Abstract
Key message
Thirty genes involved in GA and ABA metabolism and signalling were identified, and the expression profiles indicated that they play crucial roles in the bud activity-dormancy transition in tea plants.
Abstract
Gibberellin (GA) and abscisic acid (ABA) are fundamental phytohormones that extensively regulate plant growth and development, especially bud dormancy and sprouting transition in perennial plants. However, there is little information on GA- and ABA-related genes and their expression profiles during the activity-dormancy transition in tea plants. In the present study, 30 genes involved in the metabolism and signalling pathways of GA and ABA were first identified, and their expression patterns in different tissues were assessed. Further evaluation of the expression patterns of selected genes in response to GA3 and ABA application showed that CsGA3ox, CsGA20ox, CsGA2ox, CsZEP and CsNCED transcripts were differentially expressed after exogenous treatment. The expression profiles of the studied genes during winter dormancy and spring sprouting were investigated, and somewhat diverse expression patterns were found for GA- and ABA-related genes. This diversity was associated with the bud activity-dormancy cycle of tea plants. These results indicate that the genes involved in the metabolism and signalling of GA and ABA are important for regulating the bud activity-dormancy transition in tea plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To withstand unfavourable environmental factors such temperature stress, changes in day length and nutrient deficiency, perennial evergreen plants enter dormancy and later regrow under favourable conditions (Cooke et al. 2012; Horvath et al. 2003; Olsen 2010; Rohde and Bhalerao 2007; Tanino 2004; Tanino et al. 2010). Because this activity-dormancy cycle is important for plant survival and reproduction, the regulation of the mechanism underlying this cycle has gained much attention. Phytohormones, especially gibberellin (GA) and abscisic acid (ABA), have been demonstrated to play important roles in the activity-dormancy cycle (Cooke et al. 2012; Druart et al. 2007; Olsen 2010; Ruttink et al. 2007), but the expression profiles of GA- and ABA-related genes during the natural bud activity-dormancy cycle have not been elucidated. In general, GA and ABA play antagonistic roles in the regulation of plant growth and development. Increased ABA levels induce the onset of bud and seed dormancy, whereas increases in GA levels release buds and seeds from dormancy (Cooke et al. 2012; Druart et al. 2007; Zheng et al. 2015). The metabolism and signalling of GA and ABA are well characterized and have been broadly identified in many plant species (Du et al. 2015; Pearce et al. 2015; Xue et al. 2008; Wang et al. 2015). In addition, the response to GA and ABA is known to be controlled by several genes involved in GA and ABA metabolism and signal transduction; however, the expression profiles of these genes in perennial evergreen plants during winter dormancy are largely unknown.
The tea plant (Camellia sinensis (L.) O. Kuntze) is a perennial evergreen woody plant whose tender leaves and buds are processed as tea for drinking. The bud activity-dormancy conversion influences tea plant growth under stress, including high temperature and/or drought stress in the summer and cold and/or drought stress in the winter. Importantly, by controlling the tea bud flush time, the conversion also affects the economic output of tea products, especially during early spring tea production, as earlier-harvested tea usually costs relatively more than does late-harvested tea (Hao et al. 2017; Wang et al. 2014). The regulation of bud dormancy in tea plants is, therefore, a key aspect of tea plant research. To elucidate the mechanism underlying bud dormancy, dynamic changes in phytohormone levels (i.e., auxin, GA and ABA) and in the levels of phenols, polyamines, and reactive oxygen species have been investigated; the results suggest that all of these components are correlated with tea plant bud dormancy (Kakkar and Nagar 1997; Nagar and Kumar 2000; Nagar and Sood 2006; Vyas et al. 2007). Furthermore, a number of genes that are differentially expressed in dormant and active buds were also identified using subtractive hybridization methods and transcriptome analysis. The results showed that the expression patterns of certain genes, including CsGA20ox, are associated with the activity-dormancy transition (Krishnaraj et al. 2011; Paul et al. 2014; Paul and Kumar 2011; Thirugnanasambantham et al. 2013; Wang et al. 2014). Recently, we performed RNA-Seq analysis of buds at different dormancy stages and identified several regulatory pathways (Hao et al. 2017); the results of this analysis suggested that GA- and/or ABA-responsive pathways play critical roles in tea plant dormancy. GA and ABA were previously shown to influence bud dormancy in tea plants (Barua 1969). However, how GA- and ABA-related gene expression is regulated during the bud activity-dormancy cycle in the winter-spring season is unclear.
In the current study, 30 genes encoding key enzymes involved in the metabolism and signalling pathways of GA and ABA were identified. The evaluated genes exhibited tissue-specific expression patterns in different tea plant organs. In addition, they showed differential accumulation profiles in response to treatment of the plants with exogenous GA and ABA. Moreover, we systematically investigated the expression patterns of these genes during the winter–spring season from 2013 to 2015. The results provide a better understanding of the contributions of GA and ABA to the activity-dormancy cycle of tea plants.
Materials and methods
Plant materials and bud collection
In this study, 15-year-old ‘Longjing43’ tea plants cultivated in the natural tea garden of the Tea Research Institute, Chinese Academy of Agricultural Sciences (TRI, CAAS, N 30°18′, E120°10′), Hangzhou, China were used. The lateral buds were periodically sampled and imaged for analysis from November to March of 2013–14 and 2014–15. The collected buds were separated in triplicate as biological replicates and immediately frozen in liquid nitrogen, after which they were stored at − 80 °C until needed for RNA isolation. The daily temperature from October to March of 2013–14 and 2014–15 was recorded and analysed. For tissue-specific analyses, roots, mature leaves, stems, flowers and buds were collected from 3-year-old tea plants. The materials were frozen in liquid nitrogen and stored at − 80 °C until needed for RNA extraction.
GA3 and ABA treatment
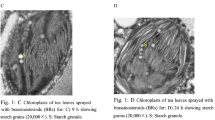
To investigate the effects of GA3 and ABA on the expression of GA- and ABA-related genes, tea plants were treated with various concentrations of GA3 and ABA for varying amounts of time. 4-year-old tea plants growing in the greenhouse under natural day length and a constant temperature of 25 ± 1 °C were selected for phytohormone treatment analysis. To examine the time course of the response to phytohormones, 100 μM GA3 and 100 μM ABA were sprayed onto the leaves of the tea plants at 9:30 a.m., and the 3rd and 4th leaves from the apical buds were collected after 0, 3, 6, 12 and 24 h. To initiate the concentration-dependent response analysis, 0, 50, 100, and 150 μM GA3 or ABA were sprayed onto the tea plant leaves, and the leaves were sampled 3 h later. The collected leaves were frozen in liquid nitrogen and stored at − 80 °C until needed for gene expression analysis.
Identification of GA- and ABA-related genes in tea plant
In the present study, the genes annotated as ent-kaurene synthase (KS), ent-kaurene oxidase (KO), ent-kaurene acid oxidase (KAO), gibberellin 20-oxidase (GA20ox), gibberellin 3-oxidase (GA3ox), gibberellin 2-oxidase (GA2ox), GA receptor (GID1) and GA repressor (DELLA) were identified from our previous transcriptomes and from data on the tea plant cultivar ‘Longjing43’ in the NCBI database. Moreover, the annotated unigenes that encode key enzymes involved in ABA metabolism (zeaxanthin epoxidase (ZEP), 9-cis-epoxycarotenoid dioxygenase (NCED), short-chain dehydrogenase/reductase (SDR), abscisic aldehyde oxidase (AAO) and CYP707A) and in signal transduction involving PP2C and PYL were also selected from the transcriptome of the ‘Longjing43’ cultivar. To verify the annotations of the sequences and query their putative full-length open reading frames (ORFs), the selected sequences were subjected to Basic Local Alignment Search Tool (BLAST)X searches in the NCBI database. For sequences not containing a complete ORF, rapid amplification of cDNA ends (RACE)-PCR was used to amplify the full-length sequences. The tested genes in this study were named according to the results of BLASTP searches.
In silico analysis of tea plant genes
Sequence identities were detected by performing clustal omega multiple alignment using EMBL-EBI. To analyse the phylogenetic tree of the tea plant and other plant amino acid sequences, the sequences were aligned using ClustalW, and trees were constructed by the neighbour-joining method and 1000 bootstraps using MEGA 5.0. The ProtParam tool (https://web.expasy.org/protparam/) was used to predict the molecular weights and theoretical pIs of the putative gene products.
Expression analyses of GA- and ABA-related genes
Total RNA was extracted from the tea plant samples according to the method of Yue et al. (2014). First-strand cDNA was synthesized using a PrimeScript™ RT reagent kit with the gDNA Eraser System (TAKARA Bio Inc., Dalian, China) according to the manufacturer’s protocol. For quantitative real-time PCR (qRT-PCR) detection, specific primers were designed, and the PTB gene was selected as a housekeeping gene due to its stable expression level in plants that received different treatments and in various plant tissues (Hao et al. 2014). The primers used in qRT-PCR are listed in Table A1. The qRT-PCR assays were performed using a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with SYBR Green reagents (TAKARA Bio Inc.) according to the product manual. 20-microlitre reaction mixtures that contained 1.0 μl of cDNA (equivalent to ∼ 50 ng of cDNA), 0.4 μl of each primer, 0.4 μl of ROX Reference Dye II and 10 μl of SYBR Premix Ex Taq II reagent were used to determine the gene transcription levels. The PCR conditions were as follows: initial denaturation at 95 °C for 30 s, 40 cycles of dissociation at 95 °C for 5 s, annealing and extension at 60 °C for 34 s, and termination by melting curve analysis as recommended by the manufacturer. All assays were performed with three biological replicates, each of which consisted of three technical replicates, and quantified using the 2−ΔΔCt or 2−ΔCt method as described by Livak and Schmittgen (2001).
Results
Tea plant bud dormancy and meteorological analysis during the winter–spring season
Daily recording of the temperature from October to March of 2013–15 indicated that the temperature showed similar fluctuation patterns in the three consecutive years. In October, the majority of the average daily temperatures were above 15 °C. The temperature gradually decreased beginning in late October, and it reached its lowest point during the period from late December to January (during this period, the majority of the daily average temperatures were below 5 °C). The temperature then slowly increased, and the average temperature was maintained at 15 °C (Fig. A1). The growth of tea plant buds was arrested, and the plants became dormant, as the temperature decreased from November to December; dormancy then persisted throughout the cold season. In late February, the dormancy of the tea buds was released, and the buds started to grow in March (Fig. A1). In general, the tea plant buds that reached the stage of ‘one bud and one leaf’ or the stage of ‘one bud and two leaves’ during mid- and late March of each year are plucked to produce tea in Hangzhou city. Hence, in this study, the sample points from November to March were classified into active/dormant stages as follows: November was defined as dormancy stage 1 (DS-1), December was defined as dormancy stage 2 (DS-2), January was defined as dormancy stage 3 (DS-3), February was defined as active stage 1 (AS-1), and two time points in March were defined as active stages 2 and 3 (AS-2 and AS-3), respectively.
Genes involved in GA metabolism and signalling
GA is successively biosynthesized from geranylgeranyl diphosphate (GGDP) by terpene synthases (TPSs), cytochrome P-450 monooxygenases (P450s), and 2-oxoglutarate-dependent dioxygenases (2ODDs) (Yamaguchi 2008). The candidate genes encoding the key enzymes in the GA biosynthetic pathway (CsKS, CsKO, CsKAO, CsGA20ox, and CsGA3ox), as well as the catabolism-related gene CsGA2ox in the tea plant, were selected from our annotated transcriptome database and from other databases. Nine genes encoding these key enzymes were identified, and the full-length cDNA sequences of these genes were cloned using RACE-PCR (Table 1). CsKS, a member of the TPS superfamily, was highly similar (71%) and closely related to CmKS (AEF32083) from Castanea mollissima (Table 1 and Fig. A2). CsKO and CsKAO, which belong to the p450 superfamily, shared high similarity (greater than 70%) with grape VvKO (AFD54196) and Sesamum indicum SiKAO (XP_011085042), respectively.
CsGA20oxs, CsGA3oxs and CsGA2oxs are members of the 2ODD protein superfamily. Two CsGA20ox genes shared 54.2% identity in amino acid sequence. They were separated into different clades, and their sequences were shown to be closely related to the sequences of these proteins from other Camellia plants, including Camellia lipogenesis and Camellia reticulata (Fig. A2-d). CsGA3ox-1 shared 79.8% amino acid identity with that of CsGA3ox-2, and both of these genes clustered into the same clade in the phylogenetic tree (Fig. A2-e). CsGA2ox-1 and CsGA2ox-2 had low similarity (48.1%) with each other but were classified into different groups (Fig.A1-f). All of the GA oxidase genes were regarded as members of the PcbC superfamily (Table 1).
In addition, three putative GID1 and four DELLA genes were isolated using the RACE-PCR method. Both CsGID1s belong to the abhydrolase superfamily (Ueguchi-Tanaka et al. 2005). The number of amino acid residues in CsGID1s ranges from 341 to 349. CsGID1a and CsGID1c were in the same cluster as AtGID1a (AT3G05120) and AtGID1c (AT5G27320), whereas CsGID1b and AtGID1b (AT3G63010) were classified into another clade (Fig. A2-g).
Four putative DELLA proteins contained a DELLA motif and a GRAS domain in the N-terminal and C-terminal regions, respectively, of their amino acid sequences. Both CsDELLA3 and CsDELLA4 were in the same clade as all five AtDELLAs, whereas CsDELLA1 and CsDELLA2 were grouped into another clade that contained other plant DELLAs (Fig. A2-h). All of these DELLA proteins possessed conserved DELLA, THYNP, VHID and RVER motifs, which are critical for binding GID1s (Murase et al. 2008).
Genes involved in ABA metabolism and signalling
ABA is first biosynthesized from zeaxanthin by ZEP and is then successively catalysed by NCED, SDR, and AAO. ABA can be inactivated by hydroxylation by ABA 8′-hydroxylase (CYP707A) (Finkelstein 2013; Wang et al. 2015). In the present study, three putative full-length sequences of CsZEP genes were identified in our annotated transcriptome database. CsZEP1 had a 2 004-bp ORF encoding 667 amino acid residues; this ORF was longer than those of CsZEP2 and CsZEP3 (Table 1). CsZEP1 and CsZEP3 were grouped into the same clade in the phylogenetic tree (Fig. A3-a).
Two chloroplast-like CsNCED genes, CsNCED1 and CsNCED2, were identified in tea plants. They encoded proteins of equivalent length and molecular weight (Table 1). Both were closely related to the AtNCEDs and were in the same clade; however, they were in different subgroups (Fig. A3-b).
CsSDR contained an 834-bp ORF encoding 277 amino acid residues (Table 1). It was closely related to the SDR of Citrus sinensis (NP_001275796) and Bixa orellana (AMJ39494) (Fig. A3-c). CsAAO had a 4 092-bp ORF (Table 1).
Five homologous genes of CsCYP707As were identified in the tea plant transcriptome database. The length of the proteins encoded by these genes ranges from 466 (CsCYP707A1) to 531 (CsCYP707A5) amino acid residues. All of the CsCYP707As genes belong to the P450 superfamily and encode proteins with relatively high isoelectric points (Table 1). These genes were separated into different clades in the phylogenetic tree (Fig. A3-e).
Two key components of ABA signalling, CsPYL and CsPP2C, were also selected and analysed in tea plants (Table 1).
Analysis of tissue-specific gene expression
The qRT-PCR approach was used to investigate the transcript abundance of the identified genes in the roots, stems, leaves, flowers and buds of tea plants to determine the tissue-specific expression levels of the individual genes. Although expression of all 30 genes was detected in all tissues, the transcript levels of individual genes varied widely among tissues (Fig. 1). In general, almost all of the studied genes were expressed at lower levels in stems than in roots, leaves, flowers and buds. Interestingly, the GA-related genes such as CsGA20ox-1/-2, CsGA3ox-1/-2, CsGID1a/b/c and CsDELLA1/2/3/4 showed preferential expression in flowers and/or buds, whereas CsKO, CsKAO, and CsGA3ox-2 were relatively highly expressed in roots. In contrast, the ABA-related genes such as CsZEP1/2/3, CsNCED1/2, and CsCYP707A1 were mainly expressed in both roots and leaves. The expression levels of both CsSDR and CsAAO were higher in leaves, flowers and buds than in stems. CsCYP707As, CsPP2C and CsPYL8 were also differentially expressed among the tested tissues.
Tissue-specific expression analysis of GA- and ABA-related genes. The transcript levels of each gene in the roots, leaves, stems, flowers and buds were determined using qRT-PCR. The results are presented as the average of three replicates and were calculated using the 2−ΔCt method; CsPTB served as a housekeeping gene. The average log2 values of three replicates were used to generate the heat map using R software. Green represents low expression, and red denotes high expression
Expression analysis of GA-related genes in response to GA3 treatment
The expression patterns of the GA-related genes in plants subjected to GA3 treatment were analysed. In plants treated with GA3 at various concentrations ranging from 50 to 150 μM, the expression levels of CsKAO, CsKO and CsKS were up-regulated. Under these conditions, CsGA20ox-2 expression was also induced. The key genes involved in the catabolism of bioactive GA (CsGA2ox-1/-2) were dramatically upregulated in response to GA3 treatment; however, the expression of CsGA3ox-1/-2 and CsGA20ox-1 was repressed. CsGID1b and CsGID1c were differentially expressed after treatment with GA3 at various concentrations, whereas CsGID1a expression was not affected. With the exception of the induction of CsDELLA3 expression, the expression of other CsDELLAs decreased in response to GA3 treatment.
Analysis of the time course of gene expression in response to 100 μM GA3 showed that the expression of CsKAO, CsKO, CsGA20ox-2, CsGA2ox-1/-2 and especially CsGA2ox-1 and CsGA3ox-1 was upregulated during the 24-h exposure of the plants to GA3 (Fig. 2b). The expression of CsKS did not change significantly during the 24-h GA3 treatment. However, the expression levels of CsDELLA1, CsDELLA2 and CsDELLA4 and of three CsGID1s were repressed during the 24-h treatment.
Analysis of the expression of GA-related genes in response to GA3 and ABA treatment. The expression patterns of GA-related genes in the leaves 3 h after treatment with 0, 50, 100, and 150 μM GA3 (a) and ABA (c) were analysed using qRT-PCR. In addition, the expression profiles of the tested genes during the 24-h treatment in response to 100 μM GA3 (b) and ABA (d) were determined using qRT-PCR. The results are presented as the average of three replicates and were calculated using the 2−ΔΔCt method; the heat map was generated using R software. CsPTB served as a housekeeping gene. Green represents low expression, and red denotes high expression
Expression analysis of GA-related genes in response to ABA treatment
On exposure of the plants to various concentrations of ABA, the expression of CsKS, CsKAO, and CsKO was significantly up-regulated (Fig. 2c). CsGA20ox-2 was induced by 100 and 150 μM ABA, whereas the expression level of CsGA20ox-1 was dramatically elevated by 100 μM ABA. CsGA3ox-1 was significantly upregulated by ABA treatment, whereas CsGA3ox-2 was considerably downregulated by 50 and 150 μM ABA (Fig. 2c). In contrast, high ABA levels (100 and 150 μM) significantly repressed CsGA2ox-2 transcription, whereas the expression level of CsGA2ox-1 increased in response to 50 and 150 μM ABA (Fig. 2c). CsGID1s were somewhat repressed by ABA; CsGID1b in particular exhibited significantly reduced expression. In contrast, with the exception of CsDELLA4, which exhibited no significant change in response to ABA treatment, the expression levels of CsDELLA1, CsDELLA2 and CsDELLA3 markedly increased after exposure of the plants to several ABA concentration conditions (Fig. 2c).
The expression of CsKO, CsKS and CsKAO was consistently upregulated during 24 h of treatment with 100 μM ABA (Fig. 2d). In addition, transcription of CsGA3ox-1, CsGA20ox-1 and CsGA20ox-2 was markedly induced during the 24-h treatment, especially in the samples collected at the 24-h time point. Nevertheless, the expression of CsGA2ox-1 and CsGA2ox-2 was clearly repressed as the treatment time progressed for 24 h. CsGA3ox-2 also exhibited reduced expression at the 6- and 12-h time points. During the 24-h ABA treatment, the expression of CsGID1s was notably repressed, especially after 12 h. Conversely, the majority of the CsDELLA genes were induced after short-term treatment (3–6 h), but their expression was suppressed after longer treatment (12–24 h).
Analysis of the expression of ABA-related genes in response to GA3 treatment
The majority of the genes involved in the ABA metabolic pathway, including CsAAO, CsSDR, CsZEP-1/-2/-3, CsCYP707A4, CsCYP707A5 were significantly repressed by 50 and/or 100 μM GA3 treatment after 3 h. Interestingly, the different expression patterns of CsCYP707A2 and CsCYP707A3 that were observed showed that CsCYP707A2 was markedly downregulated but CsCYP707A3 was markedly upregulated in response to various ABA concentrations. In contrast, both CsNCED1 and CsNCED2 were significantly upregulated by 50 and 150 μM GA3, whereas their expression did not change significantly when the plants were treated with 100 μM GA3. The expression of CsPP2C was induced and that of CsPYL8 was repressed in response to different GA3 concentrations, but these changes were not significant (Fig. 3a).
Analysis of the expression of ABA-related genes in response to GA3 and ABA treatment. The expression patterns of ABA-related genes in the leaves 3 h after treatment with 0, 50, 100, and 150 μM GA3 (a) and ABA (c) were analysed using qRT-PCR. In addition, the expression profiles of the tested genes during the 24-h treatment in response to 100 μM GA3 (b) and ABA (d) were determined using qRT-PCR. The results are presented as the average of three replicates and were calculated using the 2−ΔΔCt method; the heat map was generated using R software. CsPTB served as a housekeeping gene. Green represents low expression, and red denotes high expression
During 24-h treatment with 100 μM GA3, the expression of the majority of the studied genes, including CsZEPs, CsSDR, CsCYP707A2, CsCYP707A4, CsPP2C and CsPYL8, was repressed. In contrast, the transcript abundance of CsAAO, CsCYP707A1 and CsNCED2 increased to a maximum at the 12-h time point (Fig. 3b).
Analysis of the expression of ABA-related genes in response to ABA treatment
The expression of ABA-related genes in response to ABA treatment was also investigated. As shown in Fig. 3c, the CsAAO, CYP707A3, CYP707A5, CsPP2C, CsNCED1 and CsNCED2 genes were upregulated under different ABA concentrations. The transcript abundance of CsNCED1, CsNCED2 and CYP707A5 was dramatically induced by treatment with 50 and 150 μM ABA. In contrast, the transcription of CsSDR and CYP707A2 was significantly suppressed by ABA. The transcription of CsZEP1, CsZEP2, CsZEP3 and CsCYP707A4 did not change significantly in response to ABA treatment.
The expression levels of CsAAO, CsCYP707A1, CsCYP707A5, CsNCED2 and CsNCED1 were upregulated and persisted at significantly high levels during the 24-h treatment with 100 μM ABA (Fig. 3d). In contrast, the expression of CsSDR and CsCYP707A2 was repressed after 12 h of treatment, but several genes (CsPYL8, CsZEP1, CsZEP2, CsCYP707A4, and CsPP2C) exhibited low transcript abundance at the 12-h time point. The transcript abundance of CsZEP3 was not affected by ABA treatment in the 24-h time course assay (Fig. 3d).
Analysis of the expression of GA-related genes during the activity-dormancy cycle of the winter–spring season
Throughout the activity-dormancy cycle of 2013–14, the expression levels of genes that regulate the synthesis of bioactive GA, including CsKAO and CsKO, were dramatically downregulated during bud dormancy (from DS-1 to DS-3). When the buds began to be active (AS-1 to AS-3), these genes were significantly upregulated (Fig. 4a). Interestingly, the expression of CsKS decreased considerably at AS-3 (27 Mar 2014). An opposite transcription profile was observed for CsGA20oxs and CsGA3oxs. The genes of CsGA20ox-1 and CsGA3ox-2 were induced and their expression was maintained at high levels in the period of bud dormancy stages (DS-1 to DS-3); however, the expression levels of CsGA20ox-2 and CsGA3ox-1 were dramatically repressed and were low at these stages. Regarding the expression of bioactive GA catabolic genes, CsGA2ox-1 was considerably suppressed, but the expression of CsGA2ox-2 did not change significantly during DS-1 to DS-3; however, these genes displayed low levels of expression during AS-1 to AS-3 (Fig. 4a). Interestingly, the expression of three CsGID1s was downregulated, and the lowest level was recorded during AS-3 (27 Mar 2014). The expression of CsDELLAs gradually decreased during bud dormancy (DS-1 to DS-3) but then increased during bud active stages (AS-1 to AS-3) (Fig. 4a). With the exception of CsKAO, CsKO and CsDELLA1, the transcription of the studied genes was significantly repressed at bud sprouting (AS-3).
Expression patterns of GA-related genes in activity-dormancy during the winter–spring seasons of 2013–2014 (b) and 2014–2015 (c). A schematic of the GA metabolic pathway in higher plants is shown in (a). The dates of 2 Nov 2013, 1 Dec 2013 and 2 Jan 2014 were defined as bud dormancy stage 1 (DS-1), DS-2 and DS-3. 14 Feb 2014, 3 Mar 2014 and 27 Mar 2014 were designed as bud active stage 1 (AS-1), AS-2 and AS-3, respectively. Correspondingly, the sampling dates of 4 Nov 2014, 2 Dec 2014 and 5 Jan 2015 were expressed as DS-1, DS-2 and DS-3, and 5-Feb 2015, 3 Mar 2015 and 26 Mar 2015 were defined as AS-1, AS-2 and AS-3, respectively
As expected, the expression patterns of CsKAO, CsGA3ox-1/-2, CsGA20ox-1/-2, CsGA2ox-1/-2, CsGID1a/b/c and CsDELLA1/2/3/4 observed in 2014–15 were somewhat similar to the patterns observed in 2013–14 (Fig. 4b). Nevertheless, the expression levels of CsGA20ox-2, CsGID1a/b/c, CsDELLA1/2 increased during the bud dormancy stages (DS-1–DS-3) (Fig. 4b).
Analysis of the expression of ABA-related genes in the activity-dormancy cycle of the winter–spring season
The expression of ABA-related genes in tea plant lateral buds exhibited two different patterns to some extent based on the expression profiles in both 2013–14 and 2014–15. The expression of the majority of genes, including CsNCED2, CsZEP1/2/3, CsCYP707A-2/3/4/5, CsPP2C and CsPYL8, increased during the bud dormancy period (DS-1 to DS-3). During the bud active stages (AS-1–AS-3), the transcript abundance of these genes decreased (Fig. 5a, b). In contrast, the expression of CsSDR and CsAAO was repressed during the bud dormancy-active stages in both two-year cycles. Different expression profiles of CsNCED1 and CsCYP707A-1 during the bud dormancy-active cycle were observed in 2013–14 and 2014–15 (Fig. 5a, b).
Expression patterns of ABA-related genes in activity-dormancy during the winter–spring seasons of 2013–2014 (a) and 2014–2015 (b). The dates of 2 Nov 2013, 1 Dec 2013 and 2 Jar 2014 were defined as bud dormancy stage 1 (DS-1), DS-2 and DS-3. 14 Feb 2014, 3 Mar 2014 and 27 Mar 2014 were designed as bud active stage 1 (AS-1), AS-2 and AS-3, respectively. Correspondingly, the sampling dates of 4 Nov 2014, 2 Dec 2014 and 5 Jan 2015 were expressed as DS-1, DS-2 and DS-3, and 5 Feb 2015, 3 and 26 Mar 2015 were defined as AS-1, AS-2 and AS-3, respectively
Discussion
GA and ABA are broadly involved in plant growth and development, including shoot growth, flowering, and seed and bud dormancy. Numerous studies based on physiological and biochemical detection and molecular biology have shown that bud dormancy-active cycles are controlled by GA and ABA in many plants. In tea plants, GA and/or ABA were shown to participate in bud dormancy using content detection and transcriptome analyses (Hao et al. 2017; Paul et al. 2014). To explore how these two phytohormones regulate tea bud dormancy, the genetic information and detailed expression patterns of GA- and ABA-related genes during the dormancy-active cycle of the winter–spring season should be preferentially taken into account.
Genes involved in the metabolism and signalling of GA and ABA in tea plants
In this study, 30 genes involved in the metabolism and signalling pathways of GA and ABA were identified in tea plants (Table 1). Sixteen of the identified genes were predicted to encode key regulatory enzymes involved in GA biosynthesis (CsKS, CsKO, CsKAO, CsGA20oxs, and CsGA3oxs) and deactivation (CsGA2oxs), GA receptors (GID1s) and major signal repressors of DELLAs (Ueguchi-Tanaka et al. 2007; Yamaguchi 2008). Fourteen of the genes encoded enzymes in the ABA metabolism pathway, including CsZEPs, CsNCEDs, CsSDR, CsAAO and CsCYP707As and the ABA receptor complex of CsPP2C and CsPYL8.
Among these genes, KS, KO and SDR might be transcribed from a single gene in tea plants because no additional sequences were found in the transcriptome data in the NCBI database. Similarly, it was shown that only one KS and one KO gene are present in Arabidopsis and that only one KAO gene is present in maize (Song et al. 2011). It is suggested that these single genes not only play critical roles in GA and ABA metabolism but that they have been conserved during the evolution of GA and ABA in plants. In contrast, the majority of proteins involved in GA and ABA metabolism and signalling are encoded by multigene families. For instance, there are five GA20oxs and NCEDs genes in Arabidopsis, indicating that the genetic regulation of the downstream pathway of GA and ABA metabolism might be more complicated than that of the upstream pathway. Considering its large genome size (3.02 Gb, 25 times larger than the genome of Arabidopsis), it is likely that additional genes could be isolated from the tea plant. In addition, the proteins involved in GA and ABA signalling are mainly encoded by multigene families. In Arabidopsis, there are 3 GID1s, 5 DELLAs, and 80 PP2Cs (Wang et al. 2011; Xu et al. 2008), suggesting that these genes have redundant roles and partially specialized functions. For instance, RGA and GAI may act synergistically to repress GA-mediated internode elongation, abaxial trichome initiation and leaf expansion, while both RGL1 and RGL2 participate in seed germination regulation (Wang et al. 2011). Many of the GA- and ABA-related genes of tea plants have not previously been identified. The tea plant genome has recently been reported (Xia et al. 2017). However, we searched this genome data and discovered that many genes do not contain a full-length coding sequence. Since both GA and ABA have fundamental functions in various growth and developmental processes and stress responses, the key genes and gene family members, including CsGA20oxs, CsGA3oxs, CsNCEDs and CsPP2Cs, should be identified and subjected to systematic investigation.
Differential expression patterns of target genes in multiple organs
In this study, the expression profiles of target genes in roots, leaves, stems, flowers and buds were evaluated. The genes expressed in all five organs indicate that GA and ABA are needed to ensure the vegetative growth and reproductive development of tea plants. Many genes, especially GA-related genes, were preferentially expressed in flowers and buds, whereas several genes were expressed at low levels in roots and stems (Fig. 1). The growth and development of plants, especially in actively dividing and elongating tissues, are regulated by GA. Phytohormone responses facilitate the majority of the physiological and biochemical reactions that occur in growing tissues such as flowers and buds to keep the cell rapidly dividing and elongating (Carles and Fletcher 2003; Regnault et al. 2014). In addition, bioactive GA are primarily produced at the site of their action (Kaneko et al. 2003; Yamaguchi 2008), resulting in higher transcript abundance of the majority of GA-related genes in elongating and dividing tissues. In addition, the expression of GA-related genes, especially GA20oxs, GA3oxs and GA2oxs, exhibits tissue-specific patterns within a single tissue and at specific developmental stages (Pearce et al. 2015; Roumeliotis et al. 2013; Ye et al. 2015), indicating that flowering and bud development in tea plants is also controlled by GA.
Interestingly, the majority of ABA-related genes were generally highly expressed in roots and leaves, whereas a few genes, such as CsSDR, CsAAO and CsCYP707A-5, were highly expressed in flowers and/or buds (Fig. 1). ABA is synthesized predominantly in the vascular parenchyma cells of leaves and root tips and is then translocated to its site of action in plants (Antoni et al. 2011; Boursiac et al. 2013; Merilo et al. 2015; Umezawa et al. 2010). This was confirmed by our results based on the tissue-specific expression patterns of the key genes associated with ABA metabolism in tea plants. ABA is an essential signalling molecule under stress conditions and regulates stomatal opening and closure to control transpirational water loss as a defence against various stress stimuli (Bomke et al. 2008; Huerta et al. 2009; Xiao et al. 2010), and leaves and roots are major organs for these defence mechanisms. Tan et al. (2003) reported that five AtNCEDs localized to different organs, including roots, flowers and seeds, in Arabidopsis and suggested that the developmental control of ABA synthesis involves localized patterns of NCED gene expression. Therefore, the difference between the tissue-specific expression profiles of GA- and ABA-related genes indicates that GA and ABA play distinct roles in the regulation of tea plant growth and development. Although they belong to the same gene family, these genes were differentially expressed. For instance, CsGA20ox-1 and CsGA20ox-2 were predominantly expressed in buds and flowers, respectively, whereas both CsDELLA-3 and CsDELLA-4 displayed high transcription levels in flowers and buds. These differences suggest that single genes in the same gene family might play distinct physiological roles and/or have redundant functions in the same tissues or organs.
Feedback regulation of target genes in response to GA3 and ABA treatment
The biosynthesis of bioactive GA is feedback-regulated by exogenous GA3 treatment mainly due to the control of the expression of 2ODD-type genes. For instance, after GA3 treatment, certain GA20oxs and GA3oxs genes were repressed (also known as negative feedback regulation), and some GA2oxs genes were induced (also known as positive forward regulation) (Carrera et al. 1999; Du et al. 2015; Griffiths et al. 2006; Mitchum et al. 2006; Thomas et al. 1999). The expression of CsGA3ox-2 and CsGA20ox-1 was consistently downregulated, whereas CsGA2ox-1 and CsGA2ox-2 expressions were up-regulated at specific GA3 concentrations and during the 24-h treatment (Fig. 2a, b). However, both CsGA3ox-1 and CsGA20ox-2 exhibited increased transcription in leaves in response to GA3, which suggested that the feedback regulation of certain genes involved in GA metabolism might vary by gene family member (Carrera et al. 1999; Du et al. 2015; Yamaguchi et al. 2001). In contrast to CsGA20oxs, CsGA3oxs and CsGA2oxs, all CsKO, CsKAO and CsKS genes were upregulated by GA3 treatment, indicating that these types of genes might not be controlled by feedback regulation, as was previously reported in wheat (Huang et al. 2012) and Salvia miltiorrhiza (Du et al. 2015). These results also reflect the complexity of the underlying mechanism of GA biosynthesis in plants. Hence, exogenous GA3 treatment could compensate for the effect of bioactive GA by upregulation of the expression levels of CsGA3oxs and CsGA20oxs and by downregulation of CsGA2oxs. In contrast, aside from the increased expression of CsDELLA3 after GA3 treatment, both CsGID1s and the remaining CsDELLA genes were downregulated, similar to previously reported results (Gao et al. 2012; Griffiths et al. 2006; Liu et al. 2016; Shen et al. 2015; Yano et al. 2015). The difference in the gene expression patterns suggested that different members of the GID1s and DELLAs gene families fulfil distinct roles in the plant developmental process (Gallego-Giraldo et al. 2014; Voegele et al. 2011). In addition, GA signalling might also be feedback-regulated by exogenous GA3 (Griffiths et al. 2006; Li et al. 2013; Zentella et al. 2007). GA promote plant growth by inducing the degradation of the growth-repressing DELLA proteins; DELLAs both upregulate GA synthesis genes and downregulate GA deactivation (GA2oxs) genes (O’Neill et al. 2010; Zentella et al. 2007). DELLA proteins are transcription factors that regulate the expression of numerous genes during plant growth and development (de Lucas et al. 2008). Ravindran et al. (2017) showed that increased GA levels cause degradation of RGL2, a DELLA protein, repressing GATA12 expression and thereby releasing dormancy. Furthermore, with respect to GA signalling, decreases in the transcript abundance of GID1s and DELLAs repress the formation of the GA-GID1-DELLA complex.
ABA modulates GA biosynthesis and responses by controlling the expression of genes involved in the GA biosynthesis pathway and signal transduction. We found that the majority of bioactive GA synthesis-related genes were upregulated upon ABA treatment. For instance, the expression of CsGA20ox-1, CsGA20ox-1, and CsGA3ox-1, and especially of CsGA20ox-1, was significantly induced. ABA also upregulated CsKS, CsKAO and CsKO transcription but repressed the expression of CsGA2oxs genes (Fig. 2c, d). Similar results have been widely observed in other plants. However, GA and ABA are mutually antagonistic. Transcriptome analysis results show that ABA treatment inhibits GA-related genes (Li et al. 2015; Yang et al. 2014). Moreover, overexpression of some ABA-related genes (e.g., ABI and ABF) lowered the transcript abundance of GA metabolism genes (Muniz Garcia et al. 2014; Shu et al. 2013, 2016). In addition, both CsGID1s were downregulated under ABA conditions, suggesting that ABA also inhibits the GA response partly by suppressing the transcript abundance of GA receptors, whereas promotion and/or inhibition of CsDELLAs were observed in response to ABA treatment. It has recently been demonstrated that DELLA acts as a hub that integrates multiple signal responses to regulate plant growth and development by linking the cross-talk between GA, ABA, ethylene and environmental stimuli (Achard et al. 2003; Golldack et al. 2013; Jiang and Fu 2007). The cross-talk functions of DELLA between GA and ABA suggest that the expression of various CsDELLAs plays a distinct role in GA and/or ABA signalling.
In contrast to the effects of ABA on the expression of GA-related genes, most of the genes involved in the ABA synthesis pathway were repressed by GA3 treatment (Fig. 3a, b). Surprisingly, CsNCEDs were upregulated by both low (50 μM) and high (150 μM) concentrations of GA3; however, the opposite expression pattern was observed in response to moderate GA3 concentrations during the 24-h treatment, suggesting that the regulation of transcription of GA- and ABA-related genes depends not only on the concentration of GA3 but also on the duration of treatment. NCEDs catalyse the key step of ABA biosynthesis, which results in enhanced ABA action. In Arabidopsis, the induction or mutation of AtNCED genes controls endogenous ABA accumulation, which in turn influences GA-dependent seed germination (Lefebvre et al. 2006; Martinez-Andujar et al. 2011; Seo et al. 2016), suggesting that the antagonism between GA3 and ABA is partially based on the fact that each transcriptionally regulates the biosynthetic pathway of the other. However, endogenous ABA levels are controlled by multiple factors. Whether or not GA3 treatment affects the metabolism of ABA requires further investigation.
ABA controls the expression of numerous genes involved in its metabolic and signal transduction pathways. In this study, we observed that several genes, including CsSDR, CsZEP1, CsZEP2, CsCYP707A2, CsPP2C and CsPYL8, displayed downregulated expression profiles in response to different ABA concentrations and/or during the 24-h treatment period, whereas CsAAO, CsCYP707A1, CsCYP707A5, and CsNCED2 showed increased transcript abundance (Fig. 3c, d). This diversity of expression patterns has been previously reported (Lou et al. 2017; Saito et al. 2004; Wang et al. 2009; Zhang et al. 2016). It is largely due to the fact that each enzyme is encoded by multiple genes, and one type of gene may even have distinct roles in different tissues/organs (Lefebvre et al. 2006; Nonogaki et al. 2014; Schwarz et al. 2015; Tan et al. 2003). Interestingly, we found that CsNCED1 and CsNCED2 had similar expression patterns; however, the inconsistencies between these patterns in response to some ABA treatments suggested that these genes might play different roles in the ABA response. ABA can amplify ABA biosynthesis and signalling via a positive feedback mechanism that is mainly mediated by NCED expression (Nonogaki and Nonogaki 2017; Nonogaki et al. 2014). Our results showed that exogenous ABA treatment induces CsNCED expression. Although the content of endogenous ABA was not measured, we infer that CsNCED2 plays an important role in ABA-positive feedback regulation based on the upregulation of CsNCED gene expression.
Expression of GA- and ABA-related genes is correlated with the activity-dormancy transition of the winter–spring season
The antagonism of GA and ABA modulates the bud activity-dormancy cycle. In general, increasing ABA and decreasing GA induce the onset of dormancy; the opposite conditions facilitate the breaking of dormancy (Cooke et al. 2012; Horvath et al. 2003; Meier et al. 2012; Paul and Kumar 2011; Rohde and Bhalerao 2007; Wang et al. 2015). Similar changing trends in ABA and GA expression have been detected in dormant buds during the winter–spring season in different species of tea plants (Nagar and Kumar 2000). In the present study, we investigated the expression patterns of GA- and ABA-related genes during winter bud activity-dormancy changes in two consecutive years. We hypothesized that the expression profiles of GA- and ABA-related genes would be upregulated and/or downregulated in a manner consistent with the plant’s activity-dormancy cycles.
Compared with the expression patterns of GA-related genes, the expression patterns of ABA-related genes were highly correlated with the activity-dormancy cycle, suggesting that ABA might be more closely related to bud dormancy than GA (Khalil-Ur-Rehman et al. 2017; Li et al. 2003). Additional studies have recently shown that ABA controls bud activity-dormancy by modifying metabolic processes. The expression patterns of several genes, including NCEDs and CYP707As, in various species such as pear, grape and peach are similar to those in tea plants (Parada et al. 2016; Tuan et al. 2017; Vergara et al. 2017; Wang et al. 2015; Zheng et al. 2015). Exogenous application of ABA promotes bud dormancy and inhibits its release, whereas overexpression of NCED genes contributes to ABA accumulation and inhibits seed germination (Lefebvre et al. 2006; Martinez-Andujar et al. 2011; Seo et al. 2016; Wang et al. 2009). Based on the results, we obtained by studying tea plant gene expression in plants treated with ABA and GA3, we conclude that ABA accumulation may be predominantly controlled by the ABA synthesis gene CsNCED2 and the catabolic genes CsCYP707As (Fig. 5). Upregulation of CsNCED2 from November (DS-1) to the following January (DS-3) might contribute to the accumulation of ABA to high levels and promote the ABA response. Although this process does not trigger bud dormancy initiation and maintain the dormant state, it may be beneficial for withstanding cold temperature stress in winter.
In addition to the critical role played by GA in seed dormancy, GA also modulate bud dormancy by integrating metabolic processes and signal transduction (Cooke et al. 2012). The content of GA3 decreases during the dormancy period but increases dramatically at the bud flush stage, and exogenously applied GA3 can promote dormancy release during tea production (Barua 1969; Nagar and Kumar 2000). Exogenous application of bioactive GA promotes bud dormancy release by mediating changes in energy metabolism, protein metabolism, cell structure, cell division, and signalling and transcription pathways (Hansen et al. 1999; Zhuang et al. 2013). We found that the majority of GA-related genes were differentially expressed during the winter dormancy period and that the expression of these genes was maintained at a low level during the dormancy release period (Fig. 4), similar to the results of the transcriptome analysis by Hao et al. (2017). Similarly, Barros et al. (2012) reported that flower bud break in almond is accompanied by decreased expression of PdGA20ox under natural conditions, suggesting that repressed transcription of GA20ox plays an important role in bud break. However, it has been reported that increases in GA20oxs and GA30xs expression are correlated with bud burst (Choubane et al. 2012). These inconsistent results might be due to interspecies differences. The tea plant is an adeciduous plant, whereas species such as pear, peach and poplar are deciduous. Tea plant bud dormancy is strongly influenced by many environmental factors, and the regulation of bud dormancy among these plants differs (Hao et al. 2017).
Interestingly, Nagar and Kumar (2000) reported that the content of free GA decreased significantly during bud dormancy and that the content of conjugated GA dramatically increased; however, in the bud break stage, the content of free GA markedly increased, but conjugated GA markedly decreased. These results suggest that a reversal in the levels of conjugated and free GA plays an essential role in tea plant bud break. Moreover, phytohormones, especially GA, are closely related to the release of tea bud dormancy (Barua 1969; Jeyaraj et al. 2014; Thirugnanasambantham et al. 2013; Wang et al. 2014). Based on our analysis of the expression patterns of GA-related genes in the present study (Fig. 5), we hypothesized that the accumulation of GA that causes the dormancy break of tea plant buds might ultimately originate from GA biosynthesis but that dormancy break is mainly due to the release of conjugated GA within the tea plant. ABA can also be inactivated by conjugation to another molecule, such as by esterification of ABA to ABA-glucose ester, for storage or transport in plants (Finkelstein 2013). Moreover, we postulate that the dynamic changes in ABA during the activity-dormancy cycle mainly result from the biosynthesis and catabolic regulation of ABA. Finally, a potential model of bud active/dormancy controlled by GA and ABA is proposed (Fig. 6). The activity-dormancy transition primarily depends on the balance in the fluctuations of GA and ABA levels resulting from the differential expression of their related genes.
Potential model of bud activity/dormancy controlled by GA and ABA balance. The bud activity-dormancy transition of tea plant during winter–spring season (red) was controlled by a series of environment factors such as the temperature fluctuations (orange). In this shift, GA (blue)- and ABA (purple)-related genes were differentially expressed that results in the alterations of metabolism and siganalling of GA and ABA, thereby regulating the bud dormancy. The expression patterns of GA- and ABA-related genes identified in this study were summarized. Red and green arrows indicated the upregulated expression and downregulated expression, respectively
Conclusion
Although numerous studies have demonstrated that GA and ABA play crucial roles in the regulation of plant growth and developmental, the genes involved in their metabolism and signalling pathways, as well as their expression patterns, are unclear. In the current study, 30 genes involved in the metabolism and signalling pathways of GA and ABA were first cloned and characterized in tea plants. Tissue-specific expression revealed that GA-related genes were somewhat predominantly expressed in the flowers and tea buds, whereas high transcript levels of ABA-related genes were present in the roots and leaves. Under GA3 and ABA treatments, the expression levels of CsKS, CsKAO, CsKO, CsGA20ox-2, and CsNECD2 were up-regulated, whereas the expression levels of CsGID1b, CsSDR, CsPYL8, and CsCYP707A2 were repressed. The changes in CsGA20oxs, CsGA3oxs, CsGA2oxs and CsNCEDs showed feedback regulation in response to GA3 and ABA. Analysis of gene expression during the active growth-dormancy-sprouting transitions showed that some genes, including CsKAO, CsKO, CsGA20oxs, CsGA3oxs, CsGA2oxs, CsDELLAs, CsZEPs, CsNCEDs, CsCYP707As, CsPP2C and CsPYL8, were closely related to tea plant bud dormancy, indicating that bud dormancy in tea plants is regulated by GA and ABA. In summary, the important roles of GA and ABA signalling in the activity-dormancy cycles of tea plants were elucidated, and several genes involved in the regulation of bud dormancy were identified. These genes warrant further investigation.
Author contribution statement
XW and YY conceived and designed the experiments. CY, HC, XH, JZ, WQ, YG and NY performed the experiments and analysed the data. CY and HC wrote the paper. XW and YY revised and approved the final manuscript. All authors reviewed the manuscript.
References
Achard P, Vriezen WH, Van Der Straeten D, Harberd NP (2003) Ethylene regulates Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell 15:2816–2825
Antoni R, Rodriguez L, Gonzalez-Guzman M, Pizzio GA, Rodriguez PL (2011) News on ABA transport, protein degradation, and ABFs/WRKYs in ABA signaling. Curr Opin Plant Biol 14:547–553
Barros PM, Goncalves N, Saibo NJ, Oliveira MM (2012) Cold acclimation and floral development in almond bud break: insights into the regulatory pathways. J Exp Bot 63:4585–4596
Barua DN (1969) Seasonal dormancy in tea (Camellia sinensis L.). Nature 224:514–514
Bomke C, Rojas MC, Gong F, Hedden P, Tudzynski B (2008) Isolation and characterization of the gibberellin biosynthetic gene cluster in Sphaceloma manihoticola. Appl Environ Microbiol 74:5325–5339
Boursiac Y, Leran S, Corratge-Faillie C, Gojon A, Krouk G, Lacombe B (2013) ABA transport and transporters. Trends Plant Sci 18:325–333
Carles CC, Fletcher JC (2003) Shoot apical meristem maintenance: the art of a dynamic balance. Trends plant Sci 8:394–401
Carrera E, Jackson SD, Prat S (1999) Feedback control and diurnal regulation of gibberellin 20-oxidase transcript levels in potato. Plant Physiol 119:765–774
Choubane et al (2012) Photocontrol of bud burst involves gibberellin biosynthesis in Rosa sp. J Plant Physiol 13:1271–1280
Cooke JE, Eriksson ME, Junttila O (2012) The dynamic nature of bud dormancy in trees: environmental control and molecular mechanisms. Plant Cell Environ 35:1707–1728
de Lucas M et al (2008) A molecular framework for light and gibberellin control of cell elongation. Nature 451:480–484
Druart N et al (2007) Environmental and hormonal regulation of the activity-dormancy cycle in the cambial meristem involves stage-specific modulation of transcriptional and metabolic networks. Plant J 50:557–573
Du Q, Li C, Li D, Lu S (2015) Genome-wide analysis, molecular cloning and expression profiling reveal tissue-specifically expressed, feedback-regulated, stress-responsive and alternatively spliced novel genes involved in gibberellin metabolism in Salvia miltiorrhiza. BMC Genom 16:1087
Finkelstein R (2013) Abscisic acid synthesis and response. Arabidopsis book 11:e0166
Gallego-Giraldo C, Hu J, Urbez C, Gomez MD, Sun TP, Perez-Amador MA (2014) Role of the gibberellin receptors GID1 during fruit-set in Arabidopsis. Plant J 79:1020–1032
Gao Y, Chen J, Zhao Y, Li T, Wang M (2012) Molecular cloning and expression analysis of a RGA-like gene responsive to plant hormones in Brassica napus. Mol Biol Rep 39:1957–1962
Golldack D, Li C, Mohan H, Probst N (2013) Gibberellins and abscisic acid signal crosstalk: living and developing under unfavorable conditions. Plant Cell Rep 32:1007–1016
Griffiths J et al (2006) Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18:3399–3414
Hansen E, Olsen JE, Junttila O (1999) Gibberellins and subapical cell divisions in relation to bud set and bud break in Salix pentandra. J Plant Growth Regul 18:167–170
Hao X, Horvath D, Chao W, Yang Y, Wang X, Xiao B (2014) Identification and evaluation of reliable reference genes for quantitative real-time PCR analysis in tea plant (Camellia sinensis (L.) O. Kuntze). Int J Mol Sci 15:22155–22172
Hao X, Yang Y, Yue C, Wang L, Horvath DP, Wang X (2017) Comprehensive transcriptome analyses reveal differential gene expression profiles of Camellia sinensis axillary buds at para-, endo-, ecodormancy, and bud flush stages. Front Plant Sci 8:553
Horvath DP, Anderson JV, Chao WS, Foley ME (2003) Knowing when to grow: signals regulating bud dormancy. Trends Plant Sci 8:534–540
Huang Y, Yang W, Pei Z, Guo X, Liu D, Sun J, Zhang A (2012) The genes for gibberellin biosynthesis in wheat. Funct Integr Genom 12:199–206
Huerta L, Garcia-Lor A, Garcia-Martinez JL (2009) Characterization of gibberellin 20-oxidases in the citrus hybrid Carrizo citrange. Tree Physiol 29:569–577
Jeyaraj A, Chandran V, Gajjeraman P (2014) Differential expression of microRNAs in dormant bud of tea [Camellia sinensis (L.) O. Kuntze]. Plant Cell Rep 33:1053–1069
Jiang C, Fu X (2007) GA action: turning on de-DELLA repressing signaling. Curr Opin Plant Biol 10:461–465
Kakkar RK, Nagar PK (1997) Distribution and changes in endogenous polyamines during winter dormancy in tea [Camellia sinensis L. (O) Kuntze]. J Plant Physiol 151:63–67
Kaneko M, Itoh H, Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Ashikari M, Matsuoka M (2003) Where do gibberellin biosynthesis and gibberellin signaling occur in rice plants? Plant J 35:104–115
Khalil-Ur-Rehman M, Sun L, Li CX, Faheem M, Wang W, Tao JM (2017) Comparative RNA-seq based transcriptomic analysis of bud dormancy in grape. BMC Plant Biol 17:18
Krishnaraj T, Gajjeraman P, Palanisamy S, Subhas Chandrabose SR, Azad Mandal AK (2011) Identification of differentially expressed genes in dormant (banjhi) bud of tea (Camellia sinensis (L.) O. Kuntze) using subtractive hybridization approach. Plant Physiol Biochem 49:565–571
Lefebvre V et al (2006) Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J 45:309–319
Li C, Junttila O, Heino P, Palva ET (2003) Different responses of northern and southern ecotypes of Betula pendula to exogenous ABA application. Tree Physiol 23:481–487
Li A, Yang W, Li S, Liu D, Guo X, Sun J, Zhang A (2013) Molecular characterization of three GIBBERELLIN-INSENSITIVE DWARF1 homologous genes in hexaploid wheat. J Plant Physiol 170:432–443
Li L et al (2015) Transcriptomic insights into antagonistic effects of gibberellin and abscisic acid on petal growth in Gerbera hybrida. Front Plant Sci 6:168
Liu B et al (2016) Silencing of the gibberellin receptor homolog, CsGID1a, affects locule formation in cucumber (Cucumis sativus) fruit. New Phytol 210:551–563
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 – ∆∆CT method. Methods 25:402–408
Lou Y, Sun H, Li L, Zhao H, Gao Z (2017) Characterization and primary functional analysis of a Bamboo ZEP Gene from Phyllostachys edulis. DNA Cell Biol. https://doi.org/10.1089/dna.2017.3705
Martinez-Andujar C, Ordiz MI, Huang Z, Nonogaki M, Beachy RN, Nonogaki H (2011) Induction of 9-cis-epoxycarotenoid dioxygenase in Arabidopsis thaliana seeds enhances seed dormancy. PNAS 108:17225–17229
Meier AR, Saunders MR, Michler CH (2012) Epicormic buds in trees: a review of bud establishment, development and dormancy release. Tree Physiol 32:565–584
Merilo E, Jalakas P, Laanemets K, Mohammadi O, Horak H, Kollist H, Brosche M (2015) Abscisic acid transport and homeostasis in the context of stomatal regulation. Mol Plant 8:1321–1333
Mitchum MG et al (2006) Distinct and overlapping roles of two gibberellin 3-oxidases in Arabidopsis development. Plant J 45:804–818
Muniz Garcia MN, Stritzler M, Capiati DA (2014) Heterologous expression of Arabidopsis ABF4 gene in potato enhances tuberization through ABA-GA crosstalk regulation. Planta 239:615–631
Murase K, Hirano Y, Sun TP, Hakoshima T (2008) Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature 456:459–463
Nagar PK, Kumar A (2000) Changes in endogenous gibberellin activity during winter dormancy in tea (Camellia sinensis (L.) O. Kuntze). Acta Physiol Plant 22:439–443
Nagar PK, Sood S (2006) Changes in endogenous auxins during winter dormancy in tea (Camellia sinensis L.) O. Kuntze. Acta Physiol Plant 28:165–169
Nonogaki M, Nonogaki H (2017) Prevention of preharvest sprouting through hormone engineering and germination recovery by chemical biology. Front Plant Sci 8:90
Nonogaki M, Sall K, Nambara E, Nonogaki H (2014) Amplification of ABA biosynthesis and signaling through a positive feedback mechanism in seeds. Plant J 78:527–539
O’Neill DP et al (2010) Regulation of the gibberellin pathway by auxin and DELLA proteins. Planta 232:1141–1149
Olsen JE (2010) Light and temperature sensing and signaling in induction of bud dormancy in woody plants. Plant Mol Biol 73:37–47
Parada F, Noriega X, Dantas D, Bressan-Smith R, Perez FJ (2016) Differences in respiration between dormant and non-dormant buds suggest the involvement of ABA in the development of endodormancy in grapevines. J Plant Physiol 201:71–78
Paul A, Kumar S (2011) Responses to winter dormancy, temperature, and plant hormones share gene networks. Funct Integr Genom 11:659–664
Paul A, Jha A, Bhardwaj S, Singh S, Shankar R, Kumar S (2014) RNA-seq-mediated transcriptome analysis of actively growing and winter dormant shoots identifies non-deciduous habit of evergreen tree tea during winters. Sci Rep 4:5932
Pearce S et al (2015) Heterologous expression and transcript analysis of gibberellin biosynthetic genes of grasses reveals novel functionality in the GA3ox family. BMC Plant Biol 15:130
Ravindran P, Verma V, Stamm P, Kumar P (2017) A novel RGL2–DOF6 complex contributes to primary seed dormancy in Arabidopsis thaliana by regulating a GATA transcription factor. Mol Plant 10:1307–1320
Regnault T, Daviere JM, Heintz D, Lange T, Achard P (2014) The gibberellin biosynthetic genes AtKAO1 and AtKAO2 have overlapping roles throughout Arabidopsis development. Plant J 80:462–474
Rohde A, Bhalerao RP (2007) Plant dormancy in the perennial context. Trends Plant Sci 12:217–223
Roumeliotis E, Kloosterman B, Oortwijn M, Lange T, Visser RG, Bachem CW (2013) Down regulation of StGA3ox genes in potato results in altered GA content and affect plant and tuber growth characteristics. J Plant Physiol 170:1228–1234
Ruttink T et al (2007) A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell 19:2370–2390
Saito S, Hirai N, Matsumoto C, Ohigashi H, Ohta D, Sakata K, Mizutani M (2004) Arabidopsis CYP707As encode (+)-abscisic acid 8′-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiol 134:1439–1449
Schwarz N, Armbruster U, Iven T, Bruckle L, Melzer M, Feussner I, Jahns P (2015) Tissue-specific accumulation and regulation of zeaxanthin epoxidase in Arabidopsis reflect the multiple functions of the enzyme in plastids. Plant Cell Physiol 56:346–357
Seo M, Kanno Y, Frey A, North HM, Marion-Poll A (2016) Dissection of Arabidopsis NCED9 promoter regulatory regions reveals a role for ABA synthesized in embryos in the regulation of GA-dependent seed germination. Plant Sci 246:91–97
Shen Q, Cui J, Fu XQ, Yan TX, Tang KX (2015) Cloning and characterization of DELLA genes in Artemisia annua. Genet Mol Res 14:10037–10049
Shu K et al (2013) ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in arabidopsis. PLoS Genet 9:e1003577
Shu K et al (2016) ABI4 mediates antagonistic effects of abscisic acid and gibberellins at transcript and protein levels. Plant J 85:348–361
Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty DR (2003) Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J 35:44–56
Tanino KK (2004) Hormones and endodormancy induction in woody plants. J Crop Improv 10:157–199
Tanino KK, Kalcsits L, Silim S, Kendall E, Gray GR (2010) Temperature-driven plasticity in growth cessation and dormancy development in deciduous woody plants: a working hypothesis suggesting how molecular and cellular function is affected by temperature during dormancy induction. Plant Mol Biol 73:49–65
Thirugnanasambantham K, Prabu G, Palanisamy S, Chandrabose SR, Mandal AK (2013) Analysis of dormant bud (Banjhi) specific transcriptome of tea [Camellia sinensis (L.) O. Kuntze] from cDNA library revealed dormancy-related genes. App Bioch Biotech 169:1405–1417
Thomas SG, Phillips AL, Hedden P (1999) Molecular cloning and functional expression of gibberellin 2-oxidases, multifunctional enzymes involved in gibberellin deactivation. PNAS 96:4698–4703
Tuan PA, Bai S, Saito T, Ito A, Moriguchi T (2017) Dormancy-associated MADS-box (DAM) and abscisic acid pathway regulate pear endodormancy through a feedback mechanism. Plant Cell Physiol. https://doi.org/10.1093/pcp/pcx074
Ueguchi-Tanaka M et al (2005) GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437:693–698
Ueguchi-Tanaka M, Nakajima M, Motoyuki A, Matsuoka M (2007) Gibberellin receptor and its role in gibberellin signaling in plants. Annu Rev Plant Biol 58:183–198
Umezawa T, Nakashima K, Miyakawa T, Kuromori T, Tanokura M, Shinozaki K, Yamaguchi-Shinozaki K (2010) Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport. Plant Cell Physiol 51:1821–1839
Vergara R, Noriega X, Aravena K, Prieto H, PÉREZ FC (2017) ABA represses the expression of cell cycle genes and may modulate the development of endodormancy in grapevine buds. Front Plant Sci, 8: 812
Voegele A, Linkies A, Muller K, Leubner-Metzger G (2011) Members of the gibberellin receptor gene family GID1 (GIBBERELLIN INSENSITIVE DWARF1) play distinct roles during Lepidium sativum and Arabidopsis thaliana seed germination. J Exp Bot 62:5131–5147
Vyas D, Kumar S, Ahuja PS (2007) Tea (Camellia sinensis) clones with shorter periods of winter dormancy exhibit lower accumulation of reactive oxygen species. Tree Physiol 27:1253–1259
Wang F, Deng X (2011) Plant ubiquitin-proteasome pathway and its role in gibberellin signaling. Cell Res 6:1286–1294
Wang X, Wang Z, Dong J, Wang M, Gao H (2009) Cloning of a 9-cis-epoxycarotenoid dioxygenase gene and the responses of Caragana korshinskii to a variety of abiotic stresses. Gene Genet Syst 84:397–405
Wang X et al (2014) Identification of differential gene expression profiles between winter dormant and sprouting axillary buds in tea plant (Camellia sinensis) by suppression subtractive hybridization. Tree Genet Genom 10:1149–1159
Wang D et al (2015) Expression of ABA metabolism-related genes suggests similarities and differences between seed dormancy and bud dormancy of peach (Prunus persica). Front Plant Sci 6:1248
Xia EH et al (2017) The tea tree genome provides insights into tea flavor and independent evolution of caffeine biosynthesis. Mol Plant 10:866–877
Xiao YH et al (2010) Gibberellin 20-oxidase promotes initiation and elongation of cotton fibers by regulating gibberellin synthesis. J Plant Physiol 167:829–837
Xue T et al (2008) Genome-wide and expression analysis of protein phosphatase 2C in rice and Arabidopsis. BMC Genom 9:550
Yamaguchi S (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59:225–251
Yamaguchi S, Kamiya Y, Sun T (2001) Distinct cell-specific expression patterns of early and late gibberellin biosynthetic genes during Arabidopsis seed germination. Plant J 28:443–453
Yang C, Liu J, Dong X, Cai Z, Tian W, Wang X (2014) Short-term and continuing stresses differentially interplay with multiple hormones to regulate plant survival and growth. Mol Plant 7:841–855
Yano K, Aya K, Hirano K, Ordonio RL, Ueguchi-Tanaka M, Matsuoka M (2015) Comprehensive gene expression analysis of rice aleurone cells: probing the existence of an alternative gibberellin receptor. Plant Physiol 167:531–544
Ye H et al (2015) Map-based cloning of seed dormancy1–2 identified a gibberellin synthesis gene regulating the development of endosperm-imposed dormancy in rice. Plant Physiol 169:2152–2165
Yue C et al (2014) Molecular cloning and expression analysis of tea plant aquaporin (AQP) gene family. Plant Physiol Biochem 83:65–76
Zentella R et al (2007) Global analysis of della direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 19:3037–3057
Zhang Z et al (2016) MsZEP, a novel zeaxanthin epoxidase gene from alfalfa (Medicago sativa), confers drought and salt tolerance in transgenic tobacco. Plant Cell Rep 35:439–453
Zheng C, Halaly T, Acheampong AK, Takebayashi Y, Jikumaru Y, Kamiya Y, Or E (2015) Abscisic acid (ABA) regulates grape bud dormancy, and dormancy release stimuli may act through modification of ABA metabolism. J Exp Bot 66:1527–1542
Zhuang W, Gao Z, Wang L, Zhong W, Ni Z, Zhang Z (2013) Comparative proteomic and transcriptomic approaches to address the active role of GA4 in Japanese apricot flower bud dormancy release. J Exp Bot 64:4953–4966
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31370690, 31600555), the Natural Science Foundation of Fujian Province (2017J01616), and the Earmarked Fund for China Agriculture Research System (CARS-19), and the Chinese Academy of Agricultural Sciences through an Innovation Project for Agricultural Sciences and Technology (CAAS-ASTIP-2017-TRICAAS).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Prakash P. Kumar.
Electronic supplementary material
The online version of this article contains supplementary material, which is available to authorized users
299_2017_2238_MOESM1_ESM.docx
Fig. A1 Daily temperatures at the sampling sites and morphological changes in lateral buds on the sampling dates. The average, maximum (Max) and minimum (Min) temperatures from 1 Oct. to 31 Mar. of 2013-14 (a) and 2014–15 (b) were recorded. In 2013–14, buds were collected on 2 Nov. 2013, 1 Dec. 2013, 2 Jan. 2014, 14 Feb. 2014, 3 Mar. 2014 and 27 Mar. 2014; in 2014–15, samples were collected on 4 Nov. 2014, 2 Dec. 2014, 5 Jan. 2015, 5 Feb. 2015, 3 Mar. 2015 and 26 Mar. 2015. The time points at which the samples were taken are indicated by arrows, and the morphological changes in the lateral buds on the sampling dates are shown. Fig. A2 Neighbour-joining phylogeny of tea plant genes encoding proteins involved in GA metabolism and signalling pathways and those of other plants. a, ent-kaurene synthase (KS); b, ent-kaurene oxidase (KO); c, ent-kaurene acid oxidase; d, gibberellin 20 oxidase (GA20ox); e, gibberellin 3-oxidase (GA3ox); f, gibberellin 2-oxidase (GA2ox); g, gibberellin receptor (GID1s); h, DELLA proteins (DELLAs). Fig. A3 Neighbour-joining phylogeny of tea plant genes encoding proteins involved in ABA metabolism and signalling pathways and those of other plants. a, zeaxanthin epoxidase (ZEP); b, 9-cis-epoxycarotenoid dioxygenase (NCED); c, short-chain dehydrogenase/reductase (SDR); d, abscisic aldehyde oxidase (AAO); e, ABA 8′-hydroxylase (CYP707A); f, pyrabactin resistance-like proteins (PYLs); g, protein phosphatase 2Cs (PP2Cs). Table A1 Primers used in qRT-PCR analysis (DOCX 1628 KB)
Rights and permissions
About this article
Cite this article
Yue, C., Cao, H., Hao, X. et al. Differential expression of gibberellin- and abscisic acid-related genes implies their roles in the bud activity-dormancy transition of tea plants. Plant Cell Rep 37, 425–441 (2018). https://doi.org/10.1007/s00299-017-2238-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-017-2238-5