Abstract
The global agricultural system has been badly affected by adverse environmental changes in the past few years. These changes, including the rise of abiotic and biotic stressors negatively altered the growth and physiology of crop plants. Abiotic stresses, such as salinity, temperature extremes, drought, and heavy metals/metalloids, are major environmental constraints limiting crop growth, productivity, and sustainability worldwide. These stresses adversely affect plant metabolic activities and redox homeostasis, eventually leading to a reduction in plant growth and development. Plant growth regulators (PGRs) play a key role in regulating plants developmental processes and defensive responses under adverse environmental conditions. Among PGRs, gibberellic acid (GA3), an endogenous tetracyclic diterpenoid plant hormone, regulates many growth and developmental aspects of crop plants. GA3 plays a pivotal role in mitigating abiotic stresses induced-perturbations in plants by modulating various physio-biochemical and molecular processes. Based on recent reports, this review article describes the role of exogenously applied GA3 in improving seed germination, phenotypic characteristics, metabolic processes, yield and quality components, and post-harvest life of fruits, vegetables, and flowers. In this article, we summarize research concerning GA3 biosynthesis and signaling and discuss the potential role of GA3 in mediating tolerance to various abiotic stresses. Moreover, the present article enlightens the current research concerning the signaling pathway in gibberellin and gibberellin-mediated crosstalk with other plant hormones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants face myriad environmental stresses broadly divided into abiotic and biotic stresses. Abiotic stresses like heavy metals, water scarcity, salinity, and varying temperature are the main environmental restraints that adversely hamper phenotypic features, physio-biochemical processes, and production of crop plants (Zhu 2016; Shah et al. 2021, 2022a, b). Plants are equipped with various inherent physio-biochemical and molecular mechanisms to overcome these perturbations. These mechanisms include alteration in gene expression, synthesis of special proteins, maintaining ionic balance, accumulating osmolytes, and enhancing antioxidant defense machinery (Sharma et al. 2022). Numerous efforts have been practiced to influence the endurance of plants against environmental calamities. Among them, the supplementation of plant growth regulators (PGRs), including auxins, brassinosteroids, abscisic acid, ethylene, cytokinins, gibberellins (GAs), jasmonic acid, nitric oxide, polyamines, salicylic acid and strigolactones play a regulatory role in boosting defensive responses in plants (Islam et al. 2021; Sabagh et al. 2021). The use of PGRs has become a common practice for improving the productivity and quality of horticultural and agricultural crop production and also for the mitigation of abiotic stresses (Shah et al. 2023). PGRs are small chemical messenger molecules that influenced growth, physiological and biochemical features, and plant genotypic functions (Rademacher 2015; El Sabagh et al. 2022). Among PGRs, GAs, an important phytohormone/plant growth regulator, modulates several growth and developmental processes in crop plants. Gibberellin comprises several compounds belonging to tetracyclic diterpenoid carboxylic acid groups. Gibberellins, generally recognized as gibberellic acid (GA3), was first identified by Western scientists in the 1950s. In Asia, rice farmers know about a fungal disease called bakanae disease or foolish seedlings that have led to the identification of GAs (Gupta and Chakrabarty 2013; Taiz et al. 2015). Gibberellic acid produced from Gibberella fujikuroi can be commercially used for horticultural and agricultural purposes. It improved the growth and productivity of orchards, crops, and ornamental plants. The first GA was identified (GA1) in runner bean seeds in 1958. However, most plants that produce GA, like GA1 and GA4, have diverse roles in their physiological functions. Gibberellic acid induces growth and development processes in crop plants, including cell expansion, cell division, seed germination, mobilization of endosperm storage reserves, internode elongation, transition to flowering, sex expression, and development of fruits (Schwechheimer 2008; Rodrigues et al. 2012; Othman and Leskovar 2022). It has also been suggested that the treatment of GA3 extends the post-harvest life of fruits, vegetables, and flowers by delaying senescence, inhibiting chlorophyll degradation, and increasing the antioxidant system (Kuchi et al. 2017).
Gibberellin is actively involved in plant mechanisms associated with imparting tolerance to stress in crop plants by improving ion homeostasis, membrane permeability, antioxidant system, osmolyte accumulation, and expression of stress-mitigation genes (Emamverdian et al. 2020; Nagar et al. 2021). Gibberellin takes part in key tolerance responses of plants against abiotic stresses such as by increasing plant antioxidant activities that scavenge deleterious ROS. Studies by several workers proposed that exogenous applications of GA3 help plants to nullify the damaging consequences of environmental stresses (Hamayun et al. 2010; Elahi et al. 2022). The exogenous application of GA3 improves growth, photosynthetic pigments, net photosynthetic rate, enzyme activities, mineral nutrient acquisition, and yield efficiency of several crop plants under adverse environmental conditions (Afroz et al. 2006; Abdel-Hamid and Mohamed 2014; Rady et al. 2021; Shahzad et al. 2021). The developmental processes in the plant life cycle indicate coordinated alterations in molecular mechanisms of plant growth through complex signaling networking and synchronized participation of various hormone signaling components. It has been confirmed that the relations between various phytohormones are necessary in integrating and remodeling plant growth and enhancing their stress resistance mechanism. GA3 interacts with other PGRs both synergistically as well as antagonistically to modulate many plants metabolic processes (Weiss and Ori 2007; Shaki et al. 2019; Banerjee and Roychoudhury 2019; Abbas et al. 2022).
Considering the diverse functions of GA3 in the life cycle of plants in view, the present article aims to timely review the biosynthesis, signaling, and role of GA3 in the growth and development of crop plants. In this article, we have also focused on GA3-mediated abiotic stress tolerance in crop plants. Moreover, an attempt has also been made to discuss molecular insight regarding the crosstalk of GA3 with other PGRs in plants.
Biosynthesis
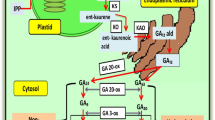
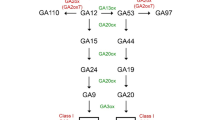
Gibberellins are naturally occurring phytohormone produced in many parts of plants, including germinating seeds, developing seeds, young leaves, and internodes. Gibberellins encompass a large group of plant growth substances with different functions during the entire life cycle of higher plants. The rate of GA biosynthesis and catabolism regulates how the GA hormone pool occurs in plants in a tissue and developmentally mediated manner. The GA biosynthetic pathways and catabolism are under strict genetic control (Taiz et al. 2015). The biosynthetic pathway of GA illustrated in (Fig. 1.) starts from geranyl–geranyl diphosphate (GGPP). The GGPP was synthesized from isopentenyl diphosphate (IPP), a 5-carbon central intermediate for many isoprenoid/terpenoid compounds. Two routes generate the IPP: the methyl erythritol phosphate (MEP) pathway in plastids and the mevalonic acid (MVA) pathway in the cytosol (Kasahara et al. 2002; Hedden and Thomas 2012). The GA biosynthetic pathway can be categorized into three phases (Hedden 2020). The first phase occurred in plastids where GGPP was changed into ent-kaurene catalyzed by two enzymes: the ent-copy-diphosphate synthase (CPS) and the ent-kaurene synthase (KS) (Van Schie et al. 2007; Taiz et al. 2015). In Arabidopsis thaliana L., enzymes CPS and KS are transcribed by single locus GA1 and GA2, respectively (Yamaguchi et al. 1998), while Oryza sativa L. by OsCPS1 and OsKS1 respectively (Sakamoto et al. 2004). The second phase takes place in the endoplasmic reticulum (ER); ent-kaurene is oxidized to GA12-aldehyde catalyzed by cytochrome P450 monooxygenases including ent-kaurene oxidase (KO) and ent-kaurenoic acid oxidase (KAO). The GA12-aldehyde is transformed into GA12 (Hedden et al. 2002; Davidson et al. 2006). In A. thaliana L., KO is located at the chloroplast membrane and KAO at ER, facilitating ent-kaurene exit from plastid and oxidizing it when entered ER. The KAO enzyme is encoded by two genes, KAO1 and KAO2 (Regnault et al. 2014), and the KO enzyme is encoded by OsKO1, OsKO2, and OsKO5 genes in A. thaliana L. and Oryza sativa L., respectively (Zhang et al. 2020). GA12 undergoes hydroxylation to form GA53. The third phase occurred in the cytosol in which GA12 and GA53 served as precursors for the non-13-hydroxylation pathway and 13-hydroxylation pathway to form GA9 and GA20, respectively catalyzed by GA20-oxidase (GA20ox). The last stage of biosynthesis is the 3 β-hydroxylation of GA9 and GA20 mediated by GA3-oxidase (GA3ox) to form GA4 and GA1, respectively, bioactive GAs in plants (Salazar-Cerezo et al. 2018; Hedden 2020). Moreover, the bioactive GA4 and GA1 are inactivated by GA2-oxidase (GA2ox). The GA2-oxidase enzyme is encoded by seven genes, viz., GA2ox1, GA2ox2, GA2ox3, GA2ox4, GA2ox6, GA2ox7, and GA2ox8. The GA2-oxidase is a key enzyme in controlling GAs concentration during plant growth and development and responding to adverse environmental conditions (Li et al. 2019).
Signaling
Gibberellins regulate many features of plant physiology by modulating transcriptional and post-transcriptional changes at the cellular level (Schwechheimer 2008). There are many components that are involved in signaling responses regulated by GAs. The signaling of GA in plants involves a homeostatic balance between the gene expression involved in the GA biosynthesis, GA receptor, and enzyme concentration that inactivates bioactive GA (Sun and Gubler 2004; Daviere and Achard 2013). The crucial part in GA signaling is the GA receptor, i.e., GID1 (gibberellin insensitive dwarf 1) identified from Oryza sativa L. GID1 is a soluble protein located in the cytosol and nucleus, has a C-terminal domain for GA binding and a flexible N-terminal domain (Griffiths et al. 2006; Ueguchi-Tanaka et al. 2007). The DELLA proteins (consisting of an N-terminal DELLA/TVHYNP motif and a C-terminal GRAS domain) are negative regulators of GA signaling in many crop plants and restrain the growth of plants (Yoshida et al. 2014). Moreover, DELLA prevents the binding of phytochrome interacting factors (PIFs) to their promoter sites and thereby interferes with their transcriptional activity. Gibberellin binds to its receptor GID1, and the GID1 undergoes conformational changes and allows the binding of the DELLA repressor protein, forming the GID1-GA-DELLA complex. The GID1-GA-DELLA complex is recognized by SCFGID2/SLY1 (skp1-cullin F-box), an E3 ubiquitin ligase that triggers polyubiquitylation and degrades DELLA repressor protein through 26S proteasome. The GID2 and SLY1 (SLEEPY1) are a subunit of F-box protein and deactivate repressors (such as SPY1, GAI, and RGA) of the GA signaling pathway in the presence of GA in the cell. After the degradation of the DELLA repressor resulted in the activation of transcription factors PIFs such as PIF3 and PIF4 and bHLH (basic helix-loop-helix), these transcription factors thus activated GA-regulated genes to cause a rapid change in gene expression and regulate biological response (Hartweck 2008; Hirano et al. 2008; Taiz et al. 2015). Additionally, two GATA family transcription factors GNC (GLUCOSE NITROGEN CARBON) and CGA1/GNL (CYTOKININ-INDUCED GATA FACTOR1/GNC-LIKE) were considered redundant regulators of plant growth. The GNC and GNL expression is repressed by PIFs mediated by GAs and requires the GID1-GA complex and degradation of DELLA. GA modulates many characteristics of plant physiology, including embryo, seed and root development, seed germination, leaf expansion, elongation of the stem, pollen maturation, and floral development (Richter et al. 2010; Schwechheimer 2012). Ramesh and Kumar (2006), Hamayun et al. (2010), and Saleem et al. (2020) suggested that supplementation of GA3 significantly promoted seed vigor, plant height, plant biomass, antioxidant system, and photosynthesis of plants under changing environmental conditions. A diagrammatic illustration of mode of action of GA in plant cell is given in Fig. 2.
Growth and Development
Gibberellin plays a vital role in improving diverse facets of growth and development such as seed germination, morphological attributes, physiological and biochemical responses, yield, and quality traits of various crop plants (Fig. 3). Findings of several workers suggested that supplementation of GA3 influences every phase during the life cycle of plants. These studies have been summarized in below sections.
Seed Germination
Seed germination is an essential step in the lifespan of seed plants. It is controlled by many environmental aspects (light, moisture, and temperature, etc.) and by endogenous phytohormones (Abscisic acid and GA3). The period of seed dormancy and the optimum moment to induce germination is critical for improving plant lifecycle and preventing potential threats in the first phases of seedling development. It is well known that GA is the main hormone intricated in seed dormancy breakdown, and the ABA/GA balance is the main regulator of seed dormancy and germination. Gibberellic acid positively regulates seed germination, whereas ABA maintains seed dormancy (Kim and Park 2008; Ravindran and Kumar 2019). Gibberellin promotes seed germination by increasing proteasome degradation of RGL2 (a DELLA repressor that stops germination), whereas ABA-induced AB15 (a leucine zipper transcription factor) that repressed seed germination (Piskurewicz et al. 2008). Endogenous GA acts as a positive regulator in this process to trigger the expression of the hydrolytic enzyme (especially α-amylase) in the aleurone layer of cereal grains, which substantially degrades the endospermic starch reserve (Damaris et al. 2019). Studies have revealed that both endogenous GA and the exogenous application of GA promote seed germination in crop plants, including Oryza sativa L. and Capparis ovata L. (Vieira et al. 2002; Soyler and Khawar 2007). Balaguera-Lopez et al. (2008) described that pre-sowing of Solanum lycopersicum L. seeds in 900 mg/L GA3 led to a higher proportion of seed germination over the control. Roychowdhury et al. (2012) also investigated the effect of pre-sowing seed treatment of GA3 on seed dormancy. They observed that Dianthus caryophyllus L. seeds soaked in 20 ppm GA3 suppressed the effect of seed dormancy by significantly increasing seed germination percentage over the control. Exogenously applied GA3 at 150 µM promotes seed germination in Triticum aestivum L. by significantly improving the α-amylase activity, rate of germination, and seedling height compared with the control (Wang et al. 2016). Further, Cornea-Cipcigan et al. (2020) suggested that seeds of Cyclamen species soaked in 50 mg/L GA3 significantly increased their germination percentage, germination time, and seedling vigor index. It can be summarized from the above studies that GA3 acts as a natural regulator in the processes intricate in seed germination by stimulating the hydrolytic enzyme that plays a key role in breaking seed dormancy.
Growth
Gibberellic acid improved plant growth by triggering cell division and elongation process, transitions from meristem to shoot growth, juvenile to adult leaf stage, vegetative to flowering and determining sex expression. Several observations revealed that GA3 plays a vital role in controlling various plant growth characteristics. For example, it improves vegetative growth, root, and stem elongation, plant biomass, and leaf area enlargement of many crop plants. The supply of 10–5 M GA3 on Brassica juncea L. seedlings enhanced dry plant mass, leaf area per plant, and relative growth rate compared with water spray control (Khan et al. 2002). The supplementation of 10–8 M GA3 on the foliage of Lycopersicum esculentum L. and 400 ppm GA3 on Ixora coccinea L. enhanced their plant height, branch and leaf fresh and dry weight (Khan et al. 2006; Gad et al. 2016). The seedlings of Papaver somniferum L. treated with 10–6 M significantly promoted plant height and dry weight (Khan et al. 2007). The leaf applied 300 ppm GA3 on Araucaria heterophylla L., and 10 µM GA3 on Mentha arvensis L. seedlings significantly increased its plant height, branch length, stem thickness and root length (Gul et al. 2006; Bose et al. 2013). The seed priming of Solanum lycopersicum L. with 900 mg/L GA3 and Cicer arietinum L. with 10–6 M improved plant height, leaf and root fresh weight and total dry mass of the plant (Balaguera-Lopez et al. 2008; Mazid 2014). The pre-treated bulb of Polianthes tuberosa L. with 150 pm GA3 significantly enhanced its plant height, leaves per plant, length, and width (Rani and Singh 2013). The supplementation of 400 mg/L enhanced plant height, tillers per plant, secondary branches and total dry weight of Linum usitatissimum L. (Rastogi et al. 2013). Zang et al. (2016) evaluated that spray of 500 mg/L GA3 enhanced leaf area, leaf fresh and dry weight of Vaccinium virgatum L. Similarly, leaf applied 1.2 ml/L GA3 increased total plant height and internode length of Hibiscus cannabinus L. (Muniandi et al. 2018). Leilah and Khan (2021) foliar application of 240 mg/L GA3 on Beta vulgaris L. improved root length, diameter and fresh weight and foliage fresh weight. Further, the spray of 10–5 M GA3 increased plant height, fresh weight and dry weight of nodules per plant of Cicer arietinum L. (Rafique et al. 2021). The reviewed literature suggested that appropriate concentration of GA3 application significantly enhanced the growth characteristics of plants by inducing a transition from meristem to shoot growth, plant biomass, leaf expansion, internode elongation, juvenile to the adult phase, vegetative to the flowering stage, defined sex expression and improved overall developmental phase of a plant.
Physio-Biochemistry
Gibberellins are reported to alleviate the harmful effects of salinity by increasing the nutrient-use efficiency, enzymatic activity, chlorophyll content, and absorption of mineral nutrients leading to improved plant physiological functions. The researchers suggested that the exogenous supplementation of GA3 improved the physio-biochemistry of plants by enhancing the physio-biochemical attributes of many crop plants. The supplementation of 10–8 M GA3 enhanced chlorophyll content, photosynthetic rate (PN), and CA activity of treated plants over the control plant (Hayat et al. 2001). The seed treatment of Nigella sativa L. with 10–5 M GA3 enhanced chlorophyll content, CA activity, stomatal conductance (gs), PN, protein content, and nitrate reductase (NR) activity (Shah 2007). The Brassica juncea L. seedlings treated with 10–5 M GA3 improved chlorophyll content, PN, gs, NR and CA activity (Siddiqui et al. 2008). Khan et al. (2009) indicated that seed treatment and foliage spray with 10–6 M GA3 enhanced chlorophyll content, PN, CA activity and mineral nutrient content in Linum usitatissimum L. The supply of 75 mg/L GA3 increased PN, internal CO2 concentration (Ci), and total chlorophyll content in Artemisia annua L. (Aftab et al. 2011). The supplementation of 0.7 mM GA3 improved chlorophyll, carotenoid, and total soluble sugar content in Gladiolus communis L. (Sajjad et al. 2014). The spray of GA3 at 10–5 M on Oryza sativa L. augmented its chlorophyll, proline and leaf-soluble protein content (Khan et al. 2016). Rai et al. (2017) demonstrated that foliar application of 5 mL/plant GA3 improved the net assimilation rate, NR activity and sucrose content in Saccharum officinarum L. The seedlings of Vigna radiata L. treated with 50 ppm GA3 increased contents of amino acids, chlorophyll, glucose and protein and activity of NR (Baliah et al. 2018). Wang et al. (2019) found that a foliar spray of 0.1 mM GA3 enhanced chlorophyll and nutrient acquisition in Abelmoschus esculentus L. The foliar feeding of 200 mg dm−3 GA3 improved CO2 assimilation intensity, transpiration rate (E), photosynthetic water-use efficiency, PN, gs and Ci in Amarine tubergenii L. (Salachna et al. 2020). Further, Rafique et al. (2021) observed treatment of 10–5 M GA3 enhanced leghaemoglobin and chlorophyll content in Cicer arietinum L. The exogenous application of 10 µM GA3 kg−1 soil improved PN, photosynthetic sulfur use efficiency and glutathione content in Vigna radiata L. (Hasan et al. 2020). Rady et al. (2021) investigated that treatment of GA3 on Vicia faba L. seedlings increased relative water content (RWC), total chlorophyll and carotenoid contents, soluble sugar, protein, ascorbate, glutathione and total phenolic content and nutrient acquisition. Shahzad et al. (2021) determined that foliar application of 100 ppm GA3 augmented chlorophyll content, soluble protein and total phenolic contents in Oryza sativa L. In summary, GA3 enhanced crop plants' physiological and metabolic processes by increasing photosynthetic pigment synthesis, enzymatic activities, protein and sugar content, mineral nutrient uptake, non-enzymatic antioxidant content and photosynthetic efficiency.
Yield and Quality
GA3 not only improves phenotypic traits and metabolic facets but also augments crop plant yield and quality characteristics. Zang et al. (2016) evaluated that spray of 500 mg/L GA3 enhanced the number of inflorescences/plants, flower number/inflorescence, fertile seed number and fruit weight of Vaccinium virgatum L. than the control plant. Further, the supplementation of 75 ppm GA3 influenced the flowers/plant, total fruit/plant, fruit set and total fruit yield in Fragaria ananassa L. (Sharma and Singh 2009). The seed treatment and foliar spray of 10–6 M GA3 improved capsules/plant, seeds/capsule, 1000-seed weight and seed yield/plant in Linum usitatissimum L. (Khan et al. 2010b, a). The foliar treatment of 50 mg/L GA3 improved the number of buds, fruit set, average fruit weight and yield in Syzygium samarangense L. (Moneruzzaman et al. 2011). Nkansah et al. (2012) proposed that spray of 25 ppm GA3 increased fruit set, number of fruits per cluster, fruit weight and yield in Mangifera indica L. The foliar spray of 400 mg/L GA3 increased capsule per plant, seeds per capsule and seed yield in Linum usitatissimum L. (Rastogi et al. 2013). Abd El-Razek et al. (2013) reported that foliar application of 100 mg/L increased fruit length and diameter, seed weight, oil content, fruit weight, and yield in Olea europaea L. Pan et al. (2013) noticed that supply of 20 mg/L GA3 enhanced panicle number, number of spikelets/panicles, grain filling percentage and 1000-grain weight of Oryza sativa L. The foliar spray of 50 ppm GA3 improved the number of pods/plants, pod length and pod fresh and dry weight of Abelmoschus esculentus L. (Shahid et al. 2013). The seed treatment of Cicer arietinum L. with 10–6 M GA3 enhanced pod number, seed yield/plant, and seed protein content (Mazid 2014). Akand et al. (2016) suggested that foliar application of 60 ppm influenced the number of the flower cluster and fruit per plant, dry matter of fruits, and yield of Lycopersicon esculentum Mill. The foliar treatment of 5 ppm GA3 improved fruit weight, fruit width, and fruit length in Rubus sp. (Colak 2018). Rahman et al. (2019) indicated that supplementation of 75 ppm GA3 increased flower and fruit clusters per plant, fruit number and weight per plant, and fruit yield t/ha in Solanum lycopersicum L. Abbas et al. (2020) reported that foliar spray of 1.0 and 1.2 g/L GA3 increased the number of fruits, fruit weight and yield in Momordica charantia L. Talat et al. (2020) found that spray of 25 and 45 ppm GA3improved fruit weight and juice weight in Citrus reticulata L. The foliar application of 240 mg/L GA3 on Beta vulgaris L. enhanced foliage yield, sucrose content, and sugar yield t/ha (Leilah and Khan 2021). Rafique et al. (2021) also described that a spray of 10–5 M GA3 increased the number of pods/plants, 50 seeds weight, seed protein, and carbohydrate content, and grain yield in Cicer arietinum L. The above studies indicated that GA3 significantly improved yield and quality components, including pollen maturation, development of flowers, fruits and seeds, and seed and oil yield of crop plants.
Post-Harvest Life of Fruits, Vegetables and Flowers
Fruits and vegetables are essential for human nutrition and are immensely suggested for a healthy diet. They are a rich source of energy, minerals, vitamins, antioxidants, dietary fibers, and other phytochemicals. On the other hand, flowers facilitate the reproduction of flowering plants, produce seeds, and pass genetic information to the next generation. However, improper post-harvest handling, like poor storage and transportation facilities and the premature senescence of flowers because of adverse climatic conditions, resulted in a worldwide economic loss (Yahia and Carrillo-Lopez 2018). To overcome this problem, it is desirable to maintain both the quality and quantity of consumable commodities through various healthy and eco-friendly post-harvest quality safeguarding strategies. The exogenous supply of GA3 is an efficacious approach that enables a longer shelf-life for fruits and vegetables (Kuchi et al. 2017). The soaking of Toona sinensis with 100 mg/L GA3 remarkably reduced its browning and decaying and alleviated the chilling stress-induced injuries by maintaining a higher level of reducing sugar, proline and increasing enzymatic and no-enzymatic antioxidants activity over the water treatment (Hu et al. 2018a, b). The addition of GA310−6 M to mineral nutrient solutions in a floating hydroponic system at the preharvest stage showed positive effects by retarding senescence and increasing the shelf life of Lactuca sativa L. and Eruca sativa Mill. After harvest (Miceli et al. 2019). The comparative transcriptome analysis of harvested leaves tissue of Brassica rapa L. showed that the activity of several differentially expressed genes like chlorophyll catabolic genes BrPPH and BrRCCR, bioactive GA degradation gene BrGA20 × 1 and NAC transcription factor BrNACo87 which are involved in chlorophyll and GA degradation, respectively during leaf senescence were significantly inhibited by the foliar treatment of 100 µM GA3 (Fan et al. 2021). The fruits of Capsicum annum L. dipped in 2 ppm GA3for 30 s and then stored at 1 ℃ resulting in a delay in the change of skin color, total ascorbate and phenolic content and an increase in antioxidant activities and provided a longer shelf life to its green fruits (Panigrahi et al. 2017; Maurya et al. 2020). The browning of Lactuca sativa L. leaves was significantly inhibited during cold storage by the treatment of 0.1 mg/L GA3, and it further enhanced the content of soluble protein and sugar, decreasing the activity of polyphenol oxidase and peroxidase (Tian et al. 2014). The fruits of Abelmoschus esculentus L. immersed in 0.1 g/LGA3 solution could postpone its senescence and maintain fruit quality and chlorophyll content.GA3 downregulate the expression of genes such as AeNOL, AeNYC, AeSGR, AeCLH, AePAO, AePPH, AeRCCR, and AeHCAR involved in chlorophyll degradation (Xiao et al. 2022). The treatment of GA3 at 0.1 mmol dm−3 delayed leaf yellowing and increased flower longevity of Lilium longiflorum (Rabiza-Swider et al. 2015). The cut flowers of Anthurium andraeanum L. sprayed with 144 µM GA3 extended the vase life of flowers, delayed senescence and increased the phenol content and activity of polyphenol oxidase, peroxidase, and superoxide dismutase (do Nascimento et al. 2018). The spray of 150 ppm GA3 significantly enhanced the freshness of flowers and delayed aging in Chrysanthemum morifolium L. (Singh and Bala 2018).
Amelioration of Abiotic Stresses
Abiotic stresses (such as salinity, drought, heavy metals, and varying temperature) cause severe effects on the growth, physiological and biochemical processes, and productivity of crop plants. Gibberellic acid is an important chemical messenger that modulates various cellular processes to induce plant tolerance against abiotic stresses. Studies suggested that the exogenous application of GA3 alleviates the negative effects of abiotic stresses by improving the physio-biochemical processes of plants (Fig. 4). In this section, findings regarding GA3-mediated responses to various abiotic stresses in several crop plants are discussed below.
GA3 and Salt Stress
Soil salinity is a major environmental threat that impairs the growth and development of crop plants by causing osmotic, ionic, and oxidative stress, metabolic disorder, and nutrient deficiency (Isayenkov and Maathuis 2019; Islam et al. 2021). The potential of GA3 in imparting tolerance to salt stress has been studied in various crop plants, and there is ample evidence that GA3 protect plant species from salinity-induced damages by maintaining membrane stability, ion homeostasis, upregulating antioxidant enzyme activities, maintaining compatible solute concentration, protecting photosystems and also inducing expression of stress genes. For example, Hamayun et al. (2010) reported that 5 µM GA3 spray attenuated the salt stress-induced effect on growth and photosynthesis by significantly increasing endogenous phytohormones level, leaf chlorophyll content, daidzein and genistein content in Glycine max L. Further, Khan et al. (2010a, b) observed that 10–6 M GA3 application overcomes salt toxicity by improving growth, antioxidant system, OP accumulation, and mineral nutrient contents in Linum usitatissimum L. Ali et al. (2012) found that treatment of 10–6 M GA3 reversed salt stress-induced effect on growth performance of Hibiscus sabdariffa L. by improving its biomass and CA activity. Iqbal and Ashraf (2013) and Younesi and Moradi (2014) demonstrated that pre-treatment of Triticum aestivum L. and Medicago sativa L. seeds with GA3 alleviated salt stress-induced inhibitory effect on their performance by improving significantly seed germination, plant biomass, photosynthetic efficiency, ion homeostasis, antioxidant defense system, endogenous hormonal homeostasis while decreasing lipid peroxidation. Further, Shaddad et al. (2013) reported that 100 ppm GA3spray treatment confers salt stress tolerance in Triticum aestivum L. by improving the contents of photosynthetic pigments, carbohydrates, amino acids, proteins and proline. Similarly, the foliar application of 100 mg/L GA3 mitigated the negative effects of salt stress by increasing plant biomass, water status, chlorophyll content, OP content, antioxidant enzyme activities and mineral nutrient acquisition in Rosa damascina L. (Ali et al. 2014). Besides, Tsegay and Andargie (2018) studied the effect of pre-treatment of 0.2 g/L GA3 on seed germination and growth performance of three crops viz., Zea mays L., Pisum sativum L. and Lathyrus sativus L., with salinity stress. They observed that GA3 pre-treatment reversed the salt-induced effect by significantly enhancing germination percentage, shoot and root length, root fresh and dry weight and reduced mean germination time. Moreover, Wang et al. (2019) revealed that a spray of GA3 at 0.1 mM mitigated the harmful effects of salt stress in Abelmoschus esculentus L. by improving antioxidant enzyme activities, proline content and by decreasing electrolyte leakage and lipid peroxidation. Chauhan et al. (2019) and Zhu et al. (2019) found that pre-treatment of Avena sativa L. and Sorghum bicolor L. seeds with GA3 relieved salt stress-induced effect on seed germination and crop growth by enhancing water uptake, seed germination percentage, length of the radical and plant biomass. Further, Moula et al. (2020) reported that foliar feeding of GA3 improved the performance of Olea europaea L. growth under salt stress by significantly enhancing leaf chlorophyll content, photosynthetic assimilation and mannitol content. Furthermore, the foliage of Solanum lycopersicum L. treated with GA3 (1.4 µM) ameliorated salt-induced osmotic and oxidative stress damages by increasing glycine betaine and proline content, glutathione and ascorbate content and enzymatic antioxidants (Siddiqui et al. 2020). Moreover, Ghani et al. (2021a, b) investigated the foliar application of GA3 (100 mg/L) nullified salinity effect on the growth of Allium cepa L. by significantly enhancing growth parameters and total soluble protein content. Based on the above-appraised literature, it may be summarized that GA3 plays a pivotal role in imparting salt tolerance in crop plants by enhancing membrane permeability, OP accumulation, nutrient acquisition, ion homeostasis, and activities of antioxidants while limiting lipid peroxidation and ROS generation.
GA3 and Water Stress
Water (drought) stress is an acute environmental cue that affects various plant processes, leading to reduced crop productivity worldwide (Kaur and Asthir 2017; Seleiman et al. 2021). It causes several interrelated physio-biochemical disorders that are detrimental to plants by disrupting cellular metabolism and causing cell damage through ionic and oxidative stress. The most severe repercussions of water stress are the gradual/rapid water loss through stomata, leading to cell dehydration and cell/tissue death. Evidence suggests that GA3 plays a pivotal role in improving the performance of plants under drought/water stress. Studies have shown that exogenous GA3 mitigates the water stress-generated effects, as seen in Brassica napus L., Zea mays L., and many other crop plants (Li et al. 2010; Al-Shaheen and Soh 2016; Khan et al. 2016). Pre-treatment of Zea mays L. seeds with GA3 (500 mg/L) attenuated drought-induced effect on growth by improving germination rate, dry seedling weight, germination and vigor index, antioxidant enzyme activities and chlorophyll and OP content (Yuan et al. 2014). Further, Al-Shaheen and Soh (2016) demonstrated that foliar spray of 100 and 300 ppm GA3 improved Zea mays L. growth performance under drought stress by improving photosynthetic pigment and OP concentration and seed protein content. In another study, Khan et al. (2020a, b, c) also observed that GA3 minimized the severity of drought stress in Brassica napus L. by enhancing antioxidant enzymes and OP concentration activities. They also reported GA3 mediated increase in protein contents, glucosinolate, unsaturated fatty acids (erucic, oleic, linoleic, and linolenic acid), and saturated fatty acid (palmitic acid). Further, Miri et al. (2021) reported that 120 ppm GA3 spray treatment attenuated the reduction in yield of Vigna unguiculata L., under drought stress by improving LRWC, photosynthetic pigments, 100-seed weight, and seed yield. Moreover, Rady et al. (2021) studied the effect of foliar application of 20 mg/L GA3on drought stressed Vicia faba L. plants. They observed that foliar application of GA3 nullified drought stress-induced perturbations by increasing growth, water use efficiency, photosynthetic pigments, LRWC, soluble sugars, membrane stability index, OP content, and antioxidant enzyme activities in Vicia faba L. From the above observations, it can be inferred that GA3 minimized water stress-induced effects in crop plants by enhancing soluble sugar content, membrane permeability, fatty acids content, OP accumulation, and antioxidant enzyme activities.
GA3 and Heavy Metal Stress
Soil contaminated with hazardous heavy metals (HMs) has become a subject of concern for sustainable agriculture. HMs stress causes serious threats to crop plant productivity by altering growth and developmental processes, leading to the death of plants. Soil can receive HMs from industrial effluents, urban run-off, burning liquid/toxic fuel, sewage waste disposal, domestic garbage dump, etc. Plants ameliorate the toxic effects of HMs through various mechanisms, including chelation (mediated by phytochelatins) and sub-cellular compartmentalization (Ghori et al. 2019). There is ample literature available on the significance of GA3 in reducing HMs stressed-induced impact in plant species. For instance, exogenously applied GA3 was reported to ameliorate the harmful effects of cadmium and lead in Lupinus albus L. by significantly improving leaf chlorophyll, soluble protein, carbohydrate, and proteases, amylases, and catalases activity (Sharaf et al. 2009). Further, exogenously applied 10–8 M GA3 mediated increased proline accumulation and activities of antioxidant enzymes were reported to ameliorate the nickel stress-induced damages in Triticum aestivum L. (Siddiqui et al. 2011). Increased antioxidant enzyme activities and OP accumulation in copper-stressed Spinacia oleracea L., were also reported (Gong et al. 2021). In another study, Zhu et al. (2012) reported that leaf applied improved cadmium stress tolerance by decreasing lipid peroxidation and expression of cadmium uptake-related gene-IRT1in Arabidopsis thaliana L. Amri et al. (2016) studied the ameliorative effect of pre-treatment of Hordeum vulgare L. seeds with 0.5 µM GA3 for 96 h in the presence of cadmium toxicity. They observed that exogenous application of GA3 improved tolerance to cadmium stress by increasing hydrolytic enzymes, sugar and amino acid content of the endosperm and mobilization of starch and protein from endosperm to seedling roots. Kaya et al. (2020) demonstrated that GA3 mediated enhanced contents of endogenous hydrogen sulfide, leaf calcium and potassium, and proline ameliorated the severity of boron toxicity in Solanum lycopersicum L. Moreover, GA3 (50 ppm) improved tolerance to lead toxicity by significantly increasing biomass, photosynthetic pigment, phenolic content, and antioxidant enzyme components in Daucus carota L. (Ghani et al. 2021a, b). Additionally, exogenously applied GA3 was evidenced to alleviate the oxidative stress damages by enhancing the antioxidant defense system, OP accumulation, photosynthetic efficiency, and growth in copper-exposed Corchorus capsularis L. and Pisum sativum L. plants (Saleem et al. 2020; Javed et al. 2021) and cadmium exposed Vigna radiata L. and Pisum sativum L. (Hasan et al. 2020; Sun et al. 2020). In conclusion, GA3 alleviated HMs toxicity in crop plants by improving endogenous hormones level, OP accumulation, and antioxidant enzyme activities.
GA3 and Temperature Stress
Global warming leads to variations in temperature. High and low temperatures have become a potential environmental cue that negatively affects many physio-biochemical and metabolic processes in plants, including germination of seeds, seedling growth, photosynthesis, protein structure, enzymes activities, membrane stability, and cell/tissue death (Mathur et al. 2014; Szymanska et al. 2017). Various studies have shown that supplementation of GA3 differentially benefits crop plants exposed to extreme temperatures by enhancing their inherent defense system (Haroun et al. 2018; Hu et al. 2018a, b; Aziz and Peksen 2020). In Raphanus sativus L., seed priming with GA3 (900 µM) improves seed germination, coleoptile, and radicle length, and fresh weight of seedlings, thus improving high-temperature stress tolerance (Cavusoglu and Kabar 2007). Similarly, Li et al. (2013) reported that pre-sowing seed treatment with GA3 (5 µmol/L) alleviated chilling stress toxicity by improving seed germination rate, germination index, lengths, and weights of radical and coleoptiles and starch degradation in Triticum aestivum L. Furthermore, GA3 treatment maintained redox homeostasis in Gladiolus hortulanus L. under variable temperatures by increasing membrane stability, antioxidant enzyme activities, and decreasing membrane leakage (Saeed et al. 2014). In Solanum lycopersicum L., GA3 (0.5 mM) treatment alleviates the toxic effects of cold stress in Solanum lycopersicum L. fruits by modulating the accumulation of endogenous hormones (Ding et al. 2015 and Zhu et al. 2016). In another study, Xie et al. (2018) reported that GA3-mediated low-temperature tolerance was associated with the upregulated expressions of UDP-glucose pyrophosphorylase, granule-bound starch synthase, β-amylase, ADP-glucose pyrophosphorylase, and invertase inhibitor genes in Solanum tuberosum L. In addition, the heat stress-induced inhibitory effect on Phoenix dactylifera L. performance was reversed by GA3 treatment, which regulates endogenous salicylic acid, abscisic acid accumulation, and activities of antioxidant enzymes (Khan et al. 2020a, b, c). From the above-cited literature, it may be summarized that GA3 mitigated the effects of varying temperatures in crop plants by regulating endogenous hormone levels, OP accumulation, ion homeostasis, and antioxidant defense system.
Gibberellin Crosstalk with Other Plant Growth Regulators
PGRs are structurally different compounds, but their signaling network interacts with each other both antagonistically or synergistically to control numerous phenotypic and developmental features in plants under both stressful and stressed free environments (Kohli et al. 2013). The interaction among PGRs (Fig. 5) showed that they could co-coordinately moderate genetic machinery and defense system to improve plants endurance against adverse environmental conditions (Khan et al. 2020a, b, c). The interaction between GAs and other PGRs such as abscisic acid (Weiss and Ori 2007), auxin (Hu et al. 2018a, b), brassinosteroids (Tong et al. 2014), cytokinin (Subbaraj et al. 2010), ethylene (Achard et al. 2007), jasmonic acid (Peng et al. 2009), nitric oxide (Bethke et al. 2007), polyamines (Verdolin et al. 2021), salicylic acid (Xie et al. 2007) and strigolactones (Ito et al. 2017) has been established under both stressed free and stressed conditions.
A schematic model of gibberellin crosstalk with other phytohormones in regulating physiological and molecular responses of crop plants. GA and ABA showed an antagonistic behavior during seed germination. The Aux/IAA and ARF proteins up regulate the gene expression involved in GA metabolism. The BRASSINAZOLE RESISTANT1 (BZR1) is a transcription factor that modulates gene expression in response to brassinosteroid. The DELLA protein GAI inhibits the activity of BZR1. GA inhibits the effects of cytokinin. Ethylene reduced the level of GA and enhanced the DELLA repressor protein accumulation. The JASMONATE ZIM-domain (JAZ) interacts and inhibits DELLA protein and activates bHLH factor PHYTOCHROME INTERACTING FACTOR 3 (PIF3) in the GA signaling pathway. Nitric oxide enhanced the accumulation of DELLA protein and negatively affecting the GID1 receptor of GA. GA (gibberellin), SA (salicylic acid), PA (polyamines), CK (cytokinin), JA (jasmonates), ST (strigolactones), BR (brassinosteroids), NO (nitric oxide), ABA (abscisic acid), GID1 (GIBBERELLIN INSENSITIVE DWARF 1), ARF (auxin response factor) and EIN3 (ETHYLENE INSENSITIVE 3). Sharp arrows indicate positive regulation and blunt arrows show negative regulation
Abscisic Acid
Abscisic acid (ABA) is a sesquiterpene plant hormone that regulates plant growth and developmental phases, including seed development and maturation and synthesis of protein and compatible solutes to tolerate adversities of the environment (Kumar et al. 2022). GA and ABA interaction help in sustaining the balance between germination and seed dormancy which plays an essential role in stress resistance. GA and ABA exhibited antagonistic behavior during seed germination. GA promotes seed germination, whereas ABA retards germination (Golldack et al. 2013). In Oryza sativa L., two WRKY genes (OsWRKY51 and OsWRKY71) are inducible for ABA and repressible for GA in embryo and aleurone cells. The WRKY genes established signaling crosstalk between ABA and GA (Xie et al. 2006). GA enhanced net carbon fixation and transport, but ABA only improved sugar transport in roots and berries of Vitis vinifera L. (Moreno et al. 2011). ABSCISIC ACID INSENSITIVE 4 (ABI4) gene regulates the primary seed dormancy by moderating the balance between ABA and GA biogenesis in A. thaliana L. (Shu et al. 2013). The GIBBERELLIN INSENSITIVE DWARF 1 (GID1), a receptor of GA, modulates stomatal development and patterning in Oryza sativa L. GID1 mutant exhibited a decrease in biosynthesis of endogenous ABA. GID1 improved submergence tolerance by regulating the consumption of carbohydrates and its function dependent on ABA and GA signaling (Du et al. 2015). The exogenous application of ABA on Cucumis melo L. induced low-temperature tolerance by improving growth characteristics and endogenous GA and SA levels (Kim et al. 2016). Yue et al. (2018) found that exogenous application of ABA and GA on Camellia sinensis L. enhanced the expression of CsKS, CsKAO, CsKO, CsGA20ox-2, and CsNECD2 and repressed CsGID1b, CsSDR, CsPYL8, and CsCYP707A2 genes. These genes expression showed that GA and ABA controlled bud dormancy. Guo et al. (2018) revealed that a Triticum aestivum L. specific microRNA 9678 (miR9678) expressed in the scutellum of germinating seeds reduced the GA levels, but ABA enhanced the activity of miR9678. Its overexpression delayed the germination of seeds. Xu et al. (2021a) indicated that exogenous application of ABA and GA regulates bulblet development in Lycoris radiata L. GA inhibits bulblet development by down-regulating the expression of carbohydrate metabolism enzymes encoding genes such as LrSUS1, LrSUS2, and AGPase whereas ABA promoted bulblet development by increasing endogenous auxin content and activities of starch synthesis enzymes including SSS and GBSS.
Auxins
Auxins are a group of essential phytohormones involved in numerous plant growth and developmental events (Zhao et al. 2010). GA and auxin interactively regulate growth and physio-biochemical processes in plants. For instance, GA and auxin promoted fruit initiation in Solanum lycopersicum L. High levels of auxin or GA induce parthenocarpy in plants. The repressor of GA signaling, like SlDELLA, and auxin signaling components, such as SlIAA9 and SlARF7, mediate the interaction between them to control fruit initiation (Jong et al. 2011; Hu et al. 2018a, b). The Aux/IAA (indole acetic acid) and ARF proteins upregulate the gene expression involved in GA metabolism, particularly AtGA20ox and AtGA2ox genes in A. thaliana L. (Frigerio et al. 2006). GA improved auxin concentration in the stem of Populus tremula L. by promoting polar auxin transport. The IAA and GA induced cell and organ growth (Bjorklund et al. 2007). Willige et al. (2011) observed that GA controls the PIN protein level in A. thaliana L., which is required to transport auxin in plants. Richter et al. (2013) indicated that GA biosynthetic deficient A. thaliana L. showed delayed flowering because GA promotes the synthesis of ARF2 protein. The ARF controls the auxin responses at the cellular level to control plant developmental phases. Shuai et al. (2017) investigated that pre-sowing seed treatment with 10 µM IAA inhibits seed germination of Glycine max L. by increasing the biosynthesis of ABA and impairing GA biosynthesis. The qPCR assay showed that genes involved in ABA biosynthesis were upregulated, whereas GA biosynthetic genes were downregulated. Further, the AFR gene (SlARF5) plays an essential role in modulating the signaling pathways of both auxin and GA in Solanum lycopersicum L. during development and fruit set (Liu et al. 2018). Yoneda et al. (2018) suggested that GA and auxin improve steviol glycoside concentration in Stevia rebaudiana L. In Glycine max L., the combined application of 100 µM GA and IAA induced hypocotyl elongation and promoted their endogenous level (Jiang et al. 2020). Kou et al. (2021) observed that exogenous application of 20 mg/L IAA induced stalk elongation and expansion of cell walls by regulating the expression of genes encoding expansin protein and increasing endogenous IAA and GA contents in Brassica rapa L. The auxin response factor (ARF6 and ARF8) interacts with DELLA and promotes the senescence of cambium (Ben-Targem et al. 2021). Liang et al. (2021) revealed that the co-application of 50 µM IAA and GA3 minimized cadmium toxicity in Sedum alfredii L. by increasing plant biomass, chlorophyll content, potassium content, and decreasing malondialdehyde.
Brassinosteroids
Brassinosteroids (BRs), steroidal plant hormones, play an essential role in regulating plant growth and physio-biochemical processes (Fariduddin et al. 2014). GA and BRs exhibited signaling pathways crosstalk to control plant growth and developmental processes. The BRASSINAZOLE RESISTANT1 (BZR1) is a transcription factor that modulates gene expression in response to BRs. The DELLA protein GAI inhibits the activity of BZR1 and regulates the hypocotyl elongation in A. thaliana L. (Gallego-Bartolome et al. 2012). The OsGSR1, a member of GAST gene family (GA-stimulated transcript), expression is inhibited by BRs and stimulated by GA. Transgenic Oryza sativa L. plant containing OsGSR1 RNAi transcript shows low endogenous BRs. However, OsGSR1 is a positive regulator for BRs and GA signaling (Wang et al. 2009). BRs modulate cell elongation in Oryza sativa L. by regulating GA metabolism. Especially, BRs induced the expression of the GA biosynthetic gene, D18/GA3ox-2, and improved cell elongation (Tong et al. 2014). Both GA and BRs regulate seed traits like mesocotyl length in Zea mays L. (Hu et al. 2017). The miRNA (OsmiR396d) is involved in the interaction of GA and BRs signaling pathways. The overexpression of OsmiR396d enhanced BRs signaling and impeded the biosynthesis and signaling of GA. Interestingly BZR1 improved the accumulation of OsmiR396d in Oryza sativa L. (Tang et al. 2018). Further, the OsmiR159d-OsGAMYBL2 coordinates the functions of GA and BRs in the growth and development of plants by modulating the expression of genes that participated in GA and BRs biosynthesis and signaling (Gao et al. 2018). Que et al. (2018) investigated that exogenous application of 0.5 mg/L 24-epibrassinolide on Daucus carota L. promoted cell elongation, GA level and cellulose deposition in its petiole. Zheng et al. (2019) observed that foliar application of 3 mg/L 28-HBR on Malus pumila L. seedlings upregulated the expression of genes involved in the biosynthesis of auxin and GA and enhanced plant growth. In Solanum lycopersicum L., BRs modulate ovule number by affecting GA biosynthesis and stabilizing DELLA protein that stimulates ovule primordial initiation (Barro-Trastoy et al. 2020). The late embryogenesis abundant (LEA) protein plays an essential role in dehydration tolerance during seed development. Mutation in the LEA33 gene affects grain size and seed germination in Oryza sativa L. LEA proteins may form a molecular regulatory network between GA and BRs signaling pathways to regulate seed germination (Li et al. 2020). Jiang et al. (2020) proposed that GA regulates hypocotyl elongation in Glycine max L. by promoting auxin and BRs function. Xiong et al. (2021) revealed that GA and BRs controlled seed germination and embryo growth and promoted glutelin protein mobilization in Oryza sativa L.
Cytokinins
Cytokinins (CK) are an important chemical messenger that plays a key role in the modulation of the plant cell cycle and various developmental processes (Cortleven et al. 2019). A negative regulation has been established between these two hormone response pathways. However, their exclusive signaling interaction to regulate developmental processes is poorly understood. GA inhibits the effects of CK. SPINDLY (SPY) is a negative regulator of GA signaling that enhances CK responses in A. thaliana L. SPY behaves as a repressor for GA signaling and regulator for cytokinin response (Greenboim-Wainberg et al. 2005). CK and GA alleviate bud dormancy and promote flowering and branching in Zantedeschia sp. (Subbaraj et al. 2010). A dominant-active DELLA1 protein controlled the GA signaling and improved cytokinin response1 (CRE1) dependent CK pathway to regulate nodulation in Medicago truncatula (Fonouni-Farde et al. 2017).
Ethylene
Ethylene (ET) is a gaseous signaling molecule that regulates many growth and physiological processes in plants (Matilla-Vazquez and Matilla 2014). ET inhibits root growth while promoting apical hook formation in A. thaliana L. by hampered degradation of DELLA repressor protein (Achard et al. 2003). ET reduced the level of GA and enhanced the DELLA repressor protein accumulation. It also delays flowering in A. thaliana L. by repressing floral meristem-identity genes viz., LEAFY (LFY) and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1) (Achard et al. 2007). The expression of the C-repeat/drought-responsive element binding factor (CBF1/DREB1b) gene (a member of APETALA2/ETHYLENE RESPONSE FACTOR family transcription factor) improves tolerance against low temperature but also restrains growth by permitting the accumulation of DELLA (Achard et al. 2008). The SNORKEL1 and SNORKEL2 are ethylene response factors that permitted Oryza sativa L. to adapt in deep water. The product of these genes induced internode elongation through GA signaling (Hattori et al. 2009). DELLA protein interacts with the DNA-binding domain of the ETHYLENE INSENSITIVE 3/EIN3-LIKE 1 (EIN3/EIL1) and inhibits the expression of the HOOKLESS 1 (HLS1) gene. GA stimulates apical hook formation in association with ET by promoting the expression of HLS1 in A. thaliana L. (An et al. 2012).
Jasmonic Acid
Jasmonic acid (JA) is a crucial growth-regulating substance that modulates diverse developmental processes of plants (Ruan et al. 2019). GA and JA showed both antagonistic and synergistic interactions to regulate plant growth and development. The JASMONATE ZIM-domain (JAZ) interacts and inhibits DELLA protein and activates bHLH factor PHYTOCHROME INTERACTING FACTOR 3 (PIF3) in the GA signaling pathway, while JA promoted JAZ degradation and free DELLA protein that restrains PIF3 and inhibit GA induced hypocotyl elongation (Yang et al. 2012). Exogenous treatment of JA improved endogenous GA content under salt stress in Oryza sativa L. (Seo et al. 2005). GA and JA modulate trichome initiation and stamen development in A. thaliana L. (Qi et al. 2014; Song et al. 2014). Further, GA regulates the expression of JA biosynthetic genes such as DAD1 and LOX1 to enhance the synthesis of JA, and a high level of JA stimulates the expression of MYB21, MYB24, and MYB54 to induce stamen filament development (Peng et al. 2009). GA promotes the biosynthesis of JA by regulating the expression of MYB21, MYB24, and MYB57 genes, which are contributed to stamen development in A. thaliana L. (Cheng et al. 2009). JA antagonizes the biosynthesis of GA by inhibiting the activities and accumulation of GA20ox and GA13ox that are involved in the biosynthesis of GA. It has been indicated that the high level of JA inhibits GA biosynthesis in the stem of Nicotiana attenuata L. (Heinrich et al. 2013). Um et al. (2018) studied that JAZ interacts with DELLA repressor proteins, including SLENDER RICE 1 (SLR1), in GA signaling. The protein OsJAZ9 inhibits JA responses and stimulates GA responses in Oryza sativa L. showed antagonistic crosstalk between GA and JA. The combined application of JA and GA3 on Cicer arietinum L. reduced cadmium contamination by increasing photosynthetic characteristics, enzymatic activities, osmolyte accumulation, and mineral nutrition (Ahmad et al. 2021).
Polyamines
Polyamines (PA) is a group of aliphatic amines that act as a plant growth regulator and participate in several developmental aspects of plants (Gonzalez et al. 2021). The exact signaling interplay between GA and PA is still not well understood. However, few studies reported that GA and PA coordinately play a role in plants growth and metabolic processes. The exogenous application of spermidine (Spd) improved drought tolerance in Agrostis stolonifera L. by improving RWC, chlorophyll content, antioxidant enzyme activities, endogenous PA levels, and reduced IAA and GA3 accumulation level (Li et al. 2015; Krishnan and Merewitz 2017). Li et al. (2018) revealed that exogenous application of Spd alleviated drought stress in Zea mays L. by enhancing photochemical efficiency, synthesis of ATP, and endogenous PA, IAA, and GA3 levels. Qin et al. (2019) also reported that the application of Spd stimulated floral induction in Malus domestica L. by increasing the activity of MdGA2ox2 that reducing GA3 level. The flowering-related gene like LEAFY also increased by Spd. The spray treatment of PA and GA3 on Impatiens hawkeri L. increased plant height, leaf numbers, plant biomass, and flower buds (Verdolin et al. 2021). The combined application of Spd and GA3 improved endogenous PA contents, PA biosynthetic enzyme activities, developmental stages of flowering, and delayed flower senescence in Rhododendron simsii L. (Xu et al. 2021b). Zandona et al. (2021) found that exogenous application of GA3 on Calibrachoa sellowiana L. breaks seed dormancy to promote seed germination and improve endogenous PA content.
Nitric Oxide
Nitric oxide (NO) is a key endogenous signaling molecule that regulates plant diverse physiological and biochemical processes (Wani et al. 2021a). Studies suggested that a possible interaction between GA and NO has existed that modulates various developmental events in plants, including seed germination, hypocotyl elongation, root growth, and growth of pollen tubes (Asgher et al. 2017). In A. thaliana L., NO up-regulates the GA signaling pathway that leads to improved protein storage in seed (Bethke et al. 2007). The NO-deficient A. thaliana L. mutant showed longer hypocotyl than wild-type ones. Interestingly, exogenous treatment of NO reduced hypocotyl length by enhancing the accumulation of DELLA protein and negatively affecting the GID1 receptor of GA (Lozano-Juste and Leon 2011). NO and other plant hormones, including GA, enhance aluminum stress tolerance in plants by maintaining their endogenous hormonal equilibrium (He et al. 2012). NO enhances the DELLA protein concentration and negatively impacts GA signal transduction (Freschi 2013). Moreover, NO and GA3 eliminated seed dormancy and induced germination in Amaranthus retroflexus L. (Kepczynski et al. 2017). NO is a crucial component in various plant hormone signaling networks, including GA, to moderate multiple metabolic functions in plants under adverse environmental conditions (Singhal et al. 2021).
Salicylic Acid
Salicylic acid (SA) is a small phenolic compound that plays a regulatory role in various physiological processes of plants (Hayat and Ahmad 2007). GA and SA are essential in plants' various growth and physiological processes. Recent studies emphasize that a complex network of interaction existed between two hormones, but still, limited literature has been found. SA inhibits seed germination in Zea mays L., A. thaliana L., and Hordeum vulgare L. Further, SA and WRKY genes regulate the activity of α-amylase. GA promotes α-amylase production in aleurone cells but is inhibited by SA. In the aleurone cells of Hordeum vulgare L. seed, the expression of HvWRKY38 is downregulated by GA but upregulated by ABA and SA (Xie et al. 2007). The application of GA3 on A. thaliana L. minimized the inhibitory effect of salt and heat stresses and improved seed germination, seedling growth, and expression of isochorismate synthase1 and PR1 genes involved in the regulation of biosynthesis and function of SA (Alonso-Ramirez et al. 2009). The seedlings of Solanum lycopersicum L. treated with SA mitigated chilling injury by enhancing antioxidant enzyme activities and regulating GAs metabolism (Ding et al. 2016). The exogenous application of SA on Carthamus tinctorius L. ameliorated salinity stress by improving mineral nutrient content, endogenous IAA and GA levels and expression of SOS1 and NHX1 genes (Shaki et al. 2019). The co-application of GA3 and SA on Brassica juncea L. enhanced growth and yield parameters, including seed yield and oil content (Ijaz et al. 2019). In Ajuga integrifolia L. SA application increased plant biomass and phytochemical production, including phenolics, flavonoids and antioxidants, while GA3 behave antagonistically to downregulate their production (Abbasi et al. 2020). The exogenously applied GA3 promoted the biosynthesis of SA, resulting in a high level of SA that improved plant defense responses against environmental cues (Emamverdian et al. 2020). Furthermore, the combined application of GA3 and SA on Echinacea purpurea L. improved plant height, biomass, yield, and oil content (Hasan-beigi 2021).
Strigolactones
Strigolactones (SLs) are a group of carotenoid-derived plant growth regulators that modulate many physiological processes, including root development and shoot branching (Wani et al. 2021b). GA and SLs may interact to regulate plant growth and developmental aspects, but their complex crosstalk is poorly understood. It has been found that GA is considered a regulator of SLs biosynthesis. Moreover, GA signaling regulates SLs biosynthesis by controlling the SLs biosynthetic gene expression (Ito et al. 2017; Marzec 2017). SLs stimulate the interaction between SLs receptor DWARF14 (D14) and a DELLA protein SLENDER1 (SLR1), exhibiting crosstalk between GA and SLs signaling pathways (Nakamura et al. 2013). The Oryza sativa L. GA biosynthetic mutant was insensitive to a synthetic analogue of SLs treatment, while the wild type responded to that treatment (Marzec 2017). GA and SL synergistically controlled the expression of GA2ox2 and SUPPRESSOR OF max2 1-LIKE8 (SMXL8) genes to regulate seedling growth of A. thaliana L. (Lantzouni et al. 2017). The defect in the biosynthesis or signaling of SLs resulted in dwarfism in Oryza sativa L. but could be restored by GA3 treatment. The transcription level of cell division and cell elongation-related genes was enhanced by GA3 treatment leading to increased shoot elongation (Zou et al. 2019).
Conclusion and Future Prospective
The constant rise in food demand due to expanding population, depleted natural resources, and climatic uncertainty is a major concern worldwide. Among the natural calamities, abiotic stress is a significant factor that adversely affects the growth and productivity of economically important crops. Plant growth regulators play a significant role in providing tolerance against abiotic stresses and improving the growth and production of crop plants. One of the important and widely discussed phytohormones is gibberellins, which are essential in improving various aspects of the horticultural crop's plant growth, development, and post-harvest life. Gibberellins enhance the abiotic stress tolerance in crop plants by modulating many gene expressions which influence the levels of antioxidants (enzymatic and non-enzymatic), osmolytes, and several other proteins and enzymes. Gibberellins interact with other plant growth regulators to improve plant growth and physiological processes. From the above-reviewed literature, it may be concluded that exogenous application of gibberellic acid increased morphological traits, physiological and metabolic processes, and yield and quality components of crop plants under diverse environmental conditions. However, in the future, there is a need to conduct further research on this hormone to explore its potential role and use it as a management tool for increasing the growth, productivity, and shelf life of valuable crops. Furthermore, the comprehensive knowledge to understand the relationship of gibberellins with other phytohormones under salt stress conditions are need to be widely investigated.
References
Abbas M, Imran F, Iqbal Khan R, Zafar-ul-Hye M, Rafique T, Jameel Khan M, Datta R (2020) Gibberellic acid induced changes on growth, yield, superoxide dismutase, catalase and peroxidase in fruits of bitter gourd (Momordica charantia L.). Horticulturae 6:72. https://doi.org/10.3390/horticulturae6040072
Abbas HMK, Askri SMH, Ali S, Fatima A, Xue SD, Muhammad Z, Zhong YJ (2022) Mechanism associated with Brassinosteroids crosstalk with gibberellic acid in plants. Brassinosteroids signalling. Springer, Singapore, pp 101–115
Abbasi BH, Ullah MA, Nadeem M, Tungmunnithum D, Hano C (2020) Exogenous application of salicylic acid and gibberellic acid on biomass accumulation, antioxidant and anti-inflammatory secondary metabolites production in multiple shoot culture of Ajuga integrifolia Buch. Ham. ex D. Don. Ind Crops Prod 145:112098. https://doi.org/10.1016/j.indcrop.2020.112098
Abd El-Razek E, Hassan HSA, Gamal El-Din KM (2013) Effect of foliar application with salicylic acid, benzyladenine and gibberellic acid on flowering, yield and fruit quality of olive trees (Olea europaea L.). Middle-East Sci Res 14:1401–1406
Abdel-Hamid AM, Mohamed HI (2014) The effect of the exogenous gibberellic acid on two salt stressed barley cultivars. Eur Sci. J 10
Achard P, Vriezen WH, Van Der Straeten D, Harberd NP (2003) Ethylene regulates Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell 15:2816–2825
Achard P, Baghour M, Chapple A, Hedden P, Van Der Straeten D, Genschik P, Harberd NP (2007) The plant stress hormone ethylene controls floral transition via DELLA-dependent regulation of floral meristem-identity genes. Proc Natl Acad Sci USA 104:6484–6489
Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P (2008) The cold-inducible CBF1 factor–dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 20:2117–2129. https://doi.org/10.1105/tpc.108.058941
Afroz S, Mohammad F, Hayat S, Siddiqui MH (2006) Exogenous application of gibberellic acid counteracts the ill effect of sodium chloride in mustard. Turk J Biol 29:233–236
Aftab T, Khan MMA, Idrees M, Naeem M (2011) Optimizing nitrogen levels combined with gibberellic acid for enhanced yield, photosynthetic attributes, enzyme activities, and artemisinin content of Artemisia annua. Front Agric China 5:51–59. https://doi.org/10.1007/s11703-011-1065-7
Ahmad P, Raja V, Ashraf M, Wijaya L, Bajguz A, Alyemeni MN (2021) Jasmonic acid (JA) and gibberellic acid (GA3) mitigated Cd-toxicity in chickpea plants through restricted cd uptake and oxidative stress management. Sci Rep 11:1–17. https://doi.org/10.1038/s41598-021-98753-8
Akand MH, Hem KM, Kumar Bhagat S, Moonmoon JF, Moniruzzaman M (2016) Growth and yield of tomato as influenced by potassium and gibberellic acid. Bull Inst Trop Agric Kyushu Univ 39:083–094
Ali HM, Siddiqui MH, Basalah MO, Al-Whaibi MH, Sakran AM, Al-Amri A (2012) Effects of gibberellic acid on growth and photosynthetic pigments of Hibiscus sabdariffa L. under salt stress. Afr J Biotechnol 11:800–804
Ali EF, Bazaid SA, Hassan FAS (2014) Salinity tolerance of Taif roses by Gibberellic acid (GA3). Int J Sci Res 3:184–192
Alonso-Ramírez A, Rodríguez D, Reyes D, Jiménez JA, Nicolás G, López-Climent M, Nicolás C (2009) Evidence for a role of gibberellins in salicylic acid-modulated early plant responses to abiotic stress in Arabidopsis seeds. Plant Physiol 150:1335–1344. https://doi.org/10.1104/pp.109.139352
Al-Shaheen MR, Soh A (2016) Effect of proline and Gibberellic Acid on the qualities and qualitative of Corn (Zea maize L.) under the influence of different levels of the water stress. Int J Sci Res Pub 6:752–756
Amri B, Khamassi K, Ali MB, da Silva JAT, Kaab LBB (2016) Effects of gibberellic acid on the process of organic reserve mobilization in barley grains germinated in the presence of cadmium and molybdenum. S Afr J Bot 106:35–40. https://doi.org/10.1016/j.sajb.2016.05.007
An F, Zhang X, Zhu Z, Ji Y, He W, Jiang Z, Guo H (2012) Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings. Cell Res 22:915–927
Asgher M, Per TS, Masood A, Fatma M, Freschi L, Corpas FJ, Khan NA (2017) Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ Sci Pollut Res 24:2273–2285
Aziz T, Pekşen E (2020) Seed priming with gibberellic acid rescues chickpea (Cicer arietinum L.) from chilling stress. Acta Physiol Plant 42:1–10. https://doi.org/10.1007/s11738-020-03124-x
Balaguera-López HE, Cárdenas-Hernández JF, Álvarez-Herrera JG (2008) Effect of gibberellic acid (GA3) on seed germination and growth of tomato (Solanum lycopersicum L.). Int Symp Tomato Tropics. https://doi.org/10.1766/ActaHortic.2009.821.15
Baliah NT, Sheeba PC, Mallika S (2018) Encouraging effect of gibberellic acid on the growth and biochemical characters of green gram (Vigna radiata L.). J Global Biosci 7:5522–5529
Banerjee A, Roychoudhury A (2019) The regulatory signaling of gibberellin metabolism and its crosstalk with phytohormones in response to plant abiotic stresses. In: Plant signaling molecules, Woodhead Publishing. pp. 333–339
Barro-Trastoy D, Carrera E, Baños J, Palau-Rodríguez J, Ruiz-Rivero O, Tornero P, Pérez-Amador MA (2020) Regulation of ovule initiation by gibberellins and brassinosteroids in tomato and Arabidopsis: two plant species, two molecular mechanisms. The Plant J 102:1026–1041. https://doi.org/10.1111/tpj.14684
Ben-Targem M, Ripper D, Bayer M, Ragni L (2021) Auxin and gibberellin signaling cross-talk promotes hypocotyl xylem expansion and cambium homeostasis. J Exp Bot 72:3647–3660. https://doi.org/10.1093/jxb/erab089
Bethke PC, Libourel IG, Aoyama N, Chung YY, Still DW, Jones RL (2007) The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol 143:1173–1188
Björklund S, Antti H, Uddestrand I, Moritz T, Sundberg B (2007) Cross-talk between gibberellin and auxin in development of Populus wood: gibberellin stimulates polar auxin transport and has a common transcriptome with auxin. The Plant J 52:499–511
Bose SK, Yadav RK, Mishra S, Sangwan RS, Singh AK, Mishra B, Sangwan NS (2013) Effect of gibberellic acid and calliterpenone on plant growth attributes, trichomes, essential oil biosynthesis and pathway gene expression in differential manner in Mentha arvensis L. Plant Physiol Biochem 66:150–158. https://doi.org/10.1016/j.plaphy.2013.02.011
Cavusoglu K, Kabar K (2007) Comparative effects of some plant growth regulators on the germination of barley and radish seeds under high temperature stress. Eurasian J Biosci 1:1–10
Chauhan A, AbuAmarah BA, Kumar A, Verma JS, Ghramh HA, Khan KA, Ansari MJ (2019) Influence of gibberellic acid and different salt concentrations on germination percentage and physiological parameters of oat cultivars. Saud J Biol Sci 26:1298–1304. https://doi.org/10.1016/j.sjbs.2019.04.014
Cheng H, Song S, Xiao L, Soo HM, Cheng Z, Xie D, Peng J (2009) Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis. PLoS Genet 5:e1000440
Çolak AM (2018) Effect of melatonin and gibberellic acid foliar application on the yield and quality of Jumbo blackberry species. Saud J Biol Sci 25:1242–1246. https://doi.org/10.1016/j.sjbs.2018.06.008
Cornea-Cipcigan M, Pamfil D, Sisea CR, Mărgăoan R (2020) Gibberellic acid can improve seed germination and ornamental quality of selected cyclamen species grown under short and long days. Agronomy 10:516. https://doi.org/10.3390/agronomy10040516
Cortleven A, Leuendorf JE, Frank M, Pezzetta D, Bolt S, Schmülling T (2019) Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ 42:998–1018
Damaris RN, Lin Z, Yang P, He D (2019) The rice alpha-amylase, conserved regulator of seed maturation and germination. Int J Mol Sci 20:450. https://doi.org/10.3390/ijms20020450
Davidson SE, Reid JB, Helliwell CA (2006) Cytochromes P450 in gibberellin biosynthesis. Phytochem Rev 5:405–419. https://doi.org/10.1007/s11101-006-9005-5
Davière JM, Achard P (2013) Gibberellin signaling in plants. Development 140:1147–1151. https://doi.org/10.1242/dev.087650
de Jong M, Wolters-Arts M, García-Martínez JL, Mariani C, Vriezen WH (2011) The Solanum lycopersicum AUXIN RESPONSE FACTOR 7 (SlARF7) mediates cross-talk between auxin and gibberellin signalling during tomato fruit set and development. J Exp Bot 62:617–626
Ding Y, Sheng J, Li S, Nie Y, Zhao J, Zhu Z, Tang X (2015) The role of gibberellins in the mitigation of chilling injury in cherry tomato (Solanum lycopersicum L.) fruit. Postharvest Biol Tech 101:88–95. https://doi.org/10.1016/j.postharvbio.2014.12.001
Ding Y, Zhao J, Nie Y, Fan B, Wu S, Zhang Y, Tang X (2016) Salicylic-acid-induced chilling-and oxidative-stress tolerance in relation to gibberellin homeostasis, C-repeat/dehydration-responsive element binding factor pathway, and antioxidant enzyme systems in cold-stored tomato fruit. J Agric Food Chem 64:8200–8206
do Nascimento Simões A, Diniz NB, da Silva Vieira MR, Ferreira-Silva SL, da Silva MB, Minatel IO, Lima GPP (2018) Impact of GA3 and spermine on postharvest quality of anthurium cut flowers (Anthurium andraeanum) cv. Arizona. Sci Hort 241:178–186
Du H, Chang Y, Huang F, Xiong L (2015) GID1 modulates stomatal response and submergence tolerance involving abscisic acid and gibberellic acid signaling in rice. J Integr Plant Biol 57:954–968. https://doi.org/10.1111/jipb.12313
El Sabagh A, Islam MS, Hossain A, Iqbal MA, Mubeen M, Waleed M, Abdelhamid MT (2022) Phytohormones as growth regulators during abiotic stress tolerance in plants. Front Agron 4:4. https://doi.org/10.3389/fagro.2022.765068
Elahi NN, Raza S, Rizwan MS, Albalawi BFA, Ishaq MZ, Ahmed HM, Ditta A (2022) Foliar application of gibberellin alleviates adverse impacts of drought stress and improves growth, physiological and biochemical attributes of Canola (Brassica napus L.). Sustainability 15:78. https://doi.org/10.3390/su15010078
Emamverdian A, Ding Y, Mokhberdoran F (2020) The role of salicylic acid and gibberellin signaling in plant responses to abiotic stress with an emphasis on heavy metals. Plant Signal Behav 15:1777372. https://doi.org/10.1080/15592324.2020.1777372
Fan ZQ, Wei W, Tan XL, Shan W, Kuang JF, Lu WJ, Chen JY (2021) A NAC transcription factor BrNAC087 is involved in gibberellin-delayed leaf senescence in Chinese flowering cabbage. Postharvest Biol Tech 181:111673
Fariduddin Q, Yusuf M, Ahmad I, Ahmad A (2014) Brassinosteroids and their role in response of plants to abiotic stresses. Biol Plant 58:9–17. https://doi.org/10.1007/s10535-013-0374-5
Fonouni-Farde C, Kisiala A, Brault M, Emery RN, Diet A, Frugier F (2017) DELLA1-mediated gibberellin signaling regulates cytokinin-dependent symbiotic nodulation. Plant Physiol 175:1795–1806
Freschi L (2013) Nitric oxide and phytohormone interactions: current status and perspectives. Front Plant Sci 4:398. https://doi.org/10.3389/fpls.2013.00398
Frigerio M, Alabadí D, Pérez-Gómez J, García-Cárcel L, Phillips AL, Hedden P, Blázquez MA (2006) Transcriptional regulation of gibberellin metabolism genes by auxin signaling in Arabidopsis. Plant Physiol 142:553–563. https://doi.org/10.1104/pp.106.084871
Gad MM, Abdul-Hafeez EY, Ibrahim OHM (2016) Foliar application of salicylic acid and gibberellic acid enhances growth and flowering of Ixora coccinea L. plants. J Plant Prod 7:85–91
Gallego-Bartolomé J, Minguet EG, Grau-Enguix F, Abbas M, Locascio A, Thomas SG, Blázquez MA (2012) Molecular mechanism for the interaction between gibberellin and brassinosteroid signaling pathways in Arabidopsis. Proceed Natl Acad Sci USA 109:13446–13451
Gao J, Chen H, Yang H, He Y, Tian Z, Li J (2018) A brassinosteroid responsive miRNA-target module regulates gibberellin biosynthesis and plant development. New Phytol 220:488–501. https://doi.org/10.1111/nph.15331
Ghani MA, Abbas MM, Ali B, Aziz R, Qadri RWK, Noor A, Ali S (2021) Alleviating role of gibberellic acid in enhancing plant growth and stimulating phenolic compounds in carrot (Daucus carota L.) under lead stress. Sustainability 13:12329. https://doi.org/10.3390/su132112329
Ghani MA, Mushtaq A, Khurram ZİAF, Basharat ALİ, Jahangir MM, Khan RW, Anam NOOR (2021b) Exogenously applied GA3 promotes plant growth in onion by reducing oxidative stress under saline conditions. J Agri Sci 27:122–128
Ghori NH, Ghori T, Hayat MQ, Imadi SR, Gul A, Altay V, Ozturk M (2019) Heavy metal stress and responses in plants. Int J Environ Sci Tech 16:1807–1828. https://doi.org/10.1007/s13762-019-02215-8
Golldack D, Li C, Mohan H, Probst N (2013) Gibberellins and abscisic acid signal crosstalk: living and developing under unfavorable conditions. Plant Cell Rep 32:1007–1016
Gong Q, Li ZH, Wang L, Zhou JY, Kang Q, Niu DD (2021) Gibberellic acid application on biomass, oxidative stress response, and photosynthesis in spinach (Spinacia oleracea L.) seedlings under copper stress. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-13745-5
Gonzalez ME, Jasso-Robles FI, Flores-Hernández E, Rodríguez-Kessler M, Pieckenstain FL (2021) Current status and perspectives on the role of polyamines in plant immunity. Ann Appl Biol 178:244–255
Greenboim-Wainberg Y, Maymon I, Borochov R, Alvarez J, Olszewski N, Ori N, Weiss D (2005) Cross talk between gibberellin and cytokinin: the Arabidopsis GA response inhibitor SPINDLY plays a positive role in cytokinin signaling. Plant Cell 17:92–102
Griffiths J, Murase K, Rieu I, Zentella R, Zhang ZL, Powers SJ, Thomas SG (2006) Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18:3399–3414. https://doi.org/10.1105/tpc.106.047415
Gul H, Khattak AM, Amin N (2006) Accelerating the growth of Araucaria heterophylla seedlings through different gibberellic acid concentrations and nitrogen levels. J Agric Biol Sci 1:25–29
Guo G, Liu X, Sun F, Cao J, Huo N, Wuda B, Yao Y (2018) Wheat miR9678 affects seed germination by generating phased siRNAs and modulating abscisic acid/gibberellin signaling. Plant Cell 30:796–814. https://doi.org/10.1105/tpc.17.00842
Gupta R, Chakrabarty SK (2013) Gibberellic acid in plant: still a mystery unresolved. Plant Signal Behav 8:e25504. https://doi.org/10.4161/psb.25504
Hamayun M, Khan SA, Khan AL, Shin JH, Ahmad B, Shin DH, Lee IJ (2010) Exogenous gibberellic acid reprograms soybean to higher growth and salt stress tolerance. J Agric Food Chem 58:7226–7232. https://doi.org/10.1021/jf101221t
Haroun S, Gamel R, Bashasha J, Aldrussi I (2018) Protective role of β-sitosterol or gibberellic acid to Lycopersicum esculentum cultivars under temperature stress. Egypt J Bot 58:233–247
Hartweck LM (2008) Gibberellin signaling. Planta 229:1–13. https://doi.org/10.1007/s00425-008-0830-1
Hasan S, Sehar Z, Khan NA (2020) Gibberellic Acid and sulfur-mediated reversal of cadmium-inhibited photosynthetic performance in mungbean (Vigna radiata L.) involves nitric oxide. J Plant Growth Regul 39:1605–1615. https://doi.org/10.1007/s00344-020-10175-4
Hasan-beigi H, Saidi M, Mohammadi M (2021) Effects of gibberellic acid and salicylic acid application on morphophysiological characteristics and essential oil yield of Echinacea purpurea (L.) Moench. Iran J Med Aroma Plants Res 36:1038–1051. https://doi.org/10.22092/IJMAPR.2021.342971.2795
Hattori Y, Nagai K, Furukawa S, Song XJ, Kawano R, Sakakibara H, Ashikari M (2009) The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 460:1026–1030. https://doi.org/10.1038/nature08258
Hayat S, Ahmad A, Mobin M, Fariduddin Q, Azam ZM (2001) Carbonic anhydrase, photosynthesis, and seed yield in mustard plants treated with phytohormones. Photosynthetica 39:111–114. https://doi.org/10.1023/A:1012456205819
Hayat S, Ali B, Ahmad A (2007) Salicylic acid: biosynthesis, metabolism and physiological role in plants. In: Hayat S, Ahmad A (eds) Salicylic acid: A plant hormone. Springer, Dordrecht, pp 1–14
He H, He L, Gu M (2012) Interactions between nitric oxide and plant hormones in aluminum tolerance. Plant Signal Behav 7:469–471. https://doi.org/10.4161/psb.19312
Hedden P (2020) The current status of research on gibberellin biosynthesis. Plant Cell Physiol 61:1832–1849. https://doi.org/10.1093/pcp/pcaa092
Hedden P, Thomas SG (2012) Gibberellin biosynthesis and its regulation. Biochem J 444:11–25. https://doi.org/10.1042/BJ20120245
Hedden P, Phillips AL, Rojas MC, Carrera E, Tudzynski B (2002) Gibberellin biosynthesis in plants and fungi: a case of convergent evolution. J Plant Growth Regul 20:319–331. https://doi.org/10.1007/s003440010037
Heinrich M, Hettenhausen C, Lange T, Wünsche H, Fang J, Baldwin IT, Wu J (2013) High levels of jasmonic acid antagonize the biosynthesis of gibberellins and inhibit the growth of Nicotiana attenuata stems. The Plant J 73:591–606. https://doi.org/10.1111/tpj.12058
Hirano K, Ueguchi-Tanaka M, Matsuoka M (2008) GID1-mediated gibberellin signaling in plants. Trend Plant Sci 13:192–199. https://doi.org/10.1016/j.tplants.2008.02.005
Hu S, Sanchez DL, Wang C, Lipka AE, Yin Y, Gardner CA, Lübberstedt T (2017) Brassinosteroid and gibberellin control of seedling traits in maize (Zea mays L.). Plant Sci 263:132–141. https://doi.org/10.1016/j.plantsci.2017.07.011
Hu J, Israeli A, Ori N, Sun TP (2018a) The interaction between DELLA and ARF/IAA mediates crosstalk between gibberellin and auxin signaling to control fruit initiation in tomato. Plant Cell 30:1710–1728. https://doi.org/10.1105/tpc.18.00363
Hu Z, Weijian L, Yali F, Huiquan L (2018b) Gibberellic acid enhances postharvest toon sprout tolerance to chilling stress by increasing the antioxidant capacity during the short-term cold storage. Sci Hort 237:184–191. https://doi.org/10.1016/j.scienta.2018.04.018
Ijaz M, Sher A, Sattar A, Shahid M, Nawaz A, Ul-Allah S, Saqib M (2019) Response of canola (Brassica napus L.) to exogenous application of nitrogen, salicylic acid and gibberellic acid under an arid climate. Soil Environ. https://doi.org/10.25252/SE/19/71619
Iqbal M, Ashraf M (2013) Gibberellic acid mediated induction of salt tolerance in wheat plants: Growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environ Exp Bot 86:76–85. https://doi.org/10.1016/j.envexpbot.2010.06.002
Isayenkov SV, Maathuis FJ (2019) Plant salinity stress: many unanswered questions remain. Front Plant Sci 10:80. https://doi.org/10.3389/fpls.2019.00080
Islam S, Zaid A, Mohammad F (2021) Role of triacontanol in counteracting the ill effects of salinity in plants: a review. J Plant Growth Regul 40:1–10. https://doi.org/10.1007/s00344-020-10064-w
Ito S, Yamagami D, Umehara M, Hanada A, Yoshida S, Sasaki Y, Asami T (2017) Regulation of strigolactone biosynthesis by gibberellin signaling. Plant Physiol 174:1250–1259. https://doi.org/10.1104/pp.17.00301
Javed T, Ali MM, Shabbir R, Anwar R, Afzal I, Mauro RP (2021) Alleviation of copper-induced stress in pea (Pisum sativum L.) through foliar application of gibberellic acid. Biology 10:120. https://doi.org/10.3390/biology10020120
Jiang H, Shui Z, Xu L, Yang Y, Li Y, Yuan X, Du J (2020) Gibberellins modulate shade-induced soybean hypocotyl elongation downstream of the mutual promotion of auxin and brassinosteroids. Plant Physiol Biochem 150:209–221. https://doi.org/10.1016/j.plaphy.2020.02.042
Kasahara H, Hanada A, Kuzuyama T, Takagi M, Kamiya Y, Yamaguchi S (2002) Contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthesis of gibberellins in Arabidopsis. J Biol Chem 277:45188–45194. https://doi.org/10.1074/jbc.M208659200
Kaur G, Asthir B (2017) Molecular responses to drought stress in plants. Biol Plant 61:201–209. https://doi.org/10.1007/s10535-016-0700-9
Kaya C, Sarıoğlu A, Ashra M, Alyemeni MN, Ahmad P (2020) Gibberellic acid-induced generation of hydrogen sulfide alleviates boron toxicity in tomato (Solanum lycopersicum L.) plants. Plant Physiol Biochem 153:53–63. https://doi.org/10.1016/j.plaphy.2020.04.038
Kępczyński J, Cembrowska-Lech D, Sznigir P (2017) Interplay between nitric oxide, ethylene, and gibberellic acid regulating the release of Amaranthus retroflexus seed dormancy. Acta Physiol Plant 39:1–13. https://doi.org/10.1007/s11738-017-2550-2
Khan NA, Mir R, Khan M, Javid S (2002) Effects of gibberellic acid spray on nitrogen yield efficiency of mustard grown with different nitrogen levels. Plant Growth Regul 38:243–247. https://doi.org/10.1023/A:1021523707239
Khan MMA, Gautam C, Mohammad F, Siddiqui MH, Naeem M, Khan MN (2006) Effect of gibberellic acid spray on performance of tomato. Turk J Biol 30:11–16
Khan MMA, Khan R, Singh M, Nasir S, Naeem M, Siddiqui MH, Mohammad F (2007) Gibberellic acid and triacontanol can ameliorate the opium yield and morphine production in opium poppy (Papaver somniferum L.). Acta Hortic 756:289–298. https://doi.org/10.1766/ActaHortic.2007.756.30
Khan MN, Mohammad F, Siddiqui MH (2009) Pre-sowing seed treatment and foliar application of gibberellic acid improve seed and fibre yield by inducing net photosynthetic rate and carbonic anhydrase activity of linseed genotypes. Int J Plant Dev Biol 3:34–38
Khan MN, Mohammad F, Siddiqui MH, Naeem M (2010a) Gibberellic acid mediated co-ordination of calcium and magnesium ameliorate physiological activities, seed yield and fibre yield of Linum usitatissimum L.—a dual-purpose crop. Physiol Mol Biol Plants 16:333–341
Khan MN, Siddiqui MH, Mohammad F, Naeem M, Khan MMA (2010b) Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol Plant 32:121–132. https://doi.org/10.1007/s11738-009-0387-z
Khan SU, Gurmani AR, Jalal-Ud-Din QA, Abbasi KS, Liaquat M, Ahmad Z (2016) Exogenously applied gibberellic acid, indole acetic acid and kinetin as potential regulators of source-sink relationship, physiological and yield attributes in rice (Oryza sativa) genotypes under water deficit conditions. Int J Agric Biol 18:139–145
Khan A, Bilal S, Khan AL, Imran M, Shahzad R, Al-Harrasi A, Lee IJ (2020) Silicon and gibberellins: synergistic function in harnessing ABA signaling and heat stress tolerance in date palm (Phoenix dactylifera L.). Plants 9:620. https://doi.org/10.3390/plants9050620
Khan MN, Khan Z, Luo T, Liu J, Rizwan M, Zhang J, Hu L (2020) Seed priming with gibberellic acid and melatonin in rapeseed: Consequences for improving yield and seed quality under drought and non-stress conditions. Ind Crops Prod 156:112850. https://doi.org/10.1016/j.indcrop.2020.112850
Khan N, Bano A, Ali S, Babar MA (2020c) Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul 90:189–203
Kim SG, Park CM (2008) Gibberellic acid-mediated salt signaling in seed germination. Plant Signal Behav 3:877–879. https://doi.org/10.4161/psb.3.10.6247
Kim YH, Choi KI, Khan AL, Waqas M, Lee IJ (2016) Exogenous application of abscisic acid regulates endogenous gibberellins homeostasis and enhances resistance of oriental melon (Cucumis melo var. L.) against low temperature. Sci Hort 207:41–47. https://doi.org/10.1016/j.scienta.2016.05.009
Kohli A, Sreenivasulu N, Lakshmanan P, Kumar PP (2013) The phytohormone crosstalk paradigm takes center stage in understanding how plants respond to abiotic stresses. Plant Cell Rep 32:945–957. https://doi.org/10.1007/s00299-013-1461-y
Kou E, Huang X, Zhu Y, Su W, Liu H, Sun G, Song S (2021) Crosstalk between auxin and gibberellin during stalk elongation in flowering Chinese cabbage. Sci Rep 11:1–9
Krishnan S, Merewitz EB (2017) Polyamine application effects on gibberellic acid content in creeping bentgrass during drought stress. J Am Soc Hort Sci 142:135–142
Kuchi VS, Kabir J, Siddiqui MW (2017) Gibberellins: the roles in pre-and postharvest quality of horticultural produce. In: Siddiqui MW and Ali A (Eds) Postharvest management of horticultural crops, Apple Academic Press pp. 181–230
Kumar S, Shah SH, Vimala Y, Jatav HS, Ahmad P, Chen Y, Siddique KH (2022) Abscisic acid: Metabolism, transport, crosstalk with other plant growth regulators, and its role in heavy metal stress mitigation. Front Plant Sci 13:972856. https://doi.org/10.3389/fpls.2022.972856
Lantzouni O, Klermund C, Schwechheimer C (2017) Largely additive effects of gibberellin and strigolactone on gene expression in Arabidopsis thaliana seedlings. The Plant J 92:924–938. https://doi.org/10.1111/tpj.13729
Leilah AA, Khan N (2021) Interactive effects of gibberellic acid and nitrogen fertilization on the growth, yield, and quality of sugar beet. Agronomy 11:137. https://doi.org/10.3390/agronomy11010137
Li Z, Lu GY, Zhang XK, Zou CS, Cheng Y, Zheng PY (2010) Improving drought tolerance of germinating seeds by exogenous application of gibberellic acid (GA3) in rapeseed (Brassica napus L.). Seed Sci Tech 38:432–440. https://doi.org/10.1525/sst.2010.38.2.16
Li X, Jiang H, Liu F, Cai J, Dai T, Cao W, Jiang D (2013) Induction of chilling tolerance in wheat during germination by pre-soaking seed with nitric oxide and gibberellin. Plant Growth Regul 71:31–40. https://doi.org/10.1007/s10725-013-9805-8
Li Z, Zhou H, Peng Y, Zhang X, Ma X, Huang L, Yan Y (2015) Exogenously applied spermidine improves drought tolerance in creeping bentgrass associated with changes in antioxidant defense, endogenous polyamines and phytohormones. Plant Growth Regul 76:71–82. https://doi.org/10.1007/s10725-014-9978-9
Li L, Gu W, Li J, Li C, Xie T, Qu D, Wei S (2018) Exogenously applied spermidine alleviates photosynthetic inhibition under drought stress in maize (Zea mays L.) seedlings associated with changes in endogenous polyamines and phytohormones. Plant Physiol Biochem 129:35–55. https://doi.org/10.1016/j.plaphy.2018.05.017
Li C, Zheng L, Wang X, Hu Z, Zheng Y, Chen Q, Zhang Y (2019) Comprehensive expression analysis of Arabidopsis GA2-oxidase genes and their functional insights. Plant Sci 285:1–13. https://doi.org/10.1016/j.plantsci.2019.04.023
Li QF, Zhou Y, Xiong M, Ren XY, Han L, Wang JD, Liu QQ (2020) Gibberellin recovers seed germination in rice with impaired brassinosteroid signalling. Plant Sci 293:110435. https://doi.org/10.1016/j.plantsci.2020.110435
Liang Y, Xiao X, Guo Z, Peng C, Zeng P, Wang X (2021) Co-application of indole-3-acetic acid/gibberellin and oxalic acid for phytoextraction of cadmium and lead with Sedum alfredii Hance from contaminated soil. Chemosphere 285:131420
Liu S, Zhang Y, Feng Q, Qin L, Pan C, Lamin-Samu AT, Lu G (2018) Tomato AUXIN RESPONSE FACTOR 5 regulates fruit set and development via the mediation of auxin and gibberellin signaling. Sci Rep 8:1–16
Lozano-Juste J, León J (2011) Nitric oxide regulates DELLA content and PIF expression to promote photomorphogenesis in Arabidopsis. Plant Physiol 156:1410–1423. https://doi.org/10.1104/pp.111.177741
Marzec M (2017) Strigolactones and gibberellins: a new couple in the phytohormone world? Trends Plant Sci 22:813–815. https://doi.org/10.1016/j.tplants.2017.08.001
Mathur S, Agrawal D, Jajoo A (2014) Photosynthesis: response to high temperature stress. J Photochem Photobiol Biol 137:116–126. https://doi.org/10.1016/j.jphotobiol.2014.01.010
Matilla-Vazquez MA, Matilla AJ (2014) Ethylene: Role in plants under environmental stress. In: Ahmad P, Wani MR (eds) Physiological mechanisms and adaptation strategies in plants under changing environment. Springer, New York, pp 189–222
Maurya VK, Ranjan V, Gothandam KM, Pareek S (2020) Exogenous gibberellic acid treatment extends green chili shelf life and maintain quality under modified atmosphere packaging. Sci Hortic 269:108934
Mazid M (2014) Seed priming application of gibberellic acid on growth, biochemical, yield attributes and protein status of chickpea (Cicer arietinum L. cv. DCP 92–3). Int J Genet Eng Biotechnol 5:17–22
Miceli A, Vetrano F, Sabatino L, D’Anna F, Moncada A (2019) Influence of preharvest gibberellic acid treatments on postharvest quality of minimally processed leaf lettuce and rocket. Horticulturae 5:63
Miri M, Ghooshchi F, Tohidi-Moghadam HR, Larijani HR, Kasraie P (2021) Ameliorative effects of foliar spray of glycine betaine and gibberellic acid on cowpea (Vigna unguiculata L. Walp.) yield affected by drought stress. Arab J Geosci 14:1–9. https://doi.org/10.1007/s12517-021-07228-7
Moneruzzaman KM, Hossain ABMS, Normaniza O, Boyce AN (2011) Growth, yield and quality responses to gibberellic acid (GA3) of Wax apple Syzygium samarangense var. Jambu air madu fruits grown under field conditions. Afr J Biotechnol 10:11911–11918
Moreno D, Berli FJ, Piccoli PN, Bottini R (2011) Gibberellins and abscisic acid promote carbon allocation in roots and berries of grapevines. J Plant Growth Regul 30:220–228. https://doi.org/10.1007/s00344-010-9186-4
Moula I, Boussadia O, Koubouris G, Hassine MB, Boussetta W, Van Labeke MC, Braham M (2020) Ecophysiological and biochemical aspects of olive tree (Olea europaea L.) in response to salt stress and gibberellic acid-induced alleviation. S Afr J Bot 132:38–44. https://doi.org/10.1016/j.sajb.2020.04.022
Muniandi SKM, Hossain MA, Abdullah MP, Ab Shukor NA (2018) Gibberellic acid (GA3) affects growth and development of some selected kenaf (Hibiscus cannabinus L.) cultivars. Ind Crops Prod 118:180–187. https://doi.org/10.1016/j.indcrop.2018.03.036
Nagar S, Singh VP, Arora A, Dhakar R, Singh N, Singh GP, Shiv Ramakrishnan R (2021) Understanding the role of gibberellic acid and paclobutrazol in terminal heat stress tolerance in wheat. Front Plant Sci 12:692252
Nakamura H, Xue YL, Miyakawa T, Hou F, Qin HM, Fukui K, Asami T (2013) Molecular mechanism of strigolactone perception by DWARF14. Nature Commun 4:1–10. https://doi.org/10.1038/ncomms3613
Nkansah GO, Ofosu-Anim J, Mawuli A (2012) Gibberellic acid and naphthalene acetic acid affect fruit retention, yield and quality of Keitt mangoes in the coastal savanna ecological zone of Ghana. Am J Plant Physiol 7:243–251
Othman YA, Leskovar DI (2022) Foliar application of gibberellic acid improves yield and head phenolic compounds in globe artichoke. Sci Hort 301:111. https://doi.org/10.1016/j.scienta.2022.111115
Pan S, Rasul F, Li W, Tian H, Mo Z, Duan M, Tang X (2013) Roles of plant growth regulators on yield, grain qualities and antioxidant enzyme activities in super hybrid rice (Oryza sativa L.). Rice 6:1–10. https://doi.org/10.1186/1939-8433-6-9
Panigrahi J, Gheewala B, Patel M, Patel N, Gantait S (2017) Gibberellic acid coating: A novel approach to expand the shelf-life in green chilli (Capsicum annuum L.). Sci Hortic 225:581–588
Peng J (2009) Gibberellin and jasmonate crosstalk during stamen development. J Integr Plant Biol 51:1064–1070. https://doi.org/10.1111/j.1744-7909.2009.00881.x
Piskurewicz U, Jikumaru Y, Kinoshita N, Nambara E, Kamiya Y, Lopez-Molina L (2008) The gibberellic acid signaling repressor RGL2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. Plant Cell 20:2729–2745. https://doi.org/10.1105/tpc.108.061515
Qi T, Huang H, Wu D, Yan J, Qi Y, Song S, Xie D (2014) Arabidopsis DELLA and JAZ proteins bind the WD-repeat/bHLH/MYB complex to modulate gibberellin and jasmonate signaling synergy. Plant Cell 26:1118–1133. https://doi.org/10.1105/tpc.113.121731
Qin L, Zhang X, Yan J, Fan L, Rong C, Mo C, Zhang M (2019) Effect of exogenous spermidine on floral induction, endogenous polyamine and hormone production, and expression of related genes in ‘Fuji’apple (Malus domestica Borkh.). Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-49280-0
Que F, Khadr A, Wang GL, Li T, Wang YH, Xu ZS, Xiong AS (2018) Exogenous brassinosteroids altered cell length, gibberellin content, and cellulose deposition in promoting carrot petiole elongation. Plant Sci 277:110–120
Rabiza-Świder J, Skutnik E, Jędrzejuk A, Łukaszewska A, Lewandowska K (2015) The effect of GA3 and the standard preservative on keeping qualities of cut LA hybrid lily ‘Richmond.’ Acta Sci Pol Hortorum Cultus 14:51–64
Rademacher W (2015) Plant growth regulators: backgrounds and uses in plant production. J Plant Growth Regul 34:845–872. https://doi.org/10.1007/s00344-015-9541-6
Rady MM, Boriek SH, El-Mageed A, Taia A, Seif El-Yazal MA, Ali EZ, Abdelkhalik A (2021) Exogenous gibberellic acid or dilute bee honey boosts drought stress tolerance in Vicia faba by rebalancing osmoprotectants, antioxidants, nutrients, and phytohormones. Plants 10:748. https://doi.org/10.3390/plants10040748
Rafique M, Naveed M, Mustafa A, Akhtar S, Munawar M, Kaukab S, Salem MZ (2021) The combined effects of gibberellic acid and rhizobium on growth, yield and nutritional status in chickpea (Cicer arietinum L.). Agronomy. https://doi.org/10.3390/agronomy11010105
Rahman MS, Saki MJ, Hosain MT, Rashid S (2019) Cumulative effect of zinc and gibberellic acid on yield and quality of tomato. Int J Biosci 14:350–360
Rai RK, Tripathi N, Gautam D, Singh P (2017) Exogenous application of ethrel and gibberellic acid stimulates physiological growth of late planted sugarcane with short growth period in sub-tropical India. J Plant Growth Regul 36:472–486. https://doi.org/10.1007/s00344-016-9655-5
Ramesh B, Kumar B (2006) Response of induced dwarf mutants of barley to exogenous application of gibberellic acid. Barley Genet Newsletter 36:3–5
Rani P, Singh N (2013) Impact of gibberellic acid pretreatment on growth and flowering of tuberose (Polianthes tuberosa L.) cv. Prajwal J Trop Plant Physiol 5:33–41
Rastogi A, Siddiqui A, Mishra BK, Srivastava M, Pandey R, Misra P, Shukla S (2013) Effect of auxin and gibberellic acid on growth and yield components of linseed (Linum usitatissimum L.). Crop Breed Appl Biotechnol 13:136–143
Ravindran P, Kumar PP (2019) Regulation of seed germination: The involvement of multiple forces exerted via gibberellic acid signaling. Mol Plant 12:24–26
Regnault T, Daviere JM, Heintz D, Lange T, Achard P (2014) The gibberellin biosynthetic genes At KAO 1 and At KAO 2 have overlapping roles throughout Arabidopsis development. Plant J 80:462–474. https://doi.org/10.1111/tpj.12648
Richter R, Behringer C, Müller IK, Schwechheimer C (2010) The GATA-type transcription factors GNC and GNL/CGA1 repress gibberellin signaling downstream from DELLA proteins and PHYTOCHROME-INTERACTING FACTORS. Genes Develop 24:2093–2104. https://doi.org/10.1101/gad.594910
Richter R, Behringer C, Zourelidou M, Schwechheimer C (2013) Convergence of auxin and gibberellin signaling on the regulation of the GATA transcription factors GNC and GNL in Arabidopsis thaliana. Proc Nat Acad Sci 110:13192–13197
Rodrigues C, Vandenberghe LPDS, de Oliveira J, Soccol CR (2012) New perspectives of gibberellic acid production: a review. Crit Rev Biotechnol 32:263–273. https://doi.org/10.3109/07388551.2011.615297
Roychowdhury R, Mamgain A, Ray S, Tah J (2012) Effect of gibberellic acid, kinetin and indole 3-acetic acid on seed germination performance of Dianthus caryophyllus (Carnation). Agri Conspec Sci 77:157–160
Ruan J, Zhou Y, Zhou M, Yan J, Khurshid M, Weng W, Zhang K (2019) Jasmonic acid signaling pathway in plants. Int J Mol Sci 20:2479
Sabagh AE, Hossain A, Islam MS, Iqbal MA, Amanet K, Mubeen M, Erman M (2021) Prospective role of plant growth regulators for tolerance to abiotic stresses. In: Aftab T, Hakeem KR (eds) plant growth regulators. Springer, Cham, pp 1–38
Saeed T, Hassan I, Abbasi NA, Jilani G (2014) Effect of gibberellic acid on the vase life and oxidative activities in senescing cut gladiolus flowers. Plant Growth Regul 72:89–95. https://doi.org/10.1007/s10725-013-9839-y
Sajjad Y, Jaskani MJ, Ashraf MY, Qasim M, Ahmad R (2014) Response of morphological and physiological growth attributes to foliar application of plant growth regulators in gladiolus ‘white prosperity’. Pak J Agric Sci 51:123–129
Sakamoto T, Miura K, Itoh H, Tatsumi T, Ueguchi-Tanaka M, Ishiyama K, Matsuoka M (2004) An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol 134:1642–1653. https://doi.org/10.1104/pp.103.033696
Salachna P, Mikiciuk M, Zawadzińska A, Piechocki R, Ptak P, Mikiciuk G, Łopusiewicz Ł (2020) Changes in growth and physiological parameters of× amarine following an exogenous application of gibberellic acid and methyl jasmonate. Agronomy 10:980. https://doi.org/10.3390/agronomy10070980
Salazar-Cerezo S, Martínez-Montiel N, García-Sánchez J, Pérez-y-Terron R, Martínez-Contreras RD (2018) Gibberellin biosynthesis and metabolism: A convergent route for plants, fungi and bacteria. Microbiol Res 208:85–98. https://doi.org/10.1016/j.micres.2018.01.010
Saleem MH, Fahad S, Adnan M, Ali M, Rana MS, Kamran M, Hussain RM (2020) Foliar application of gibberellic acid endorsed phytoextraction of copper and alleviates oxidative stress in jute (Corchorus capsularis L.) plant grown in highly copper-contaminated soil of China. Environ Sci Pollut Res 27:37121–37133. https://doi.org/10.1007/s11356-020-09764-3
Schwechheimer C (2008) Understanding gibberellic acid signaling—are we there yet? Curr Opin Plant Biol 11:9–15. https://doi.org/10.1016/j.pbi.2007.10.011
Schwechheimer C (2012) Gibberellin signaling in plants–the extended version. Front Plant Sci 2:107. https://doi.org/10.3389/fpls.2011.00107
Seleiman MF, Al-Suhaibani N, Ali N, Akmal M, Alotaibi M, Refay Y, Battaglia ML (2021) Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10:259
Seo HS, Kim SK, Jang SW, Choo YS, Sohn EY, Lee IJ (2005) Effect of jasmonic acid on endogenous gibberellins and abscisic acid in rice under NaCl stress. Biol Plant 49:447–450
Shaddad MAK, Mostafa D (2013) Role of gibberellic acid (GA3) in improving salt stress tolerance of two wheat cultivars. Int J Plant Physiol Biochem 5:50–57
Shah SH (2007) Physiological effects of pre-sowing seed treatment with gibberellic acid on Nigella sativa L. Acta Bot Croat 66:67–73
Shah SH, Islam S, Parrey ZA, Mohammad F (2021) Role of exogenously applied plant growth regulators in growth and development of edible oilseed crops under variable environmental conditions: a review. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-021-00606-w
Shah SH, Islam S, Mohammad F (2022a) Sulphur as a dynamic mineral element for plants: a review. J Soil Sci Plant Nutr 22:2118–2143
Shah SH, Parrey ZA, Islam S, Tyagi A, Ahmad A, Mohammad F (2022b) Exogenously applied sulphur improves growth, photosynthetic efficiency, enzymatic activities, mineral nutrient contents, yield and quality of Brassica juncea L. Sustainability 14:14441. https://doi.org/10.3390/su142114441
Shah SH, Islam S, Alamri S, Parrey ZA, Mohammad F, Kalaji HM (2023) Plant growth regulators mediated changes in the growth, photosynthesis, nutrient acquisition and productivity of mustard. Agriculture 13:570. https://doi.org/10.3390/agriculture13030570
Shahid MR, Amjad M, Ziaf K, Jahangir MM, Ahmad S, Iqbal Q, Nawaz A (2013) Growth, yield and seed production of okra as influenced by different growth regulators. Pak J Agric Sci 50
Shahzad K, Hussain S, Arfan M, Hussain S, Waraich EA, Zamir S, El-Esawi MA (2021) Exogenously applied gibberellic acid enhances growth and salinity stress tolerance of maize through modulating the morpho-physiological. Biochem Mol Attribut Biomol 11:1005. https://doi.org/10.3390/biom11071005
Shaki F, Maboud HE, Niknam V (2019) Effects of salicylic acid on hormonal cross talk, fatty acids profile, and ions homeostasis from salt-stressed safflower. J Plant Interact 14:340–346. https://doi.org/10.1080/17429145.2019.1635660
Sharaf AEMM, Farghal II, Sofy MR (2009) Role of gibberellic acid in abolishing the detrimental effects of Cd and Pb on broad bean and lupin plants. Res J Agric Biol Sci 5:668–673
Sharma RR, Singh R (2009) Gibberellic acid influences the production of malformed and button berries, and fruit yield and quality in strawberry (Fragaria × ananassa Duch.). Sci Hortic 119:430–433. https://doi.org/10.1016/j.scienta.2008.11.002
Sharma M, Kumar P, Verma V, Sharma R, Bhargava B, Irfan M (2022) Understanding plant stress memory response for abiotic stress resilience: Molecular insights and prospects. Plant Physiol Biochem 179:10–24. https://doi.org/10.1016/j.plaphy.2022.03.004
Shu K, Zhang H, Wang S, Chen M, Wu Y, Tang S, Xie Q (2013) ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in Arabidopsis. PLoS Genet 9:e1003577. https://doi.org/10.1371/journal.pgen.1003577
Shuai H, Meng Y, Luo X, Chen F, Zhou W, Dai Y, Shu K (2017) Exogenous auxin represses soybean seed germination through decreasing the gibberellin/abscisic acid (GA/ABA) ratio. Sci Rep 7:1–11
Siddiqui MH, Khan MN, Mohammad F, Khan MMA (2008) Role of nitrogen and gibberellin (GA3) in the regulation of enzyme activities and in osmoprotectant accumulation in Brassica juncea L. under salt stress. J Agron Crop Sci 194:214–224. https://doi.org/10.1111/j.1439-037X.2008.00308.x
Siddiqui MH, Al-Whaibi MH, Basalah MO (2011) Interactive effect of calcium and gibberellin on nickel tolerance in relation to antioxidant systems in Triticum aestivum L. Protoplasma 248:503–511. https://doi.org/10.1007/s00709-010-0197-6
Siddiqui MH, Alamri S, Alsubaie QD, Ali HM (2020) Melatonin and gibberellic acid promote growth and chlorophyll biosynthesis by regulating antioxidant and methylglyoxal detoxification system in tomato seedlings under salinity. J Plant Growth Regul 39:1488–1502. https://doi.org/10.1007/s00344-020-10122-3
Singh T, Bala M (2018) Effect of foliar spray of benzyl adenine, gibberellic acid and putrescine on post-harvest keeping quality of chrysanthemum. Agric Res J 55:386–388
Singhal RK, Jatav HS, Aftab T, Pandey S, Mishra UN, Chauhan J, Ahmed S (2021) Roles of nitric oxide in conferring multiple abiotic stress tolerance in plants and crosstalk with other plant growth regulators. J Plant Growth Regul. https://doi.org/10.1007/s00344-021-10446-8
Song S, Qi T, Wasternack C, Xie D (2014) Jasmonate signaling and crosstalk with gibberellin and ethylene. Curr Opin Plant Biol 21:112–119. https://doi.org/10.1016/j.pbi.2014.07.005
Soyler D, Khawar KM (2007) Seed germination of caper (Capparis ovata var. Herbacea) using α naphthalene acetic acid and gibberellic acid. Int J Agric Biol 9:35–38
Subbaraj AK, Funnell KA, Woolley DJ (2010) Dormancy and flowering are regulated by the reciprocal interaction between cytokinin and gibberellin in Zantedeschia. J Plant Growth Regul 29:487–499
Sun TP, Gubler F (2004) Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol 55:197–223. https://doi.org/10.1146/annurev.arplant.55.031903.141753
Sun K, Yue Y, Zhang H, Yang N, Wen D, Li X, Wang K (2020) Potential of gibberellic acid (GA3) and uniconazole for enhancing the cd absorption efficiency of maize (Zea mays L.). Pol J Environ Stud 30:851–861
Szymańska R, Ślesak I, Orzechowska A, Kruk J (2017) Physiological and biochemical responses to high light and temperature stress in plants. Environ Exp Bot 139:165–177. https://doi.org/10.1016/j.envexpbot.2017.05.002
Taiz L, Zeiger E, Møller IM, Murphy A (2015). Plant physiology and development, 6th ed. Sinauer Associates Incorporated.
Talat H, Shafqat W, Qureshi MA, Sharif N, Raza MK, ud Din S, Jaskani MJ, (2020) Effect of gibberellic acid on fruit quality of Kinnow mandarin. J Glob Innov Agri Soc Sci 8:59–63
Tang Y, Liu H, Guo S, Wang B, Li Z, Chong K, Xu Y (2018) OsmiR396d affects gibberellin and brassinosteroid signaling to regulate plant architecture in rice. Plant Physiol 176:946–959
Tian W, Lv Y, Cao J, Jiang W (2014) Retention of iceberg lettuce quality by low temperature storage and postharvest application of 1-methylcyclopropene or gibberellic acid. J Food Sci Technol 51:943–949
Tong H, Xiao Y, Liu D, Gao S, Liu L, Yin Y, Chu C (2014) Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice. Plant Cell 26:4376–4393
Tsegay BA, Andargie M (2018) Seed priming with gibberellic acid (GA3) alleviates salinity induced inhibition of germination and seedling growth of Zea mays L., Pisum sativum Var. abyssinicum A. Braun and Lathyrus sativus L. J Crop Sci Biotechnol 21:261–267. https://doi.org/10.1007/s12892-018-0043-0
Ueguchi-Tanaka M, Nakajima M, Katoh E, Ohmiya H, Asano K, Saji S, Matsuoka M (2007) Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin. Plant Cell 19:2140–2155. https://doi.org/10.1105/tpc.106.043729
Um TY, Lee HY, Lee S, Chang SH, Chung PJ, Oh KB, Choi YD (2018) JASMONATE ZIM-DOMAIN PROTEIN 9 interacts with SLENDER RICE 1 to mediate the antagonistic interaction between jasmonic and gibberellic acid signals in rice. Front Plant Sci 9:1866
Van Schie CC, Ament K, Schmidt A, Lange T, Haring MA, Schuurink RC (2007) Geranyl diphosphate synthase is required for biosynthesis of gibberellins. Plant J 52:752–762. https://doi.org/10.1111/j.1365-313X.2007.03273.x
Verdolin LG, Mariz BL, Dias LLC (2021) Gibberellin and polyamines effects in growth and flowering of New Guinea impatiens. Ornam Hortic 27:247–254
Vieira AR, Vieira MDGGC, Fraga AC, Oliveira JA, Santos CDD (2002) Action of gibberellic acid (GA3) on dormancy and activity of alpha-amylase in rice seeds. Rev Bras De Sementes 24:43–48
Wang L, Wang Z, Xu Y, Joo SH, Kim SK (2009) OsGSR1 is involved in crosstalk between gibberellins and brassinosteroids in rice. Plant J 57:498–510
Wang LL, Chen XY, Yang Y, Wang Z, Xiong F (2016) Effects of exogenous gibberellic acid and abscisic acid on germination, amylases, and endosperm structure of germinating wheat seeds. Seed Sci Technol 44:64–76. https://doi.org/10.15258/sst.2016.44.1.09
Wang YH, Zhang G, Chen Y, Gao J, Sun YR, Sun MF, Chen JP (2019) Exogenous application of gibberellic acid and ascorbic acid improved tolerance of okra seedlings to NaCl stress. Acta Physiol Plant 41:93. https://doi.org/10.1007/s11738-019-2869-y
Wani KI, Naeem M, Castroverde CDM, Kalaji HM, Albaqami M, Aftab T (2021a) Molecular mechanisms of nitric oxide (NO) signaling and reactive oxygen species (ROS) homeostasis during abiotic stresses in plants. Int J Mol Sci 22:9656. https://doi.org/10.3390/ijms22179656
Wani KI, Zehra A, Choudhary S, Naeem M, Khan M, Castroverde CDM, Aftab T (2021b) Mechanistic insights into strigolactone biosynthesis, signaling, and regulation during plant growth and development. J Plant Growth Regul 40:1836–1852
Weiss D, Ori N (2007) Mechanisms of cross talk between gibberellin and other hormones. Plant Physiol 144:1240–1246
Willige BC, Isono E, Richter R, Zourelidou M, Schwechheimer C (2011) Gibberellin regulates PIN-FORMED abundance and is required for auxin transport–dependent growth and development in Arabidopsis thaliana. Plant Cell 23:2184–2195
Xiao X, Yang M, Dong W, Zhou C, Shi L, Chen W, Li S (2022) Gibberellic acid inhibited chlorophyll degradation in post-harvest okras. Postharvest Biol Technol 190:111951
Xie Z, Zhang ZL, Zou X, Yang G, Komatsu S, Shen QJ (2006) Interactions of two abscisic-acid induced WRKY genes in repressing gibberellin signaling in aleurone cells. Plant J 46:231–242. https://doi.org/10.1111/j.1365-313X.2006.02694.x
Xie Z, Zhang ZL, Hanzlik S, Cook E, Shen QJ (2007) Salicylic acid inhibits gibberellin-induced alpha-amylase expression and seed germination via a pathway involving an abscisic-acid-inducible WRKY gene. Plant Mol Biol 64:293–303. https://doi.org/10.1007/s11103-007-9152-0
Xie Y, Onik JC, Hu X, Duan Y, Lin Q (2018) Effects of (S)-carvone and gibberellin on sugar accumulation in potatoes during low temperature storage. Molecules 23:3118. https://doi.org/10.3390/molecules23123118
Xiong M, Chu L, Li Q, Yu J, Yang Y, Zhou P, Liu Q (2021) Brassinosteroid and gibberellin coordinate rice seed germination and embryo growth by regulating glutelin mobilization. Crop J 9:1039–1048
Xu J, Li Q, Li Y, Yang L, Zhang Y, Cai Y (2021a) Effect of exogenous gibberellin, paclobutrazol, abscisic acid, and ethrel application on bulblet development in Lycoris radiata. Front Plant Sci 11:2287. https://doi.org/10.3389/fpls.2020.615547
Xu Q, Li H, Liu S, Huang W, Xian X, Li Q, Pan Y (2021b) Gibberellin and spermidine synergistically regulate polyamine metabolism during Rhododendron flower development. https://doi.org/10.21203/rs.3.rs-188291/v1
Yahia EM, Carrillo-Lopez A (2018) Postharvest physiology and biochemistry of fruits and vegetables. Woodhead publishing.
Yamaguchi S, Sun TP, Kawaide H, Kamiya Y (1998) The GA2 locus of Arabidopsis thaliana encodes ent-kaurene synthase of gibberellin biosynthesis. Plant Physiol 116:1271–1278. https://doi.org/10.1104/pp.116.4.1271
Yang DL, Yao J, Mei CS, Tong XH, Zeng LJ, Li Q, He SY (2012) Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc Nat Acad Sci USA 109:E1192–E1200. https://doi.org/10.1073/pnas.1201616109
Yoneda Y, Shimizu H, Nakashima H, Miyasaka J, Ohdoi K (2018) Effect of treatment with gibberellin, gibberellin biosynthesis inhibitor, and auxin on steviol glycoside content in Stevia rebaudiana Bertoni. Sugar Tech 20:482–491
Yoshida H, Hirano K, Sato T, Mitsuda N, Nomoto M, Maeo K, Ueguchi-Tanaka M (2014) DELLA protein functions as a transcriptional activator through the DNA binding of the indeterminate domain family proteins. Proc Nat Acad Sci 111:7861–7866. https://doi.org/10.1073/pnas.1321669111
Younesi O, Moradi A (2014) Effect of priming of seeds of Medicago sativa" Bami" with gibberellic acid on germination, seedlings growth and antioxidant enzymes activity under salinity stress. J Hortic Res 22:167
Yuan Z, Wang C, Li S, Li X, Tai F (2014) Effects of different plant hormones or PEG seed soaking on maize resistance to drought stress. Can J Plant Sci 94:1491–1499
Yue C, Cao H, Hao X, Zeng J, Qian W, Guo Y, Wang X (2018) Differential expression of gibberellin-and abscisic acid-related genes implies their roles in the bud activity-dormancy transition of tea plants. Plant Cell Rep 37:425–441. https://doi.org/10.1007/s00299-017-2238-5
Zandoná LO, Lando AP, Goeten D, Steiner N (2021) The control over physiological dormancy break by gibberellins in Calibrachoa sellowiana (Sendtn.) Wijsman seeds are associated with polyamines. Acta Physiol Plant 43:1–14
Zang YX, Chun IJ, Zhang LL, Hong SB, Zheng WW, Xu K (2016) Effect of gibberellic acid application on plant growth attributes, return bloom, and fruit quality of rabbiteye blueberry. Sci Hortic 200:13–18. https://doi.org/10.1016/j.scienta.2015.12.057
Zhang H, Li M, He D, Wang K, Yang P (2020) Mutations on ent-kaurene oxidase 1 encoding gene attenuate its enzyme activity of catalyzing the reaction from ent-kaurene to ent-kaurenoic acid and lead to delayed germination in rice. PLoS Genet 16:e1008562. https://doi.org/10.1371/journal.pgen.1008562
Zhao Y (2010) Auxin biosynthesis and its role in plant development. Ann Rev Plant Biol 61:49–64
Zheng L, Gao C, Zhao C, Zhang L, Han M, An N, Ren X (2019) Effects of brassinosteroid associated with auxin and gibberellin on apple tree growth and gene expression patterns. Hortic Plant J 5:93–108. https://doi.org/10.1016/j.hpj.2019.04.006
Zhu JK (2016) Abiotic stress signaling and responses in plants. Cell 167:313–324. https://doi.org/10.1016/j.cell.2016.08.029
Zhu XF, Jiang T, Wang ZW, Lei GJ, Shi YZ, Li GX, Zheng SJ (2012) Gibberellic acid alleviates cadmium toxicity by reducing nitric oxide accumulation and expression of IRT1 in Arabidopsis thaliana. J Hazard Mater 239:302–307. https://doi.org/10.1016/j.jhazmat.2012.08.077
Zhu Z, Ding Y, Zhao J, Nie Y, Zhang Y, Sheng J, Tang X (2016) Effects of postharvest gibberellic acid treatment on chilling tolerance in cold-stored tomato (Solanum lycopersicum L.) fruit. Food Bioproc Tech 9:1202–1209. https://doi.org/10.1007/s11947-016-1712-3
Zhu G, An L, Jiao X, Chen X, Zhou G, McLaughlin N (2019) Effects of gibberellic acid on water uptake and germination of sweet sorghum seeds under salinity stress. Chil J Agri Res 79:415–424
Zou X, Wang Q, Chen P, Yin C, Lin Y (2019) Strigolactones regulate shoot elongation by mediating gibberellin metabolism and signaling in rice (Oryza sativa L.). J Plant Physiol 237:72–79. https://doi.org/10.1016/j.jplph.2019.04.003
Acknowledgements
SHS and SI are thankful to University Grant Commission, New Delhi, India for providing the research fellowships.
Funding
The funding was provided by University Grants Commission of India (IN), 18PHDBT030, Sajad Hussain Shah.
Author information
Authors and Affiliations
Contributions
SHS and SI: generated the idea and wrote and drafted the article. MHS: reviewed and edited the manuscript and FM designed and supervised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors announce that no competing interest exists.
Additional information
Handling Editor: M.Iqbal R. Khan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, S.H., Islam, S., Mohammad, F. et al. Gibberellic Acid: A Versatile Regulator of Plant Growth, Development and Stress Responses. J Plant Growth Regul 42, 7352–7373 (2023). https://doi.org/10.1007/s00344-023-11035-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-11035-7