Abstract
Physiological growth of late planted sugarcane crop is restricted by high temperature and a short growth period. This causes considerable reduction in crop and sucrose yields. Improving physiological growth within the short period is, therefore, highly desirable. Two field experiments were undertaken to determine the effect of exogenous applications of Ethrel and gibberellic acid (GA3) on sprouting, shoot population and physiological growth. Sugarcane setts were soaked overnight in Ethrel before planting. Foliar application of GA3 was performed at 90, 120 and 150 days after planting (DAP). Ethrel soaking led to 100% sprouting and high settling population at 20 DAP, due to a significant increase in bud moisture and activities of acid invertase (AI), indole acetic acid oxidase (IAAO), adenosine triphosphatase (ATPase), superoxide dismutase (SOD) and nitrate reductase (NR) activity in vivo. Early sprouting increased the growth period to 245 days compared to 220 days in the unsoaked setts. The applications increased leaf area (57%), leaf area index (76%), leaf area ratio (71%), leaf area duration (48%), biomass duration (52%) and net assimilation rate (69.64%) at the grand growth stage. The changes led to increased shoot numbers (26.3%), internodal numbers stalk−1 (40.74%), internodal length (40%), internodal girth (46.15%) and stalk length (42%) at the harvest stage. The stimulated physiological growth augmented dry matter content, oBrix and purity of cane juice by 24.2, 3 and 0.3%, respectively. The study demonstrates that the induction of higher shoot numbers together with increased leaf area index (LAI) and stalk elongation within a short growth period through Ethrel soaking and gibberellic acid applications is positively associated with enhanced dry matter and sucrose contents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sugarcane (Saccharum officinarum L.) is one of the most productive plant species, represented by stout, jointed, fibrous stalks that potentially produce 40–70 tonnes of dry weight ha−1year−1, depending on crop growth duration (Bakker 1999). The crop is planted from culm pieces containing axillary buds positioned above each node. The phytomers originating from buds pass through four growth stages, that is, germination, tillering, grand growth and maturity, before the commercial component is harvested (Moore and Botha 2014). When the culms are cut from the plant, initiation of sprouting of axillary buds is not automatic. Sprouting of buds requires both the correct temperature (20–30 °C) and a moist environment (Donaldson 2009). The germination stage lasts until 45 days and is followed by the tillering stage. Tillering is the process of side shoots emerging from the axillary buds of the existing culm to form additional culms. Tillers arise at the base of the plant from the axillary buds on internodes that have undergone little expansive growth. Tillering is a major yield determining process as optimal yield depends on establishment of a sufficient tiller density (Bell and Garside 2005). The tillering stage lasts for around 120 days and vegetative growth involves several orders of tillering (van Dillewijn 1952). The tillering stage is followed by the grand growth stage of around 70–170 days, where internodes start their expansion until the leaf attached at its base is fully expanded. Elongation is completed at the individual cell and internode level by the time younger leaves have fully expanded (Rae and others 2006). The leaves on each internode serve as major source organs, whereas stalk internodes function as sink organs (Howell 1998). The structural development and elongation of culms is followed by the maturity stage, in which sucrose accumulation begins in the lower internodes while the internodes at the top of the culm expand, for about 90 days until harvest of the crop (Fernandis and Benda 1985).
In India, sugarcane is planted in October (autumn), February (spring) and May (late) with growth duration of 420, 360 and 270 days, respectively (Kapur and others 2011, Table 1). Though planting in October and February is favourable for obtaining high cane yield, farmers prefer to plant sugarcane in late May after the wheat harvest, as a part of the prominent crop rotation sequence of rice–wheat–sugarcane–ratoon–moong (Bhullar and others 2002). However, the temperature in late May is high and desiccating (40–43 °C, Table 1 ). The germination stage in late planted sugarcane, therefore, coincides with high temperature, exposing buds to temperature about 10–13 °C higher than their optimal growing conditions. This causes severe losses in soil and sett moisture contents (Yadav and others 1997). Plants exposed to temperatures about 5 °C above their optimal growing conditions exhibit a characteristic set of cellular and metabolic responses required for the plants to survive under high-temperature conditions (Guy 1999). These effects include rapid and excessive accumulation of reactive oxygen species and abscisic acid (Maestri and others 2002), accompanied by a decrease in synthesis of normal proteins and accelerated transcription and translation of heat-shock proteins (HSPs; Bray and others 2000). The overproduced reactive oxygen species (ROS) react directly with lipids, proteins and nucleic acids and cause lipid peroxidation-mediated membrane injury, protein degradation, enzyme inactivation, deoxyribonucleic acid (DNA) strand disruption and moisture deficitd in cells (Liu and Huang 2000; Maestri and others 2002). The cellular and metabolic changes prevailing at germination stage impose severe limitations on the early germination pattern, subsequent settling establishment and restrict the crop growth period to 220 days (Yadav and others 1997). The restricted crop growth period suppresses physiological growth of leaves and shoots at the tillering stage, stem elongation and dry matter accumulation during the grand growth stage and sucrose accumulation at the harvest stage (Lingle 1999).
High temperature also reduces CO2 influx for photosynthesis, net photosynthetic rate and dry matter partitioning and the ability to utilize photosynthates and restricts tiller formation (Oh-e and others 2007). Under normal conditions, a synchronism exists between the mother shoot and tillers and further, between tillers themselves. High temperature, however, imposes adverse impacts on synchronism and mobilization of assimilates and nutrients amongst tillers, causing a severe reduction in their numbers (Bita and Gerats 2013). Decreased tiller numbers reduce productivity, as tillers per plant at an early stage determine the number of millable canes, which is the key component of cane yield (Bell and Garside 2005). Physiologically, high temperatures also affect leaf development, leaf characteristics and internodal elongation at the tillering stage (Bonnett and others 2006). The base temperature for stem elongation has been calculated as 16–18 °C (Lingle 1999). Temperatures above 36 °C and reduced moisture availability led to shorter internodes and stalk length (Bonnett and others 2006). A short grand growth period imposes adverse effects on canopy coverage, amount of light interception, cumulative growth rate and dry matter accumulation (Table 1, Dhawan and others 1997; Moore and Botha 2014). After the grand growth stage, physiological growth again gets restricted from October onwards until January, due to sub-optimal temperatures (Rai and others 2008). Along with, tiller mortality reduces the ratio of mother shoot:tiller to merely 1:0.5 as compared with 1:2 in autumn and spring planted sugarcane (Kapur and others 2011; Moore and others 1997). This causes severe reduction in the number of millable canes per hectare (NMC ha−1), dry matter contents and cane yields (Bhullar and others 2002; Yadav and others 1997; Bonnett and others 2006).
Several attempts have been made for improving sprouting, shoot numbers and cane yield in late planted sugarcane. Reduction in moisture deficit in cane setts and buds due to high temperature has been attempted through irrigation between planting and the germination stage. However, irrigation causes crust formation in the upper layer of soil and blocks the emergence of young settlings (Srivastava and Mahindra 2012; Yadav and others 1991). Improvement in sprouting has also been addressed through increased seed rate and altered plant geometry. A narrow row to row spacing of 60 cm was adopted with a huge seed requirement (7–8 tha−1), for accommodating increased seed rate, unlike row to row spacing of 75 cm in spring planting, where the seed requirement was 5–6 tha−1 (Singh 2000; Bhullar and others 2002). However, no significant improvement in germination, shoot numbers, cane harvest index or sugar productivity was obtained. Low yields of late planted sugarcane thus continue to be a major problem in sub-tropical India (Yadav and others 1997; Dhawan and others 1997).
In sugarcane, studies are available that provide evidence on exogenous application of plant growth regulators (PGRs), which have altered the sprouting and growth processes. Ethrel application has improved sprouting in sugarcane (Li and others 2003). External application of GA3 remarkably increased internodal length in sugarcane (Moore 1980; Pribil and others 2007). However, no information is available on combined application of Ethrel and GA3 at different growth stages for improving sprouting, enhancing shoot numbers and stimulating physiological growth within a short growth period. The objective of our study was to investigate the combined effects of Ethrel and GA3 applied at critical growth stages on sprouting, shoot numbers and physiological growth within a short growth period in late planted sugarcane. The aims were threefold: (1) to test the ability of exogenously applied Ethrel to cane buds on earliness, enhanced sprouting and initial settling numbers during the germination phase; (2) to assess the effect of phasic GA3 application on shoot numbers, leaf characteristics, internodal and stalk elongation during the tillering and grand growth phases; (3) to assess the combined effects of Ethrel and GA3 application on total dry matter content and cane juice quality.
Materials and Methods
Experimental Site and Soil Climate
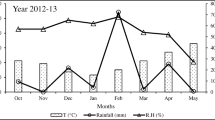
The experiment was conducted at the Indian Council of Agricultural Research (ICAR)-Indian Institute of Sugarcane Research, Lucknow, India, located at 26° 56′N, 80o52′E and 111 m above sea level. This falls in the Agro-Eco-region 4 (Northern plain and Central Highlands) and Hot Semi-arid Eco-region with Alluvial-derived (N8D2) soils (Sehgal and others 1990). The soil in the experimental field was sandy loam (13.3% clay, 24.5% silt and 62.2% sand) of Indo-Gangetic alluvial origin, very deep (>2 m), well drained, flat and classified as non-calcareous mixed hyperthermic udic ustochrept. The soil temperatures at 10 cm depth are given in Table 2. The climate of the experimental site is semi-arid, sub-tropical with hot dry summers and cold winters. The average monthly minimum and maximum temperatures during summer (April–June) range from 18.4 to 43 °C and in winter (November–February) from 7.4 to 29 °C. The average annual rainfall is 1045.5 mm and cumulative open pan evaporation is 1750 mm. Nearly 72% of the total rainfall is received through northwest monsoons during July to September (Table 2). The organic carbon (OC) content of soil was 0.48% with total nitrogen 0.069%. The available nitrogen (N), phosphorus (P), potassium (K) were 183.7, 18.7, 192 kg ha−1 in 2012–2013 and 185.6, 18.2, 190 kg ha−1 in 2013–2014.
Two crops were planted with sugarcane variety CoLk 94184 on 15th May 2012–13 and 2013–14 in different fields, prepared after wheat harvest on 6th May 2012 and 7th May in 2013, respectively, at the institute farm of the Indian Council of Agricultural Research-Indian Institute of Sugarcane Research (ICAR-IISR), Lucknow, India. Both fields with left-over wheat stubbles were irrigated and later on prepared with cultivator (once) and harrow (twice). Soil moisture of 16% was maintained in both fields during planting. Ridges and furrows were laid out at 75 cm spacing with a tractor-mounted furrow opener. The opened furrows were treated with chlorpyriphos (20% emulsifiable concentrate) for termite control.
Crop Culture and Exogenous Applications of Ethrel and GA3 at Critical Growth Stages
The sugarcane variety CoLk 94184 was planted under seven treatments in a randomized block design. Each plot (20 m × 12 m) contained 16 rows with a row to row spacing of 75 cm. In each row, five three-budded setts m−1 row length, were planted in three replications. The setts were placed in furrows with ends overlapping on each other. The treatments were T1: Unsoaked (Control), T2: Unsoaked + Water application, T3: Unsoaked + GA3 application, T4: Water soaked, T5: Water soaked + GA3 application, T6: Ethrel soaked, T7: Ethrel soaked + GA3 application (Table 3). Approximately 33,600 three-budded setts of sugarcane variety CoLk 94184 were planted in an area of 5040 m2. Prior to planting, 14,400 setts were covered with trash and left overnight for T1, T2 and T3. A total of 9600 setts were soaked in water for T4 and T5 (2400 L of water) and 9600 setts were soaked in Ethrel for T6 and T7 (2400 L of 100 ppm Ethrel). The setts were left overnight and taken out the next morning for planting. They were rinsed in Bavastine (@ 2 g L−1) prior to their planting in the furrows.
The foliar application of GA3 was performed at 90, 120 and 150 days after planting (DAP) in T3, T5 and T7. GA3 was dissolved in 0.5 cm3 of ethanol and diluted with distilled water to a concentration of 100 m mol m−3 and applied with knap sac (5 mL/plant) between 8.00 and 9.00 AM, while T2 was applied with an equal quantity of distilled water. The concentration of dissolving solvents was too low for any physiological effect on plants. The total quantity of water application and water used in the GA3 solution varied with the number of plants in every row. Ethrel and gibberellic acid were purchased from Chemical Drug House (CDH) Biochemicals, Analytical Reagent (AR) grade, with minimum assay of about 39 and 99.9%, respectively.
The crops were raised with standard agronomic cultivation practices and recommended doses of N, P, K (150:80:80 Kg ha−1). Fertilizers used were urea (46% N), single super-phosphate (6.8% P) and Muriate of Potash (46.2% K). One third of N, P and K was applied as a basal dressing in furrows at the time of planting. The remaining N was top-dressed in two equal splits at 45 and 90 DAP. Both the crops received a total of four irrigations and three inter-cultural operations. Application of insecticides was made as per recommendation for the region. The plants in all the treatments were free of pests and diseases during the experiments.
Bud Sprouting and Initial Shoot Number Determination at 20 and 45 DAP
Sprouting % was calculated by counting the number of sprouted buds out of the total planted buds. Buds with initial shoot protrusion of at least 2 mm in length were considered to be sprouted (Rai and others 2008). Bud moisture, bud dry weight and relative growth rate (RGR) were recorded with 45 buds scooped from 15 setts from respective treatments. The freshly removed buds were washed thoroughly and dried with Whatman No.1 filter paper for recording fresh weight. Bud dry weight was recorded by drying buds in a hot air oven, at 102 °C for 24 h and 80 °C for 72 h, to constant weight. RGR was computed using the formula
where ln W 1 is initial bud dry weight and ln W 2 is bud dry weight attained after time (t) in days, at 20 and 45 DAP. The numbers of settlings sprouted per plot were counted manually for recording initial plant population.
Biochemical Analysis of Cane Buds at 20 and 45 DAP
Freshly sampled bud tissues were chopped and homogenized to prepare a 10% homogenate in a chilled pestle and mortar with chilled distilled water. The homogenate was filtered through four layers of cheese cloth and then centrifuged at 8000g for 20 min at 4 °C. The supernatant obtained after centrifugation was used for estimation of reducing sugars, sucrose and total phenolic contents. Estimation of reducing sugar was done according to the method of Nelson (1944) and Somogyi (1945). Sucrose was estimated by the resorcinol thiourea method described by Roe and Papadopoulos (1954). Protein was estimated by the method of Lowry and others (1951) and acid invertase activity was assayed by the method of Hatch and Glasziou (1963). Total phenolic contents were estimated by the method described by Swain and Hillis (1999). Indole acetic acid (IAA) was determined as described by Nagar (1995) and IAAO activity was assayed by the method of Gordon and Weber (1951). The ATPase activity was assayed by the method of Fischer and Hodges (1959). Phosphorus estimation was done by following the method of Fiske and Subbarow (1925). NR activity in vivo and SOD activity were assayed by the method of Jaworski and others (1971) and Beauchamp and Fridovich (1971), respectively.
Determination of Leaf Characteristics and Shoot Development at 180 and 270 DAP
The area per leaf was calculated by the method described by Lerch and others (1977). The total leaf area of individual stalks was obtained by summation of leaf area on each stalk. Leaf area index (LAI) was calculated by multiplying the mean value of leaf area per stalk by number of stalks present in a known area. The growth parameters were individually calculated using formulae of Kvet and others (1971).
Net assimilation rate (NAR) = (W 2 − W 1) (ln L 2 − ln L 1) / [ (t 2 − t 1) (L 2 − L 1)], dry matter produced per leaf area and time units (mg cm−2 day−1)−2. With these values and shoot numbers, NAR was calculated on a land area basis.
where W and L are mean values of dry weight and leaf area at a specific time, respectively. W 1 and W 2 represent initial and final mean values of total dry weight of a stalk. L 1 and L 2 are the initial and final mean values of leaf area belonging to a stalk over the period t 2 − t 1. The growth parameters were calculated at 180 and 270 DAP. The shoot numbers in each plot were counted at 30-day intervals until 270 DAP. Stalk length, internodal length, internodal girth and internodal weight were measured with a metre scale, vernier caliper and electronic balance in 21 stalks sampled at 180 and 270 DAP.
Determination of Total Dry Matter and Juice Quality at 270 DAP
Stalk weight was recorded by chopping the stalk into small pieces. The chopped pieces were dried in an oven at 80 °C, until constant weight was achieved. Dry matter content m−2 was quantified from total number of stalks in an area of 16 m2 selected from each plot at 180 and 270 DAP. Cane juice quality was analysed from total number of stalks harvested from an area of 16 m2 at 270 DAP (Meade and Chen 1977).
Statistical Analysis
Data were analysed using the statistical product and service solution version 16.0 software (SPSS Inc, Chicago, II). One-way analysis of variance with Duncan’s Multiple Range Test (DMRT) as post hoc analysis was used to compare the means (Snedecor and Cochran 1967). Graphics were generated using Sigma Plot version 10.0 (Systat software, Inc., Point Richmond, CA). Regression analysis and correlation coefficients were calculated using MS Excel statistical tools to assess the interrelationships between treatment means across temperature among different parameters.
Results
Effect of Exogenous Application of Ethrel on Sprouting and Relative Growth Rate of Buds
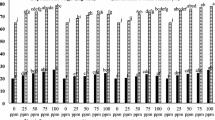
Ethrel soaking increased bud dry weight and relative growth rate by 61 and 44%, respectively, against unsoaked setts at 20 DAP. Bud moisture, bud dry weight and RGR in unsoaked and water-soaked setts were significantly less compared to Ethrel-soaked setts (Table 4). Maximum sprouting with 54,000 settings ha−1 was achieved with Ethrel soaking against no settling in unsoaked setts at 20 DAP (Fig. 1a, b). Later at 45 DAP also, there was a significant increase in bud moisture, bud dry weight, RGR and settling numbers with Ethrel soaking (Table 4). Bud moisture, bud dry weight and relative growth rate were significantly less in unsoaked setts. Sprouting of buds, therefore, was delayed up to 45 DAP in unsoaked setts (Fig. 1b). At 20 DAP, Ethrel soaking led to 100% sprouting and high settling numbers while sprouting was significantly less with unsoaked setts, even at 45 DAP (Fig. 2 a,b,c). At 45 DAP, the settling numbers increased to 55,200 ha−1 with Ethrel-soaked setts against 42,000 ha−1 in unsoaked setts. Ethrel soaking thus reduced the duration of germination to 20 days compared to 45 days in unsoaked setts and provided an additional 25 days for crop growth.
a Effect of treatments on sprouting % at 20 and 45 DAP. b Effect of treatments on settling numbers at 20 and 45 DAP. T1: Unsoaked (Control), T2: Unsoaked + Water application, T3: Unsoaked + GA3 application, T4: Water soaked, T5: Water soaked + GA3 application, T6: Ethrel soaked, T7: Ethrel soaked + GA3 application. Vertical bars represent ± SE of mean values of three replicates (n = 45). Means followed by same superscript in each parameter do not differ significantly at p = 0.05 by Duncan’s Multiple Range Test (DMRT). DAP Days after planting
Effect of Exogenous Application of Ethrel on Biochemical Changes During Sprouting and Shoot Numbers
Ethrel soaking of buds led to a fourfold increase in acid invertase (AI) activity at 20 DAP. This caused significant increase in reducing sugars and a decrease in sucrose contents. An increase of 53 and 81% in reducing sugar content and a decrease in sucrose contents by 37 and 40% were recorded in Ethrel-soaked setts at 20 and 45 DAP, respectively, against unsoaked setts. The increased reducing sugars and decreased sucrose contents due to higher AI activities at 20 DAP enhanced growth of buds and emergence of settlings in Ethrel-soaked setts (Table 5). Low AI activity, less reducing sugars and higher sucrose contents in unsoaked setts delayed sprouting and settling emergence to 45 days. NR activity in vivo, SOD and IAAO activities increased at 20 and 45 DAP in Ethrel-soaked setts against unsoaked setts. NR activity in vivo increased by 76 and 59% at 20 and 45 DAP, respectively. IAAO activity increased by 74.7 and 107% at 20 and 45 DAP, respectively. An increase of 79 and 77% in SOD activity was recorded at 20 and 45 DAP, respectively (Table 6). Althoughe enzyme activities increased significantly in water-soaked setts, they were 20% less than in Ethrel-soaked setts. The IAA contents decreased by 42 and 25% whereas total phenolic contents decreased by 32 and 51% in Ethrel-soaked setts at 20 and 45 DAP against unsoaked setts, respectively (Table 6).
Effect of Exogenous Application of Ethrel and GA3 on Leaf Characteristics and Canopy Coverage at 180 and 270 DAP
Foliar application of GA3 on shoots from Ethrel-soaked setts at 90, 120 and 150 DAP increased foliage numbers, leaf area (LA), leaf area index (LAI), leaf area duration (LAD), leaf area ratio (LAR), net assimilation rate (NAR) and biomass duration (Z) significantly, both at 180 and 270 DAP. The highest foliage numbers were recorded with Ethrel soaking and GA3 applications at 180 DAP (Fig. 3a). LA and LAI increased by 52 and 23% with Ethrel soaking and GA3 applications against unsoaked setts at 180 DAP (Fig. 3b, c). LAD, LAR, and Z increased by 48, 52 and 71%, respectively, at 180 DAP (Fig. 4a, b, d). At 270 DAP, LA, LAI, LAD and LAR were at a maximum with Ethrel soaking and GA3 applications against unsoaked setts (Figs. 3a, b, 4a, b). Net assimilation rate (NAR), which indicates the rate of increase in biomass per unit leaf area per day, was highest with Ethrel soaking and GA3 applications at 180 and 270 DAP (Fig. 4c). Biomass duration (Z, gd × 103) increased by 57 and 63% at 180 and 270 DAP, respectively (Fig. 4d).
Effect of treatments on growth parameters of sugarcane during growth cycle. a Foliage number, b Leaf area ; c Leaf area index (LAI). T1: Unsoaked (Control), T2: Unsoaked + Water application, T3: Unsoaked + GA3 application, T4: Water soaked, T5: Water soaked + GA3 application, T6: Ethrel soaked, T7: Ethrel soaked + GA3 application. Vertical bars represent ± SE of mean values of three replicates (n = 21). Means followed by same superscript in each parameter do not differ significantly at p = 0.05 by Duncan’s Multiple Range Test (DMRT). DAP Days after planting
Effect of treatments on growth parameters of sugarcane during growth cycle. a Leaf area duration (LAD), b leaf area ratio (LAR), c net assimilation rate (NAR), d biomass duration (Z). T1: Unsoaked (Control), T2: Unsoaked + Water application, T3: Unsoaked + GA3 application, T4: Water soaked, T5: Water soaked + GA3 application, T6: Ethrel soaked, T7: Ethrel soaked + GA3 application. Vertical bars represent ± SE of mean values of three replicates (n = 21). Means followed by same superscript in each parameter do not differ significantly at p = 0.05 by Duncan’s Multiple Range Test (DMRT). DAP Days after planting
Effect of Exogenous Application of Ethrel and GA3 on Shoot and Cane Juice Characteristics at 180 and 270 DAP
A significant increase in mean internodal number, internodal length and internodal weight was recorded with Ethrel soaking and GA3 applications against unsoaked setts (Fig. 5a–c). Similarly, an increase in internodal number, length and weight was recorded with water soaking and GA3 application but the shoot numbers were significantly less than with Ethrel soaking. Mean internodal numbers per stalk increased by 58% with Ethrel soaking and GA3 applications at 270 DAP (Fig. 5a). At 180 and 270 DAP, internodal length increased by 66 and 62%, respectively (Figs. 5b, 6a). Mean internodal weight was at a maximum at 180 DAP and it increased by 120% at 270 DAP. There was a significant reduction in mean internodal length with unsoaked setts without GA3 at 270 DAP (Fig. 6b).
Effect of treatments on intermodal growth parameters of sugarcane during growth cycle.a Mean internodal number; b mean internodal length; c. mean internodal weight. T1: Unsoaked (Control), T2: Unsoaked + Water application, T3: Unsoaked + GA3 application, T4: Water soaked, T5: Water soaked + GA3 application, T6: Ethrel soaked, T7: Ethrel soaked + GA3 application. Vertical bars represent ±SE of mean values of three replicates (n = 21 stalks). Means followed by same superscript in each parameter do not differ significantly at p = 0.05 by Duncan’s Multiple Range Test (DMRT). DAP Days after planting
Cane stalk length, stalk and root dry weight increased significantly with Ethrel soaking and GA3 applications both at 180 and 270 DAP. Stalk length increased by 46 and 42% and stalk dry weight increased by 77 and 78% at 180 and 270 DAP, respectively (Fig. 7a, b). Shoot numbers increased significantly by 55 and 47% at 180 and 270 DAP with Ethrel soaking and GA3 applications against unsoaked setts (Fig. 7c). Ethrel soaking and GA3 applications led to a threefold increase in root weight. Root weight was at a maximum with GA3 application against control at 180 and 270 DAP (Fig. 7d). Maximum shoot numbers in a clump were recorded with Ethrel soaking and GA3 application whereas minimum shoot numbers in a clump of sugarcane were observed with unsoaked setts without GA3 application at 270 DAP (Fig. 8a, b). The dry matter content, oBrix and purity of cane juice increased by 24.2, 3 and 0.3% in Ethrel-soaked setts with GA3 application against control at 270 DAP (Table 7).
Effect of treatments on stalks and roots of sugarcane. a Stalk length; b stalk dry weight; c shoot numbers d root weight. T1: Unsoaked (Control), T2: Unsoaked + Water application, T3: Unsoaked + GA3 application, T4 : Water soaked, T5: Water soaked + GA3 application, T6 : Ethrel soaked, T7: Ethrel soaked + GA3 application. Vertical bars represent ±SE of mean values of three replicates (n = 21 stalks). Means followed by same superscript in each parameter do not differ significantly at p = 0.05 by Duncan’s Multiple Range Test (DMRT). DAP Days after planting
Discussion
Impaired sprouting of sugarcane buds under high and desiccating temperatures (40–43 °C) affected dry matter and sucrose contents in late planted sugarcane (Yadav and others 1997). The setts containing axillary buds are highly sensitive to high temperatures which cause severe moisture loss and dysfunctional cell membranes (Finch-Savage and others 2006; Wahid and Close 2007). Moisture deficit in buds of unsoaked setts suppressed sprouting significantly. The decreased moisture level suppresses sprouting due to adverse effects on integrity and functioning of cell membranes, accelerated kinetic energy and loosening of chemical bonds within the molecules. The lipid bilayers of the cell membrane thereby become more fluid either by denaturation of proteins or by increased unsaturated fatty acids (Savchenko and others 2002). This enhances the permeability of membranes and causes severe loss of water and electrolytes (Rai and others 2008). Low bud moisture levels decreased the activities of acid invertase, IAAO, ATPase and NR activity in vivo. Poor sucrose hydrolysis, low reducing sugars and high IAA levels suppressed sprouting in unsoaked setts at 20 DAP. Singh and others (2003) reported that low enzyme activities and high IAA levels suppressed bud sprouting in sugarcane.
Ethrel soaking was very effective in increasing moisture levels and enhancing enzyme activities in buds at high temperatures. This led to 100% bud sprouting at 20 DAP. Although the buds were exposed to temperatures about 10–13 °C higher than optimal growing conditions, Ethrel minimized moisture loss in buds and maintained the optimum moisture level required for sprouting (Zhang and others 2001). Increased bud moisture improved the integrity and functions of cell membranes and provided an optimum environment required for sprouting (Rai and others 2008). Ethrel is believed to overcome the inhibitory effect of high temperatures by activating cell wall enzymes responsible for substrate digestion in several species (Hasegawa and Maruyama 1995). High bud moisture in Ethrel-soaked setts enhanced acid invertase activity, sucrose hydrolysis and reducing sugars at 20 DAP. Acid invertase activity and reducing sugars have been reported to increase in sugarcane buds that received Ethrel treatment (Li and Solomon 2003). Increased ATPase activity and NR activity in vivo improved adenosine triphosphate (ATP) and nitrogen availability for bud growth (Ye and others 2003). High IAAO and SOD activities reduced IAA contents and detoxified hydrogen peroxide (H2O2), which helped the buds to survive high-temperature stress (Zhao and others 2001). Li and Solomon (2003) reported Ethrel to be most effective at improving sugarcane bud sprouting and its growth.
Delayed and poor sprouting with unsoaked setts suggested a problem in establishment of initial shoot numbers at an early stage. Initial shoot numbers depend on adequate temperature and moisture (Singh and others 2003). Insufficient external moisture results in poor initial shoot establishment in sugarcane and several other crops (Inman-Bamber and Smith 2005). Although there was no sprouting at 20 DAP, the initial shoot numbers were limited to 42,000 shoots ha−1 at 45 DAP. The physiological growth of shoots was suppressed. Low LAI, LAD and Z resulted in poor leaf expansion and canopy development. This caused a poor balance between photosynthesis and respiration. Yadav and Prasad (1988) reported that when temperatures are not adequate, plants are compelled to go through a series of drought reactions which result in poor leaf emergence, leaf expansion, canopy development and formation of smaller internodes. Leuning and others (1991) reported that low LAI reduces light interception and radiation use efficiency in crops.
Ethrel soaking improved the initial shoot numbers both at 20 and 45 DAP. Ethrel induced early and enhanced bud sprouting, resulting in establishment of 54,000 shoots ha−1 compared to no shoots with unsoaked setts at 20 DAP. There was a significant increase in shoot numbers at 45 DAP. The shoot numbers increased to 55,200 shoots ha−1 compared to 42,000 shoots ha−1 with unsoaked setts. Our results are in agreement with Rao and others (2005), who reported that exogenous application of Ethrel has the ability to promote the axillary bud break and increases initial shoot numbers in several species. The early and enhanced sprouting with Ethrel-soaked setts provided an additional 25 days for physiological growth of initial shoots against unsoaked setts. The additional time period of 25 days with Ethrel soaking led to a significant increase in physiological growth of shoots at the tillering stage. LAI was 2.54 in shoots from Ethrel-soaked setts against 0.46 in shoots from unsoaked setts at the tillering stage (90 DAP). High LAI during the tillering stage led to development of a vast canopy and provided greater green leaf surfaces for increased light interception, radiation use efficiency and carbon fixation. van Andel (1973) reported a significant improvement in canopy development in plants treated with Ethrel. It was reported by Maddonni and others (2001) that because canopy structure is strongly related to total amount of intercepted radiation, a vast canopy structure favours dry matter accumulation for maximizing crop yield. Raji and others (1999) reported that high LAI and vast canopy at the initial growth stage improved sugarcane yield. A positive effect of early growth and positive correlation among LAI and yield in several varieties has been reported by Shimabuku and others (1980).
GA3 applications led to a substantial increase in shoot numbers and foliage numbers at 180 DAP. Leaf area ratio, leaf area duration, biomass duration and net assimilation rate increased by 71, 48, 52 and 69.64 per cent, respectively. Shoot numbers increased to 2, 20,000 shoots ha−1 and LAI increased to 6.03 against 1, 00,000 shoots ha−1 with LAI limited to 2.22 with unsoaked setts. Increased LAI enhanced photosynthetic activity and assimilate production in leaves (source). This increased source activitiy led to increased internodal (sink) demand for assimilates for preventing its catastrophic overproduction. The enhanced sink demand is fulfilled by unloading of assimilates from leaves into internodes (Zamski and Schaffer 1996). The translocated assimilates into internodes are utilized in cell division, cell elongation and internodal elongation (Moore 1980). The enhanced assimilate translocation from leaf phloem to internodes thus increased internodal numbers, internodal length, internodal girth and stalk length by 40.74, 40, 46.15 and 42%, respectively, against control. It was reported by Ho and Vasil (1983) that GA3 applications enhanced assimilate translocation from leaf phloem to internodes. Shimabuku and others (1980) reported that GA3 applications induced a significant increase in leaf sheath length for increasing assimilate translocation into internodes. High LAD maintained the leaves for a longer duration and increased assimilate production and translocation to internodes for a longer duration (Raji and others 1999). High LAD at the grand growth stage thus promoted faster and complete development of sinks for increasing dry matter accumulation within a short growth period.
The increased leaf activity and internodal length stimulated the physiological growth of shoots at 180 DAP. Moore (1980) reported a remarkable increase in internodal length through external application of GA3 in sugarcane and suggested that it was a result of rapid cell division and cell elongation. Cell elongation with GA3 applications is caused by the action of cell wall hydrolases which relax the cell wall and increase cell wall extensibility (Yang and others 1996). An additive effect of GA3 applications on sugarcane internodal and stalk elongation is also reported by Moore and Botha (2014). Takahashi and others (1986) reported that GA3 applications along with other hormones promoted rapid elongation and division of cells. Delayed and poor initial shoot numbers, low LAI, LAD, LAR, Z and NAR in unsoaked setts without GA3 application during the grand growth stage led to poor photosynthetic activity and assimilate production. Low LAI was responsible for smaller canopy development which affected the physiological growth of shoots adversely. Less green leaf surfaces decreased light interception, radiation use efficiency and carbon fixation. Low LAD caused assimilate losses through respiration resulting in shorter internodes with less weight. Shimabuku and others (1980) reported that low LAI, LAD, LAR, biomass duration and NAR are responsible for low energy conversion and suppression of physiological growth in several crops.
The stimulated physiological growth with Ethrel soaking and GA3 led to an increase of 26.3% in shoot numbers against controls at 270 DAP. This was due to a significant decrease in shoot mortality. The reduced shoot mortality and increased shoot numbers with GA3 application were due to development of a robust root system as indicated by a threefold increase in root weight. This reduced the competition for water and nutrients amongst shoots which is generally responsible for their mortality. The increased root weight improves absorption of water and mineral nutrients required for stimulated growth of shoots (van Antwerpen 1999). The shoot numbers, however, decreased significantly in controls due to 40% shoot mortality. Bell and Garside (2005) reported that about 50% of shoot mortality is due to competition for light and nutrients. Higher shoot numbers together with increased LAI and stalk elongation and reduced shoot mortality within a short growth period led to a significant increase in total dry matter, sucrose content and cane juice quality. Increased LAI and stalk elongation with GA3 applications were thus positively associated with enhanced dry matter and sucrose contents. Kromer (2000) reported that total dry matter productivity relies on the initial plant population, an appropriate carbon assimilation rate and its enhanced export from the source to sink organ.
In conclusion, our results indicate that Ethrel application exerted a protective effect on buds against high temperature and had beneficial effects on different parameters required for optimum sprouting during the germination stage (bud moisture level, activities of acid invertase, ATPase, NR activity in vivo, SOD and IAAO). The phenomenon of Ethrel action on sugarcane buds is associated with activation of cell wall enzymes responsible for maintaining the integrity and functions of cell membranes, which has positive effects on growth enzymes and metabolites required for optimum sprouting. The beneficial effect of Ethrel under high temperature also involved improvement in antioxidant capacity of buds through removal of reactive oxygen species with increased SOD activities. The results also indicated that phasic application of gibberellic acid on increased shoot numbers stimulated faster physiological growth of source and sink organs for increasing the dry matter accumulation within a short growth period, during the tillering and grand growth phases. The positive effects of GA3 application on LAI, internodal elongation and root growth within a short growth period increased the dry matter production and improved cane juice quality at the harvest stage. The combined effects of Ethrel and GA3 applications, thus precluded the adverse impacts of high temperature and a short growth period in late planted crops and increased total dry matter production. The finding suggests that use of Ethrel and gibberellic acid in late planted sugarcane crop holds strong potential to improve cane yield in sub-tropical India. Six field impact assessment trials at different locations are in progress for assessing the effects of exogenous application of Ethrel and gibberellic acid on cane yield.
References
Bakker H (1999) Sugar cane cultivation and management. Kluwer Academic, Plenum Publishers, New York, p 327
Beauchamp C, Fridovich I (1971) Super oxide dismutase: improved assays and assays applicable to acrylamide gels. Anal Biochem 44:276–287
Bell MJ, Garside AJ (2005) Shoot and stalk dynamics and yield of sugarcane crops in tropical and sub-tropical Queensland, Australia. Field Crops Res 92:231–248
Bhullar MS, Saini LK, Kapur ML, Singh S (2002) Effect of Method and Density of Planting on Growth and Yield of Late Planted Sugarcane. Sugar Tech 4(3–4):181–184
Bita CE, Gerats T (2013) Plant Tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress -tolerant crops. Front Plant Sci 4:273. Doi:10.3389/fpls.2013.00273
Bonnett GD, Hewit ML, Glassop D (2006) Effects of high temperature on the growth and composition of sugarcane internodes. Aust J Agric Res 57:1087–1095
Bray EA, Bailey-Serres J, Weretilnyk E (2000) Responses to abiotic stresses. In: Gruissem W, Buchannan BB, Jones RL (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, pp 1158–1203
Dhawan AK, Sahtiya HL, Dendsay JPS (1997) Low germination of sugarcane setts in Indian sub-tropics: Constraints and their management. Indian J SugTech 12:17–21
Donaldson RA (2009) Seasons effect on the potential of biomass and sucrose accumulation of some commercial cultivars of sugarcane. PhD Thesis, University of Kwazulu Natal South Africa pp 173
Fernandis AC, Benda GTA (1985) Distribution pattern of brix and fibre in primary stalk of sugarcane. Sugarcane 5:8–13
Finch-Savage WE, Leubner-Metzger G (2006) Seed dormancy and the control of germination. New Phytol 171:501–523
Fischer J, Hodges TK (1959) Monovalent ion stimulated adenosine triphosphatase from oat roots. Plant Physiol 44:385–395
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Gordon SA, Weber, RP (1951) Colorimetric estimation of indole acetic acid. Plant Physiol 26:192–195
Guy C (1999) Molecular response of plants to cold shock and cold acclimation. J Mol Microbial Biotechnol 1:231–242
Hasegawa R, Maruyama A (1995) The presence of two types of bcyanoalanine sythase in germinating seeds and their response to ethylene. Physiol Plant 93:713–718
Hatch MD, Glasziou KT (1963) Sugar accumulation cycle in sugarcane II. Relationship of invertase activity to sugar content & growth rate in storage tissue of plants grown in controlled environments. Plant Physiol 38(3):344–348
Ho WJ, Vasil IK (1983) Somatic embryogenesis in sugarcane (Saccharum officinarum L.) I. The morphology and physiology of callus formation and the ontogeny of somatic embryos. Protoplasm 118(3):169–180
Howell SH (1998) Molecular Genetics of plant development. Cambridge Univ Press, Cambridge, p 367
Inman-Bamber NG, Smith DM (2005) Water relations in sugarcane and response to water deficits. Field Crops Res 92(2):185–202
Jaworski EG (1971) Nitrate reductase assay in intact plant tissue. Biochem Biophys Res Commun 43:1272–1279
Kapur R, Duttamajumder SK, Rao KK (2011) A breeder’s perspective on the tiller dynamics in sugarcane. Curr Sci 100(2):183–189
Kromer E (2000) Source physiology and assimilate transport: the interaction of sucrose metabolism, starch storage and phloem export in source leaves and effects of sugar status in phloem. Aust J Plant Physiol 27:497–505
Kvet J, Ondok JP, Necas J, Jarvis PG (1971) Methods of growth analysis. Plant Photosynthetic Production. The Hague Publisher, The Hague, pp 343–391
Lerch GR, Reyes R, Garcia R, Leal P (1977) Crecimiento, desarrollo y variacio´ndelı´ndicerefractome´trico (Brix) en seisvariedades de can˜a de azu´car. Cien. Agric 1:79–105
Leuning R, Wang YP, Cromer RN (1991) Model simulations of spatial distributions and daily totals of photosynthesis in Eucalyptus grandis canopies. Oecologia 88:494–503
Li YR, Solomon S (2003) Ethephon: a versatile growth regulator for sugarcane industry. Sugar Tech 5:213–223
Li ZG, Lin YR, Lin YK, Lin JZ, Lin SL, Zhou WY (2003) Effects of foliar spray of ethephon on some enzyme activities in stem cells of sugarcane. Sugarcane 9(1):12–18
Lingle SE (1999) Sugar metabolism during growth and development in sugarcane internodes. Crop Sci 39:480–486
Liu X, Huang B (2000) Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci 40:503–510
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin-phenol reagent. J Biol Chem 193:265–275
Maddonni GA, Chelle M, Drouet JL, Andrieu B (2001) Light interception of contrasting azimuth canopies under square and rectangular plant spatial distributions: simulations and crop measurements. Field Crops Res 70:1–13
Maestri E, Klueva N, Perrotta C, Gulli M, Nguyen HT, Marmiroli N (2002) Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Mol Biol 48:667–681
Meade GP, Chen JGP (1977) Cane Sugar handbook. 10th edn, A Wiley Inter Science Publication, Wiley, New York
Moore PH (1980) Additive and non-additive effects of serial applications of GA3 on sugarcane internodal growth. Physiol Plant 49:271–276
Moore PH, Botha FC (2014) Sugarcane: physiology, biochemistry and functional biology. Wiley-Blackwell, New Jersey, pp 716
Moore PH, Botha FC, Furbank R, Grof C (1997) Potential for overcoming physio biochemical limits to sucrose accumulation intensive sugarcane production: Meeting the challenges beyond 2000. CAB Int Wallingford, UK 141–155
Nagar PK (1995) Changes in abscisic acid, phenols and indole acetic acid in bulbs of tuberose (Polianthes tuberosa L.) during dormancy and sprouting. Sci Hortic 63:77–82
Nelson NA (1944) Photometric adaptation of Somogyi method for the determination of glucose. J Biol Chem 153:315–380
Oh-e I, Saitoh K, Kuroda T (2007) Effects of high temperature on growth, yield and dry-matter production of rice grown in the paddy field. Plant Prod Sci 10:412–422
Pribil M, Hermann SR, Dun GD (2007) Altering sugarcane shoot architecture through genetic engineering: Prospects for increasing cane and sugar yield. Proc Aust Soc Sugar Cane Technol 29:251–257
Rae AL, Bonnett GD, Karno (2006) Understanding stem development and sucrose accumulation to increase CCS. Proc Aust Soc Sugarcane Technol 28:327–335
Rai RK, Singh P, Srivastava AK, Suman A (2008) Modulation of low-temperature-induced biochemical changes in bud and root band zones of sugar cane sets by potassium, zinc, and Ethrel for improving sprouting. J Agric Food Chem 56(24):11976–11982
Raji IY, Siemens JC, Bullock DG (1999) Growth analysis of soybean under no-tillage and conventional tillage systems. Agron J 91:928–933
Rao M, Krishnamurti M, Weerthaworn P (2005) Beneficial effects of ethephon on yields and sucrose productivity of sugarcane cultivars in Thailand. Sugar Tech 7:48–52
Roe JH, Papadopoulos NM (1954) The determination of fructose-6-phosphate and fructose 1,6-diphosphate. J Biol Chem 210–703
Savchenko G, Klyuchareva E, Abramchik L, Serdyuchenko E (2002) Effect of periodic heat shock on the inner membrane system of etioplasts. Russ J Plant Physiol 49:349–359
Sehgal JL, Mandal DR, Mandal C, Vadivelu S (1990) Agro-ecological regions of India. NBSS & LUP, Nagpur
Shimabuku MM, Kudo M, Tamaki K (1980) The influence of growth parameters and climatic factors on efficiencies of solar energy utilization in sugarcane. In: Lopez MB, Madrazo CM(eds.), Proc Int Soc Sugar Cane Technol Manila, Philippines pp 526–533
Singh A (2000) Influence of seed rates and row spacing’s on growth and yield of late planted cane. Sugar Tech 2(3):49–50
Singh I, Rai RK, Solomon S, Shrivastava AK (2003) Role of Indole-3-Acetic Acid in sprouting of subterranean buds in winter initiated sugarcane ratoon. Sugar Tech 5:181
Snedecor GW, Cochran WG (1967) Statistical Methods. Oxford and IBH Publ, India pp 593
Somogyi MA (1945) New reagent for determining sugar. J Biol Chem 160–61
Srivastava AK, Mahindra RK (2012) Sugarcane production: impact of climate change and its mitigation. Biodiversitas 13(4):214–227
Swain T, Hillis WE (1999) The phenolic constituents of Prunus domestica I. The quantitative analysis of phenolic constituent. J Sci Food Agric 10:63–68
Takahashi N, Yamaguchi I, Yamane H (1986) Gibberellins. In: Takahashi N (ed) Chemistry of Plant Hormones. CRC Press, Boca Raton, pp 57–151
van Dillewijn C (1952) Botany of Sugarcane. The Chronica Botanica Co Waltham Massachusetts pp 371
van Andel OM (1973) Morphological effects on vegetative plants of Poa pratensis L. of 6 Z-azauracil, 2-chloro ethyl phosphonic acid and 2 chloroethyl trimethyl ammonium chloride and their interaction with gibberellic acid. J Exp Bot 24:245–257
van Antwerpen R (1999) Sugarcane root growth and relationships to above-ground biomass. Proc. South Afr Sug Technol Ass 73:89–95
Wahid A, Close TJ (2007) Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol Plant 51:104–109
Yadav RL, Prasad SR (1988) Moisture use characteristics of sugarcane genotypes under different available soil moisture regimes in alluvial entisols. J Agric Sci 110:5–11
Yadav RL, Kumar R, Verma RS (1991) Effect of planting technique and planting density on yield of late planted sugarcane in North Central India. Exp Agric 27(03):281–286
Yadav RL, Singh RV, Singh R, Srivastava VK (1997) Effect of planting geometry and fertilizer N on nitrate leaching, NUE and sugarcane yield. Trop Agric 74:115–120
Yang T, Davies PJ, Reid JB (1996) Genetic dissection of relative roles of auxin and gibberellin in the regulation of stem elongation in intact brown peas. Plant Physiol 110:1029–1034
Ye YP, Tang J, Li YR, Li YJ, Yang LT (2003) Effects of ethephon on ATPase and invertase activities in internodes of sugarcane at boom growth stage. Chin J Trop Crops 23 (2):66–71
Zamski E, Schaffer AA (1996) Photoassimilate distribution in plants and crops: source–sink relationships. CRC Press, Boca Raton, p 928
Zhang XJ, Li YR, Lin YK (2001) Effects of different concentrations of ethephon soaking seed cane on plant growth and some physiological and biochemical characters in sugarcane. Sugar Crops China 3:9–13
Zhao ZG, Chen GC, Zhang CL (2001) Interaction between reactive oxygen species and nitric oxide in drought induced abscisic acid synthesis in root tips of wheat seedlings. Aust J Plant Physiol 28:1055–1061
Acknowledgements
We thank our Director, ICAR-IISR, for his support and encouragement. We are also thankful to University Grants Commission (UGC), New Delhi, India for their financial support under UGC Junior Research Fellowship (JRF) scheme. We extend our sincere thanks to Mr Abhilash Kumar Shukla and Mr CP Singh, farm manager for providing the weather data and farm facilities. The help provided by Mr Ram Singh, Dr Namita Arya, Mr Chatarpal, Mr. Santosh, Mr Surender and Mr Raj Kumar during the field experiment and the studies in the laboratory is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rai, R.K., Tripathi, N., Gautam, D. et al. Exogenous Application of Ethrel and Gibberellic Acid Stimulates Physiological Growth of Late Planted Sugarcane with Short Growth Period in Sub-tropical India. J Plant Growth Regul 36, 472–486 (2017). https://doi.org/10.1007/s00344-016-9655-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-016-9655-5