Abstract
Plant development and yield are severely hampered by climate change. Plants are very prone to a variety of abiotic stressors during growth, making them susceptible to destruction which can reduce the productivity by 20–60%. These stresses generate reactive oxygen species (ROS), which damage lipids, proteins, and nucleic acids. Microalgae and plant growth-promoting bacteria (PGPB) are remarkably effective at reducing the effects of salt stress and promoting plant growth, thereby increasing agricultural yield, and helping ensure global food security. Through a variety of mechanisms, including the production of phytohormones, 1-aminocyclopropane-1-carboxylic acid deaminase, exopolysaccharide, siderophores, hydrogen cyanide, extracellular polymeric substances, volatile organic compounds, and modulation of antioxidants defense machinery under abiotic stresses promote plant growth after inoculation of PGPB and microalgae. These microorganisms also maintain ion homeostasis, offer osmotic balance, stimulate genes that respond to salt and drought, rewire the metabolism, modify the transcription of ion transporter genes, and more. To counteract the negative consequences of salinity stress, this study summarizes the effects of PGPB- microalgae along with a tentative protective mechanism during salinity stress for sustainable agriculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A major challenge for agriculture is to accomplish the food demand of the increasing global population, which is currently growing at higher rate. There are various stresses, responsible for lowering the productivity of various crops [53]. Salinity is one of the major abiotic stresses which significantly reduce crop productivity. The deposition of mineral salts such as NaCl, Na2SO4, Na2CO3, MgSO4, NaHCO3, CaCO3 and CaSO4, is a major characteristic of saline soils [31]. Currently, 1125 million hectares of land around the world are affected by salinity, out of which 76 million hectares are due to human-induced salinization. The human-induced salinization activities are mining, industrial management, and high irrigation. There is about 20% of the agricultural area is highly affected by high salinity and this percentage is growing constantly [89, 113].
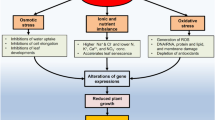
Agricultural practices such as excessive use of fertilizers, pesticides, and fungicides; unsustainable land use; and inadequate irrigation can result in salinization of the soil [42]. Additionally, applying fertilizers with high potassium and sodium contents can, under some circumstances, result in the buildup of these salts and induce soil degradation [31]. Limited rainfall and high temperatures also encourage excessive evapo-transpiration which results in an increase in the salinity of the top layers of the soil [4]. Electrical Conductivity measurements give the salinity in the soil, the permissible limit is < 0.7 dS/m. A salinity level > 3.0 dS/m is assumed to be a high and stressor that can heavily affect soil productivity. Salinity has a multidimensional impact on plants which significantly reduces agricultural output including seed germination, photosynthesis, antioxidants, membrane transport, and ethylene production. Exposure to high salt (NaCl) concentrations around the root zone increases osmotic stress and further generates ion toxicity. Osmotic stress affects water uptake, seed germination, cell elongation leaf development, quiescent lateral bud development, lateral branching, photosynthesis rate, nutrient uptake and translocation from root to shoot, interrupting the supply of carbohydrates to the meristematic regions, and exerts a negative impact on the overall growth of the plant. The high salinity causes an inflow of Na+ and Cl− into the cellular compartment which as a result causes an osmotic imbalance in plant cells [42], on the other hand, the high concentration of Na+ also inhibits the absorption of K+ which is an essential component for plant growth and development. High salinity initiates the accumulation of ROS in the biological components which consequently causes oxidative damage to various biomolecules [50]. Additionally, under salinity stress, photosystem II activity is reduced, which decreases photosynthetic efficiency [49]. Furthermore, vegetative and reproductive growth of plants are negatively impacted by a decrease in photosynthetic rate caused by salinity, which can also delay flowering, reduce fertility, and lead to decreased yield. Overall, from the stage of seed germination to seed development, salinity has a detrimental effect on the physiological and biochemical activities of the plant [108].
However, to recover saline soils, several types of physicochemical approaches are frequently used, including phytoremediation, microbial remediation, and conventional breeding methods, as well as leaching (which involves ponding the soil continuously or intermittently, sprinkling it, and eventually drying it out) and drainage (which involves adding soil amendments like gypsum). The physicochemical methods are unsustainable as they produce environmental pollution. Microbial remediation is considered a cost-effective, non-polluting, energy-saving, and widely applicable method for soil reclamation. Microbial remediation of saline soil is especially needing a certain type of halo tolerant plant growth-promoting bacteria (PGPB) and cyanobacteria such as Scytonema sp., Nostoc sp., and Anabaena sp. The microbes used in the remediation can be both endophytes and exophytes. Endophytes are a type of PGPB that reside inside their host cell and play remarkable success in enhancing the agricultural productivity of saline soils. Microorganisms that reside endophytically or exophytically have a great potential impact on the plant stress response [110]. These microbes can boost plant growth and their stress response by a variety of mechanisms such as nitrogen fixation, mineral solubilization, phytohormone synthesis, and release of siderophores (metal-chelating compounds) [60], [95]; [76]. Other genera of PGPB such as Bacillus sp., Azotobacter sp. and Pseudomonas sp. can also be very beneficial microorganisms to promote plant tolerance against saline conditions by releasing these extracellular compounds which function by the stimulation of genes related to stress, osmolytes, and antioxidant enzymes [53, 115]. Besides the release of the protecting compound to the surroundings, PGPB can also solubilize the metallic compounds and synthesize other plant hormone parameters. Hence, they are widely used worldwide for their characteristics for sustainable agriculture to minimize chemical pesticides and fertilizers over the last several decades [48, 61, 90]. Under salt stress, PGPB along with a variety of microorganisms can offer their host plants several advantages such as encouraging plant development, improving soil fertility, eliminating excess salt from plant tissues, phosphate solubilization, nitrogen fixation, and phytohormones synthesis [93, 96], [110]; [38, 119]. A proper formulation of these factors must be added to the soil to boost their viability and performance. Cyanobacteria, which are important phyllosphere and rhizosphere components, respond quickly to environmental stresses, reclaiming salt-affected desertified land and promoting vegetation cover. [66, 72, 88,89,90,91].
This review discusses the current state of knowledge about the various mechanisms used by PGPB and cyanobacteria to overcome the negative effects of salinity on plants and increase the productivity of saline soils. Numerous articles, which are considered in this review, demonstrated that bacterial and microalgal association boost plant susceptibility and releases various compounds which alleviate the tolerance level of its host in salty environments.
Effect of Salinity on the Various Components of Phyllosphere and Rhizosphere
Cell Membrane Properties
The salinity is essential for multiple biological functions; however, it is quite problematic if it is higher than the threshold values. Generally, there is a huge alteration in the composition of the cell wall of the organisms under high salinity. It can destroy the membrane’s integrity by influencing the lipid metabolism [57]. However, the overexpression of receptor-like protein kinase genes (OsRPK1) plays an important role in maintaining the plasma membrane’s H+-ATPase activity under such circumstances. Moreover, other mechanism like enforcement of cell wall by lignification, can also be adopted to tackle the saline environment. Lignin provides extra integrity to the membrane and mechanically reinforces the cell wall. There have been reports of the impact of lignin accumulation on salt tolerance in a variety of crops, including tomato, wheat, and soybean [47]. Along with the reinforcement of the cell wall, other types of pathways are also activated such as the induction of the monogalactosyl diacylglycerol synthase enzyme which maintains the integrity of the thylakoid membrane in thylakoids. High salinity can also damage the integrity by elevating the ROS levels in cell which is generally counteract by the expression of bacterial acdS gene by inhibiting the gene expression responsible for ROS production to prevent cellular damage [22]. High salinity results in the osmotic imbalance in micro-environment which is usually tackled by the mobilization of the aquaporins in the cell wall. Under salt stress, the expression of aquaporins along with the tonoplast intrinsic proteins (TIPs) and plasma membrane intrinsic proteins (PIPs) is highly under-expressed [35]. Aquaporins are the member of the family of major intrinsic proteins (MIP). It assists plants to absorb water from the soil and maintain their roots' hydraulic conductivity. Astonishingly, the role of PGPB becomes quite important in such conditions as they can nullify the effect of salinity and can over-express the aquaporins in such conditions. In doing so, they restore the plant-water relationships. For instance, it was discovered in a study that Azospirillum brasilense boosted the expression of the gene HvPIP2;1 in barley, improving plant–water relationships along with salinity tolerance [118]. In another study, Pantoea agglomera increased the expression of the gene PIP2;1 in maize and enhanced plant growth in saline environments [55]. PGPBs, such as Bacillus, Pseudomonas, and Azospirillum, are also involved in multiple plant hormone synthesis which directly influences the expression of the Aquaporins. Aquaporin intrinsic membrane proteins are currently well characterized, although only PIPs have been documented to be expressed by specific PGPB during salt stress [57, 88]. Hence, the PGPB are the pioneer components that positively control the expression of certain genes which are essential for the normal functioning of plants. As discussed earlier, under high salinity, the absorption of water and other essential elements is downgraded; however, the PGPB can restore such activities by over-expressing such pathways.
G- proteins and Ligands
The plant's local environment is formed by the multi-dimensional interaction of various microbial population, which includes bacteria and cyanobacteria, to mitigate the effects of salinity stress. This interaction majorly involves the GPCR-mediated signal transduction. GPCRs are signal transduction pathways most bacteria and cyanobacteria in the phyllosphere/rhizosphere use to detect and respond to salt stress. GPCRs on the surface of plant cells are bound by ligands such as glycine betaine, sucrose, and nodulation factors, which activate G proteins and change the expression of genes linked to the stress response. Some of the major microbes living in the phyllosphere and rhizosphere that contain GPCRs and G-proteins are B. subtilis, Synechocystis sp. PCC 6803, Nostoc sp. PCC 7120, Salinibacter ruber, and Synechocystis sp. PCC 6803 Canfora et al. [13]. Under salt stress, these microorganisms produce suitable solutes such as glycine betaine, sucrose, and glucosylglycerol, which bind to GPCRs and trigger downstream signaling pathways. Signal transduction in response to salt stress involves calmodulin, annexin, and members of the Rab family of GTPase proteins in addition to secondary messengers such as calcium ions, ROS, inositol phosphate, and phytohormones. These signaling molecules coordinate the cellular reactions of plants and associated phyllosphere and rhizosphere microbes to salt stress. Bacillus subtilis, a soil bacterium that can withstand mild salt stress, is another illustration of a bacterial species that synthesizes G-proteins in salinity [87]. A GPCR in the bacterium known as McpC interacts with glycine betaine, another suitable solute that the bacterium accumulates under salt stress. CheA is a G protein that McpC activates to control the bacterium's chemotaxis towards sources of glycine betaine [42]. A cyanobacterium, Synechocystis sp. PCC 6803, which can adapt to a variety of salt concentrations, also expresses G-proteins in high salinity. It has a GPCR called Sre1 that interacts with the compatible solute sucrose, which the cyanobacterium produces when it is under salinity stress [87, 91]. Nostoc sp. PCC 7120, a cyanobacterium that forms symbiotic relationships with plants and fungi, is another illustration of a cyanobacterial species that makes G-proteins in salinity. It has a GPCR termed NodQ that binds to nodulation factors, which are signaling molecules made by rhizobia bacteria and responsible for causing legumes to develop root nodules [31]. The major function of the G-protein expression is to enhance the symbiosis and nitrogen fixation in which the NodQ plays an important role in activating the G protein NodP. PGPB are essential for improving plants' ability to withstand salt. Through the employment of specialized transporters, PGPB actively pumps out of plant cells, reducing an accumulation of harmful ions and reducing the harm that salinity stress causes [51]. The general health and stress tolerance of the plant are influenced by the interactions with bacteria, cyanobacteria, and plants within the phyllosphere and rhizosphere. Microorganisms in the phyllosphere/rhizosphere promote plants in adjusting to and overcoming salt stress through their interactions and signaling pathways, eventually encouraging plant growth and survival. The interactions among microbes, GPCRs, signaling pathways, and PGPB within the phyllosphere and rhizosphere are crucial for mitigating the effects of salt stress and preserving plant health under demanding environmental circumstances.
Morphological Modifications
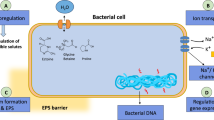
The microbes adopt various types of methodologies to combat the abiotic stresses. As it is a well-known fact that the microbes are very crucial for the behavior of the plant species as well as their response towards the environmental factor, it is very considerate to discuss the overall morphological adaptation of the microbes to sustain its activities under salinity stress. Besides retaining its ability and functionality, microbes can also secure the activity of its host under such adverse condition. Microbes are susceptible to morphological alterations brought on by salinity stress, including modifications to cell size, shape, membrane composition, and cell wall composition. These modifications can assist microorganisms in resisting osmotic pressure and preserving their cellular processes. Under salinity stress, some bacteria and cyanobacteria can produce spores, which are latent, hardy entities that can endure extreme circumstances (see Fig. 1). Spores are shielded from damage and desiccation by a thick layer of peptidoglycan and proteins [40, 53]. One such microbe is Bacillus subtilis which can produce endospores in the presence of high salt concentrations. These endospore-forming bacteria can form beneficial associations with plants and can enhance their growth and production by the mechanism of nitrogen fixation, phosphate solubilization, and plant growth promotion. Another filamentous cyanobacterium that can fix nitrogen enhance plant growth and withstand salinity is Nostoc. It can also synthesize heterocyst, which are specialized cells for nitrogen fixation [63].
Under salinity stress, some bacteria and cyanobacteria can alter the morphology of their cells, growing longer or more spherical (See Fig. 1). According to Tufail et al. [102], and Verma et al., [106], this may have an impact on the surface–to–volume ratio, which in turn affects how they exchange water and other substances with the environment. Gram-negative and halophilic, Halomonas elongate can thrive in salinities as high as 25%. Depending on the salinity of the medium, it can change the size and length of its cells. It can develop long filaments under high salinity and small rods under low salinity. Due to their survival capacity in salinity conditions, they form various compounds, which help in plant growth and protection [114]. A marine cyanobacteria, Synechococcus sp. PCC 7002 can withstand salinities up to 3.5 M NaCl, by altering the proportion of peptidoglycan in its cell wall, it can control its morphological characteristics. Its cell width increases, and its peptidoglycan layer is thicker under conditions of low salinity, whereas in conditions of high salinity, the latter is thinner [108]. A filamentous freshwater cyanobacterium known as Coleofasciculus chthonoplastes can survive in salt concentrations as high as 1.5 M NaCl. It can modify the composition and arrangement of its gas vesicles and thylakoid membranes to change its cell shape and ultrastructure. It possesses fewer and more distributed gas vesicles and thylakoids in low salinity, while more compact thylakoids and gas vesicles in high salinity [99]. Therefore, these microbes can be very efficient in reducing the salinity stress in their host. One such example is the release of promoting factors synthesized by the Synechococcus sp. PCC 7002 and Coleofasciculus chthonoplastes cyanobacteria which help to improve the productivity of many plants. These symbionts also release certain signaling compounds which can be enacted to assemble a more diverse microbiome around their host. Under salinity stress, both bacteria and cyanobacteria can alter the structure and fluidity of their phospholipids, produce more hopanoids (sterol-like molecules), and incorporate compatible solutes into their membrane, such as glycine, betaine, proline, trehalose ectoine. These changes may aid the bacteria in preserving the stability and integrity of their membranes [43]. Under salinity stress, micro-organism can modify their cell walls by enhancing the cross-linking of peptidoglycan, resulting in the synthesis of more teichoic acids (polymers of glycerol or ribitol), and increasing the amounts of lipopolysaccharides (LPS) in their outer membrane. These modifications can make the bacterium more stiff and able to withstand osmotic shock [109]. In conditions of salinity, Chlorogleopsis PCC 6912, Phormidium uncinatum, Gleocapsasp, and Nostoc commune develop extracellular sheaths that serve as water reservoirs, absorbents, and barriers against desiccation and salinity [77]. Hence, by various mechanisms, these microbes release various compounds which enhance plant growth under abiotic stresses.
Role of Microbial Extracellular Compounds in Mitigating Salt Stress
The mechanism of the tolerance differs significantly in the environment. There are two types of organisms based on their adaptability to salt stress: Halophytes and glycophytes. Glycophytes which comprise most plant species, cannot survive high salinity. In such plants, the tolerance adaptation comes due to their symbionts like microalgae, fungi, and bacteria. These microbes release certain extracellular substances which function as the osmotic balancer around the plant cell, hence, improving the plant productivity in such conditions [31, 67]. Through several mechanisms including osmotic adjustment, phytohormones synthesis, free radical protection, and the synthesis of extracellular polymeric substances (EPSs), PGPB and cyanobacteria can significantly increase salt tolerance in plants [19]. Nostoc kihlmani and Anabaena cylindrica produce EPS and enhance soil structure and increased growth of wheat plant [27]. Ansari et al. [5] demonstrated that Bacillus pumilus strain FAB10 inoculation in salinity increased EPS production, due to EPS production biofilm are developed around the root surface and enhance salt tolerance property of wheat plant. In one of the studies, it was found that extracellular products released by Scytonema hofmanni can significantly improve the development and metabolic activities of Yerua PA rice seedlings under high NaCl concentrations. Gibberellin-like compounds may be present in the cyanobacterial extracellular products and could neutralize the negative effects of salt stress [80]. Similarly, Chroococcidiopsis sp., Anabaena sp., N. muscorum, Hapalosiphon sp., and other cyanobacterial species produce growth hormones, vitamins, and peptides that help wheat plants thrive under salinity [33, 41]. The mechanisms of mitigation of salt stress in plants by these microbial floras are depicted in Fig. 2. A more elaborate discussion is presented in the further subsections.
Role of Microbial Compounds
Microbial Release of Cell Signaling Compounds
PGPB also releases certain extracellular compounds which help to recruit all the essential components around its host which are important for the plant in surviving high salinity stress. IAA is believed to be one such signaling compound. It is a naturally occurring phytohormone found in plants and algae that many bacteria can also produce and release. In the algal–bacterial co-cultivation system, C. sorokiniana exchanged Trp and thiamine as a cross-species communication for IAA produced by A. brasilense [68]. Sulfobacteria interaction with diatom (P. multiseries) is also another such example in which the conversion of Trp, released by diatoms, into IAA. Furthermore, synthesized IAA can act as the signaling molecule to encourage diatom cell development. A positive feedback loop exists when cross-kingdom signaling occurs in this way. Under diverse environmental stress, IAA notably enhanced the development of a mutualistic connection between multiple organism systems in the environment [117]. Stress-responsive genes controlled by PGPR may have an impact on plants that display induced systemic tolerance. Arabidopsis expresses many genes including BAB18 (which codes for LEA proteins) and ERD15 (which codes for the early response to dehydration) in response to P. polymyxa inoculation [31].
PGPB either directly affects plants by synthesizing phytohormones or indirectly by stimulating signaling in the host. Hence, the interaction of salt-tolerance microbes with their host on the gene level has been proven to be effective in alleviating salt stress resistance. For example, tomato (L. esculentum) expressed the codA gene of A. globiformis, which encodes choline oxidase. It stimulated the production of glycine betaine and increased the plant’s salt tolerance [12]. Another study revealed that D. natronolimnaea STR inoculation affected the transcriptional machinery essential for salinity tolerance in wheat plants by controlling genes related to the SOS pathway (gene SOS1 and SOS4) [10]. Furthermore, increased gene expression of antioxidant enzymes such as ascorbate peroxidase (APX), catalase (CAT), and superoxide dismutase (SOD), as well as higher proline content, were also reported in PGPR-inoculated wheat plants [94]. As a result, the use of beneficial stress-tolerant microorganisms not only improves the microbial composition but also improves ecological compositions. The two cyanobacterial species, N. entophytum and O. angustissima have also been used as biofertilizers for pea plants in place of the usual chemical fertilizer in salinity. Under salt stress conditions, M. aeruginosa and P. agardhii can produce cyanopeptides such as microcystins and anabaenopeptins. These compounds may affect plant physiology by modulating intracellular processes like osmotic adjustment, antioxidant defense, hormonal balance, gene expression, and metabolite synthesis [25, 29, 65].
To detect and react to environmental challenges, some eukaryotes, plants, yeast, and bacteria use two-component systems, which are sensor-responsive circuits. RppA (Regulator of photosynthesis and photopigment-related gene expression A) or Rep1 (Response regulator 1) is a response regulator protein that was studied as part of a two-part system that included a sensor histidine kinase (Hik2). These Hik2 are released by cyanobacteria. The various methods by which bacteria and cyanobacteria participate in cell signaling pathways under salinity are summarized in Table 1.
-
Signaling via ROS: ROS are highly reactive chemicals that can oxidatively harm biological components. However, ROS also function as signaling molecules that control several cellular functions, including ion transport, gene expression, and enzyme activity [86]. Salt stress causes an increase in ROS production, which in turn activates stress-responsive genes and antioxidant enzymes. To modulate salt tolerance, ROS also interacts with other signaling molecules such as Ca2+, NO, H2S, and phytohormones [103]. Antioxidants produced by bacteria and cyanobacteria can scavenge ROS and shield plant cells from oxidative damage. For instance, glutathione peroxidase (GPX) and superoxide dismutase (SOD), which lower ROS levels in plants, can be produced by cyanobacteria [26]. Enterobactter cloacae regulate the salinity stress response antioxidant enzyme in maize plant. In another study, P. boryanum and O. acuta has been shown to regulate ROS in rice leaf through the production of antioxidant enzyme peroxidase under salinity stress [114]. P. Pseudomonas enhanced agricultural outcomes through the production of proline and antioxidant enzymes in the root system and shoots of plant [17].
-
Ca2+ signaling: In plants, Ca2+ acts as a universal secondary messenger to transmit a variety of signals. Its levels are controlled by channels, pumps, and transporters present in the plasma membrane and endomembrane system. Under conditions of salt stress, salt sensors or ROS production cause Ca2+ efflux, this sets off a series of processes involving Ca2+ binding proteins such as calmodulin and calcineurin B-related proteins. These proteins influence gene expression and cellular responses by interacting with downstream targets such as protein kinases and transcription factors [2, 32]. By generating Ca2+ binding chemicals or changing Ca2+ transporters, bacteria and cyanobacteria can modify Ca2+ signaling in plants [6]. For instance, bacteria can synthesize EPS that binds Ca2+ ions and controls how readily available they are to plant cells. In a study, Bacillus aryabhattai increased Solanum lycopersicum potential to endure salt by producing exo-polysaccharides [116].
-
Signaling through phytohormones: The organic substances that control the growth and development of plants are known as phytohormones. Stress responses also involve several phytohormones, including ethylene, salicylic acid, jasmonic acid, and abscisic acid (ABA) [107]. ABA is produced when plants are under salt stress, and it builds up in plant tissues. A signaling network that includes protein kinases, phosphatases, transcription factors, and ion channels is activated when ABA binds to its receptors [45]. Stomatal closure, osmotic control, gene expression, and adaptive growth are all regulated by ABA signaling. Interacting with ABA signaling or other metabolic pathways eg: ethylene, SA, and JA also affect salt tolerance [103].
Phytohormones can be synthesized, or its biosynthesis can be controlled by bacteria and cyanobacteria in plants in harsh environment. Phytohormones-ABA synthesized in salinity stress through which plant can improve tolerance in such condition. Phytohormone are also used as plant growth regulator, they can control plant morphology such as plant height, enhance branching, promote flowering, and regulate fruit development. Bacteria, for instance, can produce IAA or ACC-deaminase, which impacts the amounts of auxin or ethylene in plants. Under salt stress, cyanobacteria can synthesize cytokinin that promote plant growth [6]. Under salinity stress circumstances, endophytes, and plant growth-promoting bacteria (PGPB) also have a substantial impact on the induction of plant signaling. Under salt stress in T. aestivum, A protophormiae (SA3) and Dietzia natronolimnaea (STR1) alter the expression of the DREB2 transcription factor and the ethylene signaling regulatory compartment (CTR1) pathway as well as increasing IAA and reducing ABA and ACC levels [8]. There are various cyanobacterial species are involved in reducing salt stress in different plant species are listed in Table 2.
Importance of Microbial Exopolysaccharide in Maintaining the Osmotic
EPS is a crucial factor since it enables crops to flourish in challenging environments. EPS consists of biopolymers such as polyester, polyamides, and polysaccharides [79]. Polysaccharides stimulate macropore development and increase soil particle adhesion, which enhances soil porosity and aeration. Soil microorganisms can improve plant nutrient uptake and raise the water potential of the soil by generating EPS around the roots [42]. The slime structure of the EPS helps to bind the soil through multiple bonding such as H–bond, cation–anion interactions etc. which helps to improve bacteria and cyanobacteria's capacity to hold water and regulate the diffusion of organic carbon sources. As a result, a slimy substance develops around the soil aggregates, protecting them from salinity and dryness. Plants that have been modified with EPS-producing bacteria exhibit resilience to salinity and water shortage [104]. Exopolysaccharides and extracellular proteins are just a couple of the polymeric compounds that some cyanobacteria can make outside of their cells [78]. Such a phenomenon has been reported by [19] where the inoculation with P. putida strain GAP-P45 in saline soil increased the aggregate stability of the soil [19]. The EPS production can be done on a large scale by using multiple organisms such as Pseudomonas, Bacillus and Paenibacillus. Additionally, after simulating rainfall, strains of Streptomyces and Penicillium significantly reduced soil erosion and loss by releasing exopolysaccharide which promotes the aggregation of soil particles.
Various genera of microbial taxa synthesize a variety of EPS which differ significantly in their composition and behavior [11, 20]. Multiple monosaccharides such as mannose, glucose, xylose etc. can be found as the monomeric subunit of these EPSs [113]. Glucose is the primary monosaccharide component of the EPS produced by Mesorhizobium sp. Semia 816, followed by galactose and mannose [81]. The monosaccharides, disaccharides and polysaccharides released by these microorganisms work as the carbon sources for another microbial component of the soil biosphere. Hence releasing saccharides, peptides, and enzymes extracellularly promotes the fixation of nitrogen and plant growth promotion, due to the salinity [15]. Some examples of EPS produced by various cyanobacteria under salinity stress are listed in Table 2. Fertilizers are a common source of salts applied to soil, and their excess can have a significant effect on soil salinization. Out of all the mineral fertilizers, potassium chloride has the greatest effect on soil salinity because plants can readily absorb potassium as a macronutrient whereas chloride, a micronutrient, remains in the soil. High concentrations of chloride ions may be detrimental to plant growth and yield, much like Na+ ion. For example, Cl− inhibits photosystem II's effectiveness, disrupting photosynthesis, and interacts with other essential plant growth anions such as PO42− and NO3− [49]. It has been demonstrated that introducing various bacterial strains into plants causes an increase in plant growth and a decrease in Cl− uptake. Reduction in Cl− ion in faba beans inoculated with a melatonin and EPS-producing Azotobacter chroococcum strain. However, because carboxyl and hydroxyl functional groups predominate in bacterial EPS, most of these EPS are negatively charged, suggesting that the drop-in chloride anion concentration in bacteria-inoculated plants may not be due to the EPS’s ability to adsorb Cl− [58]. Subsequently, these activities of the micro-engineers develop a surrounding that nullifies the effects of salinity.
The addition of these EPS-producing microbes can have a very impactful effect on plant productivity under salt stress. One of the studies used the potential of Planococcus rifietoensis RT4 and Halomonas variabilis HT1 in chickpeas and observed a many-fold improvement in the characteristics under the high salinity [75]. In saline environments, EPS may control the microsphere composition and can limit sodium (Na+) uptake by plants. Additionally, EPS plays a significant role in the development of relationships between plants and microbes [78]. It has been observed that Bacillus drentensis and Enterobacter cloacae can improve salt tolerance by enhancing nutrient and water uptake by forming biofilm in the root zone of mung beans [79]. By lowering the Na+ level that is accessible for plant uptake, EPS mitigates the negative consequences of salt stress. Microbes like Pseudomonas PS01 can use their EPS to regulate the lipoxygenase (LOX2) gene which plays a significant role in the jasmonic acid (JA) synthesis pathway. JA-pathway is the main component of the plants against multiple abiotic stresses [9]. Similarly, Pseudomonas anguilliseptica SAW24 is involved in enhancing the plant height and biomass of Faba beans under high salinity [9] while Aeromonas spp. SAL-17 and SAL-21 increase the salt tolerance in wheat plants by improving multiple physiological parameters such as proline content, nitrate reductase activity, transpiration rate, and root shoot length [59]. Cyanobacteria, specifically the species Nostoc spp. and Anabaena spp., have special qualities of binding sodium ions and forming biofilms that make them useful for remediating salt-affected soils [79].
Role of microbial-based Volatile Organic Compounds (VOCs) in Promoting Salt Tolerance in Plant
VOCs are low molecular weight, lipophilic compounds that have low boiling points and high vapor pressure. They are mainly synthesized by the microbes and have multi-dimensional functions. Indirect interactions between plants and microbes are made possible by these compounds, which enable function as short- and long-distant signals dissemination in the rhizosphere [52]. They are tiny molecules from many chemical classes that easily diffuse and evaporate through holes filled with air and water. VOCs are the perfect signal candidates in interactions with plant roots because of these characteristics [46]. It was discovered that bacteria produce over 1000 VOCs and non-organic volatile chemicals, some of which improve agricultural plant quality and yield [105]. The bacterial VOCs control the physiology and hormonal balance in the plant. It can also influence photosynthesis abilities, defense mechanisms, and nutrient uptake under harsh conditions [101]. In Arabidopsis, VOCs produced by B. subtilis GB03 activate a variety of hormonal signals, including auxin, cytokinins, brassinosteroids, gibberellins, and salicylic acid. Paraburkholderia phytofirmans produced 2-undecanone, and 3-methylbutanol, which improved plant development and resistance to salinity stress. [44, 82]. The control of the Na+ flux is the primary mechanism by which bacterial VOCs develop systemic tolerance in plants to saline conditions. These mechanisms are majorly governed by the transport proteins, including HKT1 and SOS1 [64]. Bacterial VOC promotes the expression of the HKT1 gene, which in turn speeds up the removal of sodium ions from xylem sap and plant leaves. This phenomenon has been explored by Panpatte et al. [71] and Cappellari et al. [14] where the higher biomass yield and lower sodium content are observed after the treatment with the GB03 VOC in Arabidopsis plants. The SOS3 (salt overly sensitive 3) calcium-signaling sensor may also help with salinity tolerance that is mediated by VOCs of plant growth-promoting bacteria [71]. Volatile compound-induced salt tolerance by Pseudomonas simiae strain AU was seen in soybean plants where VOCs not only reduced root Na+ levels but also boosted proline accumulation, which protects cells from osmotic stress. Additionally, the hiked levels of vegetative storage protein (VSP) along with other essential biomolecules have been observed in AU-treated plants [71]. Multi-types of biomolecules such as alcohols, Alkanes, Ketones etc. can be part of the VOCs released by Chlamydomonas reinhardtii. Due to an increase in reactive oxygen species (ROS) during salt stress, Solieria chordalis and Gymnogongrus antarcticus produced more halogenated hydrocarbons. VOCs can also function as an inhibitor to promote the survivability of plants under stress [64].
Role of Microbial Secondary Metabolites in Salinity Stress
Microbial release of secondary metabolites such as cytokinin, gibberellins, and indole acetic acid (IAA) have a very potent role in tackling salinity stress. A crucial physiological signal called ABA controls the expression of numerous genes that respond to osmotic and salt stress. These genes are responsible for controlling the water flux around the plant which is most hit under high salinity. Abiotic stressors in plants also cause them to respond with salicylic acid (SA) and brassinosteroids (BR) [37]. Under salt stress, rice seedlings produced more endogenous SA and showed an increase in the activity of SA biosynthetic enzymes. Plants can tolerate salt better in the presence of the exogenous application of SA and BR [115]. In barley, SA increased the amounts of carotenoids and chlorophyll and preserved membrane integrity in saline circumstances. BR promotes the activities of superoxide dismutase (SOD), Peroxidase (POX) and APX and enhances the production of tocopherol, ascorbate, and reduced glutathione. Synechococcus 6301 under salt stress increased cytochrome oxidase and HC/NaC antiport activities. IAA levels in Nostoc carneum TUBT04 and Nostoc carneum TUBT05 were increased in salinity [16]. Additionally, it was found that neither of the cyanobacterial strains required tryptophan as a precursor for IAA production. Gibberellins and cytokinins were also found in Anabaena cylindrica and Nostoc commune. Actinobacteria, Nocardia, Frankia, Kitasatospora, and Streptomyces are some of the bacteria that produce IAA. Abscisic acid (ABA), often known as the stress hormone is increased in the root zone when there is a water shortage due to salinity stress [42]. Under salinity, the amount of ABA rises, which aids the plant in reducing the effects of stress. In root vacuoles under salinity, ABA aids in the accumulation of compatible solutes such as proline and carbohydrates. It demonstrates that Bacillus subtilis GB03 and Pseudomonas putida R-168 enhanced the surface area of thicker roots and root dry weight in Maize seedlings under salt stress [49, 70, 97]. Bacteria can also produce gibberellins, which aid in promoting plant growth and productivity. According to [61], the overproduction of proline, ABA, trans-zeatin riboside, GA3, and IAAs led to an increase in root and shoot length and biomass when a halo-tolerant bacterium was introduced into soybean plants. While certain PGPRs are proven to metabolize ABA and have varying effects when exposed to salinity stress, other PGPRs are demonstrated to aid in its synthesis [74]. Various secondary metabolites synthesized by microbes under salt stress are listed in Table 2.
Role of Microbial-based Osmo-Protectants in Salt Tolerance in Plants
Microorganism synthesizes organic osmolytes in the cytoplasm to maintain their osmotic condition and to enhance plants’ reactions to such stress. In addition, osmoprotectants like proline, betaine, trehalose, glycine, phenols, and flavonoids were produced by helpful bacteria like Azospirillum, Burkholderia, Arthrobacter, Bacillus, Pseudomonas, and Rhizobium [108]. Additionally, osmoprotectants made by bacteria are biosynthesized quicker than those made by plants that relate to them. Heterotrophic bacteria like Pseudomonas and Stenotrophomonas strains, as well as cyanobacteria with moderate salt tolerance like Synechocystis sp. PCC 6803 and Synechococcus sp. PCC 7002, can de novo synthesize and store glucosylglycerol (GG) as an osmo-protectant. GG can be used to stabilize the proteins and enzymes such as superoxide dismutase in plants [42, 92]. According to the extensive knowledge gained through genome mining, halotolerant cyanobacteria have extended gene families and/or clusters that code for enzymes that produce specific osmoprotectants such glycine betaine (GB), betaine derivatives, and mycosporine-like amino acids (MAAs), among others [108].
Release of Microbial-based Antioxidants in Salt Stress and their Role in Salt Tolerance in Plants
In Synechocystis and Anabaena, different antioxidative mechanisms have been stimulated in response to increased saline content [82]. Under harsh conditions, multiple ROS are produced in the plant cell. ROS can damage cell integrity and significantly oxidize substances [103]. The antioxidant enzymes such as SOD, CA, GPX etc. are key machinery that tackles the ROS in the plant system. The synthesis of these enzymes can be controlled by symbionts like Pseudomonas pseudoalcaligenes and Bacillus pumilus [98]. In tomato plants which are exposed to high salt concentrations, increased SOD, CA, APX, GR, and GST activities were found which are controlled by Sphingomonas sp., Pseudomonas pseudoalcaligenes and Bacillus pumilus [42].
Even though ascorbate is one of the important antioxidants in plant cells, applying it exogenously helps plants recover from stress and reduces the negative effects of salt. Additionally, the effects of salt stress on plants are lessened by the exogenous administration of glutathione as a free radical scavenger. Other nonenzymatic antioxidants that are able to protect plants from oxidative stress include the vitamins C and E, carotenoids, and lipoic acid [82].
Conclusion
Salinity stress, in plants can have negative effects on society and the economy along with the environment. Plant growth and yields can be heavily damaged as a result of the changing environmental circumstances. Plant cells synthesize ROS as salinity stressors increase, which reduces ecological fitness. In the ensuing decades, the situation will worsen, endangering plant life. Plants enlist antioxidants to maintain a balance between the production and quenching of ROS. Through several mechanisms, PGPB can play a significant role in mitigating abiotic stress in their host. It can control many pathways such as the antioxidant system, osmotic adjustment, and metabolic reprogramming by releasing multiple extracellular compounds such as phosphate solubilization, nutrient mobilization, phytohormone and VOCs. Therefore, PGPB and cyanobacteria are excellent alternatives to conventional fertilizers because of their affordability, environmental friendliness, and sustainability to boost plant tolerance to a variety of conditions, such as salinity and drought.
References
Abbas HH, Ali ME, Ghazal FM, El-Gaml NM (2015) Impact of cyanobacteria inoculation on rice (Orize sativa) yield cultivated in saline soil. J Am Sci 11(2):13–19
Aguilera A, Klemenčič M, Sueldo DJ, Rzymski P, Giannuzzi L, Martin MV (2021) Cell death in cyanobacteria: current understanding and recommendations for a consensus on its nomenclature. Front Microbiol 12:631654
Ahmad M, Zahir ZA, Asghar HN, Asghar M (2011) Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase. Can J Microbiol 57(7):578–589
Toderich KN, Shuyskaya EV, Taha FK, Matsuo N, Ismail S, Aralova DB, Radjabov TF (2013) Integrating agroforestry and pastures for soil salinity management in dryland ecosystems in Aral Sea basin. In: Developments in soil salinity assessment and reclamation: innovative thinking and use of marginal soil and water resources in irrigated agriculture, pp 579–602
Ansari FA, Ahmad I, Pichtel J (2019) Growth stimulation and alleviation of salinity stress to wheat by the biofilm forming Bacillus pumilus strain FAB10. Appl Soil Ecol 143:45–54
Babele PK, Kumar J, Chaturvedi V (2019) Proteomic de-regulation in cyanobacteria in response to abiotic stresses. Front Microbiol 10:1315
Barnawal D, Bharti N, Maji D, Chanotiya CS, Kalra A (2014) ACC deaminase-containing Arthrobacter protophormiae induces NaCl stress tolerance through reduced ACC oxidase activity and ethylene production resulting in improved nodulation and mycorrhization in Pisum sativum. J Plant Physiol 171(11):884–894
Barnawal D, Bharti N, Pandey SS, Pandey A, Chanotiya CS, Kalra A (2017) Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol Plant 161(4):502–514
Bhagat N, Raghav M, Dubey S, Bedi N (2021) Bacterial exopolysaccharides: Insight into their role in plant abiotic stress tolerance. J Microbiol Biotechnol. https://doi.org/10.4014/jmb.2105.05009
Bharti N, Pandey SS, Barnawal D, Patel VK, Kalra A (2016) Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci Rep 6(1):34768
Bhatnagar M, Bhatnagar A (2019) Microbial diversity in normal & extreme environments, vol 1. Springer, Singapore, pp 447–496
Bhowal B, Chandra P, Saxena SC (2021) Engineering glycine betaine biosynthesis in alleviating abiotic stress effects in plants. In: Compatible solutes engineering for crop plants facing climate change. Springer, Cham, pp 97–127
Canfora L, Bacci G, Pinzari F, Lo Papa G, Dazzi C, Benedetti A (2014) Salinity and bacterial diversity: to what extent does the concentration of salt affect the bacterial community in a saline soil? PLoS ONE 9(9):e106662
Cappellari LDR, Banchio E (2020) Microbial volatile organic compounds produced by Bacillus amyloliquefaciens GB03 ameliorate the effects of salt stress in Mentha piperita principally through acetoin emission. J Plant Growth Regul 39:764–775
Casillo A, Lanzetta R, Parrilli M, Corsaro MM (2018) Exopolysaccharides from marine and marine extremophilic bacteria: structures, properties, ecological roles and applications. Mar Drugs 16(2):69
Chittapun S, Limbipichai S, Amnuaysin N, Boonkerd R, Charoensook M (2018) Effects of using cyanobacteria and fertilizer on growth and yield of rice, Pathum Thani I a pot experiment. J Appl Phycol 30:79–85
Chu TN, Tran BTH, Van Bui L, Hoang MTT (2019) Plant growth-promoting rhizobacterium Pseudomonas PS01 induces salt tolerance in Arabidopsis thaliana. BMC Res Notes 12(1):1–7
Coba de la Pena T, Redondo FJ, Manrique E, Lucas MM, Pueyo JJ (2010) Nitrogen fixation persists under conditions of salt stress in transgenic Medicago truncatula plants expressing a cyanobacterial flavodoxin. Plant Biotechnol J 8(9):954–965
Costa OY, Raaijmakers JM, Kuramae EE (2018) Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front Microbiol 9:1636
Cruz D, Vasconcelos V, Pierre G, Michaud P, Delattre C (2020) Exopolysaccharides from cyanobacteria: strategies for bioprocess development. Appl Sci 10(11):3763
Cui L, Liu Y, Yang Y, Ye S, Luo H, Qiu B, Gao X (2018) Salinity Tolerance of Picochlorum atomus and the Use of Salinity for Contamination Control by the Freshwater Cyanobacterium Pseudanabaena limnetica. PLoS ONE 8(5):63569. https://doi.org/10.1371/journal.pone.0063569
Del Carmen Orozco-Mosqueda M, Glick BR, Santoyo G (2020) ACC deaminase in plant growth-promoting bacteria (PGPB): an efficient mechanism to counter salt stress in crops. Microbiol Res 235:126439
Egamberdieva D, Jabborova D, Hashem A (2015) Pseudomonas induces salinity tolerance in cotton (Gossypium hirsutum) and resistance to Fusarium root rot through the modulation of indole-3-acetic acid. Saudi J Biol Sci 22(6):773–779
Ennab HA, El-Shemy MA, Alam-Eldein SM (2020) Salicylic acid and putrescine to reduce post-harvest storage problems and maintain quality of murcott mandarin fruit. Agronomy 10(1):115
Ferreira AHF (2006) Peptides in Cyanobacteria under different environmental conditions. Doctoral dissertation, Berlin, Techn. Univ., Diss.
Gamalero E, Glick BR (2022) Recent advances in bacterial amelioration of plant drought and salt stress. Biology 11(3):437
Gheda SF, Ahmed DA (2015) Improved soil characteristics and wheat germination as influenced by inoculation of Nostoc kihlmani and Anabaena cylindrica. Rendiconti Lincei 26:121–131
Golldack D, Lüking I, Yang O (2011) Plant tolerance to drought and salinity: stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep 30:1383–1391
Gr S, Yadav RK, Chatrath A, Gerard M, Tripathi K, Govindsamy V, Abraham G (2021) Perspectives on the potential application of cyanobacteria in the alleviation of drought and salinity stress in crop plants. J Appl Phycol. https://doi.org/10.1007/s10811-021-02570-5
Gunde-Cimerman N, Plemenitaš A, Oren A (2018) Strategies of adaptation of microorganisms of the three domains of life to high salt concentrations. FEMS Microbiol Rev 42(3):353–375
Gupta A, Mishra R, Rai S, Bano A, Pathak N, Fujita M, Kumar M, Hasanuzzaman M (2022) Mechanistic insights of plant growth promoting bacteria mediated drought and salt stress tolerance in plants for sustainable agriculture. Int J Mol Sci 23(7):3741
Hunpatin OS, Yuan G, Nong T, Shi C, Wu X, Liu H, Ning Y, Wang Q (2023) The roles of calcineurin B-like proteins in plants under salt stress. Int J Mol Sci 24(23):16958
Hussain A, Hasnain S (2011) Phytostimulation and biofertilization in wheat by cyanobacteria. J Ind Microbiol Biotechnol 38(1):85–92
Jan Z, Ali S, Sultan TW, Ahmad W (2017) The role of cyanobacteria on availability of major plant nutrients and soil organic matter to rice crop under saline soil condition. Sarhad J Agric 33(4):566–572
Jia J, Liang Y, Gou T, Hu Y, Zhu Y, Huo H, Guo J, Gong H (2020) The expression response of plasma membrane aquaporins to salt stress in tomato plants. Environ Exp Bot 178:104190
Kazamia E, Czesnick H, Nguyen TTV, Croft MT, Sherwood E, Sasso S, Smith AG (2012) Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria exhibit regulation. Environ Microbiol 14(6):1466–1476
Kazerooni EA, Maharachchikumbura SS, Adhikari A, Al-Sadi AM, Kang SM, Kim LR, Lee IJ (2021) Rhizospheric Bacillus amyloliquefaciens protects Capsicum annuum cv. Geumsugangsan from multiple abiotic stresses via multifarious plant growth-promoting attributes. Front Plant Sci 12:669693
Khan MY, Nadeem SM, Sohaib M, Waqas MR, Alotaibi F, Ali L, Zahir ZA, Al-Barakah FN (2022) Potential of plant growth promoting bacterial consortium for improving the growth and yield of wheat under saline conditions. Front Microbiol 13:958522
Kim K, Jang YJ, Lee SM, Oh BT, Chae JC, Lee KJ (2014) Alleviation of salt stress by Enterobacter sp. EJ01 in tomato and arabidopsis is accompanied by up-regulation of conserved salinity responsive factors in plants. Mol Cell 37(2):109
Klähn S, Mikkat S, Riediger M, Georg J, Hess WR, Hagemann M (2021) Integrative analysis of the salt stress response in cyanobacteria. Biol Direct 16(1):26
Kollmen J, Strieth D (2022) The beneficial effects of cyanobacterial co-culture on plant growth. Life 12(2):223
Kumar A, Singh S, Gaurav AK, Srivastava S, Verma JP (2020) Plant growth-promoting bacteria: biological tools for the mitigation of salinity stress in plants. Front Microbiol 11:1216
Laxmi KA, Kumar V, Muthukumar M, Bajpai A (2021) Morphological indicators of salinity stress and their relation with osmolyte associated redox regulation in mango cultivars. J Plant Biochem Biotechnol 30:918–929
Ledger T, Rojas S, Timmermann T, Pinedo I, Poupin MJ, Garrido T, Richter P, Tamayo J, Donoso R (2016) Volatile-mediated effects predominate in Paraburkholderia phytofirmans growth promotion and salt stress tolerance of Arabidopsis thaliana. Front Microbiol 7:1838
Lee SC, Luan S (2012) ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ 35(1):53–60
Tataranni G, Dichio B, Xiloyannis C (2012) Soil fungi-plant interaction. In: Advances in selected plant physiology aspects. InTech, pp 161–188
Liu J, Zhang W, Long S, Zhao C (2021) Maintenance of cell wall integrity under high salinity. Int J Mol Sci 22(6):3260
Lodewyckx C, Vangronsveld J, Porteous F, Moore ER, Taghavi S, Mezgeay M, der Lelie DV (2002) Endophytic bacteria and their potential applications. Crit Rev Plant Sci 21(6):583–606
Lopes MJDS, Dias-Filho MB, Gurgel ESC (2021) Successful plant growth-promoting microbes: Inoculation methods and abiotic factors. Front Sustainable Food System 5:606454
Loudari A, Latique S, Mayane A, Colinet G, Oukarroum A (2023) Polyphosphate fertilizer impacts the enzymatic and non-enzymatic antioxidant capacity of wheat plants grown under salinity. Sci Rep 13(1):11212
Lubna KMA, Asaf S, Jan R, Waqas M, Kim KM (2022) Endophytic fungus Bipolaris sp. CSL-1 induces salt tolerance in Glycine max. L via modulating its endogenous hormones, antioxidative system and gene expression. J Plant Inter 17(1):319–332
Luo H, Riu M, Ryu CM, Yu JM (2022) Volatile organic compounds emitted by Burkholderia pyrrocinia CNUC9 trigger induced systemic salt tolerance in Arabidopsis thaliana. Front Microbiol 13:1050901
Ma Y, Dias MC, Freitas H (2020) Drought and salinity stress responses and microbe-induced tolerance in plants. Front Plant Sci 11:591911
Manchanda R (2018) Significance of prognostic factor research in clinical verification. Ind J Res Homoeopath 12(1):1–3
Marulanda A, Azcón R, Chaumont F, Ruiz-Lozano JM, Aroca R (2010) Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 232:533–543
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42(6):565–572
Mishra P, Mishra J, Arora NK (2021) Plant growth promoting bacteria for combating salinity stress in plants–Recent developments and prospects: a review. Microbiol Res 252:126861
Morcillo RJ, Manzanera M (2021) The effects of plant-associated bacterial exopolysaccharides on plant abiotic stress tolerance. Metabolites 11(6):337
Nawaz MS, Arshad A, Rajput L, Fatima K, Ullah S, Ahmad M, Imran A (2020) Growth-stimulatory effect of quorum sensing signal molecule N-acyl-homoserine lactone-producing multi-trait Aeromonas spp. on wheat genotypes under salt stress. Front Microbiol. https://doi.org/10.3389/fmicb.2020.553621
Nawaz T, Saud S, Gu L, Khan I, Fahad S, Zhou R (2024) Cyanobacteria harnessing the power of microorganisms for plant growth promotion, stress alleviation, and phytoremediation in the era of sustainable agriculture. Plant Stress 11:100399
Naz N, Hameed M, Ashraf M, Ahmad R, Arshad M (2009) Eco-morphic variation for salt tolerance in some grasses from Cholistan Desert. Pakistan Pak J Bot 41(4):1707–1714
S NK (2010) Cyanobacterial reclamation of salt-affected soil, genetic engineering, biofertilisation, soil quality and organic farming. Sustainable Agric Rev 4:243–275
Normand P, Caumette P, Goulas P, Pujic P, Wisniewski-Dyé F (2015) Adaptations of prokaryotes to their biotopes and to physicochemical conditions in natural or anthropized environments. Environmental microbiology: fundamentals and applications: microbial ecology. Springer, Dordrecht, pp 293–351
Numan M, Bashir S, Khan Y, Mumtaz R, Shinwari ZK, Khan AL, Khan A, Ahmed AH (2018) Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: a review. Microbiol Res 209:21–32
Osman MEH, El-Sheekh MM, El-Naggar AH, Gheda SF (2010) Effect of two species of cyanobacteria as biofertilizers on some metabolic activities, growth, and yield of pea plant. Biol Fertil Soils 46:861–875
Pagnussat LA, Do Nascimento M, Maroniche G, Gonorazky G, Rizza LS, Creus C, Curatti L (2023) Azospirillum baldaniorum improves acclimation, lipid productivity and oxidative response of a microalga under salt stress. Algal Res 74:103192
Palacios OA, Espinoza-Hicks JC, Camacho-Dávila AA, López BR, de Bashan LE (2023) Differences in exudates between strains of chlorella sorokiniana affect the interaction with the microalga growth-promoting bacteria azospirillum brasilense: differences in exudates between strains of Chlorella sorokiniana affect the interaction with the microalga growth-promoting bacteria Azospirillum brasilense. Microbial Ecol 85(4):1412–1422
Palacios OA, Gomez-Anduro G, Bashan Y, de-Bashan, L.E. (2016) Tryptophan, thiamine and indole-3-acetic acid exchange between Chlorella sorokiniana and the plant growth-promoting bacterium Azospirillum brasilense. FEMS Microbiol Ecol 92(6):fiw77
Palaniyandi SA, Damodharan K, Yang SH, Suh JW (2014) Streptomyces sp strain PGPA39 alleviates salt stress and promotes growth of ‘Micro Tom’tomato plants. J Appl Microbiol 117(3):766–773
Paliwoda D, Mikiciuk G (2020) Use of rhizosphere microorganisms in plant production a review study. J Ecol Eng. https://doi.org/10.12911/22998993/126597
Panpatte DG, Shukla YM, Shelat HN, Vyas RV, Jhala YK (2017) Bacterial volatile organic compounds: a new insight for sustainable agriculture. Microorganisms for Green Revolution, vol 1. Springer, Singapore, pp 151–166
Panwar M, Tewari R, Nayyar H (2016) Native halo-tolerant plant growth promoting rhizobacteria Enterococcus and Pantoea sp. improve seed yield of Mungbean (Vigna radiata L.) under soil salinity by reducing sodium uptake and stress injury. Physiol Mol Biol Plants 22:445–459
Perera I, Subashchandrabose SR, Venkateswarlu K, Naidu R, Megharaj M (2018) Consortia of cyanobacteria/microalgae and bacteria in desert soils: an underexplored microbiota. Appl Microbiol Biotechnol 102:7351–7363
Qin Y, Druzhinina IS, Pan X, Yuan Z (2016) Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol Adv 34(7):1245–1259
Qurashi AW, Sabri AN (2012) Biofilm formation in moderately halophilic bacteria is influenced by varying salinity levels. J Basic Microbiol 52(5):566–572
Ramanan R, Kim BH, Cho DH, Oh HM, Kim HS (2016) Algae–bacteria interactions: evolution, ecology and emerging applications. Biotechnol Adv 34(1):14–29
Rastogi RP, Sonani RR, Madamwar D (2014) The high-energy radiation protectant extracellular sheath pigment scytonemin and its reduced counterpart in the cyanobacterium Scytonema sp. R77DM. Biores Technol 171:396–400
Richert L, Golubic S, Guédès RL, Ratiskol J, Payri C, Guezennec J (2005) Characterization of exopolysaccharides produced by cyanobacteria isolated from Polynesian microbial mats. Curr Microbiol 51:379–384
Rocha F, Esteban Lucas-Borja M, Pereira P, Muñoz-Rojas M (2020) Cyanobacteria as a nature-based biotechnological tool for restoring salt-affected soils. Agronomy 10(9):1321
Rodríguez AA, Stella AM, Storni MM, Zulpa G, Zaccaro MC (2006) Effects of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Saline systems 2:1–4
Roesler BCS, Vaz RG, Castellane TCL, de Macedo Lemos EG, Burkert CAV (2021) The potential of extracellular biopolymer production by Mesorhizobium sp. from monosaccharide constituents of lignocellulosic biomass. Biotech Lett 43:1385–1394
Saberi Riseh R, Ebrahimi-Zarandi M, Tamanadar E, Moradi Pour M, Thakur VK (2021) Salinity stress: Toward sustainable plant strategies and using plant growth-promoting rhizobacteria encapsulation for reducing it. Sustainability 13(22):12758
Sahoo RK, Ansari MW, Pradhan M, Dangar TK, Mohanty S, Tuteja N (2014) A novel Azotobacter vinellandii (SRI Az 3) functions in salinity stress tolerance in rice. Plant Signal Behav 9(7):511–523
Sapre S, Gontia-Mishra I, Tiwari S (2018) Klebsiella sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiol Res 206:25–32
Sarkar A, Pramanik K, Mitra S, Soren T, Maiti TK (2018) Enhancement of growth and salt tolerance of rice seedlings by ACC deaminase producing Burkholderia spp. MTCC 12259. J Plant Physiol 231:434–442
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24(10):R453–R462
Sharma A, Dev K, Sourirajan A, Choudhary M (2021) Isolation and characterization of salt-tolerant bacteria with plant growth-promoting activities from saline agricultural fields of Haryana, India. J Genet Eng Biotechnol 19(1):1–10
Sharma A, Maurya N, Singh SK, Sundaram S (2024) Investigation on synergetic strategy for the rejuvenation of Cr (VI) contaminated soil using biochar-immobilized bacteria and cyanobacteria consortia. J Environ Chem Eng 12(2):112034
Sharma A, Singh SK, Sundaram S (2024) Efficient biosequestration of Cr (VI) by Bacillus spp. SSAU-2: optimization, mathematical modelling, and plant growth promotion. Biochem Engineer J 204:109186
Sharma A, Singh SK, Maurya N, Tripathi SM, Jaiswal S, Agrawal M, Sundaram S (2024) Restoration of the soil fertility under Cr (VI) and artificial drought condition by the utilization of plant growth–promoting Bacillus spp. SSAU2. Int Microbiol. https://doi.org/10.1007/s10123-024-00528-4
Sharma A, Singh SK, Nath A, Sundaram S (2024) Methyl red biodegradation by novel halophilic Lactiplantibacillus plantarum SS-AU1 isolated from river Ganges. Int J Environ Sci Technol 21(10):7191–7206
Singh M, Kumar J, Singh S, Singh VP, Prasad SM (2015) Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Rev Environ Sci Biotechnol 14:407–426
Singh, N., Maurya, V., Sharma, I., Kumar, Y., Kumar, R., & Sharma, A. (2024). Combinations of PGPR with Other Soil Micro-and Macro-Organisms for Improved Stress Tolerance in Plants.
Singh RP, Jha PN (2016) Alleviation of salinity-induced damage on wheat plant by an ACC deaminase-producing halophilic bacterium Serratia sp. SL-12 isolated from a salt lake. Symbiosis 69:101–111
Singh S (2014) A review on possible elicitor molecules of cyanobacteria: their role in improving plant growth and providing tolerance against biotic or abiotic stress. J Appl Microbiol 117(5):1221–1244
Slama HB, Chenari Bouket A, Alenezi FN, Luptakova L, Baranov O, Ahadi R, Belbahri L (2023) Impacts of salt stress on the rhizosphere and endophytic bacterial role in plant salt alleviation. Int J Plant Biol 14(2):361–376
Solomon W, Mutum L, Rakszegi M, Janda T, Molnár Z (2023) Harnessing the synergy of the Cyanobacteria-plant growth promoting bacteria for improved maize (Zea mays) growth and soil health. Sustainability 15(24):16660
Sorokan A, Cherepanova E, Burkhanova G, Veselova S, Rumyantsev S, Alekseev V, Mardanshin I, Sarvarova E, Khairullin R, Benkovskaya G, Maksimov I (2020) Endophytic Bacillus spp. as a prospective biological tool for control of viral diseases and non-vector Leptinotarsa decemlineata Say. in Solanum tuberosum L. Front Microbiol 11:569457
Srivastava R, Kanda T, Yadav S, Singh N, Yadav S, Prajapati R, Kesari V, Atri N (2023) Salinity pretreatment synergies heat shock toxicity in cyanobacterium Anabaena PCC7120. Front Microbiol 14:1061927
Suarez C, Cardinale M, Ratering S, Steffens D, Jung S, Montoya AMZ, Geissler-Plaum R, Schnell S (2015) Plant growth-promoting effects of Hartmannibacter diazotrophicus on summer barley (Hordeum vulgare L.) under salt stress. Appl Soil Ecol 95:23–30
Sunita K, Mishra I, Mishra J, Prakash J, Arora NK (2020) Secondary metabolites from halotolerant plant growth promoting rhizobacteria for ameliorating salinity stress in plants. Front Microbiol 11:567768
Tufail MA, Bejarano A, Shakoor A, Naeem A, Arif MS, Dar AA, Farooq TH, Pertot I, Puopolo G (2021) Can bacterial endophytes be used as a promising bio-inoculant for the mitigation of salinity stress in crop plants? a global meta-analysis of the last decade. Microorganisms 9(9):1861
Uniyal S, Bhandari M, Singh P, Singh RK, Tiwari SP (2022) Cytokinin biosynthesis in cyanobacteria: Insights for crop improvement. Front Genet 13:933226
Upadhyay SK, Singh JS, Singh DP (2011) Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 21(2):214–222
Vasseur-Coronado M, Vlassi A, Boulois HDD, Schuhmacher R, Parich A, Pertot I, Puopolo G (2021) Ecological role of volatile organic compounds emitted by Pantoea agglomerans as interspecies and interkingdom signals. Microorganisms 9(6):1186
Verma E, Singh S, Niveshika, & Mishra, A. K. (2019) Salinity-induced oxidative stress-mediated change in fatty acids composition of cyanobacterium Synechococcus sp. PCC7942. Int J Environ Sci Technol 16:875–886
Verma V, Ravindran P, Kumar PP (2016) Plant hormone-mediated regulation of stress responses. BMC Plant Biol 16:1–10
Waditee-Sirisattha R, Kageyama H (2023) Halotolerance, stress mechanisms, and circadian clock of salt-tolerant cyanobacteria. Appl Microbiol Biotechnol 107(4):1129–1141. https://doi.org/10.1007/s00253-023-12390-x
Wang H, Li HX, Fang F, Guo JS, Chen YP, Yan P, Yang JX (2019) Underlying mechanisms of ANAMMOX bacteria adaptation to salinity stress. J Ind Microbiol Biotechnol 46(5):573–585
Wang SN, Ge SH, Zhuang LL, Zhang J (2024) Multiple pathways for the enhancement of wheat growth by Chlorella vulgaris. J Plant Growth Regul 43(2):550–562
Wang T, Li D, Tian X, Huang G, He M, Wang C, Kumbhar AN, Woldemicael AG (2024) Mitigating salinity stress through interactions between microalgae and different forms (free-living & alginate gel-encapsulated) of bacteria isolated from estuarine environments. Sci Total Environ 926:171909
Wang W, Wu Z, He Y, Huang Y, Li X, Ye BC (2018) Plant growth promotion and alleviation of salinity stress in Capsicum annuum L. by Bacillus isolated from saline soil in Xinjiang. Ecotoxicol Environ Saf 164:520–529
Xia M, Zhang S, Shen L, Yu R, Liu Y, Li J, Wu X, Chen M, Qiu G, Zeng W (2022) Optimization and characterization of an antioxidant exopolysaccharide produced by Cupriavidus pauculus 1490. J Polym Environ. https://doi.org/10.1007/s10924-021-02339-4
Yadav P, Singh RP, Rana S, Joshi D, Kumar D, Bhardwaj N, Gupta RK, Kumar A (2022) Mechanisms of stress tolerance in cyanobacteria under extreme conditions. Stresses 2(4):531–549
Yang W, Wang F, Liu LN, Sui N (2020) Responses of membranes and the photosynthetic apparatus to salt stress in cyanobacteria. Front Plant Sci 11:713
Yoo SJ, Weon HY, Song J, Sang MK (2019) Induced tolerance to salinity stress by halotolerant bacteria Bacillus aryabhattai H19–1 and B. mesonae H20–5 in tomato plants. J Microbiol Biotechnol 29(7):1124–1136
You X, Xu N, Yang X, Sun W (2021) Pollutants affect algae-bacteria interactions: a critical review. Environ Pollut 276:116723
Zawoznik MS, Ameneiros M, Benavides MP, Vázquez S, Groppa MD (2011) Response to saline stress and aquaporin expression in Azospirillum-inoculated barley seedlings. Appl Microbiol Biotechnol 90:1389–1397
Zhou L, Liu W, Duan H, Dong H, Li J, Zhang S, Zhang J, Ding S, Xu T, Guo B (2023) Improved effects of combined application of nitrogen-fixing bacteria Azotobacter beijerinckii and microalgae Chlorella pyrenoidosa on wheat growth and saline-alkali soil quality. Chemosphere 313:137409
Acknowledgements
Neetu Maurya is thankful to the UGC for providing fellowship for the financial assistance.
Author information
Authors and Affiliations
Contributions
Neetu Maurya contributed to the manuscript by drafting and writing. Abhijeet Sharma contributed to the manuscript drafting assistance to Neetu Maurya who also prepared the initial draft. Shanthy Sundaram contributed to the manuscript by reviewing and providing feedback for improvement.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest for this manuscript. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
Not applicable.
Consent of Participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maurya, N., Sharma, A. & Sundaram, S. The Role of PGPB-Microalgae interaction in Alleviating Salt Stress in Plants. Curr Microbiol 81, 270 (2024). https://doi.org/10.1007/s00284-024-03805-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-024-03805-7