Abstract
Introduction
Management of ventriculomegaly in pediatric patients with syndromic craniosynostosis (SC) requires understanding the underlying mechanisms that cause increased intracranial pressure (ICP) and the role of cerebrospinal fluid (CSF) in cranial vault expansion in order to select the best treatment option for each individual patient.
Methods
A total of 33 pediatric patients with SC requiring craniofacial surgery were retrospectively evaluated. Cases of nonsyndromic craniosynostosis and shunt-induced craniosynostosis were excluded. Six syndrome-based categories were distinguished: Crouzon syndrome, Pfeiffer syndrome, Apert syndrome, cloverleaf skull syndrome, and others (Muenke syndrome, Sensenbrenner syndrome, unclassified). All of the patients were treated surgically for their cranial deformity between 2010 and 2016. The presence of ventriculomegaly and ventriculoperitoneal (VP) shunt requirement with its impact in cranial vault expansion were analyzed. Clinical and neuroimaging studies covering the time from presentation through the follow-up period were revised. The mean postoperative follow-up was 6 years and 3 months. A systematic review of the literature was conducted through a PubMed search.
Results
Of the total of 33 patients with SC, 18 (54.5%) developed ventriculomegaly and 13 (39.4%) required ventriculoperitoneal (VP) shunt placement. Six patients (18.2%) required shunt placement previous to craniofacial surgery. Seven patients (21.2%) required a shunt after craniofacial surgery. Seven fixed pressure ventriculoperitoneal shunts and six programmable valves were placed as first choice. All patients improved their clinical symptoms after shunt placement. Aesthetic results seemed to be better in patients with programmable shunts.
Conclusions
Unless clear criteria for overt hydrocephalus are present, it is recommended to perform craniofacial surgery as a first step in the management of patients with SC in order to preserve the expansive effect of CSF for cranial vault expansion. In our experience, the use of externally programmable valves allows for the treatment of hydrocephalus while maintaining the expansive effect of CSF for the remodeling of the cranial vault. Prospective evaluations are needed to determine causality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Both alterations in cerebrospinal fluid (CSF) hydrodynamics and craniosynostosis lead to increased intracranial pressure (ICP) but with an opposite effect on the growth of the skull vault.
The aim of this study was to determine when the treatment of ventriculomegaly becomes mandatory in patients with SC and which intervention is suggested so as to maintain the expansive effect of the CSF needed to favor remodeling of the skull vault.
Material and methods
A retrospective, observational study was conducted to evaluate the management of ventriculomegaly in pediatric patients with SC at Hospital de Pediatría SAMIC “Prof. Dr. Juan P. Garrahan” in Buenos Aires, Argentina, between 2010 and 2016.
Only patients with craniosynostosis with two or more closed sutures associated with a set of facial and body abnormalities caused by a characteristic genetic disorder were included. Six syndrome-based categories were distinguished according to clinical and radiological exams (phenotypic diagnosis): Crouzon syndrome, Pfeiffer syndrome, Apert syndrome, cloverleaf skull syndrome, and others (Muenke syndrome, Sensenbrenner syndrome, unclassified).
A total of 33 patients with SC requiring craniofacial surgery were evaluated. Craniofacial surgery was defined as fronto-orbital advancement (FOA) and posterior cranial vault expansion (PCVE) consisting of biparietal-occipital cranial expansion and/or distraction with springs. The presence of ventriculomegaly and ventriculoperitoneal (VP) shunt requirement with its impact in cranial vault expansion were analyzed.
In our series, raised ICP was diagnosed by non-invasive ICP monitoring, performing clinical and neuroradiological assessment in all patients.
Additionally, a systematic review of the literature was conducted through a PubMed search of original articles, case reports, and reviews using the keywords “syndromic craniosynostosis,” “hydrocephalus,” “ventriculomegaly,” “craniofacial surgery,” and “endoscopic third ventriculostomy”.
Results
From a total of 33 patients with SC, six syndrome-based categories were distinguished: Crouzon syndrome (16), Pfeiffer syndrome (6), Apert syndrome (4), cloverleaf skull syndrome (4), and others (Muenke syndrome 1, Sensenbrenner syndrome 1, unclassified 1).
SC was associated with ventriculomegaly in 18 of the patients. In eight of these 18 patients, ventriculomegaly was observed previous to craniofacial surgery. The remaining 10 patients developed ventriculomegaly after craniofacial surgery; two after fronto-orbital advancement and eight after posterior cranial vault expansion (Table 1).
Of the total of 18 patients with syndromic ventriculomegaly, 13 required ventriculoperitoneal (VP) shunt placement (6 cases with Crouzon syndrome, 4 with Pfeiffer syndrome, 2 with cloverleaf skull syndrome, 1 unclassified) (Table 2).
Six patients required shunt placement previous to craniofacial surgery: three of them because of clinical signs (fundoscopic alterations, bulging fontanelle, Parinaud’s syndrome) and/or symptoms (headache, vomiting, sensory loss) of raised ICP and the three remaining patients because of signs of increased ICP on neuroimaging studies (progressive ventricular dilation, periependymal edema, effacement of the subarachnoid spaces and basal cisterns, poor bone quality) despite being asymptomatic. Seven patients required a shunt after craniofacial surgery due to progressive ventriculomegaly associated with signs and symptoms of raised ICP and in one patient due to an uncontrolled CSF fistula through the surgical wound after incidental dural laceration during the fronto-orbital advancement. Seven fixed pressure ventriculoperitoneal shunts and six programmable valves were placed as first choice (Table 3).
All patients improved their clinical symptoms after shunt placement. Aesthetic results were poor in patients with fixed ventriculoperitoneal shunt; in four of them, a second surgery for posterior cranial vault expansion was necessary (Table 4). On the other hand, in patients with programmable valves, no complications were seen, and no surgical revision procedures were required. Both functional and aesthetic results were good in these patients. The mean follow-up was 6 years and 3 months.
Discussion
Intracranial hypertension in SC: pathophysiology and clinical features
Intracranial hypertension is always present in SC and is multifactorial [1, 16, 17]. Understanding of its pathophysiology to make a correct diagnosis is essential.
Three factors seem to be involved in the increased ICP in these patients [2, 32, 35]:
-
A
Upper airway obstruction related to midface hypoplasia and secondary hypercapnia and vasodilation altering the mechanisms of cerebral autoregulation
-
B
Decreased cranial volume secondary to craniosynostosis
-
C
Abnormal intracranial CSF hydrodynamics that may lead to hydrocephalus
Patients with SC have been shown to have narrowing of the skull base foramina (jugular foramen) with the consequent venous congestion and increased venous pressure that causes a decrease in CSF absorption [6, 20]. Already in 1984, Sainte-Rose et al. stated that patients with craniosynostosis have increased vascular resistance leading to raised ICP, associated or not with ventriculomegaly according to the “distensibility of the cranial vault.” In this latter case, the condition is referred to as “pseudotumor cerebri.” The authors describe performing (in 3 patients) an anastomosis between the transverse sinus and the extracranial internal jugular vein bypassing the stenosis at the level of the jugular foramen and thereby decreasing the ICP and ventricular size within the period of 1 year. Nevertheless, clinical symptoms and visual impairment did not allow for a longer waiting time in two other cases, and VP shunt placement was necessary [28, 31]. Following the same line of thought, Cinalli et al. refer to a pseudotumor-like state in patients with increased ICP without ventricular dilatation due to the early and simultaneous closure of different sutures [6].
In addition, in these patients a small posterior fossa with a reduced area of the foramen magnum is observed (“crowded posterior fossa,”) especially in those with premature closure of the lambdoid sutures. Mutations in the gene coding for fibroblast growth factor receptor 2 (FGFR2) may be responsible for premature closure of lambdoid sutures and reduction in foramen magnum area, predominantly seen in children with Crouzon and Pfeiffer syndrome [8, 9], with the subsequent Chiari I malformation (CIM). This generates a posterior fossa disproportion with disappearance of the cisterna magna and herniation of the cerebellar tonsils. The cisterna magna normally functions as a shock absorber, which absorbs the pulse pressure of the CSF coming from the cranial side. If this shock-absorbing capacity is lost, the pressure wave propagated through the central canal is markedly increased creating a pressure gradient at the foramen magnum. This increased CSF output resistance contributes both to the hydrocephalus, as the outflow through the fourth ventricle is blocked [7, 10, 14], and to the syringomyelia observed in some of these patients [4, 16, 29] (Fig. 1).
In fact, of the total of 33 patients with SC of our series, 13 (39.4%) required ventriculoperitoneal (VP) shunt placement (6 cases with Crouzon syndrome, 4 with Pfeiffer syndrome, 2 with cloverleaf skull syndrome, 1 unclassified). None of the patients with Apert syndrome required VP shunt (Table 2). According to our results and in agreement with the literature, in patients with Apert syndrome, VP shunt placement is rarely necessary as they used to present with tetraventriculomegaly with no active hydrocephalus because of jugular foramen stenosis and non-reduction of foramen magnum. Cinalli et al. also explain this phenomenon by the fact that in patients with Apert syndrome, sagittal and lambdoid suture involvement is rare and usually late and that fusion of the skull base synchondroses occurs later as well [5, 6].
In our series, 11 of the 13 patients who required VP shunt placement had a posterior fossa showing craniocerebral disproportion and had been diagnosed with a Chiari I malformation (Table 4). Only two of them required decompressive surgery of the posterior fossa because of central apnea confirmed by polysomnography (PSG) (patients 5 and 7).
All these findings support that the etiology of the raised ICP in this condition is multifactorial [2, 17, 22].
Ventriculomegaly and even dilation of the temporal horns are often seen in patients with SC. Eide suggests that the size or changes in size of the cerebral ventricles are not reliable predictors of increased ICP [13]. Indeed, after craniofacial surgery increased, ventricular size is observed and should not “a priori” be considered as progressive hydrocephalus, as it may be related to the remodeling of the cranial vault and thus stabilizes thereafter [10]. In our series, we observed how fundoscopy improves regardless of the initial ventricular size after craniofacial surgery. The ventricles gain enough space to dilate after cranial vault expansion with a concomitant decrease in ICP.
Therefore, in these patients the concepts of ventriculomegaly and hydrocephalus should be well analyzed and differentiated. Ventriculomegaly is strictly defined as an isolated ventricular dilation (Evans Index ≥0.3) on neuroimaging without radiological signs of raised ICP.
The presence of ventriculomegaly is necessary but not sufficient to define hydrocephalus. Hydrocephalus is defined as progressive enlargement of the ventricle size which, in the majority of the cases, is associated with ballooning of ventricle horns, periependymal edema, and effacement of subarachnoid spaces, together with clinical signs and/or symptoms of raised ICP [3, 13, 25] (Fig. 2).
Treatment strategies
Syndromic craniosynostosis implies a functional and aesthetic disorder. The first goal is to treat intracranial hypertension, and the second goal is to restore patients’ appearance. As we already mentioned, both hydrocephalus and craniosynostosis lead to increased ICP, but their treatment may have an opposite effect on the growth of the skull vault. While the posterior and anterior cranial fossae are enlarged to restore the intracranial volume, treating hydrocephalus may lose the expansive effect of the CSF for cranial vault remodeling. Three different scenarios could be described in order to adjust the most suitable treatment option for each one.
-
I
Intracranial hypertension and slit ventricles: pseudotumor-like state.
-
II
Intracranial hypertension and non-tense ventriculomegaly.
-
III
Intracranial hypertension and hydrocephalus.
In patients who present with slit ventricles and papilledema (scenario I), an urgent decompressive craniofacial surgery is indicated in order to avoid the risk of severe or progressive visual loss.
As it was already mentioned, in patients who present with ventriculomegaly, the adequate interpretation of whether this ventriculomegaly is a non-tense ventriculomegaly or an overt hydrocephalus is essential. Ventriculomegaly per se does not require shunting (scenario II).
Hydrocephalus (scenario III) should be treated urgently with a VP shunt placement if the patient’s life is at risk and to avoid cognitive delay and visual loss (because of papilledema) and improve ventilatory disorders when they occur [17, 26, 30, 33]. Initially, in our patients, collapse of the ventricles was observed after fixed pressure shunt insertion, losing the expansive effect of the CSF for cranial vault remodeling and thereby leading to a poor cranial vault expansion (Fig. 3) [18, 27]. Of the total patients in whom a fixed pressure shunt was placed, three patients (patients 5, 16, and 30) presented with symptomatic shunt dysfunction because of blockage of the proximal catheter after ventricle collapse. Two of these patients (patients 5 and 30) suffered shunt infection due to multiple shunt revision. In one patient (patient 1), a subdural hematoma was diagnosed in a postoperative CT scan, because of overdrainage. To avoid surgery, the patient remained for 1 week in Trendelenburg position and with an abdominal girdle in order to increase the intra-abdominal pressure and reduce the CSF drainage. The hematoma remained stable and was completely reabsorbed after 1 month.
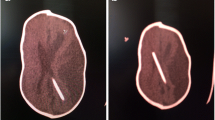
Preoperative (a) and postoperative (b) three-dimensional brain CT scan of a patient with SC associated with hydrocephalus who underwent posterior cranial vault expansion and placement of a ventriculoperitoneal shunt with a fixed pressure setting valve. Postoperative photo (c) showing poor cranial vault expansion due to the loss of the expansive effect of the CSF on cranial vault remodeling
In order to avoid a poor cranial vault expansion after fixed pressure shunt placement, we tried to maintain the cranial vault distraction with the use of springs; however, this was not enough (patient 9) [11, 19, 24]. In some patients (patient 5, 7, 9, and 16), we tried performing a second posterior cranial vault expansion in order to restore de cranial volume lost after shunt insertion with incomplete aesthetic result, except for two of them in which we achieved a good aesthetic result because we replaced the fixed pressure valves for programmable valves (patients 7 and 9) (Table 4).
As an alternative to shunt placement, Di Rocco et al. proposed the use of endoscopic third ventriculostomy (ETV). The authors consider ETV the primary surgical option in patients with complex faciocraniosynostosis complicated by hypertensive hydrocephalus in order to maintain the positive effect of the expanding CSF forces for skull enlargement. Nevertheless, they also reported a relatively high failure rate of this procedure and recommend close clinical and radiological monitoring [12].
According to other authors, however, ETV does not resolve increased ICP secondary to impairment of CSF absorption due to jugular foramen stenosis [16, 36, 37]. And added to the ventricular dysmorphism existing in many of these patients, we do not consider this procedure to be the best therapeutic option.
At our institution, during the last 5 years, in patients with signs and/or symptoms of increased ICP secondary to hydrocephalus, we have been opting for the use of programmable differential pressure valves that allow us to treat hydrocephalus while maintaining the expansive effect of CSF for the remodeling of the cranial vault by regulating the valve to the pressure that is best tolerated by the patient, with good vault expansion and aesthetic result (Fig. 4). Flow-regulated valves could be a good alternative as well, although we do not have experience with them at our center [21, 34]. We placed programmable valves in six patients as first choice (patients 3, 8, 17. 19, 20, and 29) and in other two patients in replacement of fixed pressure shunt when performing a second cranial vault expansion because of a poor aesthetic result (patients 7 and 9). The initial pressure setting was 110 mmHg when the shunt was placed for the first time. In one patient because of persisting bulging fontanelle, we lowered the pressure setting to 80 mmHg. In patients in which we replaced the fixed pressure shunt for a programmable valve while performing a second cranial vault expansion, we set the pressure in 140 mmHg in order to gain more volume with the safety that we were working with an distensible cranial vault. We achieved a good aesthetic result in these eight patients with no need of surgical shunt revision and nonsurgical-related complications.
Preoperative (a) and postoperative (b and c) brain CT scan of a patient with SC associated with hydrocephalus who underwent posterior cranial vault distraction and insertion of a ventriculoperitoneal shunt with a programmable valve resolving the hydrocephalus while maintaining the expansive effect of the CSF on the remodeling of the cranial vault with a good aesthetic outcome and cranial vault expansion
Regarding SC and CIM, we do not perform a foramen magnum osteo-ligamentary decompression, unless the patient presents with Chiari signs, such as central apneas in a PSG or characteristics pathological findings in somatosensory evoked potentials (SSEP), or symptoms (nuchalgia, swallowing disorders, paresthesia) as is not yet shown to prevent overt hydrocephalus and could add a potential risk of hindbrain herniation because of supratentorial intracranial hypertension and vascular anomalies seen in these patients [15, 23].
The different treatment strategies proposed for each scenario are summarized in Fig. 5.
In order to provide a correct follow-up of our patients, we performed a CT scan within the first week after craniofacial surgery or shunt placement. If after craniofacial surgery an asymptomatic ventricular enlargement is observed, we repeat a brain CT scan within the first month after surgery in order to diagnose progressive ventricular enlargement that would require shunt placement. For long-term assessment, we preferred complete shunt X-rays and brain and spine MRI once a year, for early diagnosis of shunt failure, syringomyelia, and to rule out signs of raised ICP. We also indicate a complete ophthalmological exam with fundoscopy after 3, 6, and 12 months of surgery and, thereafter, once a year. A neurosurgical consultation is recommended every 2 weeks during the first postoperative month and every 3 months for at least the first year.
In conclusion, management of SC may need to address the presence of intracranial hypertension, ventriculomegaly, and hydrocephalus as well as the functional and aesthetic consequences of these disorders and their treatment implications. In our opinion, unless clear criteria for decompensated hydrocephalus are present, it is recommended to perform craniofacial surgery as a first step in order to preserve the expansive effect of CSF for cranial vault expansion. Immediate and long-term assessment by a multidisciplinary craniofacial team is an important and effective way to detect signs and symptoms of intracranial hypertension in patients who have undergone or will need craniofacial reconstruction. If a patient demonstrates persistent symptoms consistent with high ICP, neuroimaging studies should be obtained to rule out hydrocephalus. In this situation, we found that treatment with programmable valves may be a good alternative, maintaining the expansive effect of CSF for remodeling of the cranial vault with excellent vault expansion. Future prospective multicenter studies would be useful to select the best treatment options for these patients.
Data availability
The authors affirm that all data and materials comply with field standards.
References
Agochukwu NB, Solomon BD, Muenke M (2012) Impact of genetics on the diagnosis and clinical management of syndromic craniosynostosis. Childs Nerv Syst 28(9):1447–1463 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4101189/
Baird L, Gonda D, Cohen S et al (2012) Craniofacial reconstruction as a treatment for elevated intracranial pressure. Childs Nerv Syst 28:411–418 https://www.ncbi.nlm.nih.gov/pubmed/22068642
Borgesen S, Gjerris F (1987) Relationships between intracranial pressure, ventricular size and resistance to CSF outflow. J Neurosurg 67:535–539 https://www.ncbi.nlm.nih.gov/pubmed/3655891
Chang H, Nagakawa H (2003) Hypothesis in the pathophysiology of syringomyelia based on simulation of cerebrospinal fluid dynamics. J Neurol Neurosurg Psychiatry 74(3):344–347 https://www.ncbi.nlm.nih.gov/pubmed/12588922
Cinalli G, Renier D, Sebag G et al (1995) Chronic tonsillar herniation in Crouzon’s and Apert’s syndromes: the role of premature synostosis of the lambdoid suture. J Neurosurg 83:575–582 https://www.ncbi.nlm.nih.gov/pubmed/7674004
Cinalli G, Saint-Rose C et al (1998) Hydrocephalus and craniosynostosis. J Neurosurg 88:209–214 https://www.ncbi.nlm.nih.gov/pubmed/9452225
Coll G, Arnaud E, Selek L et al (2012) The growth of the foramen magnum in Crouzon syndrome. Childs Nerv Syst 28:1525–1535 https://www.ncbi.nlm.nih.gov/pubmed/22872269
Coll G, Abed Rabbo F, Jecko V, Sakka L, Di Rocco F, Delion M (2019) The growth of the posterior cranial fossa in FGFR2-induced faciocraniosynostosis: a review. Neurochirurgie. 65(5):221–227
Coll G, El Ouadih Y, Abed Rabbo F, Jecko V, Sakka L, Di Rocco F (2019) Hydrocephalus and Chiari malformation pathophysiology in FGFR2-related faciocraniosynostosis: a review. Neurochirurgie. 65(5):264–268
Collmann H, Sorensen N, Kraub J (2005) Hydrocephalus in craniosynostosis: a review. Childs Nerv Syst 31:902–912 https://www.ncbi.nlm.nih.gov/pubmed/15864600
Costa M, Ackerman L, Tholpady S et al (2015)Spring-assisted cranial vault expansion in the setting of multisutural craniosynostosis and anomalous venous drainage: case report. JNS Pediatrics 1:80–85. https://doi.org/10.3171/2014.12.PEDS14604
Di Rocco F, Jucá CE, Arnaud E et al (2010) The role of endoscopic third ventriculostomy in the treatment of hydrocephalus associated with faciocraniosynostosis. J Neurosurg Pediatrics 6:17–22 https://www.ncbi.nlm.nih.gov/pubmed/20593982
Eide PK (2003) The relationship between intracranial pressure and size of cerebral ventricles assessed by computed tomography. Acta Neurochir 145:171–179 https://www.ncbi.nlm.nih.gov/pubmed/12632112
Esparza J, Hinojosa J, García Recuero I et al (2008) Surgical treatment of isolated and syndromic craniosynostosis. Results and complications in 283 consecutive cases. Neurocirugía (Astur) 19:509–529 https://www.ncbi.nlm.nih.gov/pubmed/19112545
Fearon JA (2019) Discussion: onset and resolution of Chiari malformations and hydrocephalus in syndromic craniosynostosis following posterior vault distraction. Plast Reconstr Surg 144(4):941–942
Fishman M, Hogan G, Dodge P (1971) The concurrence of hydrocephalus and craniosynostosis. J Neurosurg 34(5):621–629 https://www.ncbi.nlm.nih.gov/pubmed/4326640
Frassanito P, Palombi D, Tamburrini G (2021) Craniosynostosis and hydrocephalus: relevance and treatment modalities. Childs Nerv Syst. https://doi.org/10.1007/s00381-021-05158-z
Golinko MS, Atwood DN, Ocal E (2018) Surgical management of craniosynostosis in the setting of a ventricular shunt: a case series and treatment algorithm. Childs Nerv Syst 34(3):517–525 https://www.ncbi.nlm.nih.gov/pubmed/29110198
Goodrich JT, Tepper O, Staffenberg DA (2012) Craniosynostosis: posterior two-third cranial vault reconstruction using bioresorbable plates and PDS suture lattice in sagittal and lambdoid synostosis. Childs Nerv Syst 28(9):1399–1406 https://www.ncbi.nlm.nih.gov/pubmed/22872255
Hayward R (2005 Oct) Venous hypertension and craniosynostosis. Childs Nerv Syst 21(10):880–888
Henderson D, Budu A, Zaki H et al (2020) A comparison between flow-regulated and adjustable valves used in hydrocephalus during infancy. Childs Nerv Syst 36(9):2013–2019. https://doi.org/10.1007/s00381-020-04552-3
Khanna P, Thapa M, Lyer R et al (2011) Pictorial essay: the many faces of craniosynostosis. Indian J Radiol Imaging 21(1):49–56 https://www.ncbi.nlm.nih.gov/pubmed/21431034
Lin LO, Zhang RS, Hoppe IC, Paliga JT, Swanson JW, Bartlett SP, Taylor JA (2019 Oct) Onset and resolution of Chiari malformations and hydrocephalus in syndromic craniosynostosis following posterior vault distraction. Plast Reconstr Surg 144(4):932–940
Nowinski D, Di Rocco F et al (2012) Posterior cranial vault expansion in the treatment of craniosynostosis. Comparison of current techniques. Child Nerv Syst 28:1537–1544 https://www.ncbi.nlm.nih.gov/pubmed/22872270
Padayachi LC (2016)Non-invasive intracranial pressure assessment. Childs Nerv Syst 32(9):1587–1597. https://doi.org/10.1007/s00381-016-3159-2https://pubmed.ncbi.nlm.nih.gov/27444289/
Passi N, Degnan AJ, Levy LM (2013) MR imaging of papilledema and visual pathways: effects of increased intracranial pressure and pathophysiologic mechanisms. AJNR Am J Neuroradiol 34(5):919–924. https://doi.org/10.3174/ajnr.A3022
Pattisapu J, Gegg C, Olavarria G et al (2010) Craniosynostosis: diagnosis and surgical management. Atlas Oral and Maxillofacial Surg Clin North Am 18:77–91 https://www.ncbi.nlm.nih.gov/pubmed/21036311
Saint-Rose C, LaCombde J et al (1984) Intracranial venous sinus hypertension: cause or consequence of hydrocephalus in infants? J Nuerosurg 60:727–736 https://www.ncbi.nlm.nih.gov/pubmed/6707742
Schijman E (2004) History, anatomic forms, and pathogenesis of Chiari I malformations. Childs Nerv Syst 20:323–328 https://www.ncbi.nlm.nih.gov/pubmed/14762679
Schirmer CM, Hedges TR (2007) Mechanisms of visual loss in papilledema. Neurosurg Focus FOC 23(5): E5. https://doi.org/10.3171/FOC-07/11/E5 https://thejns.org/focus/view/journals/neurosurg-focus/23/5/foc-07_11_e5.xml
Sprujit B, Tasker RC, Driessen C et al (2016) Abnormal transcranial Doppler cerebral blood flow velocity and blood pressure profiles in children with syndromic craniosynostosis and papilledema. J Craniomaxillofac Surg 44:465–470 https://www.ncbi.nlm.nih.gov/pubmed/26857754
Tamburrini G, Caldarelli M, Massimi L et al (2012) Complex craniosynostoses: a review of the prominent clinical features and the related management strategies. Childs Nerv Syst 28:1511–1523 https://www.ncbi.nlm.nih.gov/pubmed/22872268
Thiele-Nygaard AE, Foss-Skiftesvik J, Juhler M (2020) Intracranial pressure, brain morphology and cognitive outcome in children with sagittal craniosynostosis. Childs Nerv Syst 36(4):689–695. https://doi.org/10.1007/s00381-020-04502-z
Thomale UW, Gebert AF, Haberl H, Schulz M (2013) Shunt survival rates by using the adjustable differential pressure valve combined with a gravitational unit (proGAV) in pediatric neurosurgery. Childs Nerv Syst 29(3):425–431. https://doi.org/10.1007/s00381-012-1956-9
Won Choi J, Young Lim S, Shin HJ (2016) Craniosynostosis in growing children: pathophysiological changes and neurosurgical problems. J Korean Neurosurg Soc 59(3):197–203 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4877540
Yadav Y, Mukerji G et al (2009) Complex hydrocephalus (combination of communicating and obstructive type): an important cause of failed endoscopic third ventriculostomy. BMC Research Notes 2:137 https://bmcresnotes.biomedcentral.com/articles/10.1186/1756-0500-2-137
Yadav Y, Parihar V et al (2012) Endoscopic third ventriculostomy. J Neurosci Rural Pract 3(2):163–173 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3409989
Code availability
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This is a retrospective observational study. The Research Ethics Committee of the Hospital de Pediatría SAMIC “Prof. Dr. Juan P. Garrahan” has confirmed that no ethical approval is required.
Consent to participate
Not applicable.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images in Figs. 1, 2, 3, 4, and 5.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric Neurosurgery
Rights and permissions
About this article
Cite this article
Tcherbbis Testa, V., Jaimovich, S., Argañaraz, R. et al. Management of ventriculomegaly in pediatric patients with syndromic craniosynostosis: a single center experience. Acta Neurochir 163, 3083–3091 (2021). https://doi.org/10.1007/s00701-021-04980-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-021-04980-3