Abstract
Water–rock interaction is one of the prime factors affecting the fluoride contents of surface and groundwater. If fluoride concentration of drinking water has been neglected, excess fluoride can cause serious dental and medical problems on human health, which is well known at Golcuk-Isparta region. In the research area, Egirdir lake, Golcuk lake and surrounding springs have been utilized as drinking water sources. Golcuk lake water and surrounding groundwaters have high fluoride content (1.4–4.6 mg/l), which is above the WHO standards. Fluoride is predominantly supplied by dissolution of fluoride within the fluormicas of volcanics during the circulation of water. Fluoride concentrations of waters have shown variations for dry and rainy seasons depending on the degree of interaction between groundwater and volcanic rocks. It tends to decrease in rainy seasons and increase in dry seasons for all years. In this study, temporal variations and spatial distribution of fluoride concentration in public water system of Isparta were investigated to get benefit using GIS techniques from1990 to 2003 years. Extremely fluoride concentrations were measured in the public water system in 1990 at almost every district of the city. In 2003, fluoride content of the public water system decreased in some district of city due to drinking water has started obtaining from Egirdir lake in 1995. The fluoride contents of Isparta drinking water ought to be modified with suitable mixture of lake waters and groundwater point of view to health impact.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoride is the most known electronegative element. It is highly reactive and not found in the elemental state in nature, only in solid salts or fluoride ions in aqueous solution (Sreedevi et al. 2006). Naturally occurring fluoride in groundwater has been the subject of numerous studies in a variety of geologic settings.
Especially, the effects of fluoride on human health are well-studied. The maximum tolerance limit of fluoride in drinking water specified by the World Health Organization (WHO 1984) is 1.5 mg/l. Many epidemiological studies of possible adverse effects of the long-term ingestion of fluoride via drinking water have been carried out. These studies clearly establish that fluoride primarily produces effects on skeletal tissues (bones and teeth). Low concentrations provide protection against dental caries, especially in children. Fluoride may give rise to mild dental fluorosis at drinking water concentrations between 0.9 and 1.2 mg/l (Dean 1942). As a rough approximation, for areas with a temperature climate, manifest dental fluorosis occurs at lower concentrations in the drinking water because of the greater amounts of water consumed (Cao and Li 1992).
Fluoride can also have more serious effects on skeletal tissues. Skeletal fluorosis (with adverse changes in bore structure) is observed when drinking water contains 3–6 mg of fluoride per liter. Crippling skeletal fluorosis develops where drinking water contains over 10 mg of fluoride per liter (WHO 1984). Long-term accumulation of fluoride likely also affects thyroid function (Balabolkin et al. 1995), may have neurological effects (Mullenix et al. 1995), may induce reproductive problems (Dominguez et al. 1995) and may affect the pineal gland (Luke 1994). Fluoride’s well-know genotoxic properties may be showing up in the population as an increased risk for various cancers (Tohyama 1996; Zeiger et al. 1993).
Many investigations have been made related to origin and hydrogeochemistry of high fluoride in groundwater for the different regions of the world (Kundu et al. 2001; Carrillo-Rivera et al. 2002; Rukah and Alsokhny 2004; Gupta et al. 2005; Jacks et al. 2005; Karro et al. 2006; Ozsvath 2006; Gou et al. 2006; Sreedevi et al. 2006; Valenzuela-Vasquez et al. 2006). In these researches, groundwaters have studied on water samples obtaining from springs and dug wells, and determining theirs high fluoride contents have originated from water–rock interaction and regional thermal water mixing. In this research, fluorine contents and temporal variations were investigated using GIS techniques in the public water system of Isparta city as a different research work of previous works. Regional hydrogeology, origin and hydrogeochemistry of high fluoride contents were also carried out in this study as a case investigation.
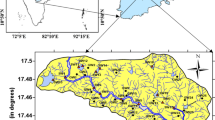
The research area is situated within the Lake District in the southwest of Turkey (Fig. 1). For along time, fluorine problem related to drinking water of Isparta region have been well known. Published studies were predominantly concentrated on the existence of fluoride in water and rocks, fluorosed human teeth at the Isparta area (Ayhan 1983; Pekdeger et al. 1992; Irlayıcı 1993; Coban et al. 2001). Dental fluorosis causes high amounts of fluoride (1.15–3.6 mg/l) in drinking water has been observed since 1954 in the Isparta (Samsar 1983). Samsar (1983) reported existence of fluorosis at a ratio 100% among the children in the Isparta. Ermis et al. (2003) were determined increasing the water fluoride levels were associated with higher prevalence and severity of dental fluorosis and had no influence on caries experience among children in their study doing on fluorosed teeth were collected from Isparta.
Geological map (were taken Yalcınkaya 1989)
Negative effects of fluoride in drinking water have been well known matter in Isparta since previous years. However, any research work on fluoride control within drinking water has not yet been performed. In this study, in order to control fluoride, temporal variations of fluoride contents for public water system and effected regions of residential areas have been examined for 1990–2003 years. The understanding of the origin and distribution of fluoride within water may be useful in devising water exploitation, sustainable management strategies and health risk analysis. Risk analysis plays an important role for environmental health. Currently, it is a primary tool for providing the quantitative data aimed at identifying and mitigating environmental health hazards (Kay 1999). Therefore, hydrogeology of research area and hydrogeochemistry of fluoride waters have also been investigated.
Methodology
The data was obtained in this study by following the methods. Water samples were collected in different periods. Samples were safely stored in two polyethylene bottles. One of the bottles was acidified with suprapure HNO3 for determination of cations analyses and another was kept unacidified for the anion analyses. Temperature, pH and electrical conductivity (EC) were also measured in the field. The major chemical constituents were analyzed at the ACME Laboratory (Canada-ISO 9002 Acceredited Co.). HCO3 was determined volumetrically and SO4 by the gravimetric method in the laboratory of Environmental Engineering Department in Suleyman Demirel University (Isparta/Turkey). Fluoride analyses of drinking waters were measured Scott Sanchis colourmetric method using Zr-Alizarine Red solution (TSE 1997) and Orion ion electrometer. Previous fluoride data of drinking water were obtained from the State Hydraulic Works (SHW) and Public Health Laboratory (PHL), Isparta, Turkey.
The detection of disease clusters, where high incidence rates are observed around a specific location, has benefited from the development of methods and tools in GIS. Therefore, fluoride contents of Isparta public for water system were evaluated using GIS techniques. ArcGIS software, Spatial Analyst extension and inverse distance weight (IDW) interpolation methods were applied throughout research evaluations. For the research, Quickbird satellite four band multispectral 2.44 m resolution image acquired on the date 15 July 2006 was used. Quickbird satellite is an orthorectified, radiometrically calibrated, corrected for sensor and platform-induced distortions, and mapped to a UTM Zone 36 N, ED50 datum.
Geology and hydrogeology
Isparta city is located in the Lake District of Turkey, and its bedrocks consists Neogene volcanic products of Golcuk volcanism and Eocene and Miocene sediments, Isparta ophiolitic complex, allochthonous and autochthonous limestone. Geological units in the research area and their hydrogeological properties are described in Fig. 1.
Ophiolitic complex is composed of mafic-ultramafic rocks such as serpentinite, harzburgite, gabbro, peridotite and pelagic-terrigeneous sediments as radiolarite, chert, limestone, shale, sandstone (Robertson 1993). The ophiolitic complex has an impermeable rock property. Allochthonous and autochthonous limestones contain some folds–cracks and they are also permeable karstic aquifer. Eocene sediments consist of sandstone, shale, clayey limestone and cherty limestone. Miocene clastics have been formed from the intercalation of sandstone, claystone, marl, shale and overlain by conglomerate which has polygenic pebbles (Gormus and Ozkul 1995). Eocene clastic sediments were deposited as flysch facies and impermeable rock unit. Golcuk volcanites consist of andesite fractured and undergoes partially alteration. Golcuk pyroclastics consist of interbedded with thin-medium layered sediments in the form of sandstone, claystone, marl, tuff and tuffite. Alluvium deposits have occurred dominantly unconsolidated clay, silt, sand and gravel constituents derived from surrounding volcanic units.
Recharge of groundwater mainly occurs via vertical seepage of meteoric water in the research area. Aquifer units discharging the springs are mainly Miocene clastics, Golcuk pyroclastics and volcanic rocks. This pyroclastics were slightly permeable due to tuff levels. They keep in stock some groundwater and its different permeabilities cause a number of aquifers and aquitards lying one above the other. Furthermore, andesite also keeps in stock some groundwater within its fractures. In addition, springs discharged from conglomerate and shale levels within the Miocene sediments.
Hydrogeochemistry
To follow the research analysis, approximately total 80 samples were collected from waters based on different years for the research area from Golcuk-Egirdir lake water and public water system of Isparta city. Until 1995 from 1962, all of the drinking waters of Isparta city have been obtained from Golcuk lake and Andik spring. Later, due to increasing demand of water, additional drinking water has also been obtained from Egirdir Lake which is located northeast of Isparta, since 1995. Recently, Golcuk lake water and Andik spring water have been only given as drinking water for particular districts of Isparta city.
Spring locations to take water samples for the chemical analyses are presented in a geological map form in Fig. 1. The results of the chemical analyses and water types of the spring-lake waters are listed in Table 1. pH values of spring waters are ranged from 6.2 to 8.3, indicating alkaline nature. Electrical conductivity (EC) values of spring waters were also determined. Their values are between about 544 and 143 μS/cm and temperature were measured between 8.1 and 14.4°C. pH values of Golcuk lake water 7.8–8.2 and electrical conductivity values were measured as 235 and 290 μS/cm. Electrical conductivity values of Egirdir lake are ranged from 402 to 440 μS/cm. The major ions of waters are shown as a Piper triangular diagram in Fig. 2. Based on the Piper classification, spring and lake waters are bicarbonates. Classification of waters was based on the principles of International Association of Hydrogeologists (IAH 1979). The contents of major ions for waters changes are related to their interaction rocks. As a result of water–rock interaction, Na and K ions have increased in the springs discharged from tuff level, due to cation exchange reaction with increasing Na+ concentrations and Ca2+ concentration decreases (Table 1).
The correlation matrixes between the major ions calculated with Aquacem 3.7 computer code are presented in Table 2. Fluoride content showed a positive correlation with a sodium concentration and a strong negative correlation including calcium and bicarbonate concentrations. This situation was also presented in Fig. 3 showing the relationships among F with Ca, Mg, HCO3, Na, K, pH values. Concentration of fluoride in the rock, long residence time of rock water interaction and Ca and HCO3 contents of groundwater are the important factors to determine the degree of fluoride dissolution (Kundu et al. 2001). The solubility of fluoride in waters increases in relation with high concentrations of Na+.
Fluoride concentrations of springs are shown in a geological map with circular diagrams which prepared in different scales according to fluoride content (Fig. 4). Apparently, these contents could change the related to geological structure. The fluoride contents of springs discharged from tuff levels are between 3.71 and 5.62 mg/l. The springs discharged from Golcuk pyroclastic rocks bearing volcanic level also contain a range of 1.81–3.95 mg/l. The fluoride contents of springs discharged from Miocene clastics are between 0.39 and 1.02 mg/l (Table 1; Fig. 5). Generally, fluoride content in groundwater usually depends on rock type, interaction period with host rock, as well as the dissolution kinetics for fluorite, apatite or silicate minerals (Sreedevi et al. 2006).
The fluoride contents of drinking water in Isparta region is strikely related to circulation of water within volcanic and pyroclastic rocks. However, fluoride contents of pyroclastics are much lower than that of volcanics. Mica (phlogopite/biotite), apatite and amphibole are the main fluoride carrying minerals in the research area. The fluormicas (predominantly fluorphlogopite) is predominant the main fluoride source mineral. Hence, high fluoride is attributed to abundance of micas (Coban et al. 2001). However, micas are very efficient at removing fluoride from a melt. Overhead explained data show that, fluoride contents of water are increased with the interaction of water–volcanic rocks (Fig. 5).
Generally, flour generally migrates in form of F− ion in the alkaline nature. Under the circumstances, migrate of flour is supervised by calcium within the water. The solubility limits of fluorite and calcite provide a natural control for water composition. Mineral saturation index for calcite and fluorite were calculated as discharge temperature and pH by the Solmineq 88 (Kharaka et al. 1988) computer code (Table 1). The plot of calcite saturation index (SIc) and fluorite saturation index (SIf) are shown in Fig. 6. Most of the samples were almost over-saturated respect to fluorite. Some of the samples discharged from Miocene clastic and limestone units were only over-saturated respect to calcite.
In different periods, the water samples taken from the Golcuk Lake and Andik stream, supplied drinking water of Isparta city, were examined and the results of fluoride analyses are given in Table 3. The fluoride contents of Golcuk lake water are between 1.4 and 2.65 mg/l, and of the Andik stream water are between 2.61 and 5.3 mg/l (Table 3). The higher fluoride content of the Andik mobile water is possibly originated from more interaction time with volcanic rocks, relative to Golcuk stagnant water.
In addition, Table 3 indicates the fluoride content of the Golcuk and Andik waters decreases in rainy periods while increasing the dry periods for all the years (Fig. 7a, b). The presence of dissolved fluorine is possibly only under favorable physico-chemical conditions and when residence time is long enough. Therefore, the water quantity and residence time were important factors playing a major role in increasing F− concentration of water in a dry period. The residence time of waters with the aquifer materials also significantly regulates the F− concentrations in groundwater (Rommohana Rao et al. 1993; Wodeyar and Sreenivasan 1996; Saxena and Ahmed 2001; Subba Rao 2003). Because, the dissolution rates of fluoride minerals are generally slow (Gaus et al. 2002) and fluoride solubility increase as a relationship with residence time. In the rainy periods, spring waters generally have a sudden discharging tendency as connected with increase of rainfall. Speed of water is usually faster than the dry period. Therefore, the residence time between water with aquifers is short and F− content of groundwater is less in the rainy period. The discrimination is more strike for Andik stream water, considering the precipitation fall into region (Fig. 7a, b).
Spatial distribution of fluoride for drinking water
As in many areas of GIS researches, this type of work could supply a benefit from recent developments in relatively basic and inexpensive equipment (Wright et al. 2004). Considerable recent attention has focused on health in urban areas. GIS has also been used to help predict future public health risks such as those arising from extremely GIS. It has a useful role to play in policy-related studies of health care utilization and accessibility (Arcury et al. 2005; Wang and Luo 2005).
The fluoride contents of public water system in Isparta for health impact assessment were also evaluated using GIS techniques. The fluoride contents of public water system were measured at random locations of Isparta city in 1990 and in 2003 by PHL (PHL 1990–2005). Using this data, the spatial distribution maps for fluoride on the development plan of city have been established for the years of 1990 and 2003. These maps have been prepared using inverse distance weighted (IDW) interpolation methods with ArcGIS Spatial Analyst extension and overlaying with Quickbird satellite image (Figs. 8, 9).
Extreme fluoride values were then measured in public water system. According to 1990 data, the fluoride contents of all drinking waters are ranged between 1.42 and 2.47 mg/l. Overall drinking water of Isparta city were currently taken from Golcuk lake and Andık stream. All of the measured fluoride contents are rather exceeding than the upper limit of fluoride concentration (1.5 mg/l) advised by WHO. In 2003, drinking water was supplied from Egirdir Lake, Golcuk Lake and Andik stream for different districts of Isparta as unmixed and partially mixed. In this year, fluoride contents of drinking water have generally decreased and suitable to WHO. But, the fluoride contents of domestic-drinking water, only obtained from Andik water in some district of city, increased up to 3.6 mg/l (Fig. 9). In addition, fluoride contents of Isparta domestic-drinking water are given in Table 4. The fluoride contents decreased up to 93% ratio depending on the mixing with the different sources of water—the two lakes and the stream. On the other hand, increasing fluoride contents was observed for two drinking water locations because of getting from only Andik stream in 2003 (Table 4).
Health impact assessment
According to upstairs knowledge’s, the high fluoride concentration has notified in determining the public water system of Isparta city. Nowadays, some regions for Isparta city are also threated of health risk although Egirdir lake water has been used as widespread in the city. Therefore, it is suggested that, urgently fluoride poisoning can be prevented or minimized by using alternative water sources, by removing excessive fluoride from drinking waters.
For the study area, alternative water sources could be a surface water and groundwater containing low fluoride. Alluvium is the most important aquifer in Isparta plain located in the city residential area. Wells having irrigation purpose are extant. The measured flow-rates of wells in the alluvium aquifer range between 10 and 40 l/s (Irlayıcı 1993). But, favorable areas which may obtain groundwater for drinking water of Isparta residential area are limited to take into consideration of contamination due to urbanization. The enough quantity drinking water for Isparta obtaining from this area is impossible. Furthermore, the fluoride content of groundwater recharging from volcanic rocks in the Isparta plain was determined as 1.1 and 2 mg/l (Irlayıcı 1993). Therefore, utilizing from surface waters is more reasonable. The drinking water of Isparta city is still partially supplied from Egirdir lake. The fluorine contents of Egirdir lake water are appropriate to WHO drinking water standards. It is suggested that, fluoride contents of Isparta drinking water should be modified by suitable mixed waters of both Egirdir lake having less fluoride and Golcuk lake, or Egirdir lake and Andik water.
Another alternative is also a defluoridation of water. Basically there are two approaches for treating water supplies to remove fluoride; flocculation and adsorption. Hydrate aluminum salts, a coagulant commonly used for water treatment, is used to flocculate fluoride ions in the water. Adsorption, the other approach is to filter the water down through a column packed with a strong adsorbent, such as activated alumina (Al2O3), activated charcoal, or ion exchange resins. But, both the community and household defluoridation systems have pros and cons (Meenakshi 2006). Furthermore, method of defluoridation water was expensive and it has application difficulty.
Conclusion and discussion
High fluoride is a prominent aspect of Golcuk-Isparta region, SW Turkey and causes dental problems. Fluoride in natural waters in the study area originated from the solution of apatite and more commonly from the solution of fluoride-bearing micas and amphiboles. The fluormicas (predominantly fluorphlogopite) is a predominant the main fluoride source mineral. Mainly aquifer units which discharged springs are Miocene clastics, Golcuk pyroclastics and volcanic rocks.
The fluoride contents of springs discharged from volcanic rocks, Golcuk pyroclastic and Miocene clastic are changed between 3.71–5.62, 1.81–3.95 and 0.39–1.02 mg/l, respectively. The fluoride contents of pyroclastic are much lower than that of volcanic. In addition, fluoride concentration changes for dry-rainy seasons as related to the degree of interaction between water and volcanic rocks. Fluoride contents tend to decrease in rainy seasons and to increase in dry seasons for all years. In the research area, fluoride concentrations of groundwater increase in related to residence time in the volcanic-proclastic rocks aquifer thereby having longer contact time for dissolution of fluoride bearing minerals.
In the research area, groundwater is classified as a bicarbonate type from Piper diagrams. Fluoride content showed positive correlation with sodium and negative correlation with calcium concentration. The groundwater analyses showed an over-saturated position respect to fluorite. Some of the samples discharged from Miocene clastic and limestone units were only over-saturated with respect to calcite.
All of the drinking water for Isparta city has been obtained from Golcuk lake and Andik spring from 1962 to 1995, later, it has also been obtained from Egirdir Lake since 1995. Extreme fluoride values were measured in public water system. The fluoride contents of Isparta public water system on behalf of health impact assessment were evaluated by GIS techniques. The some districts of Isparta city affecting high-fluoride water were determined. The drinking water of Isparta city is still partially supplied from Egirdir lake. The fluorine contents of Egirdir lake water are appropriate to WHO drinking water standards. It is suggested that, fluoride contents of Isparta drinking water should be modified by suitable mixed waters of both Egirdir lake having less fluoride and Golcuk lake, or Egirdir lake and Andik water.
References
Arcury TA, Gesler WM, Preisser JS, Sherman J, Spencer J, Perin J (2005) The effects of geography and spatial behaviour on health care utilization among the residents of a rural region. Health Serv Res 40:135–155
Ayhan E (1983) Sulardaki fluorun muhtemel kokeni. A U I Muh Haftasi Smp Turk 15:49–65 (in Turkish)
Balabolkin MI, Mikhailets ND, Labouskaia RN, Chernousova NV (1995) The inter-relationship of the thyroid and immune statuses of workers with long-term fluoride expose. Ter Arkh 67(1):2–41
Cao SR, Li YF (1992) The evaluation of indoor air quality in areas of endemic fluorosis caused by coal combustion. In: Proceedings of the XIX Conference of the International Society for Fluoride Research, Kyoto
Carrillo-Rivera JJ, Cardona A, Edmunds WM (2002) Use of abstraction regime and knowledge of hydrogeological conditions to control high-fluoride concentration in abstracted groundwater: San Luis Potosi basin, Mexico. J Hydrol 261:24–47
Cengiz M, Kır E, Kır I (1998) Investigation of the dispersion of nitrate, nitrite and fluoride in the drinking water sources and lake in Isparta. J Sci Institute of Suleyman Demirel University Isparta 3:69–80 (in Turkish)
Coban H, Caran S, Gormus M (2001) Origin of fluoride within the Afyon-Isparta volcanic district, SW Turkey: is fluormica the key? In: Cidu (ed) Water–rock interaction. Swets & Zeitlinger, Lisse (90 2651 824 2)
Dean HT (1942) Epidemiological studies in the United States. In: Moulton FR (ed) Fluoride and dental health. American Association for the Advancement of Science, AAAS Publication No: 19, Washington DC
Dominguez L, Diaz A, Fornes MW, Mayorga LS (1995) Reagents that activate GTP-binding proteins trigger the acrosome reaction in human spermatozoa. Int J Androl 18(4):203–207
Ermis RB, Koray F, Akdeniz G (2003) Dental caries and fluorosis in low- and high-fluoride areas in Turkey. Quintessence Int 34(5):354–360
Gaus I, Shand P, Gale IN, Williams AT, Eastwood JC (2002) Geochemical modeling of fluoride concentration changes during aquifer storage and recovery (ASR) in the Chalk aquifer, Wessex, England. Q J Eng Geol Hydrogeol 35(2):203–208
Gormus M, Ozkul M (1995) Stratigraphy of the area between Gonen-Atabey (Isparta) and Aglasun (Burdur). J Sci Institute of Suleyman Demirel University 1:43–64, Isparta (in Turkish)
Gou Q, Wang Y, Ma T, Ma R (2006) Geochemical processes controlling the elevated fluoride concentrations in groundwaters of the Taiyuan Basin, Northern China. J Geochem Explor GEXPLO-04434
Gupta SK, Deshpande RD, Agarwal M, Raval BR (2005) Origin of high fluoride in groundwater in the North Gujarat-Cambay region. India Hydrogeol J 13:596–605
IAH (International Association of Hydrogeologist) (1979) Map of mineral and thermal water of Europe, Scale 1:500,000. International Association of Hydrogeologist, London
Irlayıcı A (1993) Hydrogeology of Isparta plain and environmental problems related to groundwater. M Sc Thesis, Suleyman Demirel University, Isparta (in Turkish)
Jacks G, Bhattacharya P, Chaudhary V, Singh KP (2005) Controls on the genesis of some high-fluoride groundwaters in India. Appl Geochem 20:221–228
Karro E, Indermitte E, Saava A, Haamer K, Marandi A (2006) Fluoride occurrence in publicly supplied drinking water in Estonia. Environ Geol 50(3):389–396
Kay BH (1999) Water resources: Health, Environment and Development, E&FN SPON an imprint of Routledge, 249p (ISBN 0 419 22290 1)
Kharaka YK, Gunter WD, Affarwall PK, Perkins EH, De Braall JD (1988) SOLMINEQ (a computer program code for geochemical modelling of water–rock interactions. In: US Geological Survey Water Investigations, Report 88-4227
Kundu N, Panigrahi MK, Tripathy S, Munshi S, Powell MA, Hart BR (2001) Geochemical appraisal of fluoride contamination of groundwater in the Nayagarh District of Orissa, India. Environ Geol 41:451–460
Luke JA (1994) Effect of fluoride on the physiology of the pineal gland. Caries Res, pp 28204
Meenakshi RC M (2006) Fluoride in drinking water and its removal. J Hazard Mater B137:456–463
Mullenix PJ, Denbesten PK, Schunior A, Kernan WJ (1995) Neurotoxicity of sodium fluoride in rats. Neurotoxicol Teratol 17(2):77–169
Ozsvath DL (2006) Fluoride concentrations in a crystalline bedrock aquifer Marathon County, Wisconsin. Environ Geol 50(1):132–138
Pekdeger A, Ozgur O, Schneider HJ (1992) Hydrogeochemistry of fluoride in shallow aqueous systems of the Golcuk area, SW Turkey. In: Proceedings of the 7th international symposium on water–rock interaction, Utah, pp 821–824
Public Health Laboratory (1990–2005) Results analyses of the fluoride content of waters in the Isparta city (in Turkish)
Robertson AHF (1993) Mesozoic-Tertiary sedimentary and tectonic evolution of Neotethyan carbonate platforms, margins and small ocean basins in the Antalya Complex, southwest Turkey. Spec Publs Int Ass Sediment 20:415–465
Rommohana Rao NV, Suryaprakasa Rao K, Schuiling RD (1993) Fluoride distribution in waters of Nalgonda district, Andhra Pradesh, India. Environ Geol 21:84–89
Rukah YA, Alsokhny K (2004) Geochemical assessment of groundwater contamination with special emphasis on fluoride concentration, North Jordan. Chemie der Erde Geochem 64:171–181
Samsar E (1983) Fluoroz. Akdeniz Universitesi Isparta Muh Fak 1 Muh Haft Isparta pp 45–48 (in Turkish)
Saxena VK, Ahmed S (2001) Dissolution of fluoride in groundwater: a water-rock interaction study. Environ Geol 40(9):1084–1087
Sreedevi PD, Ahmed S, Made B, Ledoux E, Gandolfi JM (2006) Association of hydrogeological factors in the temporal variations of fluoride concentration in a crystalline aquifer India. Environ Geol 50(1):1–11
State Hydraulic Works (SHW) (1999–2006) Results analyses of the lake waters (in Turkish)
Subba Rao N (2003) Groundwater quality: focus on fluoride concentration in rural parts of Guntur district, Andhra Pradesh,India. Hydrol Sci J 48(5):835–847
Tohyama E (1996) Relationship between fluoride concentration in drinking water and mortality rate from uterine cancer in Okinawa prefecture, Japan. J Epidemiol 6(4):184–191
TSE (1997) Turkish water intended for human consumption standards (in Turkish). Standard No. 266, Turkish Standard Institute, Ankara
Valenzuela-Vasquez L, Ramırez-Hernandez J, Reyes-Lopez J, Sol-Uribe A, Lazaro-Mancilla O (2006) The origin of fluoride in groundwater supply to Hermosillo City, Sonora, Mexico. Environ Geol 51:17–27
Wodeyar BK, Sreenivasan G (1996) Occurrence of fluoride in the groundwaters and its impact in Peddavankahalla Basin, Bellary district, Karnataka, India—a preliminary study. Curr Sci 70:71–74
World Health Organization (1984) Fluoride and fluorides, Environmental Health Criteria No: 36, Geneva
Wright JA, Gundry SW, Genthe B, Preez M, Moyo S, Potgieter N, Ndamba J (2004) Use of handheld computers for collecting water quality data in developing countries. Water Int 29:517–522
Wang FH, Luo W (2005) Assessing spatial and nonspatial factors for healthcare access: towards an integrated approach to defining health Professional shortage areas. Health Place 11:131–146
Yalcınkaya S (1989) Geology of Isparta—Aglasun (Burdur). PhD thesis, Istanbul University
Zeiger E, Shelby MD, Witt KL (1993) Genetic toxicity of fluoride. Environ Mol Mutagen 21(4):309–318
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davraz, A., Sener, E. & Sener, S. Temporal variations of fluoride concentration in Isparta public water system and health impact assessment (SW-Turkey). Environ Geol 56, 159–170 (2008). https://doi.org/10.1007/s00254-007-1148-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-007-1148-1