Abstract
Introduction
Secondary craniosynostosis subsequent to shunting is one of the late complications of ventricular shunt placement in the early childhood. Several interventions have been used to treat high intracranial pressure associated with this condition. This study aimed to evaluate the patients’ clinical symptoms and head circumference before and after a method of decompressive craniotomy, coined as external–internal cranial expansion (EICE).
Methods
A retrospective study was conducted, and the patients who had undergone EICE for the treatment of post-shunt craniosynostosis between 2010 and 2020 were enrolled. This approach was a combination of a hinge multiple-strut decompressive craniectomy and internal cranial flap thinning by drill. Data, extracted from medical records, were used to evaluate the patients’ symptoms and head circumferences before and 12 months after surgery.
Results
A total of 16 patients were enrolled in the study, of which eight were females. Before the surgery, 9 patients (56.2%) suffered from visual impairment, and all had intractable headache. Papilledema was recorded in all, with 3 cases having optic disc paleness. After cranial expansion, only two patients had headaches, diagnosed as migraine-type and psychosomatic headaches, respectively. In two patients, progressive visual impairments got worsening after surgery, which would be due to severe preoperative optic nerve atrophy. Patients’ head circumferences significantly increased after the surgery (mean of 48.97 ± 4.28 cm vs. 45.78 ± 4.31 cm; P value < 0.0001).
Conclusion
In lower resource countries, where newer technologies like distraction osteogenesis is not easily available, external–internal cranial expansion can be considered an effective alternative for patients with post-shunt craniosynostosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ventriculoperitoneal shunts (VP shunts) are considered the main treatment of hydrocephalus since 1950s [1, 3]. Several long- and short-term complications, including infection, subdural hematoma, pneumocephalus, shunt malfunction, dehiscence, and fracture are associated with shunt treatments [2, 3]. Slit ventricle syndrome (SVS), associated with compactness of ventricles secondary to an early phase of overdrainage, occurs in a minority of patients years after VP shunting in infancy [4, 5]. In symptomatic cases, SVS is presented with intermittent and chronic headaches, while it can be associated with secondary craniosynostosis in more severe cases [6,7,8]. Post-shunt craniosynostosis is one of the long-term complications of shunting in young ages, caused by cerebrospinal fluid (CSF) overdrainage and early closure of the skull sutures [9, 10]. In this situation, increased thickness of skull and secondary synostosis may lead to increase in intracranial pressure (ICP) [11,12,13].
Besides symptomatic treatment, several surgical interventions have been proposed for the management of post-shunt craniosynostosis. Different techniques of cranial expansion and decompression have been described [14,15,16]. Although newer techniques like distraction osteogenesis have evolved during the recent decades [17, 18], conventional decompressive surgeries still play a crucial role in lower resource countries where newer methods are not easily available. In the current series, we reported the changes in the head circumference and clinical symptoms of patients with craniocerebral disproportion due to post-shunt craniosynostosis who underwent a modified technique of decompressive craniectomy, coined as external–internal cranial expansion (EICE).
Methods and materials
Study design and setting
The study was retrospectively designed. Patients with craniocerebral disproportion due to post-shunt craniosynostosis who underwent EICE between 2010 and 2020 were enrolled. The patients’ identifications were confidential, and the study was approved by the institutional Ethics Committee (Ethics Code; IR.TUMS.MEDICINE.REC.1398.654).
Study population
Sixteen patients, operated on for post-shunt craniosynostosis, were retrospectively enrolled. Most patients with symptomatic SVS were managed in outpatient setting by medical therapy or lumbar puncture. In total, 51 cases needed admission for intracranial hypertension. All patients were initially checked for shunt malfunction and underwent revision if needed. Those who remained symptomatic despite well-functioning shunts were considered as craniocerebral disproportion and planned for cranial expansion.
The patients’ clinical data including age at the time of initial shunt surgery, symptoms (headache, papillary edema, and impaired vision), head circumference at the time of craniosynostosis surgery, head circumference 12 months after surgery, and clinical improvement of headaches and visual symptoms were extracted from the clinical documents or gathered by in-person contact with the patients or their guardians.
Statistical analysis
IBM™ SPSS® statistics software was used for statistical analysis. Quantitative data were expressed as mean and standard deviation and qualitative data as number and percentage. The Kolmogorov–Smirnov test was used to determine whether the values of patients’ head circumferences are distributed normally. Since the distribution was normal (P value = 0.2), a paired sample T-test was used to evaluate the difference in the head circumferences before and after surgery. The level of significance was considered as P value < 0.05.
Surgical procedure
Patients with craniocerebral disproportion due to post-shunt craniosynostosis, who remained symptomatic despite having a functional shunt, underwent “external–internal cranial expansion” (EICE). The surgical approach, defined by the senior author, F. N., included a combination of multiple-strut hinge decompressive craniectomies for “external expansion” and internal cranial flaps thinning by drill for “internal expansion” (Fig. 1). This combined approach was aimed to address both “suture closure” and “calvarial thickening,” respectively. Under general anesthesia and in supine position, a bicoronal incision was made and supraperiosteal dissection was performed from glabella toward inion. Periosteal flaps were reflected downward in both sides and kept to use after final reconstruction. A bifrontal craniotomy and three to four biparietal strip osteotomies were made, followed by reducing internal thickness of each skull flap by drilling with burr (Fig. 1). As VP shunts are mostly implanted from the occipital entry site in our center, one of the posterior biparietal struts was adjusted so that the ventricular catheter remained in place. The frontal flap was remodeled with slightly anterior inclination to increase the volume of cranial vault. The biparietal flaps were fixed 1 cm above the skull base from one side using microplates, while the other side was left mobile without any fixation. The fixed side for the next strut was alternately changed (Fig. 2). Metal microplates were routinely used, except for 2 patients who were younger than 36 months and underwent EICE using absorbable plates. The periosteal flaps were used to cover the reconstructed bone flaps. Skin closure was done with overtraction in most cases, because of increased calvaria volume.
External–internal cranial expansion approach, consisting of a combination of hinge multiple-strut decompressive craniectomies and internal cranial flap scraping using a microdrill. A Periosteal flaps were reflected downward in both sides, following a bicoronal incision and supraperiostal dissection from the glabella to inion. B, C A bifrontal craniotomy and four biparietal strip osteotomies were made. The most posterior biparietal strut was adjusted according to the implanted VP shunt*. D The internal thickness of each skull flap was reduced using a microdrill. E, F The frontal flap was replaced with slightly anterior inclination, and biparietal struts were fixed 1 cm above the skull base. The periosteal flaps were used to cover the reconstructed bones
Schematic view of surgical reconstruction: forward inclination of the bifrontal flap, and alternate fixation of biparietal strips 1 cm above the skull base from one side while the other side being left free without fixation. Adjustment of the posterior biparietal strut to keep the ventricular catheter in place can be seen in right side
Postoperative surveillance
The patients were given postoperative care in intensive care unit (ICU). They were evaluated in outpatient clinics 2 weeks after discharge and then every 2 months for the next 12 months. Since all patients were cases of hydrocephalus with repeated exposure to irradiation for diagnostic computed tomography (CT) scans, postoperative radiologic re-assessment was not mandatory and only performed on clinical indication.
Results
A total of 16 patients were retrospectively enrolled. They all were cases of post-shunt craniosynostosis and craniocerebral disproportion with persistent symptoms of intracranial hyperattention, despite receiving conservative treatments or shunt revision.
Clinical data of patients are tabulated in Table 1. All patients had shunt placement for primary hydrocephalus during their infancy. The mean age at the time of first shunt placement was 2.56 ± 1.77 months, ranged from less than 1 to 8 months. At the time of cranial decompression, the patients’ mean age was 75.81 ± 38.39 months. Preoperative symptoms included intractable headache and vomiting, papilledema, visual impairment, loss of consciousness, seizure, ophthalmoplegia, and facial palsy (Table 1).
Headache
All patients had headache accompanied with papilledema which remained resistant to medical therapy, lumbar puncture, and shunt revision. The headache in all cases had chronic and intermittent features with on and off fluctuations. The mean duration of headache before EICE was 8.12 ± 2.78 months. After surgery, only two patients suffered from headache (13.3%), while papilledema has been resolved. One of these patients was diagnosed with psychosomatic headaches (case#1), and the other had migraine-type headaches (case#10).
Visual impairments
Preoperative visual records revealed papilledema in all patients and progressive visual impairments in 9 cases (56.2%). Three cases had optic disc pallor. New-onset ophthalmoplegia was detected in 3 patients, consisting of 2 cases with 6th nerve palsy and one case of unilateral ptosis with contralateral 6th palsy.
After expansile surgery, 2 patients with severe visual loss continued to have visual deterioration which would be due to severe optic nerve atrophy. Both patients had shown disc pallor on fundoscopy prior to surgery, and their conditions did not improve after decompression. Papilledema was resolved in other 14 patients within 12 months after EICE surgery (Table 1). The 6th nerve palsy was resolved in one patient (case#8) but remained the same in an infant with bilateral involvement (case#4). The adolescent with unilateral 6th nerve palsy and contralateral ptosis (case#13) improved significantly after surgery.
Seizure, developmental regression, loss of consciousness, and facial palsy
Three cases developed new-onset seizure in preoperative period. Seizure was postoperatively controlled in 2 patients (cases #1 and #7). The other patient (case#12) was a case of post-traumatic hydrocephalus in infancy who suffered from developmental delay. He presented with symptomatic SVS at the age of 5 years. He was scheduled for expansile surgery because of visual impairment, but just the day before surgery, he suddenly developed seizure and loss of consciousness. The patient underwent EICE in an emergent setting. The level of consciousness improved and headache was relieved, but optic atrophy and visual loss persisted and seizure was not well controlled.
The youngest patient in this series (case#4) developed symptomatic craniocerebral disproportion with motor regression and bilateral 6th nerve palsy, less than 1 year since the initial shunting. After EICE surgery, motor regression improved somewhat, but bilateral 6th nerve palsy persisted.
One patient (case#16) preoperatively developed a unilateral peripheral facial palsy (House-Brackman grade V), which improved to grade II within the first month after EICE and completely relieved after 6 months.
Head circumference
The mean head circumference was 45.78 ± 4.31 cm preoperatively, which reached to 48.97 ± 4.28 cm within 12 months of EICE surgery. The rate of increase ranged from 3.5% to 12.5% (7.02 ± 2.48). The difference between the preoperative and postoperative circumferences was statistically significant (P value < 0.0001).
According to the surgery records, the thickness of skull had abnormally were increased in all patients, being 2.5 cm in the thickest parts. The thickness was obviously higher in the posterior sections. However, objective measurements of thickness were not recorded.
Postoperative complications
According to data extracted from the files, all 16 patients underwent intraoperative transfusion and 11 cases (68.7%) needed postoperative transfusion. No new seizure or neurological deficits were detected during postoperative period. One patient (case#16) developed postoperative pulmonary edema which was successfully managed. None of the patients needed further shunt revision or extra interventions. Wound dehiscence occurred in 3 patients, indicating wound closing tension and potential need to tissue expanders allowing for better closure at the wound edges. There was no intra or postoperative mortality in this series.
A case of shunt independence
One female patient (case#11) with persistent symptoms despite several shunt revisions was planned for EICE surgery. However, she developed shunt infection in the last shunt surgery and the parents refused a new shunt implantation. She underwent EICE surgery followed by close observation in ICU for any sign of intracranial hypertension. The symptoms recovered and she remained shunt-independent in 5-year follow-up.
Discussion
History
Pappas in 1963 cited that post-shunt craniosynostosis is a consequence of successful CSF diversion [19]. The very first description of subtemporal craniectomy after multiple ventricular catheter revisions in small ventricles was published in 1974 by Epstien [15]. The term “cephalocranial disproportion” was initially coined by Hoffman in 1976 to describe acquired Chiari-like anomaly long after CSF shunt insertion [20]. Hyde-Rowan used the term “slit ventricle syndrome” in a paper describing re-expansion of small ventricles following insertion of high resistance valves and anti-siphon devices [21]. Afterward, multiple lines of evidence were published to demonstrate the pathophysiology of slit ventricles, shunt-related craniosynostosis, and craniocerebral disproportion.
In addition to sutural fusion, calvarial thickness has been a matter of interest since the introduction of CSF shunts [22]. Moseley designated the term “hyperostosis cranii ex vacuo” to differentiate ubiquitous calvarial thickening due to shunt insertion, in contrast to hyperostosis frontalis interna [23].
SVS spectrum and craniocerebral disproportion
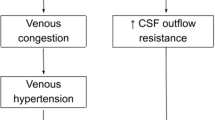
SVS is an important and hard-to-treat late complication of CSF shunting in early childhood. Episodic headaches in patients harboring CSF shunts with small ventricles on neuroimaging comprise the clinical definition of SVS. Although many shunted patients may develop slit-like ventricles, the rate of symptomatic slit ventricles remains low [1]. In his breakthrough study in 1993, Rekate suggested that SVS could be divided into five clinical entities, requiring different therapeutic approaches [24]. These five clinical presentations included low ICP phase, intermittent shunt malfunction, elevating ICP with non-functioning shunt, elevating ICP with functioning shunt (the so-called craniocerebral disproportion), and headache unrelated to shunt function. Over the next years, multiple theories have been proposed about the clinical and pathophysiological spectrum of SVS. Siphoning, ventricular collapse and CSF isolation, acquired craniocerebral disproportion, and venous congestion are among the main theories described [25].
The fourth entity of the above-mentioned spectrum, “elevating ICP with functioning shunt,” is defined as “craniocerebral disproportion” [22], which may compromise interior cranial volume and cause intracranial hypertension due to post-shunt craniosynostosis and calvarial thickening. Hence, different expansile surgeries have been developed to address this pathology [26].
Conventional methods of treatment
Diverse therapeutic approaches used for SVS with or without craniocerebral disproportion can imply to elusive pathophysiology of this problem. Sandler et al. summarized the therapeutic methods into two broad categories: drainage procedures and expansile procedures [26, 27]. Rekate proposed different therapeutic approaches for five clinical subtypes of SVS. Low-pressure headaches may respond to antisiphon device insertion or higher valve resistance. Cisternal or lumbar shunts may be an option for intermittent ICP rises, while antisiphon devices or upgrading the valve resistance may be applied in this subcategory. Persistent high ICP may denote either true ventricular catheter obstruction, for which shunt revision is required, or craniocerebral disproportion. Insertion of a new shunt in a less collapsible area (lumbar, cisternal) and various expansile techniques are the options available for the latter. Such “expansile” procedures aim to increase the internal cranial volume either by bone removal (such as foramen magnum decompression, suturectomy, or subtemporal craniectomy) or by cranial vault remodeling (as cranial morcellation). The last category includes headaches unrelated to ICP and shunt function, which can be treated with anti-migraine therapy [24].
Normally, brain is entrapped in a fixed-size container with minor compliance to overpressuring events such as catheter closure, leading to accumulated pressure inside the brain parenchyma and CSF. Calvarium opening can increase the expansibility of inner contents and avert the compression of vital neurovascular structures (e.g., optic nerve) and devices (proximal VP catheter) by limiting the frequency of ICP fluctuations [15]. Lower rates of post-decompression shunt obstruction, as observed in the current series and other reports, are the clues of such hypothesis. Since expansile surgeries increases the compliance of intra-axial structures and decreases the frequency of both hypotensive and hypertensive events [14, 28], headaches are usually relieved postoperatively.
A relatively new decompression technique, distraction osteogenesis, proposed by Hirabayashi et al. in 1998, uses gradual distraction to correct primary craniosynostosis [17]. Later, Park et al. apply this technique to treat post-shunt craniosynostosis [18]. Even though, such technologies may not be easily available in lower resource countries and modifications of the conventional expansile procedures are still demanded in these areas.
External–internal cranial expansion
This study evaluated the effects of EICE method, as a combination of multiple-strut hinge decompressive craniectomies and internal cranial flap thinning by drilling. The main purpose of this approach was addressing both suture synostosis and skull thickening to achieve a notable increase in the internal volume of the calvaria. In our experiment, considering the mean preoperative and postoperative head circumference of 45.78 and 48.97 cm, the radius of the skull would be 7.29 cm and 7.79 cm, respectively. Hence, it can be inferred from the estimated values that, after 12 months of EICE procedure, the radius of calvaria increased by 0.5 cm on average. Accordingly, the calculated preoperative and postoperative volume will be 1,622.8 cm3 and 1,980.0 cm3. Nevertheless, as the calvarium is not a complete sphere, the estimated volumes are not presentative of the accurate volume of calvaria. Yet, the ratio of preoperative to postoperative estimates can be applicable, indicating 20% increase in volume. Theoretically, 20% increase in cranial volume would result in 20% decrement of ICP (Fig. 3). Apart from sophisticated calculations of the diameters and volumes, a well-stablished philosophy is that at least some extents of reducing ICP roots in disrupting the integrity of the closed “box” of skull. Indeed, these calculations need to be validated through volumetric studies. Even though, in this series, routine postoperative brain CT was not performed to avoid extra irradiation in growing children.
Substantial improvement of headache and visual impairments, estimated increase in the head circumference, and no major morbidity or mortality in this series indicate that EICE modification could be a potentially effective alternative in patients with post-shunt craniosynostosis. Post-operative optic atrophy was observed just in patients who had irreversible preoperative optic nerve damage which led to permanent visual impairment, highlighting the importance of close observation and on-time interventions for at-risk patients. The procedure resulted in shunt independence in one patient, so can be considered a potential alternative for repeated shunt revisions in some cases of sever SVS and recurrent symptomatic shunt obstruction. Nonetheless, to be suggested even in thoroughly selected patients, this approach needs to be evaluated in higher number of patients under ICP monitoring.
Limitations, strengths, and future perspective
The current series revealed safety and efficacy of EICE method for post-shunt craniosynostosis over 12 months of follow-up. Even though, this study is subject to several limitations, including inherent drawbacks of the retrospective design. Moreover, the sample size is too small and heterogenous. Patients of different ages and preoperative conditions were included, while clinical response, postoperative course, and changes in the diameter and rate of head growth may vary by age. Postoperative neuroimaging and volumetric studies were not routinely performed for objective measurements of the internal diameter of skull and size of ventricles. Intraoperative measurements of cranial flaps thickness were not available. Postoperative assessments were evaluated just for 12 months after surgery, while long-term growth indices may have a different course, particularly when patients are at different ages.
Post-shunt craniosynostosis is a rare and late consequence of VP shunting. In the current series, we tried to evaluate the safety and efficiency of a modified expansile surgery in a low-resource setting where access to newer less invasive techniques is limited. Further efforts with larger sample size and longer follow-up, using volumetric studies and objective intraoperative and postoperative measurements, can better delineate all positive and negative aspects of this approach.
Conclusion
External–internal cranial expansion can be considered a safe and potentially effective intervention for patients with post-shunt craniosynostosis. This approach can expand the intracranial volume, increase the pressure buffering capacity of brain, decrease the ICP fluctuations, and improve the longevity of VP device. Although this approach can be suggested as a promising alternative where other modern techniques are not accessible, there is still need for future studies on other technical aspects and safety measures.
Availability of data and material
Queries about the data should be directed to the corresponding author.
References
Albright AL, Tyler-Kabara E (2001) Slit-ventricle syndrome secondary to shunt-induced suture ossification. Neurosurgery 48(4):764–769. https://doi.org/10.1097/00006123-200104000-00013 (discussion 769–770)
Aoki N (1990) Lumboperitoneal shunt: clinical applications, complications, and comparison with ventriculoperitoneal shunt. Neurosurgery 26(6):998–1003 (discussion 1003–1004)
Aschoff A, Kremer P, Hashemi B, Kunze S (1999) The scientific history of hydrocephalus and its treatment. Neurosurg Rev 22(2–3):67–93. https://doi.org/10.1007/s101430050035 (discussion 94–65)
Baskin JJ, Manwaring KH, Rekate HL (1998) Ventricular shunt removal: the ultimate treatment of the slit ventricle syndrome. J Neurosurg 88(3):478–484. https://doi.org/10.3171/jns.1998.88.3.0478
Selman WR, Spetzler RF, Wilson CB, Grollmus JW (1980) Percutaneous lumboperitoneal shunt: review of 130 cases. Neurosurgery 6(3):255–257. https://doi.org/10.1227/00006123-198003000-00005
Epstein F, Lapras C, Wisoff JH (1988) ‘Slit-ventricle syndrome’: etiology and treatment. Pediatr Neurosci 14(1):5–10
Olson S (2004) The problematic slit ventricle syndrome. A review of the literature and proposed algorithm for treatment. Pediatr Neurosurg 40(6):264–269. https://doi.org/10.1159/000083738
Ryoo HG, Kim SK, Cheon JE, Lee JY, Wang KC, Phi JH (2014) Slit ventricle syndrome and early-onset secondary craniosynostosis in an infant. Am J Case Rep 15:246–253. https://doi.org/10.12659/ajcr.890590
Faulhauer K, Schmitz P (1978) Overdrainage phenomena in shunt treated hydrocephalus. Acta Neurochir (Wien) 45(1–2):89–101. https://doi.org/10.1007/bf01774384
Weinzweig J, Bartlett SP, Chen JC, Losee J, Sutton L, Duhaime AC, Whitaker LA (2008) Cranial vault expansion in the management of postshunt craniosynostosis and slit ventricle syndrome. Plast Reconstr Surg 122(4):1171–1180. https://doi.org/10.1097/PRS.0b013e3181858c84
Gault DT, Renier D, Marchac D, Jones BM (1992) Intracranial pressure and intracranial volume in children with craniosynostosis. Plast Reconstr Surg 90(3):377–381. https://doi.org/10.1097/00006534-199209000-00003
Kabbani H, Raghuveer TS (2004) Craniosynostosis. Am Fam Physician 69(12):2863–2870
Martínez-Lage JF, Ruiz-Espejo Vilar A, Pérez-Espejo MA, Almagro MJ, de San R, Pedro J, Felipe Murcia M (2006) Shunt-related craniocerebral disproportion: treatment with cranial vault expanding procedures. Neurosurg Rev 29(3):229–235. https://doi.org/10.1007/s10143-006-0022-z
Eide PK, Helseth E, Due-Tønnessen B, Lundar T (2001) Changes in intracranial pressure after calvarial expansion surgery in children with slit ventricle syndrome. Pediatr Neurosurg 35(4):195–204. https://doi.org/10.1159/000050421
Epstein FJ, Fleischer AS, Hochwald GM, Ransohoff J (1974) Subtemporal craniectomy for recurrent shunt obstruction secondary to small ventricles. J Neurosurg 41(1):29–31. https://doi.org/10.3171/jns.1974.41.1.0029
Gough J, Walker DG, Theile R, Tomlinson FH (2005) The role of cranial expansion for craniocephalic disproportion. Pediatr Neurosurg 41(2):61–69. https://doi.org/10.1159/000085158
Hirabayashi S, Sugawara Y, Sakurai A, Harii K, Park S (1998) Frontoorbital advancement by gradual distraction. Technical note J Neurosurg 89(6):1058–1061. https://doi.org/10.3171/jns.1998.89.6.1058
Park DH, Chung J, Yoon SH (2009) The role of distraction osteogenesis in children with secondary craniosynostosis after shunt operation in early infancy. Pediatr Neurosurg 45(6):437–445. https://doi.org/10.1159/000277618
Kloss JL (1968) Craniosynostosis secondary o ventriculoatrial shunt. Am J Dis Child 116(3):315–317. https://doi.org/10.1001/archpedi.1968.02100020317015
Hoffman HJ, Tucker WS (1976) Cephalocranial disproportion. A complication of the treatment of hydrocephalus in children. Childs Brain 2(3):167–176
Hyde-Rowan MD, Rekate HL, Nulsen FE (1982) Reexpansion of previously collapsed ventricles: the slit ventricle syndrome. J Neurosurg 56(4):536–539. https://doi.org/10.3171/jns.1982.56.4.0536
Yoon MK, Parsa AT, Horton JC (2013) Skull thickening, paranasal sinus expansion, and sella turcica shrinkage from chronic intracranial hypotension. J Neurosurg Pediatr 11(6):667–672. https://doi.org/10.3171/2013.2.Peds12560
Moseley JE, Rabinowitz JG, Dziadiw R (1966) Hyperostosis cranii ex vacuo. Radiology, 87(6):1105-1107 passim. https://doi.org/10.1148/87.6.1105
Rekate HL (1993) Classification of slit-ventricle syndromes using intracranial pressure monitoring. Pediatr Neurosurg 19(1):15–20. https://doi.org/10.1159/000120694
Ros B, Iglesias S, Linares J, Cerro L, Casado J, Arráez MA (2021) Shunt overdrainage: reappraisal of the syndrome and proposal for an integrative model. J Clin Med 10(16). https://doi.org/10.3390/jcm10163620
Sandler AL, Goodrich JT, Daniels LB 3rd, Biswas A, Abbott R (2013) Craniocerebral disproportion: a topical review and proposal toward a new definition, diagnosis, and treatment protocol. Childs Nerv Syst 29(11):1997–2010. https://doi.org/10.1007/s00381-013-2257-7
Di Rocco C, Velardi F (2003) Acquired Chiari type I malformation managed by supratentorial cranial enlargement. Childs Nerv Syst 19(12):800–807. https://doi.org/10.1007/s00381-003-0837-7
Burger R, Duncker D, Uzma N, Rohde V (2008) Decompressive craniotomy: durotomy instead of duroplasty to reduce prolonged ICP elevation. Acta Neurochir Suppl 102:93–97. https://doi.org/10.1007/978-3-211-85578-2_19
Author information
Authors and Affiliations
Contributions
Zohreh Habibi contributed to the supervision, interoperation of data, and final approval. Farid Faraji contributed to the data gathering and contribution to drafting. Esmaeil Mohammadi contributed to the statistical analysis. Keyvan Tayebi Meybodi critically revised the manuscript. Sepehr Ramezani contributed to drafting of the manuscript. Faezeh Aghajani contributed to drafting of the manuscript. Farideh Nejat contributed to the conceptualization and final approval.
Corresponding author
Ethics declarations
Ethics approval
Institutional ethical approval code is IR.TUMS.MEDICINE.REC.1398.654. The study adhered to the tenets of the Declaration of Helsinki.
Consent to participate
Informed consent to use clinical data for research purposes had been taken from parents at admission time.
Consent for publication
Consent for publication was taken from parents, conditioning that the patients’ identity is not recognizable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Habibi, Z., Faraji, F., Mohammadi, E. et al. External–internal cranial expansion to treat patients with craniocerebral disproportion due to post-shunt craniosynostosis: a case series. Childs Nerv Syst 39, 953–961 (2023). https://doi.org/10.1007/s00381-022-05744-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-022-05744-9