Abstract
The mechanism of Cu tolerance in plants and its control measures are of considerable significance for the remediation of Cu-contaminated soils. Gibberellic acid (GA3) is involved in plant growth and development and in the response to heavy metal stress. In the present study, changes in the biomass, oxidative stress response responses, and photosynthesis of spinach seedlings were examined under Cu stress with exogenous GA3 applied at concentrations of 0, 3, 5, 10, 20, 40, 60, or 80 mg L−1. Under Cu stress, the plant Cu concentration and oxidative damage were greater, photosynthetic parameters and biomass declined, and antioxidant enzyme activities and the proline concentration increased. However, spinach growth did not terminate, indicating that spinach seedlings had strong Cu tolerance. When low concentrations of GA3 (3–5 mg L−1) were added to Cu-stressed spinach seedlings, the damage caused by Cu stress to spinach seedlings was reduced, and the Cu tolerance of spinach seedlings was enhanced, which mainly manifested as reduced oxidation damage, an increased proline concentration, elevated antioxidant enzyme activities, decreased Cu concentration in leaves, and increased Cu concentration in roots, increased photosynthetic parameters, and an increased in the total biomass. In contrast, additions of GA3 at concentrations higher than 40 mg L−1 intensified oxidative damage and decreased the activities of antioxidant enzymes, photosynthetic parameters, and biomass. Additionally, the Cu concentration increased in leaves and decreased Cu concentration in roots, indicating that high concentrations of GA3 aggravated stress damage and severely influenced physiological functions in spinach seedlings. In summary, the application of 3–5 mg L−1 GA3 to spinach seedlings in Cu-contaminated soil can be used to reduce Cu toxicity to plants and increase Cu tolerance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution in soil has become a global concern. In China, approximately 20 million ha of farmland are contaminated by heavy metals (Li et al. 2011; Cai et al. 2019). According to the communique on the soil pollution survey conducted by the Ministry of Environmental Protection (MEP) of China and the Ministry of Land and Resources in 2014, the national total percentages of soil heavy metal pollution exceeded 16.1%. Cu was ranked fourth accounting for 2.1% of the total pollution by heavy metals (MEP 2014). The sources of excess Cu in soil were mainly mining and smelting waste (Acosta et al. 2011), garbage incineration, sewage application, and excessive application of Cu-containing pesticides and fertilizers (Możdżeń et al. 2016; Sun et al. 2018). Some reports showed that the level of soil Cu pollution reached 102.5 mg kg−1 in 31 provincial capitals in China (Zhang et al. 2018), especially in the southwest, which is a major area for large-scale mining and smelting of non-ferrous metals such as Cu, Pb, Zn, and Cd. Mining activities contaminate the surrounding via the drainage of mine waste, spillage of tailings, and dust emission, among other pathways (Cheng et al. 2009). Excess Cu in the soil can then enter the human body through the food chain threatening human health (Cai et al. 2019).
In plants, excess Cu has many detrimental consequences, such as facilitating the production of reactive oxygen species (ROS), decreasing plant biomass, inactivating enzymes, and disturbing photosynthesis (Chandrasekhar and Ray 2017; Rombel-Bryzek et al. 2017). To counteract the adverse effects of Cu stress, plants have developed various defense mechanisms, including antioxidant defense and toxic ion chelation and detoxification (Yruela 2009; Song et al. 2014). In addition, signaling molecules such as gibberellic acid (GA3), indole-3-acetic acid (IAA), and citric acid may also induce stress tolerance in plants (Agami 2016; Ben Massoud et al. 2017).
GA3, as a signaling molecule in plants, is involved in eliciting specific responses to biotic and abiotic stresses. GA3 provides protection against saline stress in tomato (Maggio et al. 2010) and wheat (Akman 2012), induces heat stress responses in winter rapeseed seedlings (Leul and Zhou 1999), and drought stress responses in tomato, pepper, and mint (Abdelkader 2015). Furthermore, GA3 is involved in alleviating heavy metal stress (Piotrowska-Niczyporuk et al. 2012), reducing heavy metal accumulation (Abd EI-Monem et al. 2009), and improving antioxidant capacities (Alonso-Ramirez et al. 2009) in plants. GA3 application can reduce oxidative stress injury in Cu-stressed pea seeds (Ben Massoud et al. 2017). Foliar spray of GA3 to Pb-stressed maize also significantly increased Pb uptake and improved plant growth (Hadi et al. 2010), and the application of 5 μmol L−1 GA3 improved root growth and reduced the Cd content and lipid peroxidation in Arabidopsis thaliana seedlings under Cd stress (Zhu et al. 2012).
Spinach (Spinacia oleracea L.; order Caryophyllales) contains large amounts of vitamins, carotenoids, organic acids, alkaline minerals, and antioxidants (Aehle et al. 2004; Lisiewska et al. 2011; Becker et al. 2014). It is characterized by having a large leaf area, high relative growth rate, and high heavy metal absorption rate (Alia et al. 2015). Irrigation of spinach with sewage water containing heavy metals resulted in higher heavy metal accumulation in the leaves of spinach seedling than in their roots (Naz et al. 2016). Similarly, spinach grown in sewage-irrigated soil (Cu2+ content of 7.93 mg L−1) showed a Cu2+ content above the standard level, whereas spinach growth and yield were significantly increased (Naz et al. 2016). Spinach tolerance mechanisms mainly include an increase in antioxidant enzyme activities and an accumulation of secondary metabolites (Gong et al. 2019). However, little is known about the interaction between GA3 and toxic heavy metals such as Cu in spinach. Therefore, we aimed to (i) compare the effects of different GA3 concentrations on the physiological mechanism of spinach seedlings under Cu stress and (ii) explore whether exogenous GA3 can alleviate Cu toxicity in spinach, and if so, determine the optimal concentration of GA3 and the possible mechanism of GA3 mediated protection. Accordingly, plant growth, oxidative damage, antioxidant enzyme activities, and levels of photosynthetic pigments, gas exchange, and chlorophyll fluorescence were evaluated in spinach seedling leaves under Cu stress with and without GA3 treatment.
Materials and methods
Culturing of test materials
The Japanese big-leaf spinach seeds used in the present study were provided by the seed breeding station of Wangzhendian, Qingxian County, Hebei Province, China. Healthy seeds were sterilized using 0.1% NaClO, rinsed with deionized water, and then evenly placed on filter paper in a Petri dish. Seeds were germinated in an incubator at 25 °C for 48 h, in the dark. Germinated seeds were selected and planted in peat soil until they had grown to approximately 10 cm. Ten seedlings showing similar growth characteristics were selected and transplanted into 53 × 23 × 18 cm (length × width × height) pots supported by a tray placed underneath. The potting matrix comprised of peat soil:perlite at a 7:3 ratio, of which 3 kg (2.1 kg dry weight) was used to fill each pot. Spinach plants were grown in an open greenhouse. Complete Hoagland nutrient solution was supplied to all pots for 20 days to establish a vigorous root system, after which the GA3 treatment began. The modified complete Hoagland nutrient solution is comprised of Ca(NO3)2·4H2O 5 mmol L−1, KNO3 5 mmol L−1, Fe2SO4·7H2O 1 mmol L−1, MgSO4·7H2O 2 mmol L−1, H3BO3 0.045 mmol L−1, MnCl·4H2O 0.01 mmol L−1, ZnSO4·7H2O 0.8 mmol L−1, CuSO4·5H2O 0.3 mmol L−1, Na2Mo2O4·2H2O 0.4 mmol L−1, and Na-EDTA·2H2O 0.02 mmol L−1 (Zhu et al. 2012; Ouzounidou and Ilias 2005).
GA3 treatment
Plants were divided into nine groups. One group, to which only 400 mL Hoagland nutrient solution was added in a single dose, was used as the control (C1). The other eight groups were provided with a one-time irrigation of 400 mL Hoagland nutrient solution containing 700 mg kg−1 Cu2+ in the form of CuSO4 (analytical reagent, Wuxi Yatai United Chemical Co. LTD). The Hoagland nutrient solution, containing 4.56 mg kg−1 Cu2+, was adjusted to pH 6.5 ± 0.3 using 0.1 mmol L−1 NaOH or HCl.
To examine the effect of GA3 on the Cu-stressed seedlings, GA3 was sprayed on the leaves of the seedlings that received Cu2+ at concentrations of 0, 3, 5, 10, 20, 40, 60, and 80 mg L−1 GA3 (guarantee reagent, Fuzhou Feijing Biotechnology Co. LTD). These eight treatments are hereafter referred to as Cu + GA3-0 (C2), Cu + GA3-3 (T1), Cu + GA3-5 (T2), Cu + GA3-10 (T3), Cu + GA3-20 (T4), Cu + GA3-40 (T5), Cu + GA3-60 (T6), and Cu + GA3-80 (T7), respectively. The GA3 solution was mixed with Tween-20 (C58H114O26, a leaf surfactant) and a total of 20 mL of GA3 was applied to each pot (one application per pot). The C2 plants were sprayed with an equal amount of water (20 mL/pot) mixed with Tween-20. Each treatment was replicated three times in a randomized block design.
Plants were watered with deionized water to maintain a weight of 3.4 ± 0.05 kg. Cultivation was conducted at a day/night temperature of 22/15 °C, respectively, under a relative humidity of 70–80%, with 14 h of illumination (light intensity of 8000 lux) and 10 h of darkness. Treatment concentrations were set according to the results of preliminary tests (Gong et al. 2019; Naz et al. 2016; Ji et al. 2015; Hadi et al., 2010; Falkowska et al. 2011). After 7 days of treatment, plant samples were collected to measure the plant stress indicators.
Plant stress indicator measurements
Determination of biomass
Seedlings were carefully removed from pots, rinsed with tap water, and then rinsed three times with deionized water. After drying with blotting paper, the leaves, stems, and roots were separated, and their fresh weight (FW) was immediately determined. Thereafter, samples were oven-dried at 105 °C for 10 min, and then at 80 °C until a constant weight was obtained, which was recorded as the dry weight (DW) (Gong et al. 2019).
Determination of Cu concentration
Seedling roots were soaked in 20 mmol L−1 Na2-EDTA solution for 3 h to remove the Cu2+ adsorbed on their surfaces and then washed repeatedly with deionized water. Samples were then oven-dried at 105 °C for 20 min and then at 80 °C for 72 h. The dried plant samples were ground and collected in a porcelain crucible, and then digested with HNO3 and HClO4 (4/1, v/v) on a hot plate. Dense yellow fumes appeared from the flask and H2O2 was added continuously until dense yellow fumes disappeared. When the samples became colorless, the porcelain crucibles were removed from a hot plate, and volume was made up to 25 mL using distilled water. Cu concentrations were determined in the School of Chemistry, Hubei University, using inductively coupled plasma atomic emission spectroscopy (Fisons ARL Accuris, Ecublens, Switzerland) (Zhu et al. 2012).
Determination of malondialdehyde and proline contents
Malondialdehyde (MDA) was determined by the thiobarbituric acid (TBA) method (Tewari et al. 2002). Fresh leaf samples (0.3 g) were collected and ground with phosphate buffer to form a slurry, which was then centrifuged at 3000 ×g for 15 min. The resulting supernatant was isolated, and after the addition of 5 mL 0.5% thiobarbituric acid (TBA), the solution was mixed by shaking while being heated at 100 °C for 10 min. The mixture was subjected to rapid cooling centrifugation, the absorbance was determined at 532 nm and 600 nm using TBA as a blank sample. The measurements were performed using a UV-1750 spectrophotometer (Shimadzu, Kyoto, Japan).
Proline content was determined by acidic ninhydrin colorimetry (Tewari et al. 2002). Fresh leaf samples (0.5 g) were weighed and mixed with 5 mL sulfosalicylic acid for extraction in a boiling water bath for 10 min, after which the mixture was allowed to cool. The resulting supernatant was isolated by suction, followed by addition of 2 mL glacial acetic acid and 4 mL acidic ninhydrin in a boiling water bath. The mixture was then allowed to cool to room temperature, after which 4 mL toluene was added. The mixture was thoroughly shaken and allowed to stand until stratified; after isolation, the upper layer absorbance was determined at 520 nm using a UV-1750 spectrophotometer (Shimadzu, Kyoto, Japan).
Determination of antioxidant enzyme activities
Superoxide dismutase (SOD, E.C. 1.15.1.1) activity was determined according to Beauchamp and Fridovich’s method (Beauchamp and Fridovich 1971). The reaction mixture contained the enzyme extract, 20 μmol L−1 riboflavin, 750 μmol L−1 nitroblue tetrazolium (NBT), 13.37 mM methionine, 0.1 mM EDTA, and 50 mmol L−1 phosphate buffer (pH 7.8). One unit of SOD activity was defined as the amount of enzyme required to inhibit 50% of the initial NBT reduction under light. The measurements were performed using a UV-1750 spectrophotometer (Shimadzu, Kyoto, Japan).
Peroxidase (POD, EC 1.11.1.7) activity was assayed in a 2.9 mL reaction mixture containing 50 μL 0.02 mol L−1 guaiacol, 0.1 mL enzyme extract, and 10 μL 0.04 mol L−1 H2O2. The optical density at 470 nm for 1 min at 25 °C was recorded using a UV-1750 spectrophotometer (Shimadzu, Kyoto, Japan) (Batish et al. 2006).
Catalase (CAT, EC 1.11.1.6) activity was assayed according to the method of Lu et al. (2018). The 3.3-mL reaction mixture contained 100 mmol L−1 phosphate buffer (pH 7.4), 0.1 mL enzyme extract, 0.5 mL 65 μmol L−1 H2O2, and 0.5 mL 32.7 mol L−1 ammonium molybdate. The absorbance of the sample was determined at 405 nm using a UV-1750 spectrophotometer (Shimadzu, Kyoto, Japan).
Total ascorbate peroxidase (APX, EC 1.11.1.11) activity was determined by monitoring the ascorbate oxidation rate at 290 nm (Nakano and Asada 1981). The protein extract was added to a 1.7 mL reaction solution, containing 50 mmol L−1 phosphate buffer (pH 7.8), 0.1 mmol L−1 EDTA, and 5 mM ascorbic acid. The reaction was initiated by the addition of 100 μL of 20 mM H2O2. The decrease in absorbance was monitored using a UV-1750 spectrophotometer (Shimadzu, Kyoto, Japan).
Determination of photosynthetic pigments, photosynthetic parameters, and chlorophyll fluorescence parameters
Chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoid contents were determined according to Hiscox and Israeltem’s methods (Hiscox and Israeltem 1979). The leaf content (per gram) was calculated according to Arnon’s formula (Arnon 1949).
The net photosynthetic rate (Pn), intercellular CO2 concentration (Ci), stomatal conductance (gs), and transpiration rate (Tr) of functional leaves were determined using a Li-6800 portable photosynthesis meter (LI-COR, Lincoln, NE, USA). Chlorophyll fluorescence parameters were determined using a PAM-2100 chlorophyll fluorometer (Walz, Wurzburg, Germany) and included minimum fluorescence (F0), maximum fluorescence after dark adaptation (Fm), photochemical quenching (qP), non-photochemical quenching (NPQ), and electron transport rate (ETR). The ratio of Fv/Fm was calculated according to the formula (Fm − F0) / Fm (Gong et al. 2019).
Data analyses
Statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). One-factor ANOVA was performed to identify statistically significant differences among treatments, followed by Duncan’s multiple comparisons (P < 0.05).
Results
Effects of Cu stress and GA3 on spinach seedlings biomass
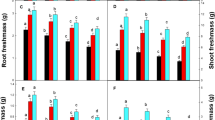
As shown in Fig. 1, Cu stress (treatment C2) significantly inhibited spinach seedling growths, as the total FW and total DW in C2 decreased by 31.9% and 41.0%, respectively (P < 0.05), compared with that of C1. When 3–5 mg L−1 GA3 was applied (treatments T1–T2), the inhibitory effects of Cu stress on spinach biomass were reversed. In treatments T1 and T2, total FW increased by 25.5% and 24.3%, respectively, while total DW increased by 27.2% and 26.8%, respectively, compared with that of C2. When high concentrations of GA3 were used (treatments T3–T7), the increment of biomass increase was reduced. In treatments T6 and T7, the total FW only increased by 10.8% and 4.4%, respectively, while the total DW only increased by 11.5% and 8.6%, respectively. The changes in the FW and DW of leaves, stems, and roots exhibited essentially the same trend as that of total biomass.
Effects of Cu stress and GA3 on Cu accumulation in spinach seedlings
Compared to seedlings of C1, Cu concentration significantly increased in leaves and roots under Cu stress (treatment C2) (Table 1), with leaves having higher concentration than roots. When GA3 was applied to Cu-stressed seedlings (treatments T1–T7), the Cu concentration decreased in leaves and increased in roots. Notably, the lowest leaf Cu concentration and the highest root Cu concentration were observed in treatment T2. In addition, compared with C2, both the decrement of Cu concentration in the leaves and the increment of Cu concentration in the roots decreased in treatments T1–T7. Similarly, the decrement of leaf Cu concentration and the increment of the root Cu concentration reached the maximum values in treatment T2.
Data are mean ± standard error (n = 3). Values within a row followed by the same letter are not significantly different (p < 0.05)
The influence of Cu stress and GA3 on lipid peroxidation and proline contents of spinach leaves
As shown in Fig. 2, Cu stress (treatment C2) significantly increased the MDA content (P < 0.05) compared with C1. When exogenous GA3 was applied (treatments T1–T7), the MDA contents were higher than that in C2, except for treatment T1 in which the MDA content was 18.2% lower than that in C2. In T6 and T7, the MDA contents increased by 75.9% and 62.6% of that in C2, respectively.
Proline content in C2 was higher than in C1 (Fig. 2b). When GA3 was added (treatments T1–T7), the proline contents showed an initial increase followed by a decrease. Proline decreased by 74.4% and 45.4% in T1 and T2, respectively, compared with that in C2. Proline content peaked in T5, increasing by 18.1% of that in C2.
Effects of Cu stress and GA3 on antioxidant enzyme activities of spinach leaves
Exposure of spinach seedlings to Cu stress (treatment C2) significantly increased the activities of antioxidant enzymes (SOD, POD, CAT, and APX), as shown in Fig. 3. However, the maximum increase occurred in treatments T1 and T2 for all four enzyme activities, with average increase of 19.3%, 127.3%, 3.7%, and 2.7%, respectively, compared with treatment C2. In treatments T5–T7, SOD, CAT, and APX activities declined to their minimum values, decreasing by 27.9%, 15.2%, 15.8%, and 35.2%, respectively, on average, compared to C2.
Effects of Cu stress and GA3 treatment on chlorophyll contents and photosynthetic characteristics of spinach leaves
The Chl a, Chl b, and carotenoid content was significantly lower under Cu stress (treatment C2) than that in C1 (P < 0.05) (Table 2). When GA3 was sprayed on Cu-stress seedlings (treatments T1–T7), Chl a contents showed a decreasing trend; however, it was higher than that under Cu stress without application of GA3 (treatment C2). However, Chl b contents exhibited a decreasing trend from T4 to T7. The highest Chl b content was observed in T1 (0.44-fold higher than in C2), and the lowest in T7, and there was no significant difference from that in C2 (P > 0.05).
When compared to C1, the Pn, gs, and Tr values decreased in treatment C2, while Ci values increased (Table 2). When GA3 was added (treatments T1–T7), the Pn value showed a trend of gradual decline, with a minimum value in T7, which was lower than that in C2. However, the gs, Tr, and Ci values first increased and then decreased, reaching maximum values in T3, in which they increased by 83.1%, 20.3%, and 87.5%, respectively, compared to C2.
Data are mean ± standard error (n = 3). Values within a row followed by the same letter are not significantly different (p < 0.05)
Effects of Cu stress and GA3 on chlorophyll fluorescence parameters of spinach leaves
Cu stress (treatment C2) significantly reduced chlorophyll fluorescence parameters of spinach leaves, and the Fv/Fm, NPQ, qP, and ETR values in C2 were 11.8%, 21.2%, 60.8%, and 77.0% lower than those in C1, respectively (Fig. 4). However, exogenous GA3 reversed the reduction of chlorophyll fluorescence parameters. When compared with values in C2, the Fv/Fm, qP, and ETR values in T1 and T2 showed the largest increments, increasing by averages of 0.14, 0.84, and 1.09-fold, respectively. The Fv/Fm values in T6 and T7 only increased by averages of 0.03, 0.24, and 0.17-fold, respectively, in comparison to in C2. The NPQ values increased significantly in T1–T5 (0.75, 1.01, 0.72, 0.91, and 0.77-fold, respectively) and decreased significantly in T6 and T7 (0.18 and 0.27-fold, respectively) compared to C2.
Discussion
High Cu concentration inhibited the physiological activity of spinach seedlings
Excess Cu affects the physiological activities of plants, leading to decreased plant growth, inhibited photosynthesis, and changes to physiological and metabolic processes (Dai et al. 2016; Chandrasekhar and Ray 2017). Our present data showed that, compared with the control treatment (C1), the addition of 700 mg kg−1 Cu (treatment C2) significantly increased the Cu, MDA, and proline content, as well as the activities of four antioxidant enzymes (SOD, POD, CAT, and APX), and decreased the chlorophyll contents, gas exchange parameters (Pn, gs, and Tr), chlorophyll fluorescence parameters (Fv/Fm, qP, NPQ, and ETR), and total biomass. Because seedlings survived, high Cu concentration led to Cu2+ accumulation and an increase in the oxidative stress response in spinach seedlings. However, seedlings resisted oxidative stress damage by increasing antioxidant enzyme activities to maintain vital functions. In addition, the content of proline, which is an osmotic regulatory compound with antioxidant effects, increased in spinach seedlings improving the antioxidant capacity of plants and chelating Cu2+ to reduce its toxic accumulation (Sharma and Dietz 2006; Dresler et al. 2014) thereby improving the Cu tolerance of spinach seedlings.
However, different plants have varying Cu tolerances. Elsholtzia haichowensis, a plant with strong Cu tolerance, can survive under soil with Cu concentrations up to 600 mg kg−1 (Chen et al. 2015) and Sedum sediforme were able to survive extreme phytotoxicity conditions in soil containing 5000 mg kg−1 Cu (Poschenrieder et al. 2001). However, the biomass and photosynthesis rate of cucumber treated with 10 μg g−1 Cu for 5 days (Vinit-Dunand et al. 2002) and the biomass of tomato treated with 5 μmol L−1 Cu decreased (Zhang et al. 2017). In the present study, although the Cu concentrations were not as high as those applied to E. haichowensis and S. sediforme, spinach seedlings showed strong Cu tolerance compared to that of tomato and cucumber observed in previous studies.
Low-concentration GA3 alleviated the damage of Cu stress to spinach seedlings
As an active member of the signal cascade involved in the induction of plant stress responses (Tuna et al. 2008), GA3 can reduce heavy metal accumulation in plants and alleviate oxidative damage when applied to plants under heavy metal stress (Zhu et al. 2012; Ben Massoud et al. 2017). The results of the present study showed that low GA3 concentrations (3–5 mg L−1) applied to Cu-stressed seedlings could significantly decrease the Cu contents in the leaves, but that in the roots significantly increased. These results indicated that GA3 applied at low concentrations reduced Cu2+ accumulation in the leaves and reduced the toxic influence of Cu (Falkowska et al. 2011), but promoted Cu2+ accumulation in the roots (Zhu et al. 2012). It is also possible that GA3 inhibited Cu2+ migration from roots to leaves (Fujita et al. 2006). The root is the main organ of plants exposed to Cu-contaminated soil, and Cu2+ enters the root through the plant’s physiological activities such as transpirational pull and active and passive water absorption, which increased Cu2+ accumulation in the roots. However, roots prevent the extensive migration of Cu2+ to the leaves through ion chelation, ion regionalization, and other routes (Yruela 2009; Song et al. 2014). Thus, when GA3 was applied, the damage caused by Cu stress in leaves was alleviated and Cu tolerance in roots was enhanced, removing excess Cu2+ from the soil. Similar results have been obtained in previous studies: with the application of 10 mg L−1 GA3, Cd concentrations increased in the stems of potato (Solanum tuberosum L.) under Cd stress, while Cd concentrations in the leaves decreased (Ji et al. 2015); when GA3 was applied to maize under Pb stress, Pb uptake by roots was significantly increased (Hadi et al. 2010). In addition, our results also indicated that the total Cu content in leaves and roots was the lowest at the low GA3concentrations (3–5 mg L−1). It may be that low GA3 concentrations aggravated root cell wall embolization and that lignification, and the cuticle of the cell wall formed protective substances; the barrier function of root apoplasts was enhanced, and thus a large number of Cu2+ were prevented from entering the plant resulting in damage caused by excess Cu2+ to be alleviated (Krishnamurthy et al. 2009; Lu et al. 2019; Yuankun et al. 2020). This is also a strong evidence that low GA3 concentrations can alleviate the damage to plants caused by Cu2+ stress.
Exogenous addition of plant hormones can improve heavy metals tolerance by reducing ROS accumulation in the membrane peroxidation reaction and enhancing the antioxidant defense ability of plants (Lu et al. 2010; Agami 2016). The MDA content is an important indicator of the degree of membrane lipid peroxidation and damage in plants (Rombel-Bryzek et al. 2017). In the present study, when low GA3 concentrations (3–5 mg L−1) were applied, the MDA contents remarkably decreased while the activities of antioxidant enzymes (SOD, POD, CAT, and APX) and biomass significantly increased. Possible reasons for this phenomenon are as follows: (i) low GA3 concentration can reduce the oxidative damage to plants caused by high Cu concentration and thus, alleviate the damage to plants caused by Cu and (ii) a low GA3 concentration may induce upregulation of antioxidant enzyme genes in plants, thus enhancing their ability to resist oxidative stress (Kaur Kohli et al. 2018). Hence, plant growth and Cu tolerance were enhanced in spinach seedlings after the addition low GA3concentrations. Similar observations have been reported in previous studies. Ji et al. (2015) found that addition of 1000 mg L−1 GA3 to black nightshade (Solanum nigrum L.) could increase plant biomass under Cd stress and promote its phytoremediation ability in Cd-contaminated soil. Zhu et al. (2012) showed that GA3 could reduce the Cd damage to lipid peroxidation in A. thaliana plants and enhance their Cd tolerance. In the present study, when low GA3 concentrations were added, the chlorophyll contents increased, as well as Pn, Ci, Fv/Fm, NPQ, qP, and ETR values. The reasons for this may be that (i) GA3, as a signaling molecule, can induce the PSII reaction center to be in an open state, allowing the use of more excitation energy for electron transmission thereby improving the electron flow efficiency of PSII (Zhang et al. 2015; Możdżeń et al. 2016); (ii) GA3 may trigger some protective mechanisms of the photosynthetic apparatus resulting in the stability of PSII (Ouzounidou and Ilias 2005); and (iii) GA3 enhances photosynthetic CO2 uptake, probably by increasing the coupling between electron transport and phosphorylation (Tamas et al. 1974; Ouzounidou and Ilias 2005), thereby promoting photosynthesis in spinach seedlings.
High concentration of GA3 inhibits physiological activities of spinach seedlings under Cu stress
The timing, concentration, and utilization techniques of exogenous plant growth regulators should be considered. Among them, concentration is an important factor affecting plant growth (Saeid and Abooalfazl 2019). In the present study, the effects of GA3 on plant growth physiology differed depending on the applied concentration (Muniandi et al. 2018), When GA3 was applied at concentrations higher than 40 mg L−1 (treatments T5–T7), the Cu contents were higher in leaves and lower in roots than that following application at when lower GA3 concentrations (treatments T1 and T2), indicating that the application of high GA3 concentrations promoted Cu2+ accumulation in leaves while reducing Cu2+ accumulation in roots. The reason for this may be that (i) GA3 can alter the concentrations of heavy metals accumulated in plants (Falkowska et al. 2011; Hadi et al. 2010); (ii) spinach leaves are large and have a high water content, which means that plants need to transport a large amount of water from roots to leaves, resulting in Cu2+ accumulation in leaves. Similarly, Khan et al. (2011) found that application of a high concentration of plant growth regulation hormones reduced growth of Salix tetrasperma Roxb. In addition, when GA3 concentrations were higher than 40 mg L−1 (treatments T5–T7), the MDA contents significantly increased, and the activities of antioxidative enzymes (SOD, POD, CAT, and APX ) decreased, and the total biomass of spinach seedlings decreased, indicating that the high GA3 concentration aggravated the damage of membrane lipid peroxidation to seedlings under Cu stress, leading to the decline of plant antioxidant defense functions and plant biomass (Muniandi et al. 2018). Similar results have been reported in previous studies. For example, CAT and APX activities decreased when 1 μmol L−1 GA3 was applied to pea (Pisum sativum L.) seedlings under Cu stress (Ben Massoud et al. 2017). Addition of 24-epibrassinolide and salicylic acid to Indian mustard (Brassica juncea L.) under Pb stress decreased SOD activity (Kaur Kohli et al. 2018).
Photosynthesis is one of the most sensitive physiological activities of plants to environmental stress, and chlorophyll fluorescence is the most affected (Diao et al. 2014; Zhang et al. 2015). In the present study, the application of GA3 at concentrations higher than 40 mg L−1 (treatments T5–T7) led to declines in Chl a and carotenoid contents compared with low GA3 concentration (treatments T1 and T2); gas exchange parameters (Pn, Ci, gs, and Tr) and chlorophyll fluorescence parameters (Fv/Fm, NPQ, qP, and ETR) were also considerably reduced. These results suggested that (i) high GA3 concentrations interfered with electron donation to photochemical reactions in PSII, leading to a reduction of the energy distributed to heat dissipation, decreasing the prevention exerted upon damaging free radicals formation (Muniandi et al. 2018) and (ii) the high GA3 concentration inhibited the growth of the leaf primordia within the terminal bud, resulting in decreases in the number and shape of leaves, the photosynthetic area, photosynthetic products, and the photosynthetic electron transfer rate (Gou et al. 2010; Wang et al. 2015). Eventually, the photosynthetic rate reduced, and physiological functions were affected.
Conclusion
Soil containing 700 mg kg−1 Cu significantly increased the Cu contents and decreased the biomass of spinach seedlings; however, the antioxidant defense ability of plants was enhanced, which enabled the seedlings to maintain their survival and demonstrate strong Cu tolerance. While the addition of 3–5 mg L−1 GA3 could alleviate the damage from Cu stress and enhance the Cu tolerance of spinach seedlings, application of GA3 at concentrations higher than 40 mg L−1 aggravated the damage caused by Cu stress, as the biomass, antioxidant enzyme activities, and photosynthesis of spinach seedlings decreased. Therefore, our results suggest that application of 3–5 mg L−1 GA3 to spinach seedlings can alleviate the toxicity of Cu-contaminated soil to plants.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Abbreviations
- FW:
-
Fresh weight
- DW:
-
Dry weight
- MDA:
-
Malondialdehyde
- TCA:
-
Trichloroacetic acid
- SOD:
-
Superoxide dismutase
- POD:
-
Peroxidase
- CAT:
-
Catalase
- APX:
-
Ascorbate peroxidase
- ROS:
-
Reactive oxygen species
- Chl a/b:
-
Chlorophyll a/b
- PSII:
-
Photosystem II
- P n :
-
Net photosynthetic rate
- g s :
-
Stomatal conductance
- C i :
-
Internal CO2 concentration
- T r :
-
Transpiration rate
- Fv/Fm:
-
Maximum quantum yield of PSII photochemistry
- qP:
-
Photochemical quenching
- NPQ:
-
Nonphotochemical quenching
- ETR:
-
Electron transport rate
- NBT:
-
Nitroblue tetrazolium
References
Abd EI-Monem MS, Ibrahim IF, Mahmoud RS (2009) Role of gibberellic acid in abolishing the detrimental effects of Cd and Pb on broad bean and lupin plants. Res J Agric Biol Sci 5:668–673
Abdelkader AF (2015) Tolerance strategies of some GA3-pretreated edible plants during growing under drought stress. Egyptian Soc ExpBiolo 11(2):217–225
Acosta JA, Faz A, Martínez-Martínez S, Zornoza R, Carmona DM, Kabas S (2011) Multivariate statistical and GIS-based approach to evaluate heavy metals behavior in mine sites for future reclamation. J Geochem Explor 109:8–17. https://doi.org/10.1016/j.gexplo.2011.01.004
Aehle E, Grandic S, Reynaudle R, Ralainirina S, Baltorarosset F, Mesnard C, Prouillet C, Maziere JC, Fliniaux MA (2004) Development and evaluation of an enriched natural antioxidant preparation obtained from aqueous spinach (Spinacia oleracea) extracts by an adsorption procedure. Food Chem 86:579–585. https://doi.org/10.1016/j.foodchem.2003.10.006
Agami RA (2016) Pre-soaking in indole-3-acetic acid or spermidine enhances copper tolerance in wheat seedlings. S Afr J Bot 104:167–174. https://doi.org/10.1016/j.sajb.2015.10.003
Akman Z (2012) Effects of GA and kinetin pre-sowing treatments on seeding emergence and seeding growth in wheat under saline conditions. J anim Vet Adv 8(2):362–367. https://doi.org/10.1016/j.fsi.2008.03.014
Alia N, Sardar K, Said M, Salma K, Sadia A, Sadaf S, Toqeer A, Miklas S (2015) Toxicity and bioaccumulation of heavy metals in spinach (Spinacia oleracea) grown in a controlled environment. Int J Environ Res Public Health 12:7400–7416. https://doi.org/10.3390/ijerph120707400
Alonso-Ramirez A, Rodriguez D, Reyes JA, Jimenez G, Nicolas M, Lopez-Climent A, Gomez-Cadenas, Nicolas C (2009) Evidence for a role of gibberellins in salicylic acid-modulated early plant responses to abiotic stress in Arabidopsis seeds. Plant Physiol 150:1335–1344. https://doi.org/10.1104/pp.109.139352
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Batish DR, Singh HP, Setia N, Kaur S, Kohli RK (2006) Effect of 2-benzoxazolinone (BOA) on seedling growth and associated biochemical changes in mung bean (Phaseolus aureus). Zeitschrift Fur Naturforschung C A J Biosci 61:709–714. https://doi.org/10.1515/znc-2006-9-1017
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Becker C, Klaering HP, Kroh LW, Krumbein A (2014) Cool-cultivated red leaf lettuce accumulates cyanidin-3-O-(6″-O-malonyl)-glucoside and caffeoylmalic acid. Food Chem 146:404–411. https://doi.org/10.1016/j.foodchem.2013.09.061
Ben Massoud M, Inès K, Ferjani EE, Chaoui A (2017) Alleviation of copper toxicity in germinating pea seeds by IAA, GA3, Ca and citric acid. J Plant Interact 13:21–29. https://doi.org/10.1080/17429145.2017.1410733
Cai L, Wang Q, Luo J, Chen L, Zhu R, Wang S, Tang C (2019) Heavy metal contamination and health risk assessment for children near a large Cu-smelter in central China. Sci Total Environ 650:725–733. https://doi.org/10.1016/j.scitotenv.2018.09.081
Chandrasekhar, C., Ray, J.G. 2017. Copper accumulation, localization and antioxidant response in Eclipta alba L. in relation to quantitative variation of the metal in soil. Acta Physiol Plant, 39. https://doi.org/10.1007/s11738-017-2508-4.
Chen J, Shafi M, Li S, Wang Y, Wu J, Ye Z, Peng D, Yan W, Liu D (2015) Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of Moso bamboo (Phyllostachys pubescens). Sci Rep 5:13554. https://doi.org/10.1038/srep13554
Cheng H, Hu Y, Luo J, Xu B, Zhao J (2009) Geochemical processes controlling fate and transport of arsenic in acid mine drainage (AMD) and natural systems. J Hazard Mater 165:13–26. https://doi.org/10.1016/j.jhazmat.2008.10.070
Dai H, Xu Y, Zhao L, Shan C (2016) Alleviation of copper toxicity on chloroplast antioxidant capacity and photosystem II photochemistry of wheat by hydrogen sulfide. Braz J Botany 39:787–793. https://doi.org/10.1007/s40415-016-0250-6
Diao M, Ma L, Wang J, Cui J, Fu A, Liu H (2014) Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. Plant Growth Regul 33:71–682. https://doi.org/10.1007/s00344-014-9416-2
Dresler S, Hanaka A, Bednarek W, Maksymiec W (2014) Accumulation of low-molecular- weight organic acids in roots and leaf segments of Zea mays plants treated with cadmium and copper. Acta Physiol Plant 36:1565–1575. https://doi.org/10.1007/s11738-014-1532-x
Falkowska M, Pietryczuk A, Piotrowaka A, Bajguz A, Grygoruk A, Czerpak R (2011) The effect of gibberellic acid (GA3) on growth, metal biosorption and metabolism of the green algae Chlorella vulgaris (Chlorophyceae) Beijerinck exposed to cadmium and lead stress. Pol J Environ Stud 20:53–59. https://doi.org/10.1002/ldr.1042
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9:436–442. https://doi.org/10.1016/j.pbi.2006.05.014
Gong Q, Wang L, Dai T, Zhou J, Kang Q, Chen H, Li K, Li Z (2019) Effects of copper on the growth, antioxidant enzymes and photosynthesis of spinach seedlings. Ecotoxicol Environ Saf 171:771–780. https://doi.org/10.1016/j.ecoenv.2019.01.016
Gou J, Strauss SH, Tsai CJ, Fang K, Chen Y, Jiang X (2010) Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. Plant Cell 22:623–639. https://doi.org/10.1105/tpc.109.073239
Hadi F, Bano A, Fuller MP (2010) The improved phytoextraction of lead (Pb) and the growth of maize (Zea mays L.): the role of plant growth regulators (GA3 and IAA) and EDTA alone and in combinations. Chemosphere. 80:457–462
Hiscox JD, Israeltem GF (1979) A method for extraction of chlorophyll from leaf tissue without maceration using dimethyl sulfoxide. Rev Can Bot 57:1332–1334. https://doi.org/10.1139/b79-163
Ji P, Tang X, Jiang Y, Tong Y, Gao P, Han W (2015) Potential of gibberellic acid 3 (GA3) for enhancing the phytoremediation efficiency of Solanum nigrum L. Bull Environ Contam Toxicol 95:810–814. https://doi.org/10.1007/s00128-015-1670-x
Kaur Kohli S, Handa N, Bali S, Arora S, Sharma A, Kaur R, Bhardwaj R (2018) Modulation of antioxidative defense expression and osmolyte content by co-application of 24-epibrassinolide and salicylic acid in Pb exposed Indian mustard plants. Ecotoxicol Environ Saf 147:382–393. https://doi.org/10.1016/j.ecoenv.2017.08.051
Khan MI, Ahmad N, Anis M (2011) The role of cytokinins on in vitro shoot production in Salix tetrasperma Roxb.: a tree of ecological importance. Trees. 25:577–584. https://doi.org/10.1007/s00468-010-0534-6
Krishnamurthy P, Ranathunge K, Franke R, Prakash HS, Schreiber L, Mathew MK (2009) The role of root apoplastic transport barriers in salt tolerance of rice (Oryza sativa L.). Planata 230(1):119–134. https://doi.org/10.1007/s00425-009-0930-6
Leul M, Zhou WJ (1999) Alleviation of Waterlogging Damage in Winter Rape by Uniconazole Application: Effects on Enzyme Activity, Lipid Peroxidation, and Membrane Integrity. J Plant Growth Regul 18(1):9–14. https://doi.org/10.1007/PL00007046
Li G, Sun G, Williams P, Nunes L, Zhu Y (2011) Inorganic arsenic in Chinese food and its cancer risk. Environ Int 37:1219–1225. https://doi.org/10.1016/j.envint.2011.05.007
Lisiewska Z, Kmiecik W, Gębczyński P, Sobczyńska L (2011) Amino acid profile of raw and as-eaten products of spinach (Spinacia oleracea L.). Food Chem 126:460–465. https://doi.org/10.1016/j.foodchem.2010.11.015
Lu Y, Jiang P, Liu S, Gan Q, Cui H, Qin S (2010) Methyl jasmonate or gibberellins A3-induced astaxanthin accumulation is associated with up-regulation of transcription of beta-carotene ketolase genes (bkts) in microalga Haematococcus pluvialis. Bioresour Technol 101:6468–6474. https://doi.org/10.1016/j.biortech.2010.03.072
Lu Q, Zhang T, Zhang W, Su C, Yang Y, Hu D, Xu Q (2018) Alleviation of cadmium toxicity in Lemna minor by exogenous salicylic acid. Ecotoxicol Environ Saf 147:500–508. https://doi.org/10.1016/j.ecoenv.2017.09.015
Lu H, Waichin L, Nora Fungyee T, Zhihong Y (2019) Effects of root morphology and anatomy on cadmium uptake and translocation in rice (Oryza sativa L.). J Environ Sci 075(001):296–306. https://doi.org/10.1016/j.jes.2018.04.005
Maggio A, Barbieri G, Raimondi G, Pascale S (2010) Contrasting effects of GA treatments to tomato plants exposed to increasing salinity RID A-5646-2011. J plant growth regul 29(1): 63–72. https://doi.org/10.1007/s00344-009-9114-7
Ministry of Environmental Protection, 2014. A national survey of soil pollution bulletin.
Możdżeń K, Wanic T, Rut G, Łaciak T, Rzepka A (2016) Toxic effects of high copper content on physiological processes in Pinus sylvestris L. Photosynthetica 55:193–200. https://doi.org/10.1007/s11099-016-0229-3
Muniandi, Sures Kumar M, Hossain MA, Abdullah MP, Ab Shukor NA (2018) Gibberellic acid (GA3) affects growth and development of some selected kenaf (Hibiscus cannabinus L.) cultivars. Ind Crop Prod 118:180–187. https://doi.org/10.1016/j.indcrop.2018.03.036
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Naz S, Anjum MA, Akhtar S (2016) Monitoring of growth, yield, biomass and heavy metals accumulation in spinach grown under different irrigation sources. Int J Agric Biol 18:689–697. https://doi.org/10.17957/ijab/15.0129
Ouzounidou G, Ilias I (2005) Hormone-induced protection of sunflower photosynthetic apparatus against copper toxicity. Biol Plant 49(2):223–228. https://doi.org/10.1016/S0079-6123(03)46004-4
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka E, Godlewska-Zylkiewicz B (2012) Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol Biochem 52:52–65. https://doi.org/10.1016/j.plaphy.2011.11.009
Poschenrieder C, Bech J, Llugany M, Pace A, Fenés E, Barceló J (2001) Copper in plant species in a copper gradient in Catalonia (North East Spain) and their potential for phytoremediation. Plant Soil 230:247–256
Rombel-Bryzek A, Rajfur M, Zhuk O (2017) The impact of copper ions on oxidative stress in garden cress Lepidium sativum. Ecol Chem Eng S 24. https://doi.org/10.1515/eces-2017-0041
Saeid R, Abooalfazl A (2019) The application of plant growth regulators to improve phytoremediation of contaminated soils. Chemosphere. 220:818–827. https://doi.org/10.1016/j.chemosphere.2018.12.203
Sharma SS, Dietz KJ (2006) The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot 57:711–726. https://doi.org/10.1093/jxb/erj073
Song Y, Zhang H, Chen C, Wang G, Zhuang K, Cui J, Shen Z (2014) Proteomic analysis of copper-binding proteins in excess copper-stressed rice roots by immobilized metal affinity chromatography and two-dimensional electrophoresis. Bio Metals 27:265–276. https://doi.org/10.1007/s10534-014-9707-x
Sun Z, Xie X, Wang P, Hu Y, Cheng H (2018) Heavy metal pollution caused by small-scale metal ore mining activities: a case study from a polymetallic mine in South China. Sci Total Environ 639:217–227. https://doi.org/10.1016/j.scitotenv.2018.05.176
Tamas I, Schwartz J, Hagin J, Simmonds R (1974) Mech Regul Plant Growth
Tewari RK, Kumar P, Sharma PN, Bisht SS (2002) Modulation of oxidative stress responsive enzymes by excess cobalt. Plant Sci 162:381–388
Tuna AL, Kaya C, Dikilitas M, Higgs D (2008) The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ Exp Bot 62:1–9. https://doi.org/10.1016/j.envexpbot.2007.06.007
Vinit-Dunand F, Epron D, Alaoui-Sossé B, Badot PM (2002) Effects of copper on growth and on photosynthesis of mature and expanding leaves in cucumber plants. Plant Sci 163:53–58. https://doi.org/10.1016/S0168-9452(02)00060-2
Wang GL, Que F, Xu Z, Wang F, Xiong A (2015) Exogenous gibberellin altered morphology, anatomic and transcriptional regulatory networks of hormones in carrot root and shoot. BMC Plant Biol 15:290–295. https://doi.org/10.1186/s12870-015-0679-y
Yruela I (2009) Copper in plants: acquisition, transport and interactions. Funct Plant Biol 36:409–430
Yuankun L, Qi T, Xinyu G, Jipeng L, Jinxing L, Yongchao L, Tingqiang L (2020) Low calcium-induced delay in development of root apoplastic barriers enhances Cd uptake and accumulation in Sedum alfredii. Sci Total Environ 25:723–734. https://doi.org/10.1016/j.scitotenv.2020.137810
Zhang Y, Xu S, Yang S, Chen Y (2015) Salicylic acid alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in two melon cultivars (Cucumis melo L.). Protoplasma. 252:911–924. https://doi.org/10.1007/s00709-014-0732-y
Zhang JH, Li Z, Sun HL, Wu H, Chen SY (2017) Adversity stress-related responses at physiological attributes, transcriptional and enzymatic levels after exposure to Cu in Lycopersicum esculentm seedlings. Sci Hortic 222:213–220. https://doi.org/10.1016/j.scienta.2017.05.027
Zhang X, Zha T, Guo X, Meng G, Zhou J (2018) Spatial distribution of metal pollution of soils of Chinese provincial capital cities. Sci Total Environ 643:1502–1513. https://doi.org/10.1016/j.scitotenv.2018.06.177
Zhu X, Jiang T, Wang Z, Lei G, Shi Y, Li G, Zheng S (2012) Gibberellic acid alleviates cadmium toxicity by reducing nitric oxide accumulation and expression of IRT1 in Arabidopsis thaliana. J Hazard Mater 239-240:302–307. https://doi.org/10.1016/j.jhazmat.2012.08.077
Funding
This work was funded by grants from key projects of the Hubei Natural Fund (innovation group) of China (2016CFA016) and the research project of Xinjiang Agricultural Vocational and Technical College (XJNZYKJ201901).
Author information
Authors and Affiliations
Contributions
Qong Gin, Ling Wang, and Jing-yi Zhou conducted the experiments, collected and analyzed the samples, and drafted the manuscript. Qun Kang and Duan-dan Niu analyzed the data and revised the manuscript. Zhao-hua Li and Qong Gin conceived and designed this work. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Responsible Editor: Philipp Gariguess
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Gong, Q., Li, Zh., Wang, L. et al. Gibberellic acid application on biomass, oxidative stress response, and photosynthesis in spinach (Spinacia oleracea L.) seedlings under copper stress. Environ Sci Pollut Res 28, 53594–53604 (2021). https://doi.org/10.1007/s11356-021-13745-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13745-5