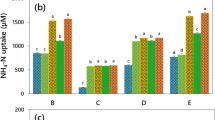
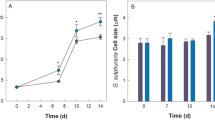
Abstract
The microalga Chlorella sorokiniana and the microalgae growth-promoting bacteria (MGPB) Azospirillum brasilense have a mutualistic interaction that can begin within the first hours of co-incubation; however, the metabolites participating in this initial interaction are not yet identified. Nuclear magnetic resonance (NMR) was used in the present study to characterize the metabolites exuded by two strains of C. sorokiniana (UTEX 2714 and UTEX 2805) and A. brasilense Cd when grown together in an oligotrophic medium. Lactate and myo-inositol were identified as carbon metabolites exuded by the two strains of C. sorokiniana; however, only the UTEX 2714 strain exuded glycerol as the main carbon compound. In turn, A. brasilense exuded uracil when grown on the exudates of either microalga, and both microalga strains were able to utilize uracil as a nitrogen source. Interestingly, although the total carbohydrate content was higher in exudates from C. sorokiniana UTEX 2805 than from C. sorokiniana UTEX 2714, the growth of A. brasilense was greater in the exudates from the UTEX 2714 strain. These results highlight the fact that in the exuded carbon compounds differ between strains of the same species of microalgae and suggest that the type, rather than the quantity, of carbon source is more important for sustaining the growth of the partner bacteria.





Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Kouzuma A, Watanabe K (2015) Exploring the potential of algae/bacteria interactions. Curr. Opin. Biotech. 33:125–129
Fuentes JL, Garbayo I, Cuaresma M, Montero Z, González-del-Valle M, Vílchez C (2016) Impact of microalgae-bacteria interactions on the production of alga biomass and associated compounds. Mar. Drugs 14:100
Herzi F, Hlaili AS, Le Poupon C, Mabrouk HH, Mounier S (2013) Characterization of exudates release by the marine diatom Skeletonema costatum exposed to copper stress: a 3D-fluorescence spectroscopy approach. Biometals 26:773–781
Huang X-G, Sx Li, Liu F-J, Lan W-R (2018) Regulated effects of Prorocentrum donghaiense Lu exudate on nickel bioavailability when cultured with different nitrogen sources. Chemosphere 197:57–64
Qi W, Mei S, Yuan Y, Li X, Tang T, Zhao Q et al (2018) Enhancing fermentation wastewater treatment by co-culture of microalgae with volatile fatty acid and alcohol-degrading bacteria. Algal. Res. 31:31–39
Yao S, Lyu S, An Y, Lu J, Gjermansen C, Schramm A (2018) Microalgae-bacteria symbiosis in microalgal growth and biofuel production: a review. J. Appl. Microbiol. 126:359–368
Lopez BR, Palacios OA, Bashan Y, Hernández-Sandoval FE, de-Bashan LE (2019) Riboflavin and lumichrome exuded by the bacterium Azospirillum brasilense promote growth and changes in metabolites in Chlorella sorokiniana under autotrophic conditions. Algal. Res. 44:101696
Palacios OA, Gomez-Anduro G, Bashan Y, de-Bashan LE (2016) Tryptophan, thiamine and índole-3-acetic acid exchange between Chlorella sorokiniana and the plant growth-promoting bacterium Azospirillum brasilense. FEMS Microbiol. Ecol. 92:fiw077
de-Bashan LE, Mayali X, Bebout BM, Weber PK, Detweiler AM et al (2016) Establishment of stable synthetic mutualism without co-evolution between microalgae and bacteria demonstrated by mutual transfer of metabolites (NanoSIMS isotopic imaging) and persistent physical interaction (Fluorescent in situ hybridization). Algal. Res. 15:179–186
González-González LM, de-Bashan LE (2021) Toward the enhancement of microalgal metabolite production through microalgae-bacteria consortia. Biology 10:282
de-Bashan LE, Moreno M, Hernandez J-P, Bashan Y (2002) Removal of ammonium and phosphorous ions from synthetic wastewater by the microalgae Chlorella vulgaris coimmobilized in alginate beads with the microalgae growth-promoting bacterium Azopirillum brasilense. Water Res. 36:2941–2948
Hernandez J-P, de-Bashan LE, Bashan Y (2006) Starvation enhances phosphorous removal from wastewater by the microalgae Chlorella spp. co-immobilized with Azospirillum brasilense. Enzyme Microb. Tech. 38:190–198
Choix FJ, de-Bashan LE, Bashan Y (2012) Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: II. Heterotrophic Cond. Enzym. Microb. Tech. 51:300–309
Peng H, de-Bashan LE, Higgins BT (2021) Comparison of algae growth and symbiotic mechanisms in the presence of plant growth promoting bacteria and non-plant growth promoting bacteria. Algal. Res. 53:102156
Palacios OA, Lopez BR, Bashan Y, de-Bashan LE (2019) Early changes in nutritional conditions affect formation of synthetic mutualism between Chlorella sorokiniana and the bacterium Azospirillum brasilense. Microbial. Ecol. 77:980–992
Bashan Y, Lopez BR, Huss VAR, Amavizca E, de-Bashan LE (2016) Chlorella sorokiniana (formely C. vulgaris) UTEX 2714, a non-thermotolerant microalga useful for biotechnological applications and as a reference strain. J. Appl. Phycol. 28:113–121
Seymour JR, Amin SA, Raina J-B, Stocker R (2017) Zooming in on the phycosphere: the ecological interface for phytoplankton-bacteria relationships. Nat. Microbiol. 2:17065
Sapp M, Schwaderer AS, Wiltshire KH, Hoppe H-G, Gerdts G, Wichels A (2007) Specie-specific bacterial communities in the phycosphere of microalgae? Microbial. Ecol. 53:683–699
Morris BEL, Henneberger R, Huber H, Moissl-Eichinger C (2013) Microbial syntrophy: interaction for the common good. FEMS Microbiol. Rev. 37:384–406
Leandro SM, Gil MC, Delgadillo I (2003) Partial characterization of exopolysaccharides exuded by planktonic diatoms maintained in batch culture. Acta. Oecol. 24:S49–S55
Kirkwood AE, Nalewajko C, Fulthorpe RR (2006) The effects of cyanobacterial exudates on bacterial growth and biodegradation of organic contaminants. Microbial. Ecol. 51:4–12
Pivokonsky M, Safarikova J, Maresova M, Pivokonska L, Kopecka I (2014) A comparison of the character of algal extracellular versus cellular organic matter produced by cyanobacterium, diatom and green alga. Water Res. 51:37–46
de-Bashan LE, Trejo A, Volker ARH, Hernandez J-P, Bashan Y (2008) Chlorella sorokiniana UTEX 2805, a heat and intense, sunlight-tolerant microalga with potential for removing ammonium from wastewater. Bioresource Technol. 99:4980–4989
González LE, Cañizares RO, Baena S (1997) Efficiency of ammonia and phosphorous removal from a Colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and Scenedesmus dimorphus. Bioresource Technol. 60:259–262
Bashan Y, Trejo A, de-Bashan LE (2011) Development of two culture media for mass cultivation of Azospirillum spp. and for production of inoculants to enhance plant growth. Biol. Fert. Soils 47:963–969
Chrzanowski TH, Crotty RD, Hubbard JG, Welch RP (1984) Applicability of fluorescein diacetate method of detecting active bacteria in freshwater. Microb. Ecol. 10:179–185
Wishart DS (2019) NMR metabolomics: a look ahead. J. Magn. Reason 306:155–161
Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K et al (2018) HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 46:D608–D617
Cui Q, Lewis IA, Hegeman D, Anderson ME, Li J, Schulte CF, Westler WM, Eghbalnia HR, Sussman MR, Markley JL (2008) Metabolite identification via the Madison metabolomics consortium database. Nat Biotechnol 26:162–164
Ulrich EL, Akutsu H, Doreleijers JF, Harano Y et al (2008) BioMagResBank. Nucleic Acids Res. 36:D402–D408
Bharti SK, Roy R (2012) Quantitative 1H NMR spectroscopy. TRAC-Trend Anal. Chem. 35:5–26
Harcombe WR, Riehl WJ, Dukovsky I, Granger BR, Betts A, Lang AH et al (2014) Metabolic resource allocation in individual microbes determines ecosystem interactions and spatial dynamics. Cell Rep. 7:1104–1115
da Silva ML, Franco AOR, Odebrecht C, Giroldo D, Abreu PC (2016) Carbohydrates produced in batch cultures of the surf zone diatom Asterionellopsis glacialis sensu lato: influence in vertical migration of the microalga and bacterial abundance. J. Exp. Mar. Biol. Ecol. 474:126–132
Smith DJ, Underwood GJC (2000) The production of extracellular carbohydrates by estuarine benthic diatoms. The effect of growth phase and light and dark treatment. J. Phycol. 36:321–333
Gruber PJ, Frederick SE, Tolbert NE (1974) Enzymes related to lactate metabolism in green algae and lower land plants. Plant Physiol. 53:167–170
Sullivan JT, Brown SD, Ronson CW (2013) The NifA-RpoN regulon of Mesorhizobium loti strain R7A and its symbiotic activation by a novel Lacl/GalR-family regulator. PLoS ONE 8:e53762
Shariati M, Lilley RMcC (1994) Loss of intracellular glycerol from Dunaliella by electroporation at constant osmotic pressure: subsequent restoration of glycerol content and associated volume changes. Plant Cell Environ. 17:1295–1304
Wang L, Huang X, Lim DJ, Carrasco-Laserma AK, Yau-Li SF (2019) Uptake and toxic effects of triphenyl phosphate on freshwater microalgae Chlorella vulgaris and Scenedesmus obliquus: Insights from untargeted metabolomics. Sci. Total Environ. 650:1239–1249
Loewus FA, Murthy PPN (2000) myo-Inositol metabolism in plants. Plant Sci. 150:1–19
Nelson DE, Koukoumanos M, Bohnert HJ (1999) Myo-Inositol-dependent sodium uptake in ice plant. Plant Physiol. 119:165–172
Tanner W (2000) The chlorella hexose/H+-symporters. Int. Rev. Cytol. 200:101–141
Morales-Sánchez D, Martinez-Rodriguez OA, Kyndt J, Martinez A (2015) Heterotrophic growth of microalgae: metabolic aspects. World J. Microb. Biot. 31:1–9
Talarsky A, Manning SR, La Claire JW (2016) Transcriptome analysis of the euryhaline alga, Prymnesium parvum (Prymnesiophyceae): effects of salinity on differential gene expression. Phycologia 55:33–44
Perez-Garcia O, Bashan Y (2015) Microalgae heterotrophic and mixotrophic culturing for bio-refining: from metabolic routes to techno-economics. In Prokop A, Bajpai R, Zappi M (eds) Algal Biorefineries Vol. 2: Products and refinery design. Switzerland: Springer International Publishing, 61–131
Lee S-M, Kim S-K, Lee N, Ahn C-Y, Ryu C-M (2020) D-Lactic acid secreted by Chlorella fusca primes pattern-triggered immunity against Pseudomonas syringae in Arabidopsis. Plant J 102:761–778
Singh VS, Dubey AP, Gupta A, Singh S, Singh BN, Tripathi AK (2017) Regulation of a glycerol-induced quinoprotein alcohol dehydrogenase by σ54 and a LuxR-Type regulator in Azospirillum brasilense SP7. J Bacteriol 199:e00035-e17
Galbraith MP, Feng SF, Borneman J, Triplett EW, de Bruijn FJ, Rossbach S (1998) A functional myo-inositol catabolism pathway is essential for rhizopine utilization by Sinorhizobium meliloti. Microbiology 144:2915–2924
Jiang G, Krishnan AH, Kim Y-W, Wacek TJ, Krishnan HB (2001) A functional myo-inositol dehydrogenase gene is required for efficient nitrogen fixation and competitiveness of Sinorhizobium fredii USDA191 to nodulate soybean (Glycine max [L.] Merr.). J. Bacteriol. 183:2595–2604
Wisniewsky-Dyé F, Lozano L, Acosta-Cruz E, Borland S, Drogue B, Prigent-Combaret C et al (2012) Genome sequence of Azospirillum brasilense CBG497 and comparative analyses of Azospirillum core and accessory genomes provide insight into niche adaptation. Genes 3:576–602
Reis VM, Baldani VLD, Baldani JI (2015) Isolation, identification and biochemical characterization of Azospirillum spp. and other nitrogen-fixing bacteria. In Cassán FD, Okon Y, Creus CM (eds) Hanbook of Azospirillum. Technical issues and protocols. Switzerland: Springer International Publishing, 3–26
Westby CA, Cutshall DS, Vigil GV (1983) Metabolism of various carbon sources by Azospirillum brasilense. J Bacteriol 156:1369–1372
O´Neil L, Mukherjee Y, Alexandre G (2018) Analyzing chemotaxis and relate behaviors of Azospirillum brasilense. Curr. Protocol. Microbiol. 48:3E.3.1-3E.3.11
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 63:541–556
Anderson CM, Parkinson FE (1997) Potential signaling roles for UTP and UDP: sources, regulation and release of uracil nucleotides. Trends Pharmacol. Sci. 18:387–392
Funding
Funding was provided by Consejo Nacional de Ciencia y Tecnología of Mexico (CONACYT Basic Science-2017, grant 284562).
Author information
Authors and Affiliations
Contributions
OP and JCEH participated equally in designing, performing the experiments, analyzing the data and writing the initial draft. AACD analyze and interpret the data from NMR, BRL assisted in the experiments and help with the initial draft of the manuscript. LEdeB discussed the experimental setting, supervised the experiments, and critically revised the article for intellectual content, including the manuscript’s final version. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This research does not involve human or animal subjects.
Competing Interests
The authors declare no competing interests.
Additional information
This study is dedicated to the memory of Dr. Yoav Bashan (1953–2018), pioneer in MGPB field and founder of Bashan Institute of Science, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Palacios, O.A., Espinoza-Hicks, J.C., Camacho-Dávila, A.A. et al. Differences in Exudates Between Strains of Chlorella sorokiniana Affect the Interaction with the Microalga Growth-Promoting Bacteria Azospirillum brasilense. Microb Ecol 85, 1412–1422 (2023). https://doi.org/10.1007/s00248-022-02026-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-022-02026-4