Abstract
ε-Poly-l-lysine (ε-PL) is a naturally-occurring l-lysine homopolymer having a broad-spectrum antimicrobial activity and used widely as a food preservative. In the present study, the combined use of immobilization and in situ product removal (ISPR) was evaluated for the production of ε-PL by Streptomyces ahygroscopicus GIM8. Results showed that ε-PL production in the flask cultures decreased from 0.84 to 0.38–0.56 g/L upon immobilization on loofah sponge with different amounts (0.5–3 g in 50 mL medium in a flask). By applying continuous ISPR to the immobilized flask cultures, ε-PL production as high as 3.51 g/L was obtained compared to 0.51 g/L of the control. A satisfactory titer of 1.84 g/L ε-PL could also be achieved with intermittent ISRP (three cycles of ISPR operation during cultivation). Further investigation showed that low levels of ε-PL retained in the broth appeared to favor its biosynthesis. In the repeated-batch fermentation in a 5 L immobilized bioreactor, with continuous ISPR, the final average ε-PL concentration and productivity were 3.35 g/L and 0.797 g/L/day, respectively, and 3.18 g/L and 0.756 g/L/day for the alternative (intermittent ISPR), in comparison to 1.16 g/L and 0.277 g/L/day with no ISPR usage. In the fed-batch fermentation with immobilized cells, the combined use of intermittent ISPR and extra nutrient feeding increased ε-PL concentration and productivity up to 24.57 g/L and 9.34 g/L/day. The fermentation processes developed could serve as an effective approach for ε-PL production and, moreover, the combination could greatly simplify downstream processing for ε-PL separation and purification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
ε-Poly-l-lysine (ε-PL), an extracellular metabolite produced by a variety of Streptomyces strains, is a homo-polyamide of l-lysine (25–35 monomers) characterized by the isopeptide bond between the α-carboxyl and ε-amino groups [1]. It has a high isoelectric point (approximately 9.0) in water due to the presence of many free amino groups along its backbone [2], and thus exhibits multi-cation characteristics under acidic and slightly alkaline conditions. Due to its polycationic nature, ε-PL exhibits antimicrobial effects against a broad spectrum of microorganisms, including bacteria, molds, yeasts, and some viruses [3, 4]. Moreover, ε-PL is biodegradable, water soluble, and thermally stable, and has non-toxicity to humans and the environment [5, 6]. The above-mentioned properties of ε-PL have enabled its application as a natural and safe preservative in various foods. Additionally, ε-PL has diverse applications in the pharmaceutical, cosmetic, and electronics industries [7].
The mechanism of ε-PL biosynthesis has attracted considerable interest since its discovery. The l-lysine monomer, which is suggested to be synthesized through the diaminopimelate pathway in the ε-PL-producing Streptomyces strains [8], has been proven to be the direct precursor for ε-PL biosynthesis [9, 10]. A recent study showed that the biosynthesis of ε-PL is ATP-dependent, and catalyzed by a highly unusual non-ribosomal peptide synthetase through a series of complex reactions [11]. Despite these improvements, the biosynthetic pathway of ε-PL has not been fully elucidated to date, which has hindered technical improvements to the fermentation performance of ε-PL-producing strains through molecular biology technologies [12]. In this context, efforts are ongoing to improve ε-PL fermentation process with a focus on strain breeding [13], culture medium optimization [14], and process regulations [15, 16]. The reported highest production of ε-PL reached 59.5 g/L in a fed-batch fermentation using a bred hyper-strain [13]. Applying an acidic pH shock strategy in the fed-batch fermentation on glycerol also generated ε-PL concentration as high as 54.70 g/L [17].
It has been reported that during fermentation of ε-PL by some Streptomyces strains, high cell density is beneficial for its production [18]. As to culture conditions, the culture medium pH is an important process parameter, which significantly affects ε-PL biosynthesis, and therefore a two-stage pH control strategy has been developed to achieve a balance between cell growth and ε-PL biosynthesis [19]. Further investigation demonstrated that acidic conditions could inactivate the ε-PL-degrading enzyme of ε-PL producers, which is suggested to be responsible for ε-PL accumulation [20]. Dissolved oxygen level also markedly affects ε-PL production, and the optimal oxygen saturation is reported to be 20% in the production stage [15].
Cell immobilization is a useful technique for enhancing the product titer and productivity during microbial fermentation. Compared to free-cell fermentation, immobilization of cells offers several obvious advantages, such as increased cell density [21], better tolerance against changing environment [22], increased resistance against toxic substances [23], reduced contamination problems [24], and repeated use of cell mass [25]. Immobilization is usually achieved by physical adsorption and polymeric gel entrapment [26]. Compared to the gel entrapment method, the adsorption method is simple to operate and its operational cost is minimal [27]. Immobilization by adsorption has been used for ε-PL fermentation, with the significant improvement of yield and the great potential for multiple use of immobilized cells [28].
Metabolic feedback inhibition is a regulatory mechanism in which the end-product inhibits its own synthesis when its concentration reaches a threshold level during fermentation. This inhibition effect is common. In situ product removal (ISPR), which involves sequestering the inhibitory product from the vicinity of fermenting cells immediately after its production, is an effective method to overcome feedback inhibition [29]. ISPR can also prevent the metabolites from exerting their toxic effects on the fermenting cells and stabilizes easily degradable products [29, 30]. Currently, there are a variety of recovery techniques, including solvent extraction, adsorption, diffusion dialysis, membrane separation, and electrodialysis [31]. Application of suitable ISPR techniques in fermentation has been shown to greatly improve the titer of several antibiotics, such as josamycin [32] and pristinamycin [33]. Previously, we had demonstrated that a resin-based ISPR method was effective in promoting ε-PL production [34].
This study aimed to enhance ε-PL production by Streptomyces ahygroscopicus GIM8 through simultaneous application of cell immobilization and ISPR techniques. Cell immobilization was employed because it can increase cell density and operational stability, and immobilized cells hold great potential to be used repeatedly. Furthermore, a cell-free culture broth as a result of immobilization would greatly facilitate in situ removal of ε-PL from the broth. In the combined system, ISPR is expected to avoid the toxic effect of ε-PL against producing cells, and also to reduce the number of steps required for product recovery. Initially, the effect of continuous or intermittent ISPR on ε-PL production was investigated in immobilized cultures in flasks. To establish a theoretic basis for the difference in yield improvement between continuous and intermittent ISPR, the effect of ε-PL on its further biosynthesis was also determined. Then, repeated-batch or fed-batch fermentation with immobilized cells and ISPR was examined for ε-PL production in a 5 L bioreactor as a preliminary study for potential industrial applications.
Materials and methods
Microorganism
The ε-PL-producing strain Streptomyces ahygroscopicus GIM8, previously isolated from soil by our group [35], was used throughout this study. It was maintained at the China Center for Type Culture Collection with collection number CCTCC M2011191.
Media
The strain was cultured in M3G medium according to Kahar et al. [19], with the following components: 50 g glucose, 5 g yeast extract, 10 g (NH4)2SO4, 1.36 g KH2PO4, 0.8 g K2HPO4, 0.5 g MgSO4·7H2O, 0.04 g ZnSO4·7H2O, and 0.03 g FeSO4·7H2O in 1 L distilled water, for culturing seed and ε-PL fermentation. The glucose feed comprised of 500 g glucose and 50 g ammonium sulfate in 1 L distilled water. The two components were separately prepared, autoclaved, and mixed after cooling. The nutrient feed was a concentrated yeast extract solution of 200 g/L, which was autoclaved at 115 °C for 30 min.
Inoculum preparation
A loop of spores scraped from the slants of 5-day-old cultures was transferred to 50 mL medium in a 250 mL shake flask, and cultured at 30 °C and 190 rpm for 20 h. The culture obtained was used as the inoculum in fermentation.
Processing of resin
Cationic ion-exchange resin D152 (Guangfu Fine Chemical Research Institute, Tianjin, China) was processed as described by Wang et al. [36], with slight modifications. Briefly, fresh D152 resins were soaked in 95% ethanol for 2 h with gentle stirring to remove the impurities, filtered and thoroughly washed with distilled water. Next, the resins were treated with three cycles of 1 M NaOH and then 1 M HCl, and finally rinsed with distilled water until neutral pH was achieved. Each of 2 g of the treated resins was wrapped in a 60-mesh nylon cloth, tied, and sterilized in distilled water. For its use in a column, the resin was packed, and then autoclaved at 121 °C for 20 min.
Immobilized-cell fermentation in flasks
The easily available, inexpensive, and renewable matrix loofah sponge (Luffa cylindrical) was used as the immobilization material. The matrix was cut into cubes with a side length of 1.2 cm. To determine the optimal loofah sponge amount for immobilized fermentation, a 250 mL flask containing 50 mL M3G medium and various amounts (0.5–3.0 g) of loofah sponge was sterilized together. After inoculation with 2 mL of the seed culture, the flasks were cultured for 4 days at 30 °C and 190 rpm. The flask lacking the loofah sponge served as the control (free-cell fermentation).
ISPR operation in flasks
For continuous ISPR, a sterilized resin bag was transferred into the flasks when the immobilized cells had initiated ε-PL biosynthesis (approximately 36 h after the start of fermentation), and maintained in the culture till the end of the fermentation. Herein, a quantity of 2 g resin in a bag used for each flask was sufficient for in situ removal of the produced ε-PL under the experimental conditions. For intermittent ISPR, a sterilized resin bag was placed into the flasks at specified time points during culturing. After complete (less than 30 min) or partial recovery of ε-PL from the broth, the bag was withdrawn for ε-PL measurement, which was designated as one cycle of ISPR operation. Such a procedure was performed repeatedly according to the experimental design.
Experimental setup for immobilized fermentation with ISPR
Figure 1 shows the schematic diagram of the experimental setup for the coupled immobilized fermentation–product recovery process. The integrated system comprises a 5 L stirred-tank bioreactor (BioSTAT B5, B. Burn Company, Germany), cylindrical loofah sponges (8 pieces), an ion-exchange column, three peristaltic pumps (Baoding Longer Precision Pump Co., Ltd., Hebei, China), a self-made 4-layer gauze filter, desorption agent reservoir, and effluent collector. Cylindrical loofah sponge with 8 cm diameter and 10 cm length (7.5 g/piece, dry weight basis) were affixed to the baffles and impellers of the bioreactor, which divided the loofah sponge bed into upper and lower sections with each section comprising four pieces of the loofah sponge. The inner diameter and length of the column were 8 cm and 75 cm, respectively, and packed with 600 g of D152 resin. The gauze filter was used to separate the cells from the broth to ensure the passage of a cell-free broth through the resin column.
Experimental setup of the integrated immobilized fermentation and product recovery system. A Air compressor; B air filter; C 5 L stirred-tank bioreactor; D cylindrical loofah sponge; E custom-made 4-layer gauze filter; F peristaltic pump; G ion-exchange column; H desorption agent reservoir; I effluent collector
Bioreactor culture conditions
For all bioreactor studies in this work, the seed culture (300 mL) was inoculated into the bioreactor containing 2.7 L M3G medium with initial pH 6.8. The aeration rate was 2.0 vvm, and temperature was maintained at 30 °C. The pH was measured online using a pH electrode. The cultures were not agitated throughout the fermentation. An anti-foaming agent KM-70 was automatically added to the culture broth to inhibit the formation of foam during the process of fermentation.
Batch fermentation
Batch fermentation with immobilized cells was performed in the integrated bioreactor system shown in Fig. 1. A bioreactor without the loofah sponge bed was used for free-cell fermentation as control. For both types of fermentation, there was no agitation and broth circulation, and the pH of the medium was not controlled. The fermentation was terminated after 6 days.
Repeated-batch fermentation with immobilization and ISPR
The apparatus shown in Fig. 1 was used. After the start of fermentation, when the pH of the culture medium decreased to 3.8, ammonia solution at 10% (v/v) was pumped into the bioreactor, and maintained this level afterwards. After 5 days, the exhausted medium was drained, and the loofah sponge with the immobilized cells was left for the second batch. The second and subsequent batches were started by replenishing the bioreactor with 3 L fresh M3G medium. The fermentation duration for all batches, except the initial batch, was 4 days. The pH control patterns were the same for all batches. To compensate for the water evaporation during each batch of culturing, the autoclaved distilled water was pumped into the bioreactor to maintain broth volume at a constant level of 3 L. Continuous ISPR was performed by continuously pumping the broth from the bioreactor through the resin column at a flow rate of 50 mL/min during the ε-PL production stage, and the ε-PL-free broth was returned to the bioreactor. Intermittent ISPR was started once the ε-PL level in the broth reached 0.2 g/L during the fermentation, and the broth was recirculated at a higher flow rate of 200 mL/min till its concentration decreased to nearly zero. Recirculation of broth was conducted intermittently.
Fed-batch fermentation with immobilization, ISPR, and extra nutrient feeding
The cultivation was conducted in the experimental setup showing in Fig. 1. After the start of fermentation, the glucose feed was fed into the culture to maintain its level at approximately 10.0 g/L when the glucose level in the broth decreased to below 10.0 g/L. For feeding of the nutrient, it was started after the medium pH decreased to 3.8, with a flow rate of 1 mL/h, and continued till the end of fermentation. The pH of broth was maintained at 3.8 since it decreased to this. The fermentation lasted for 16 days. The procedures for intermittent ISPR were the same as those for the repeated batch process. The broth did not exceed 3 L during the entire course of fermentation due to considerable evaporation, and was maintained by supplementing sterilized distilled water if necessary. Fed-batch fermentation of immobilized cells with intermittent ISPR alone or in combination with extra nutrient feeding was also carried out for comparison.
Determination of the biomass, ε-PL and residual glucose
The free biomass was measured in aliquots of 10 mL of the culture broth. The broth was sampled, filtered through a pre-weighed filter paper, washed, and then dried at 80 °C till a constant weight was obtained. The immobilized loofah sponge was collected, washed, and then dried. Immobilized biomass was calculated based on the weight difference before and after cell growth on the loofah sponge. Residual glucose levels were measured using the 3,5-dinitrosalicylic acid colorimetric method. ε-PL was analyzed using the method described by Itzhaki [37]. The resin-bound ε-PL was eluted out using 0.1 M HCl before measurement. The adsorbed ε-PL concentration was added to the ε-PL concentration in the bioreactor at a corresponding measured time point.
Statistical analysis
All data, except the results from bioreactor studies, are presented as mean ± standard deviation. The data were analyzed using a one-way analysis of variance. Significant differences were determined using Duncan’s multiple range tests (P < 0.05).
Results and discussion
Effect of immobilization by loofah sponge on cell growth and ε-PL production
Loofah sponge, which has high porosity and resistance to autoclaving, is widely used for the immobilization of Streptomyces for metabolites production [38, 39]. As shown in Table 1, the final immobilized biomass increased from 1.03 to 11.06 g/L with the increase in the supplemented amount of loofah sponge from 0.5 g to 2.0 g per flask, while the freely suspended biomass decreased correspondingly from 8.18 g/L to nearly zero. A further increase in the loofah sponge amount did not proportionally increase immobilized biomass, and free biomass still remained absent in the broth, which may be because loofah sponge amounts higher than 2.0 g/flask provided sufficient solid surfaces and inner pores to accommodate mycelial growth.
The final total biomass (including free and adsorbed) of 7.32–9.21 g/L with loofah sponge amounts below 2 g/flask was lower than that in the free-cell fermentation (10.31 g/L). With supplementation of loofah sponge amounts higher than 2 g/flask, cell growth increased by 7.27–10.86% relative to the free-cell culture. The reason for the decreased total biomass with the carrier lower than 2 g/flask is that the mycelia and loofah sponge collide with each other during shaking, which is detrimental to the growth of mycelia. Somewhat differently, the growth of Kitasatospora sp. MY 5–36 in ε-PL fermentation was reported to be enhanced through immobilization on the loofah sponge [28].
The ε-PL production decreased from 0.56 to 0.38 g/L when the loofah sponge addition was increased from 0.5 to 1.5 g/flask, while markedly improved at 2.0 g/flask, reaching 0.52 g/L. Beyond this amount, the concentration of ε-PL did not vary significantly and remained stable. Totally, the ε-PL titers obtained in all immobilized cultures were lower than that obtained in the free-cell fermentation (0.84 g/L). The decreased ε-PL production can be attributed to inefficient transfer of nutrient compositions and dissolved oxygen to the mycelia growing inside the pores of the loofah sponge. By contrast, ε-PL production could be greatly improved by immobilization in another study [28]. This inconsistency may be related to differences in the strains and loofah sponge amounts used.
Glucose and glycerol are the two major carbon sources utilized for ε-PL fermentation [14, 19]. From Table 1, glucose utilization was the highest in fermentation with free cells, and the lowest in fermentation with cells immobilized on 1.5 g of loofah sponge in a flask. This implied that glucose consumption is positively correlated with biomass growth and ε-PL synthesis.
Synergistic effect of immobilization and ISPR on ε-PL production
Simultaneous application of immobilization and ISPR is reported to synergistically enhance the production of many important fermentation products, such as fumaric acid [40], hexanoic acid [41], butanol [42], and Monascus pigment [43]. The incorporation of ISPR with immobilized fermentation at a flask-culture scale enhanced ε-PL production, displaying a synergistic effect, as shown in our previous study [44]. However, the effect of ISPR methods and recovery content on ε-PL production was not investigated in that work, and also in other studies.
As observed in Table 2, the titer of ε-PL obtained in the immobilized fermentation in flasks was only 0.52 g/L, while increased to 3.51 g/L when coupled with continuous ISPR. For the performance of intermittent ISPR for ε-PL production, the cumulative titers were 1.02, 1.41, and 1.84 g/L, respectively, when one (at 60 h), two (at 60 and 78 h), and three (at 48, 60, and 80 h) cycles of ISPR operation were performed during the fermentation. This result may mean that the continuous removal of ε-PL from broth promoted its production more effectively than intermittent ISPR. The combined effects of increased cell density, metabolic flux re-distribution, and extended cellular activity may contribute to enhanced ε-PL production for the integrated process, and the latter two mechanisms may play a critical role in the enhancement. Regarding cell growth, the number of ISPR cycles had little effect on the mycelia during fermentation.
Effect of ε-PL on its further production
To evaluate the effect of ε-PL on its further production by immobilized cells in flasks, ISPR was separately performed at 54 h and 72 h of incubation, and the ε-PL concentration after each ISPR cycle was typically retained in the range of 0.1–0.3 g/L. As shown in Table 3, the cumulative ε-PL levels were 1.14, 1.33, and 1.46 g/L, respectively, when the ε-PL levels after each ISPR cycle were maintained at 0.3, 0.2, and 0.1 g/L. This yield trend indicated that low ε-PL levels present in the broth favor ε-PL production. There were no significant differences in immobilized growth among fermentation with different levels of ε-PL retained in the broth. During butanol fermentation coupled with gas stripping, it was reported that its concentration must be controlled below 8 g/L to obtain a good titer [42]. In fermentation of succinic acid, as its inhibitory level was approximately 50 g/L, ISPR must be carried out before this level is reached [45].
Comparison of batch fermentation with free and immobilized cells
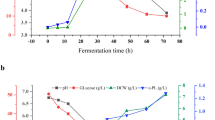
The comparative profiles of batch fermentation with free and immobilized cells in the 5 L bioreactor are shown in Fig. 2. As shown in Fig. 2A, the inoculated mycelia were rapidly adsorbed onto the loofah sponge, and no freely suspended mycelia could be observed after 6 h of fermentation, which was maintained till the end of the process. The attached mycelia exhibited abundant growth on the surfaces and inner pores of the loofah sponge matrice. For the free-cell fermentation, the cultures had a short lag phase, followed by a rapid growth phase. Maximum biomass was 7.41 g/L at 72 h, following which it started to decrease slightly, perhaps because of cell autolysis.
From Fig. 2B, the pH in both fermentations declined continuously from the time of inoculation till the end of the culturing period. The pH decline in the free-cell fermentation was faster than that in the immobilized fermentation. The pH of broth decreased to approximately 4.0 within 36 h for the free-cell fermentation, whereas this low pH could be reached only after 46 h of fermentation in the case of using the immobilized cells. This result indicates that immobilization suppressed the metabolism of the strain.
As shown in Fig. 2C, ε-PL production by free or immobilized cells was initiated after 36 or 46 h of fermentation, respectively. The highest ε-PL concentration of 1.34 g/L by free cells was shown at 96 h and did not vary significantly after this time. At 120 h, the immobilized cells yielded the highest value of 1.18 g/L ε-PL, 13.56% lower than that by free cells. The low concentration and delayed production of ε-PL may be attributed to decreased diffusion of nutrients and dissolved oxygen to the immobilized cells embedded or adsorbed in the loofah sponge. Figure 2D shows that glucose consumption rate and amount by free cells were higher than those by immobilized cells.
Performance of repeated-batch fermentation with immobilized cells and ISPR
Figure 3A shows the time courses of the repeated-batch fermentation of S. ahygroscopicus GIM8 with immobilization alone. The observed ε-PL concentration was 1.13 g/L in the initial batch, which increased to 1.28 g/L in the second batch, and started to decline in the subsequent three batches. The average ε-PL concentration (five batches) was 1.16 g/L. Recycling of immobilized cells may increase the percentage of dead cells, which may contribute to a decreased titer of ε-PL after the third batch. In the initial batch, both freely suspended mycelia and pellets were absent in the broth after a short time of inoculation due to their adsorption onto the carrier, and the broth was maintained clear till the end of cultivation. In subsequent four batches, the free biomass appeared earlier in the broth with each subsequent batch, and the final free biomass also increased, with 2.87 g/L in the fifth batch. The formation of free biomass may be caused because of the oversaturation of mycelia within the loofah sponge matrix, and because of the release of dead mycelia from the carrier, whose proportion increases with each cycle.
Profiles of ε-PL production and free biomass formation during repeated-batch fermentation of immobilized S. ahygroscopicus GIM8 with continuous or intermittent ISPR, or without use in a 5 L bioreactor. Continuous ISPR was started from 46 h in the initial batch, and from 52 h for use of intermittent ISPR
Continuous ISPR was applied to the fermentation for the evaluation of its efficiency for ε-PL improvement (Fig. 3B). The initiation of ε-PL synthesis was at 46 h post-inoculation, and then the circulation of broth for in situ removal of ε-PL was started and continued till the end of each batch. The ε-PL concentration was 3.21 g/L at the end for the initial batch. The time at which ε-PL production was initiated in subsequent batches was earlier than that in the initial batch. This is because recycling of immobilized cell mass eliminates the initial lag phase and reduces the time required for cell proliferation. The maximum ε-PL concentration was 3.73 g/L for the third batch, and decreased in the subsequent two batches. The average titer of ε-PL was 3.35 g/L. The final free biomass in the fifth batch was 3.12 g/L.
The profiles of ε-PL production and free biomass formation in the repeated-batch fermentation with immobilized cells and intermittent ISPR are shown in Fig. 3C. The ε-PL titer observed in the initial batch was 3.14 g/L and peaked in the third batch (3.63 g/L). The average yield of ε-PL (five batches) was 3.18 g/L, which was only slightly lower than that with the continuous ISPR approach (3.35 g/L). This implied that the continuous and complete in situ removal of ε-PL from the cells seemed to be unnecessary for this type of fermentation. Changes of free biomass in the fermentation with intermittent ISPR were comparable to those in the fermentation with continuous ISPR.
These findings suggested that intermittent ISPR has an effect similar to continuous ISPR in improving ε-PL production during immobilized fermentation. As intermittent ISPR has higher technical feasibility compared to continuous ISPR from an industrial application point of view, intermittent ISPR is a more suitable method for improving ε-PL production in the fermentation industry.
Performance of fed-batch fermentation with immobilized cells, intermittent ISPR and additional feeding of nutrients
Fed-batch fermentation, which has routine industrial applications, is widely used for ε-PL production [13, 19]. In fed-batch fermentation, the production of microbial metabolites could be significantly enhanced through additional supplementation of yeast extract solution to the fermentor [45, 46]. For ε-PL production, the time required for an entire fed-batch process is usually more than 7 days, and nutrients may deplete during the later culturing stages, which may be a limiting factor in improving the overall process efficiency. In this context, the impact of yeast extract supplementation on ε-PL production was evaluated during fed-batch fermentation with immobilized cells and with or without the use of intermittent ISPR.
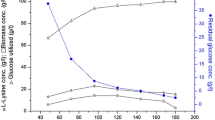
Figure 4A shows the profiles of fed-batch fermentation with the single immobilization for ε-PL production. As the fermentation proceeded, the medium pH decreased continuously and reached approximately 3.8 at 46 h. Production of ε-PL increased slowly from 46 h, and reached 2.32 g/L at the end of fermentation (12 days). Regarding the performance of loofah sponge for immobilization during fed-batch fermentation, the inoculated mycelia rapidly attached to the carrier and exhibited good growth on its surfaces and pores. The small and compact pellets were formed in the broth after 8 days, and their amount increased slightly to 2.21 g/L at the end. Continuous utilization of glucose by the mycelia was steadily observed in the growth (0–46 h) and ε-PL production (46–288 h) stages.
Profiles of fed-batch fermentation of ε-PL by immobilized S. ahygroscopicus GIM8 with intermittent ISPR and extra nutrient feeding alone or in combination, or without use in a 5 L bioreactor. Black arrows indicate the start of yeast extract solution feeding (from 46 h), and white arrows indicate the start of intermittent ISPR (from 52 h)
For the fermentation of immobilized cells with extra nutrient feeding strategy (Fig. 4B), a concentrated yeast extract solution was constantly pumped into the culture from 46 h up to 14 days of fermentation. Compared to the fermentation without yeast extract feeding (Fig. 4A), the nutrient feeding increased the formation rate of ε-PL, and also extended the effective synthesis duration up to day 13, reaching ε-PL titer of 4.45 g/L. Moreover, ε-PL concentration still increased slightly even after day 13. The decreased ε-PL formation rate after day 13 may be due to the accumulation of some inhibitory metabolites in the broth and cell aging. The appearance of free cells (after day 11) in the fermentation supplemented with yeast extract was later than that (after day 8) in the unsupplemented fermentation. The final density of free cells in the broth was low at 1.58 g/L.
As shown in Fig. 4C, by applying intermittent ISPR to the fed-batch fermentation of immobilized cells from 52 h, ε-PL production was accelerated. The total ε-PL concentration at the end of the fermentation period (12 days) was 8.52 g/L, which was 3.67-fold higher than that in the fermentation without ISPR (Fig. 4A). A small amount of free biomass at 0.44 g/L appeared in the broth after day 9, and increased slightly up to 2.53 g/L at the end of the culture period, only slightly higher than that (2.21 g/L) in the fermentation without ISPR (Fig. 4A).
The application of a combination of intermittent ISPR (from 52 h) and additional nutrient feeding (from 46 h) significantly increased the ε-PL production rate (Fig. 4D). The high-efficiency synthesis period was also markedly prolonged compared with that of the fermentation with the single nutrient feeding or intermittent ISPR. The cumulative titer of ε-PL (24.57 g/L) was 5.39-fold or 2.88-fold higher than that in the fermentation subjected to only nutrient feeding (4.56 g/L) or intermittent ISPR (8.52 g/L), respectively. The stripping of ε-PL surrounding the immobilized mycelia by intermittent ISPR, and the improved cellular activity by the yeast extract feeding may account for most of the enhancing effect. Free biomass was observed on day 14, and increased to only 1.54 g/L at the end of cultivation (16 days).
Summary and comparison with other studies
As summarized in Table 4, the ε-PL productivity of batch fermentation with free cells was higher than that with immobilized cells. The total ε-PL concentration (15.88 g/L) and productivity (0.756 g/L/day) of the repeated-batch fermentation with immobilized cells and intermittent ISPR were comparable to those (16.74 g/L and 0.797 g/L/day, respectively) of immobilized fermentation with continuous ISPR. In the fed-batch fermentation of immobilized cells with the combined use of extra nutrient feeding and intermittent ISPR, ε-PL concentration at 24.57 g/L and productivity of 1.54 g/L/day were significantly higher than those observed in other fermentation processes in this work. In a fed-batch fermentation with immobilized cells [28], the average ε-PL concentration and productivity were 34.11 g/L and 9.34 g/L/day, respectively, and in another study using a novel two-stage pH control for fed-batch fermentation [16], ε-PL concentration reached 30.11 g/L, with a productivity of 4.18 g/L/day. These reported measurements were higher than those reported in this study, which may be because of the differences in strains and culture conditions. The advantages of the developed fermentation processes in our study are obvious. First, no agitation was used, thus the cost of power consumption could be greatly reduced. Second, the leakage of intracellular nucleic acid-related substances would be minimal due to cell immobilization and the absence of shear stress, which helps to obtain high purity of ε-PL products [47].
Conclusions
In the present study, the immobilization of S. ahygroscopicus GIM8 on loofah sponge did not lead to an enhancement of ε-PL productivity, while the combined use of immobilization and ISPR exerted a synergistic effect on its production. Low levels of ε-PL in the broth favored ε-PL production. Intermittent ISPR by maintaining ε-PL in the broth at a relatively low level was almost comparable to continuous ISPR in improving ε-PL production during immobilized fermentation. Applying both strategies of intermittent ISPR and nutrient feeding to the fed-batch fermentation of immobilized cells could greatly improve ε-PL production in a 5 L bioreactor, reaching 24.57 g/L. The fed-batch fermentation with immobilized cells, intermittent ISPR, and nutrient feeding can be a promising strategy for enhancing ε-PL production.
References
Shima S, Sakai H (1977) Polylysine produced by Streptomyces. Agric Biol Chem 41:1807–1809
Hiraki J (2000) Basic and applied studies on ε-polylysine. J Antibact Antifungal Agents 23:349–354
Shima S, Fukuhara Y, Sakai H (1982) Inactivation of bacteriophages by ε-poly-l-lysine produced by Streptomyces. Agric Biol Chem 46:1917–1919
Shima S, Matsuoka H, Iwamoto T, Sakai H (1984) Antimicrobial action of ε-poly-l-lysine. J Antibiot 37:1449–1455
Pandey AK, Kumar A (2014) Improved microbial biosynthesis strategies and multifarious applications of the natural biopolymer epsilon-poly-l-lysine. Process Biochem 49:496–505
Hiraki J, Ichikawa T, Ninomiya S, Seki H, Uohama K, Seki H, Kimura S, Yanagimoto Y, Barnett JW (2003) Use of ADME studies to confirm the safety of ε-polylysine as a preservative in food. Regul Toxicol Pharmacol 37:328–340
Shih IL, Shen MH, Van YT (2006) Microbial synthesis of poly(ε-lysine) and its various applications. Bioresour Technol 97:1148–1159
Hamano Y, Nicchu I, Shimizu T, Onji Y, Hiraki J, Takagi H (2007) ε-Poly-l-lysine producer, Streptomyces albulus, has feedback-inhibition resistant aspartokinase. Appl Microbiol Biotechnol 76:873–882
Shima S, Oshima S, Sakai H (1983) Biosynthesis of ε-poly-l-lysine by washed mycelium of Streptomyces albulus No. 346. Nippon Nogeikagaku Kaishi 57:221–226
Chen XS, Ren XD, Zeng X, Zhao FL, Tang L, Zhang HJ, Mao ZG (2013) Enhancement o f ε-poly-l-lysine production coupled with precursor l-lysine feeding in glucose-glycerol co-fermentation by Streptomyces sp. M-Z18. Bioprocess Biosyst Eng 36:1843–1849
Yamanaka K, Maruyama C, Takagi H, Hamano Y (2008) ɛ-Poly-l-lysine dispersity is controlled by a highly unusual nonribosomal peptide synthetase. Nat Chem Biol 4:766–772
Shukla SC, Singh A, Pandey AK, Mishra A (2012) Review on production and medical applications of ɛ-poly-l-lysine. Biochem Eng J 65:70–81
Wang L, Chen XS, Wu GY, Li S, Zeng X, Ren XD, Tang L, Mao ZG (2017) Enhanced ε-poly-l-lysine production by inducing double antibiotic-resistant mutations in Streptomyces albulus. Bioprocess Biosyst Eng 40:271–283
Chen XS, Tang L, Li S, Liao LJ, Zhang JH, Mao ZG (2011) Optimization of medium for enhancement of ε-poly-l-lysine production by Streptomyces sp. M-Z18 with glycerol as carbon source. Bioresour Technol 102:1727–1732
Bankar SB, Singhal RS (2011) Improved poly-ε-lysine biosynthesis using Streptomyces noursei NRRL 5126 by controlling dissolved oxygen during fermentation. J Microbiol Biotechnol 21:652–658
Chen XS, Li S, Liao LJ, Ren XD, Li F, Tang L, Zhang JH, Mao ZG (2011) Production of ε-poly-l-lysine using a novel two-stage pH control strategy by Streptomyces sp. M-Z18 from glycerol. Bioprocess Biosyst Eng 34:561–567
Ren XD, Chen XS, Zeng X, Wang L, Tang L, Mao ZG (2015) Acidic pH shock induced overproduction of ε-poly-l-lysine in fed-batch fermentation by Streptomyces sp. M-Z18 from agro-industrial by-products. Bioprocess Biosyst Eng 38:1113–1125
Hirohara H, Takehara M, Saimura M, Masayuki A, Miyamoto M (2006) Biosynthesis of poly(ε-l-lysine)s in two newly isolated strains of Streptomyces sp. Appl Microbiol Biotechnol 73:321–331
Kahar P, Iwata T, Hiraki J, Park E, Okabe M (2001) Enhancement of ε-polylysine production by Streptomyces albulus strain 410 using pH control. J Biosci Bioeng 91:190–194
Kito M, Takimoto R, Yoshida T, Nagasawa T (2002) Purification and characterization of an ε-poly-l-lysine-degrading enzyme from an ε-poly-l-lysine-producing strain of Streptomyces albulus. Arch Microbiol 178:325–330
Liu Y, Liu D (2004) Kinetic study on glycerol production by repeated batch fermentation using free Candida krusei. Process Biochem 39:1507–1510
Chen CC, Lan CC, Pan CL, Huang MY, Chew CH, Hung CC, Chen PH, Victor Lin HT (2019) Repeated-batch lactic acid fermentation using a novel bacterial immobilization technique based on a microtube array membrane. Process Biochem 87:25–32
Suwannakham S, Yang ST (2005) Enhanced propionic acid fermentation by Propionibacterium acidipropionici mutant obtained by adaptation in a fibrous-bed bioreactor. Biotechnol Bioeng 91:325–337
Huang L, Lacroix C, Daba H, Simard RE (1996) Pediocin 5 production and plasmid stability during continuous free and immobilized cell cultures of Pediococcus acidilactici UL5. J Appl Bacteriol 80:635–644
Yang XH, Wang BW, Cui FN, Tan TW (2005) Production of lipase by repeated batch fermentation with immobilized Rhizopus arrhizus. Process Biochem 40:2095–2103
Dishisha T, Alvarez MT, Hatti-Kaul R (2012) Batch- and continuous propionic acid production from glycerol using free and immobilized cells of Propionibacterium acidipropionici. Bioresour Technol 118:553–562
Meleigy SA, Khalaf MA (2009) Biosynthesis of gibberellic acid from milk permeate in repeated batch operation by a mutant Fusarium moniliforme cells immobilized on loofa sponge. Bioresour Technol 100:374–379
Zhang Y, Feng XH, Xu H, Yao Z, Ouyang PK (2010) ε-Poly-l-lysine production by immobilized cells of Kitasatospora sp. MY 5–36 in repeated fed-batch cultures. Bioresour Technol 101:5523–5527
Stark D, von Stochar U (2003) In situ product removal (ISPR) in whole biotechnology during the last twenty years. Adv Biochem Eng Biotechnol 80:149–175
Pongtharangku T, Demirci A (2007) Online recovery of nisin during fermentation and its effect on nisin production in biofilm reactor. Appl Microbiol Biotechnol 74:555–562
Singhvi M, Zendo T, Gokhale D, Sonomoto K (2018) Greener l-lactic acid production through in situ extractive fermentation by an acid-tolerant Lactobacillus strain. Appl Microbiol Biotechnol 102:6425–6435
Eiki H, Gushima H, Saito T, Ishida H, Oka Y, Osono T (1988) Product inhibition and its removal on josamycin fermentation by Streptomyces narbonensis var. josamyceticus. J Biosci Bioeng 66:559–565
Zhang LJ, Jin ZH, Chen XG, Jin QC, Feng MG (2012) Glycine feeding improves pristinamycin production during fermentation including resin for in situ separation. Bioprocess Biosyst Eng 35:513–517
Liu SR, Wu QP, Zhang JM, Mo SP (2011) Production of ε-poly-l-lysine by Streptomyces sp. using resin-based, in situ product removal. Biotechnol Lett 33:1581–1585
Huang JM, Wu QP, Liu SR, Zhang JM (2011) Screening of new ε-polylysine producing strain and structure identification of its product. Microbiol China 38:871–877 (in Chinese)
Wang P, He JY, Yin JF (2015) Enhanced biocatalytic production of l-cysteine by Pseudomonas sp. B-3 with in situ product removal using ion-exchange resin. Bioprocess Biosyst Eng 38:421–428
Itzhaki FR (1972) Colorimetric method for estimating polylysine and polyarginine. Anal Biochem 50:569–574
Kar S, Swain MR, Ray RC (2009) Statistical optimization of alpha-amylase production with immobilized cells of Streptomyces erumpens MTCC 7317 in Luffa cylindrical L. sponge discs. Appl Biochem Biotechnol 152:177–188
Saudagar PS, Shaligram NS, Singhal RS (2008) Immobilization of Streptomyces clavuligerus on loofah sponge for the production of clavulanic acid. Bioresour Technol 99:2250–2253
Cao NJ, Du JX, Gong CS, Tsao GT (1996) Simultaneous production and recovery of fumaric acid from immobilized Rhizopus oryzae with a rotary biofilm contactor and an adsorption column. Appl Environ Microbiol 62:2926–2931
Roddick FA, Britz ML (1997) Production of hexanoic acid by free and immobilized cells of Megasphaera elsdenii: influence of in-situ product removal using ion exchange resin. J Chem Tech Biotechnol 69:383–391
Xue C, Zhao JB, Lu CC, Yang ST, Bai FW, Tang IC (2012) High-titer n-butanol production by Clostridium acetobutylicum JB200 in fed-batch fermentation with intermittent gas stripping. Biotechnol Bioeng 109:2746–2756
Liu J, Guo T, Luo Y, Chai X, Wu J, Zhao W, Jiao P, Luo F, Lin Q (2019) Enhancement of Monascus pigment productivity via a simultaneous fermentation process and separation system using immobilized-cell fermentation. Bioresour Technol 272:552–560
Liu SR, Wu QP, Zhang JM, Mo SP, Yang XJ, Xiao C (2012) Enhanced ε-poly-l-lysine production from Streptomyces ahygroscopicus by a combination of cell immobilization and in situ adsorption. J Microbiol Biotechnol 22:1218–1223
Li Q, Wang D, Hu G, Xing J, Su Z (2011) Integrated bioprocess for high-efficiency production of succinic acid in an expanded-bed adsorption system. Biochem Eng J 56:150–157
Yang XP, Tsao GT (1995) Enhanced acetone-butanol fermentation using repeated fed-batch operation coupled with cell recycle by membrane and simultaneous removal of inhibitory products by adsorption. Biotechnol Bioeng 47:444–450
Kahar P, Kobayashi K, Iwata T, Hiraki J, Kojima M, Okabe M (2002) Production of ɛ-polylysine in an airlift bioreactor (ABR). J Biosci Bioeng 93:274–280
Acknowledgements
This work was financially supported by the Key Cultivating Program of Ningde Normal University (2018ZDK01) and the Industry-Leading Program of the Science and Technology Bureau of Fujian Province (2015N0032).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, SR., Yang, XJ. & Sun, DF. Enhanced production of ε-poly-l-lysine by immobilized Streptomyces ahygroscopicus through repeated-batch or fed-batch fermentation with in situ product removal. Bioprocess Biosyst Eng 44, 2109–2120 (2021). https://doi.org/10.1007/s00449-021-02587-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02587-7