high positive scores indicating that concentration of copper is high in these groundwaters.
Conclusion
The present study has thus demonstrated the usefulness of factor analysis in interpreting the hydrogeochemical data. Factor score analysis has been used successfully to delineate the stations under study with various factors and the seasonal effect on the sample stations. The degree to which the groundwater composition is influenced by various factors had been assessed by examining the factor scores; the variations in factor scores can be used as an indicator of groundwater residence. The correlation studies reveals that both in the premonsoon and postmonsoon Na+ was found to have high correlation with Cl− and both the alkaline earth metal ions were found to have high correlation with SO 2+4 and Ca2+ and Mg2+ were found to have high positive correlation with bicarbonates especially Mg2+. Seasonal effect is very much seen in the case of factor 1 as it shows significant changes in the loading of variables in both the periods. The seasonal effect is found to be significant in the case of factor 2 also. The premonsoon is explicitly a nitrate factor along with high loadings on other major ions but in the case of postmonsoon it does not have any significant loading with regard to any of the major ions but the trace metals were found to acquire some significance. The areal distribution map on this factor also shows significant changes during both the monsoon periods. Factor 3 is found to be a heavy metal factor both in the pre and postmonsoon periods. From the areal distribution pattern, it is found that the wells lying in the southern part of the study area shows high positive scores with regard to the heavy metals. Factor 4 is explicitly a fluoride factor for both the seasons especially in the postmonsoon. Since the lithology of the area does not support the higher fluoride concentration, it is logical to assign it to anthropogenic activities. The areal distribution pattern for both the seasons shows that only a small region is found to have higher concentration and the majority of the wells are found to be not affected with regard to this factor. Monsoonal effect is visible in the case of factor 5 where the premonsoon is found to be a nitrite factor whereas the postmonsoon shows high loadings in CO 2−3 In general, factor analysis shows significant seasonal effect among the chemical budget of ions and the results also indicates that the dilution predominates over that of the leaching factor.
References
APHA (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Association, Washington, 1467 pp
Ashley RP, Lloyd JW (1978) An example of the use of factor analysis and cluster analysis in ground water chemistry interpretation. J Hydrol 39:355–364
AWWA (1971) Water quality and treatment. McGraw-Hill, New York
Bakac M (2000) Factor analysis applied to a geochemical study of suspended sediments from the Gediz River, western Turkey. Environ Geochem Health 22(2):93–111
Briz-Kishore BH, Murali G (1992) Factor analysis for revealing hydrochemical characteristics of a watershed. Environ Geol Water Sci 19:3–9
Brown E, Skougslad MW, Fishman MJ (1970) Methods for collection and analysis of water samples for dissolved minerals and gases. US Geological Survey, Techniques for water resources investigations, Book 5, Chapt A1
Clesceri LS, Greenberg AE, Eaton AD (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, American Water Works Association, Water Environment Federation, Washington
Dalton MG, Upchurch SB (1978) Interpretation of hydrochemical facies by factor analysis. Ground Water 10:228–233
Davis JC (2002) Statistics and data analysis in geology. Wiley, (ASIA) pte Ltd, Singapore, New York, pp 526–540
Dawdy DR, Feth JH (1967) Applications of factor analysis in study of chemistry of groundwater quality, Mojave River Valley, California. Water Resour Res 3:505–510
Douglas EB, Leo WN (1977) Hydrogeochemical relationships using partial correlation coefficient. Water Resour Bull 13:843–846
Gupta LP, Subramanian V (1994) Environmental geochemistry of the river Gomti: a tributary of the river Ganges. Environ Geol 24:235–243
Harman HH (1960) Modern factor analysis. University of Chicago Press, Chicago
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water. US Geological Survey Water Supply Paper 2254
Hitchon B, Billings GK, Klovan JE (1971) Geochemistry and origin of formation waters in the western Canada sedimentary basin-III. Factors controlling chemical composition. Geochim Cosmochim 35:567–598
Jayakumar R, Siraz L (1997) Factor analysis in hydrogeochemistry of coastal aquifers—a preliminary study. Environ Geol 31:174–177
Lawrence FW, Upchurch SB (1976) Identification of geochemical patterns in groundwater by numerical analysis. In: Saleem ZA (ed) Advances in groundwater hydrology. American Water Resources Association, USA, pp 199–214
Lawrence FW, Upchurch SB (1983) Identification of recharge areas using factor analysis. Ground Water 20:680–687
Olmez L, Jack WB, Villaume JF (1994) A new approach to understanding multiple-source groundwater contamination: factor analysis and chemical mass balance. Water Res 28:1095–1101
Rainwater FH, Thatcher LL (1960) Methods for collection and analysis of water samples. US Geological Survey Water Supply Paper 1454
Ramamohana Rao NV, Suryaprakash Rao K, Schuiling RD (1993) Fluorine distribution in waters of Nalconda District, Andhra Pradesh, India. Environ Geol 231:4–89
Razack M, Dazy J (1990) Hydrochemical characterization of groundwater mixing in sedimentary and metamorphic reservoirs with combined use of piper’s principal and factor analysis. J Hydrol 114:371–393
Reeder SW, Hitchon B, Levinson AA (1972) Hydrogeochemistry of the surface waters of the Mackenzie River drainage basin, Canada-I. Factors controlling inorganic composition. Geochim Cosmochim Acta 36:825–865
Rowell DJ (1994) Soil science: methods and applications. Longman Scientific and technical, London
Ruiz F, Gomis V, Blasco P (1990) Application of factor analysis to the study of a coastal aquifer. J Hydrol 119:169–177
Seyhan EV, Van de Caried AA, Engelen GB (1985) Multivariate analysis and interpretation of the hydrochemistry of a dolomite reef aquifer, Northern Italy. Water Resour Res 21:1010–1024
Shuxia Y, Shang J, Zhao J, Guo H (2003) Factor analysis and dynamics of water quality of the Songhua River, northeastChina. Water Air Soil Pollut 144(1):159–169
Subbarao C, Subbarao NV, Chandu SN (1996) Characterisation of ground water contamination using factor analysis. Environ Geol 28:175–180
Subba Rao N, Krishna Rao G, John Devadas D (1998a) variation of fluoride in groundwaters of crystalline terrain. J Environ Hydrol 6:3
Subba Rao N, Prakasa Rao J, Nagmalleswara Rao, Niranjan Babu P, Madhusudhana Reddy P, John Devadas D (1998b) A preliminary report on fluoride content in groundwaters of Guntur area, Andhra Pradesh, India. Curr Sci 75(3):887–888
Subramanyam V (1970) Changes in the groundwater regimen of the Neyveli Lignite field, South Arcot district, Tamil Nadu, India. Results of Research on Representative and Experimental Basins-Proceedings of the Wellington Symposium Dec’ 1970, vol 97, pp 299–311
Syed Munaf A, Hussain M, Abderrahman W (2005) Using multivariate factor analysis to assess surface/logged water quality and source of contamination at a large irrigation project at Al-Fadhli, Eastern Province, Saudi Arabia. Bull Eng Geol Environ 64:319–327
Usunoff EJ, Guzman AG (1989) Multivariate analysis in hydrochemistry. An example of the use of factor and correspondence analysis. Ground Water 17:27–34
Vengosh A, Keren R (1996) Chemical modifications of groundwater contaminated by recharge of treated sewage effluent. Contam Hydrol 23:347–360
Wayland KG, Long DT, Woodhams S, Pijanowski BC, Hyndman DW (2003) Identifying biogeochemical signatures of land use with baseflow synoptic sampling and factor analysis. J Environ Quality 32:180–190
Wodeyar BK, Srinivasan G (1996) Occurrence of fluoride in the groundwaters and its impact in Peddavankahalla basin, Bellary District, Karnataka—a preliminary study. Curr Sci 70(1):71–74
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayaprakash, M., Giridharan, L., Venugopal, T. et al. Characterization and evaluation of the factors affecting the geochemistry of groundwater in Neyveli, Tamil Nadu, India. Environ Geol 54, 855–867 (2008). https://doi.org/10.1007/s00254-007-0868-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-007-0868-6