Abstract
Purpose
Cerebrospinal fluid diversion via ventricular shunt is a common treatment for hydrocephalus. Change in cranial morphology associated with a sutural fusion has been termed shunt-related or induced craniosynostosis (SRC) or craniocerebral disproportion (CCD). We present a series of patients with SRC who underwent cranial vault remodeling (CVR) and our treatment algorithm.
Methods
Thirteen patients were retrospectively reviewed who had SRC and CVR; 92% of patients had a ventriculoperitoneal (VP) shunt placed for largely intraventricular hemorrhage of prematurity (69% of patients) at a mean age of 2.2 months. The shunt revision rate was 38.4%, and 54% of patients had a programmable shunt placed initially.
Results
The mean age at time of CVR was 3.6 years old. The most commonly affected sutures (CT confirmed) were the sagittal and coronal sutures, with three patients exhibiting pancraniosynostosis. The mean time from placement of the shunt to CT evidence of sutural fusion was 2.0 years. Abnormal head shape was noted in all 13 patients; 11 of these also had either chronic headaches, papilledema, seizures, or behavioral changes in the setting of functional shunt. Mean follow-up after the initial CVR was 3.3 years. No shunt infections were attributed to the CVR. The families of all patients were contacted and reported improvement in head shape with 60% of families reporting improvement in behavior, 75% reported improvement in headaches, and 40% reported decrease in seizure frequency or intensity. Shunt setting or type was not routinely changed after CVR.
Conclusions
Our threshold for CVR in SRC is met when shunt malfunction has been ruled out and there are (1) radiographic evidence of craniosynostosis, (2) signs of increased ICP clinically or radiographically, and (3) cranial dysmorphism, i.e., dolichocephaly. The majority of cases of SRC result in improved cranial morphology in addition to some abatement of the symptoms of increased intracranial pressure. Early involvement of an experienced craniofacial/neurosurgical team could allow for early diagnosis and intervention which may prevent progression to more severe deformities. SRC is a complex entity, with multiple etiologies, and a future study is needed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ventricular shunts are placed by neurosurgeons for hydrocephalus with a variety of indications early in life, the most common being hydrocephalus secondary to intraventricular hemorrhage (IVH) of prematurity. In developing countries, the incidence of congenital hydrocephalus, when neonatal cases of spina bifida is included, idiopathic and acquired, is three to five per 1000 live births [3]. As of 1995, 33,000 ventricular shunts were placed annually in the USA with the prevalence of these shunts greater than 125,000. Three thousand additional shunt-associated procedures are performed annually due to complications including need for revisions, infections, and general malfunctions [2]. This number is up from 16,000 shunt-related operations reported in the USA in 1988 [9] and only expected to rise with increased awareness of the condition. The increasing frequency of ventricular shunt-related procedures has led to a global cost of $1.1 billion in the USA in the year 2000 alone [12].
Generally, the flow of cerebrospinal fluid (CSF) is well controlled with ventricular shunting; however, in certain circumstances, the patient can be “overshunted” resulting in excessively decreased intracranial pressure, loss of tension across cranial sutures, and premature functional fusion of one or more of the cranial sutures [13]. Depending upon the age at shunting and when this physiologic phenomenon occurs, a shunt-induced craniosynostosis (SIC) or craniocerebral disproportion (CCD) can result. For the purposes of this report, however, we will refer to the reported phenomenon as shunt-related craniosynostosis (SRC). Slit-ventricle syndrome (SVS) is an entity that affects 1–5% of shunted patients who have both small, slit-like ventricles on radiographic studies and disabling, recurrent headaches [7], though does not speak to head morphology. SVS, however, can and does occur independently of shunt-related craniosynostosis. The incidence of SRC has been reported at 1% [14], and with the increasing use of ventricular shunts, it is anticipated that more cases of SRC will be reported.
While the phenomenon of SRC has previously been described in isolated case reports over the past few decades [1, 11, 15, 18], there is a dearth of information with regard to complications and outcomes in a larger number of cases. As such, the goal of this study is to expand upon the foundation of previous reports by performing an in-depth analysis of a higher volume of operative patients with SRC and those who have a shunt and premature fusion of their cranial sutures at Arkansas Children’s Hospital (ACH) in an effort to foster increased awareness of abnormal head shape and early involvement of the neurosurgical/craniofacial team. Here, we report a series of 13 pediatric patients and highlight one detailed case report of patients who underwent cranial vault remodeling (CVR) in the setting of a ventricular shunt.
Methods
Institutional Review Board (IRB) approval from our institution was obtained (IRB # 205018). Retrospective chart review was carried out for all patients who underwent craniosynostosis surgery between 2013 and 2016. Inclusion criteria were those that had a ventricular shunt (ventriculoperitoneal (VP), etc.) and a CVR procedure including strip craniectomies. Exclusion criteria included those patients who did not have a shunt or a shunt placed following their CVR. Head shape was extracted from the medical record either by analyzing patient photographs and/or reviewing operative notes of the primary surgeon.
A secure Redcap database was used to compile and analyze data. Descriptive statistics were used. Consent for photography for scientific publication was obtained when appropriate.
Results
Patient demographics and shunt characteristics (Table 1)
Thirteen patients were identified as having underwent CVR for SRC. Of these, 13 patients were identified that had previously undergone shunt placement with subsequent premature fusion of one or more cranial sutures. Of this subset, 10 were males and 3 were females with ages ranging from the day of birth to 0.49 years of age at initial shunt placement and a median age of 0.16 years. Reasons for shunting varied but was predominantly (75%) intraventricular hemorrhage. Eleven patients had (VP) shunts placed with all but one being right-sided and one bilateral. The other two patients had left-sided VP shunt placement. As the date of initial shunt placement spanned a nearly 16-year period, both device trends and surgeon preference contributed to the nearly equal use of both programmable and nonprogrammable shunts at initial placement.
Shunt revisions and valve adjustments
Of the 13 patients included in the study, a total of five patients underwent an operative shunt revision at some point in their care. Three out these five patients also underwent a shunt revision following CVR. The indications for shunt revision, either before or after CVR, were essentially the same. These included symptoms of shunt malfunction, including headaches, nausea, vomiting, lethargy, change in mental status, and/or radiographic evidence of shunt malfunction.
Specifically, patient #3 had three revisions beginning 5 years after CVR related to proximal and distal catheter malfunctions. The shunt valve was not changed. Patient #4 had three revisions starting 1 year after CVR due to distal shunt malfunction and noted to have significant peritoneal adhesions. The shunt was eventually changed to a ventriculoatrial shunt. Originally, he had a Delta 1.5 valve, and this was changed to a programmable shunt Strata set at 1.0 per surgeon’s preference. Patient #5 had six revisions starting 4 years following CVR due to proximal malfunctions. His shunt began at setting of 2.0, but then was lowered to 1.0, approximately 4 months prior to CVR because of dilated ventricles, vomiting, and irritability. No change in the setting was made after CVR.
The remaining two patients who underwent revisions both had nonprogrammable shunts. Patient #2 underwent revision (before CVR) approximately 1 year after initial placement for lethargy and dilated ventricles and found to have proximal shunt malfunction. Patient #8 had both extensive CVR and shunt revision at the same time, because of imaging just before planned CVR that had shown dilated ventricles and intraoperative finding of a proximal catheter malfunction. There is no evidence in our series or others to suggest that the shunt revision rate and CVR are necessarily correlated.
Regarding adjustments in those with programmable shunts, patient #12 started at a 1.5 setting but then presented with seizure-like activity, change in mental status, and a CT revealing chronic subdural hematoma. To attenuate the hematoma, the setting was increased to 2.0, 6 months prior to CVR and not changed afterwards.
Regarding radiographic findings of ventricle size, a total of four patients had slit-like ventricles evident in the most recent CT prior to CVR. Two of these patients had programmable shunt valves. Patient #13 presented to our center at 5 months of age with an initial setting of 0.5, which was increased to 1.5 after a CT scan revealed collapse of the cerebral mantle and subdural hygroma. The shunt setting was not adjusted after surgery. Patient #6 had programmable valve set at 2.0 which was not adjusted at any point but still had slit-like ventricles. The remainder of the patients did not undergo adjustment of their programmable shunt settings, either before or after CVR.
Sutural fusion and cranial vault remodeling (Table 2)
Premature closure of more than one cranial suture was seen in seven patients, with two exhibiting pancraniosynostosis of all eight of the sutures monitored. For all but one patient, records or photographs were reviewed to identify the predominant head shape just prior to CVR. Three patients had plagiocephaly, three dolichocephaly, two trigonocephaly, and two turribrachycephaly. Though head circumference and percentile was not available uniformly, microcephaly was used as descriptor in at least three patient charts we reviewed. The most commonly affected were the sagittal and right coronal sutures, each being fused in nine patients. There were nearly as many patients with single sutures affected (n = 7) as multiple sutures. A range was noted in the age of patients when there was the first CT evidence of sutural fusion. Because of the retrospective nature of the study, some of the older CTs and reports were not available for review and only 8/13 CTs were able to be analyzed. Four patients developed evidence of craniosynostosis at approximately 1 year old or younger and four between 1 and 5 years old. For the patients who developed sutural fusion prior to 1 year old along with dysmorphic shape, it may be more difficult to establish a causal relationship of shunting and sutural fusion, as these children may have developed craniosynostosis regardless if they were shunted or not.
Abnormal head shape was noted among all patients and was most often the presenting complaint from parents. In the preoperative evaluations, the neurosurgeon ensured the shunt was working properly first. In three cases, the shunts were revised prior to CVR, but both symptoms of increased intracranial pressure and abnormal head shape persisted and thus our indications for CVR surgery. Among the observed symptoms, three patients exhibited papilledema, four patients were noted by caregivers to have behavioral changes, five patients had seizures, and the most common symptom of headaches was documented in seven patients. Age at initial CVR ranged from 0.31 to 13.89 years with a median age of 2.85 and a median time from initial shunt placement to CVR of 2.57 years. Seven patients had fronto-orbital advancement (FOA), with six of these patients undergoing simultaneous anterior CVR and one in conjunction with total CVR. Total cranial vault remodeling alone was performed in four patients while the remaining two had posterior CVR and strip craniectomy, respectively. In four patients, a second CVR was necessary. Of these, three initially had FOA with anterior CVR and one had a strip craniectomy. Three of the patients had planned staged procedures knowing they would need both an anterior and a posterior procedure.
Outcomes following CVR (Table 3)
Twelve parents reported improved head shape, 3 of 5 patients previously demonstrating behavioral or cognitive changes had improved, 2 of 5 patients had improvement of seizure frequency, and 6 of 8 reported improvement in headache complaints. Only one patient’s family reported a subjectively negative effect in that their child started to stutter after the operation. Patients will continue to be followed to determine comprehensive outcomes. There were no mortalities and no incidence of shunt infection following the CVR. Of the three patients that had a shunt revision after CVR, none were seemingly related to the surgeries as they all occurred after 90 days following the CVR.
Case reports
Case 1 patient
Patient SB was born at 23 weeks gestational age due to placental rupture and suffered a grade 4 IVH requiring a VP shunt placement at DOL #1 with several intervening revisions. She also had a history of infantile spasms, chronic lung disease, kidney stones, and GERD. The initial shunt was placed at an outside hospital (OSH) and was a Medtronic programmable valve that was placed on the highest flow setting, i.e., 0.5. It was noted at her first visit to our hospital when a head CT was obtained that the left cerebral cortex was collapsing away from the inner table of the skull, see Fig. 1a, b. The setting was put to 1.5, and on the next visit, it was again changed to 2.5. The cranial sutures were noted to be normal by neurosurgery on 7-month-old visit. At her 1-year visit, she was significantly delayed with a head circumference in the 2nd percentile. The CT scan showed fused coronal and sagittal sutures and abnormal head shape. On the scan at 2 years old, she had a closure of her bilateral coronal and sagittal sutures. Plastic surgery first saw her when she was 3 years old, Fig. 2a, b, at which time she was being treated for seizures and the mom stated she was having increasing head-banging behavior. A new CT scan was obtained (Fig. 3a–d) demonstrating pancraniosynostosis and moderate left orbital retrusion, dolichocephaly, and turricephaly. The mother also stated that although delayed in the intervening months between our evaluations, she lost verbal ability. Total CVR with FOA was offered to the family with very clear expectations that this would most certainly increase intracranial volume but that her developmental course would be less predictable after the surgery.
She underwent a FOA/total CVR. No subperiosteal dissection was carried out over the area of the shunt, and perioperative antibiotics were continued throughout her hospital stay. The subperiosteal dissection is shown in Fig. 4a, b, and the removed bone flaps with severe imprinting on the inner table can be seen in Fig. 4c. Creation of the bandeau is seen in Fig. 4d. The finalized remodeled calvarium is shown immediately postop in Fig. 4e, f. Her postoperative course was unremarkable and she was discharged on postoperative day 4. In addition to improved head shape, the caregivers noticed that she regained some of her verbal ability and that the frequencies of her headaches and seizures decreased. Three-month postoperative photographs and early CT scan are shown in Figs. 5 and 6.
a, b After subperiosteal dissection of the scalp and design of the bandeau and osteotomies. Bone flaps to be removed were labeled off with a sterile pencil. c, d Removal of the frontal and parietal bone with obvious imprinting on the inner table of the skull or “copper-beaten appearance.” Early back-table reconstruction of the bandeau with absorbable plates and rivets. All the plating is on the inner table surface so as not to have the imprint of a plate in nonhair-bearing areas. e, f Remodeled bilateral supraorbital rims and total calvarium, note the shunt protected by an island of periosteum and never exposed during the entire procedure
Discussion
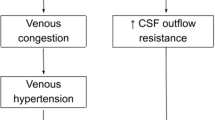
Shunt-related craniosynostosis can be an unfortunate side effect of intraventricular shunting. It is most likely due to excessive thickening of the cranial vault due to loss of tension across the dura and suture lines, although the exact mechanism is still not completely understood. SRC was first reported in the 1960s noted by radiologists on films subsequent to shunting procedures [10, 19], and since that time, there have been over 15 different names associated with the phenomenon that Sandler et al. refer to as CCD [17]. Additionally, it has been shown that up to 37% of shunted children suffer from intense and incapacitating headaches possibly associated with a related manifestation of SRC and CCD, SVS so named for the slit-like appearance of ventricles on imaging [16]. While only 1–5% of patients with radiographically small ventricles are symptomatic, it can severely impact the quality of life and is characterized by a triad of intermittent, yet severe recurrent headaches, small ventricles, and “slow refilling of shunt-pumping devices” in shunted patients that can persist for years [20]. Some authors claim that many cases of patients with SVS are actually CCD with radiologically compressed ventricles due to increased pressure and crowding in a prematurely fused cranium [16]. Due to this crowding, whether in true SVS or CCD, therapeutic approaches hinge on relief of intracranial pressure via cranial vault expansion.
Additionally, Weinzwig et al. [20] reviewed 12 cases of patients with SVS. The mean age was 4.2 years, and patients had undergone an average of nearly five shunt revisions each prior to CVR. The reported mean time for suture fusion was approximately 26.3 months (with a range of 9 to 40 months). More than half of patients (7/12) underwent shunt revisions following CVR, though at less of a rate than prior. However, the timing of the placement of a shunt in relationship to the CVR repair was not addressed. The time to develop SVS to surgical repair in this particular study was typically around 6 years [8]. In this case series, head shape was improved in all 12 patients and neurologic symptoms in 8.
In one of the largest series of eight patients with shunt-induced scaphocephaly, the authors report that in at least two of the patients with CT-proven incomplete fusion, the head shape improved. Moreover, they advocate insertion of a programmable shunt after CVR to account for the dilation of the ventricles after the acute increase in intracranial volume. In their study, the mean time from placement of the shunt to age that abnormal head shape was noted was approximately 4.75 months (range 1 to 7 months) [6].
Multiple methods to treat SRC have been shown to be successful in addition to CVR described above. Spring-assisted cranioplasty has also been shown to reduce the occurrence of shunt-induced dolichocephaly, and Davis et al. argue for the proactive early intervention in shunted patients with abnormal head shape but not yet demonstrating premature fusion to prevent this phenomenon [4]. Posterior cranial vault distraction has also been described to successfully alleviate symptoms and improve head shape [1, 5] in addition to a specific distraction technique coupled with intraoperative continuous ICP pressure monitoring in two patients to prevent underexpansion, symptomatic relapse, and future surgical interventions [17].
When to operate
Few protocols have addressed a systematic way to address a change in head shape in the face of a shunted patient. Sandler and colleagues [17] published a diagnostic therapeutic algorithm and treatment of chronic headaches in shunted patients with functioning shunts. Our approach to the indication for this procedure does not necessarily differ; however, we have found in many cases that offering patients intracranial ICP monitoring can prove logistically difficult and be equivocal in many cases. Our treatment protocol is driven by discrete radiographic findings, clinical exam, and neurological signs and symptoms. These are summarized in Table 4.
No one feature can be an absolute indication for CVR, but any criteria met in any two of three categories were present in all of the cases we presented in this series. Although all 13 patients had CT confirmed suture craniosynostosis, we would argue, in agreement with Weinzweig et al. [20], that even if the suture is not visually fused per CT scan, it may be visibly open but functionally fused due to the pathological interplay between the dura and cranial bone resulting in an abnormal head shape.
With regard to head shape, there did not appear to be one predominant shape and multisuture involvement was about as prevalent as single suture in this series. Moreover, the location of the shunt, with 10/13 being right-sided, parietal or occipital shunts, did not seem to affect which sutures were fused. We would suspect that the head shape abnormalities arise from multiple factors: extrinsic ones including head shape position in the early neonatal period, so as to have the shunt-side up, in addition to intrinsic factors such as alteration of cerebral driving force in children with IVH and perhaps alteration of CSF flow from the shunt itself. Another confounding variable is that it is difficult to determine the exact role of the shunt, as that child may have been destined to develop craniosynostosis regardless, as could be possible in the four patients from this series who had CT evidence of fusion prior to 1 year old. What can be stated, however, is that of the seven patients with multiple sutures involved, none had any of the typical syndromes associated with craniosynostosis such as Crouzon, Apert, Pfeiffer, or even Kleeblattschädel, and thus, a genetic predisposition for sutural fusion may be less likely in these cases. The absence of any known syndrome in this subgroup is suggestive that shunting played a larger role in sutural fusion.
The timing of CVR has also varied in the literature, and as demonstrated by Doorenbosch and colleagues [6], although the abnormal head shape can be recognized and diagnosed anywhere from 1 to 7 months after shunting, the group only operated on the two patients that had CT confirmed sutural fusion and abnormal head shape. From this study and others, we can surmise that head shape may begin to change within months of shunting in an infant, and CT confirmed sutural fusion anywhere between several months to several 3 years following shunting. It is evident after review of this series that further study of a larger sample size with prospective review of head shape and CT is needed to more accurately determine the timing of sutural fusion in the setting of a ventricular shunt. We did note a range in timing and this is likely based not only on when criteria were fulfilled for CVR but to some degree surgeon preference. Based on our experience and universal knowledge of cerebral growth, we would advocate for CVR prior to 3 years of age if SRC is diagnosed but certainly in at least 2 of 3 of the criteria in Table 4 are met once the diagnosis is confirmed.
From a technical perspective, the craniofacial and pediatric neurosurgeon must design the operation with very much the same criteria they would use in a nonshunted craniosynostosis patient. The goal is to leave behind bone flaps that approximate the normal contour, removing all else. Remodeling can then occur in such a way as to expand intracranial volume and provide a “normal” appearing cranium and bandeau. An exception to this may be a situation such as with patient #3, who underwent a strip craniectomy around 4 months of age (2 months after shunt placement) and would have been too young to perform a full posterior CVR. In these cases, we would continue to monitor the patients’ head shape and development and reassess the need for future surgery. Based on the inherent risk in CVR, our practice is not to operate for head shape or CCD alone, but only when abnormal head shape is present in the setting of signs or symptoms of increased ICP as enumerated in Table 4. Thus, any reshaping procedure we perform in this series and in our current practice also includes cranial vault expansion, i.e., FOA or biparietal expansion (in the case of a sagittal fusion for example) into a larger fixed volume with absorbable plates and pins. If a subperiosteal dissection is typically carried out to gain access to the skull, we recommend a “no-touch technique” with regard to the shunt itself, by leaving a periosteal island of several centimeters surrounding the shunt. Appropriate perioperative antibiotics are given and continued for the duration of the inpatient stay as a measure of prophylaxis.
Limitations and further inquiries
As this is a retrospective study, there are limitations that need to be addressed. First, the heterogeneity of the patient population. It is more challenging to draw conclusions about the relationship of shunting to sutural fusion when there were differences in shunt type, surgeon preference, timing of placement, and reason for shunting. The time span over which the initial shunts were placed was approximately 16 years. Moreover, this was not a single-surgeon study, and the decision to operate and, in some sense, the timing represent the aggregate decisions of five different neurosurgeons and three different craniofacial plastic surgeons. In addition, some data such as serial head circumference could not be reliably extracted from the medical record in many cases, making clinical interpretation difficult, if not impossible.
Role of the shunt in sutural fusion and prevention
As the only center in the State that performs ventricular shunts, we have a unique opportunity to prospectively study the phenomenon of SRC and test constantly and adjust our criteria. Using a programmable or nonprogrammable shunt did not seem to have a salient effect on which patients developed SRC and which did not. In the case of the programmable shunts, it is our practice to start with the highest valve setting possible (low flow) at initial placement that will alleviate the hydrocephalus symptoms and then change only as necessary. We do not change the shunt valve or its type and maintain the existing setting in the case of a programmable valve unless it is clinically indicated after CVR. In cases where over drainage remains to be an issue even with appropriate valve setting, other factors may be contributing such as the inherent brain compliance as we can suspect in patients #1 and #6.
.
Moreover, as in the case of many shunted infants, even despite optimal shunt function and CSF flow dynamics, the brain, and by extension the underlying dura, is abnormal and thus cranial suture pathology may occur. As the abnormal head shapes that arise may be multifactorial in nature, there may be no one definitive way to prevent the deformities observed. However, one way to prevent synostosis due to shunt over drainage may be to use a higher resistance setting in a programmable shunt or a higher pressure in a differential pressure valve at initial placement and allow larger tolerated size ventricles and expanded subarachnoid space. More importantly, increased awareness and involvement of the craniofacial/neurosurgical team early on could allow for early diagnosis and intervention and prevent evolution to more severe deformities. Future studies may be needed to further determine the role of CSF diversion, i.e., shunting, CSF dynamics, and cranial suture biology.
References
Bhadkamkar MA, Albright SB, Wolfswinkel EM, Bollo R, Buchanan EP (2015) Posterior cranial vault distraction in the treatment of shunt-induced craniosynostosis. J Craniofac Surg 26:e70–e72. https://doi.org/10.1097/SCS.0000000000001319
Bondurant CP, Jimenez DF (1995) Epidemiology of cerebrospinal fluid shunting. Pediatr Neurosurg 23:254–258 discussion 259
Chi JH, Fullerton HJ, Gupta N (2005) Time trends and demographics of deaths from congenital hydrocephalus in children in the United States: National Center for Health Statistics data, 1979 to 1998. J Neurosurg 103:113–118. https://doi.org/10.3171/ped.2005.103.2.0113
Davis C, Lauritzen CG (2008) Spring-assisted remodeling for ventricular shunt-induced cranial deformity. J Craniofac Surg 19:588–592. https://doi.org/10.1097/SCS.0b013e31816aaa60
de Lima MH, Harshbarger RJ, George TM (2013) Treatment of cephalocranial disproportion in shunt-induced slit ventricle syndrome with cranial vault distraction osteogenesis. Pediatr Neurosurg 49:187–192. https://doi.org/10.1159/000358924
Doorenbosch X, Molloy CJ, David DJ, Santoreneos S, Anderson PJ (2009) Management of cranial deformity following ventricular shunting. Childs Nerv Syst: ChNS: Off J Int Soc Pediatr Neurosurg 25:871–874. https://doi.org/10.1007/s00381-009-0842-6
Epstein F, Lapras C, Wisoff JH (1988) ‘Slit-ventricle syndrome’: etiology and treatment. Pediatr Neurosci 14:5–10
Fong KD, Warren SM, Loboa EG, Henderson JH, Fang TD, Cowan CM, Carter DR, Longaker MT (2003) Mechanical strain affects dura mater biological processes: implications for immature calvarial healing. Plast Reconstr Surg 112:1312–1327. https://doi.org/10.1097/01.PRS.0000079860.14734.D6
Gardner P, Leipzig TJ, Sadigh M (1988) Infections of mechanical cerebrospinal fluid shunts. Curr Clin Top Infect Dis 9:185–214
Kloss JL (1968) Craniosynostosis secondary to ventriculoatrial shunt. Am J Dis Child 116:315–317
Lagrange E, Schafer H (1966) Observations of the course of hydrocephalus following ventriculo-auriculostomy. Description of 3 cases with premature suture synostosis. Dtsch Med Wochenschr 91:1918–1926. https://doi.org/10.1055/s-0028-1111615
Patwardhan RV, Nanda A (2005) Implanted ventricular shunts in the United States: the billion-dollar-a-year cost of hydrocephalus treatment. Neurosurgery 56:139–144 discussion 144-135
Pudenz RH, Foltz EL (1991) Hydrocephalus: overdrainage by ventricular shunts. A review and recommendations. Surg Neurol 35:200–212
Roberts JR, Rickham PP (1970) Craniostenosis following Holter valve operation. Dev Med Child Neurol 12(Suppl 22):145+
Ryoo HG, Kim SK, Cheon JE, Lee JY, Wang KC, Phi JH (2014) Slit ventricle syndrome and early-onset secondary craniosynostosis in an infant. The Am J Case Rep 15:246–253. 10.12659/AJCR.890590
Sandler AL, Daniels LB 3rd, Staffenberg DA, Kolatch E, Goodrich JT, Abbott R (2013) Successful treatment of post-shunt craniocerebral disproportion by coupling gradual external cranial vault distraction with continuous intracranial pressure monitoring. J Neurosurg Pediatr 11:653–657. https://doi.org/10.3171/2013.2.PEDS12404
Sandler AL, Goodrich JT, Daniels LB 3rd, Biswas A, Abbott R (2013) Craniocerebral disproportion: a topical review and proposal toward a new definition, diagnosis, and treatment protocol. Childs Nerv Syst: ChNS: Off J Int Soc Pediatr Neurosurg 29:1997–2010. https://doi.org/10.1007/s00381-013-2257-7
Schendel SA, Shuer LM (1994) Multiple-suture synostosis subsequent to ventricular shunting. Plast Reconstr Surg 93:1073–1077
Strenger L (1963) Complications of ventriculovenous shunts. J Neurosurg 20:219–224. https://doi.org/10.3171/jns.1963.20.3.0219
Weinzweig J, Bartlett SP, Chen JC, Losee J, Sutton L, Duhaime AC, Whitaker LA (2008) Cranial vault expansion in the management of postshunt craniosynostosis and slit ventricle syndrome. Plast Reconstr Surg 122:1171–1180. https://doi.org/10.1097/PRS.0b013e3181858c84
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Institutional Review Board (IRB) approval from our institution was obtained (IRB # 205018). Consent for photography for scientific publication was obtained when appropriate.
Conflict of interest
The authors declare that they have no conflicts.
Rights and permissions
About this article
Cite this article
Golinko, M.S., Atwood, D.N. & Ocal, E. Surgical management of craniosynostosis in the setting of a ventricular shunt: a case series and treatment algorithm. Childs Nerv Syst 34, 517–525 (2018). https://doi.org/10.1007/s00381-017-3648-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3648-y