Abstract
The present study was undertaken to examine the salinity stress-induced physiological and biochemical alterations in the cyanobacterium Synechococcus sp. PCC 7942. Cyanobacterial cultures supplemented with different concentrations of NaCl were evaluated for growth, carbohydrate, total lipid, ROS generation, and stress biomarkers to evaluate the ROS-mediated lipid production in Synechococcus 7942. Salt concentration of 500 mM induced a five- and threefold increase in the production of carbohydrates and lipids, respectively. The fatty acids composition in terms of total quantity and oleic acid content of the investigated species was also improved as the salinity level increased from 0 to 500 mM NaCl. The data showed maximum MUFA production at 10 mM NaCl with dominance of palmitoleic acid (88.3%) and oleic acid (0.31%), whereas PUFA was found to be maximally produced at 250 mM NaCl with dominance of linoleic acid. Salt stress enhanced the accumulation of carbohydrate and total lipids and antioxidative enzymes, and modulates the fatty acids and hydrocarbon composition of cyanobacterium. Production of fatty acid and hydrocarbon under saline conditions indicates that salinity can be used as a factor to modulate the biochemical pathways of cyanobacteria toward efficient biofuel production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among the several problems related with use of fossil fuels, major one is pollution of ecosystems together with its fast depletion (Greenwell et al. 2010). An alternative strategy to minimize these problems is to develop the technology of biofuel production using suitable biological strains. Biofuels are most promising solution to the worlds fuel problem due to their sustainability and biodegradability (Kiran et al. 2016). Since the microorganisms require exogenous supplementation of carbon, utilization of these organisms for biofuel production will be a very costly approach. On the other hand, using land crops for biofuel production will minimize food crop production and increase the food cost (Machado and Atsumi 2012; Scharlemann and Laurance 2008). Cyanobacteria, one of the most diverse group of oxygenic photosynthetic prokaryotes with wide ecological tolerance, possess the ability to directly convert solar energy into renewable bioenergy and environmentally friendly biofuels (Monshupanee and Incharoensakdi 2013). They are currently being explored as a commercial biofuel feedstock due to their fast growth rate, ability to grow under several conditions such as wastewater, reduced requirement of water and other resource inputs, the possibility of not occupying arable lands for their cultivation, ability to grow under extreme environments, as well as capability to grow photoautotrophically, heterotrophically, and mixotrophically (de Farias Silva and Bertucco 2016; Gomaa et al. 2016). Thus fatty acid and hydrocarbon production by cyanobacteria seems to be a promising alternative to fossil fuel because they are more advantageous in comparison with other microalgae in terms of industrial production due to their fast growth, simple nutrient requirements (mainly water, sunlight, and CO2) and easy to genetically engineer (Heidorn et al. 2011; Huang et al. 2010; Koksharova and Wolk 2002; Ruffing 2011). The importance of using cyanobacteria for biofuel production is that they can enhance lipid production per hectare area by 100 times compared to other microorganisms (Rittmann 2008). Salt stress is one of main abiotic factor limiting the growth and productivity of cyanobacteria and plants (Allakhverdiev et al. 2001). Cyanobacteria are reported to modulate their physiological and biochemical attributes toward salt stress tolerance. Under high saline condition, genes involved in compatible solute synthesis, oxidative stress, and ion channel showed increased expression (Ludwig and Bryant 2012). Salinity stress was reported to enhance lipid accumulation in several microalgae such as Chlamydomonas sp. JSC4, Nannochloropsis, Scenedesmus sp. CCNM 1077 (Martínez-Roldán et al. 2014; Pancha et al. 2015). Microalga Dunaliella salina 1650 had also been reported to produce hydrocarbon under photoautotrophic condition (Song et al. 2008). A previous study by Pancha et al. (2015) in Scenedesmus sp. and by Yilancioglu et al. (2014) in Dunaliella sp. reported that salinity stress and nitrogen depletion, respectively, resulted in co-occurrence of ROS (reactive oxygen species) and lipid accumulation. In the present study, we have examined salinity-induced modulations in growth behavior, carbohydrate and lipid production, fatty acid and hydrocarbon content as well as level of ROS generation and antioxidative response of cyanobacterium Synechococcus sp. PCC 7942 in order to elucidate the cellular mechanism regarding ROS-induced lipid production which may be useful in biofuel production under salinity stress.
Materials and methods
Cyanobacterial strain and cultivation
The cyanobacterium Synechococcus sp. PCC 7942 used in this study was a gracious gift from Karl Forchhammer, University of Tuebingen, Germany. This strain was grown axenically in the BG11 medium supplemented with 17 mM NaNO3 and 10 mM NaHCO3 in the fluorescent cool light (85 µE m−2 s−1) at 30 °C and 7.5 pH.
Experimental design
In order to find out optimum, LC50 and lethal concentrations, growth of the cyanobacterial cells were measured treated with different concentrations of NaCl ranging from 1 to 1000 mM. It was found that 10 mM is optimum concentration, whereas 500 mM is LC50. To further investigate the salinity-induced morphological and biochemical alterations, 10, 50, 100, 250, 500 mM NaCl concentrations were tested.
Growth analysis
Cyanobacterial growth was monitored by measuring the dried cyanobacterial biomass as well as chlorophyll a content at 663 nm according to the protocol of Mc Kinney (1941).
Lipid and carbohydrate estimation
Cellular carbohydrate was estimated as per the protocol of Dubois et al. (1956) to understand the role of salinity on carbohydrate production. Total lipids were extracted according to Folch et al. (1957) and gravimetrically determined after solvent evaporation. Total lipids were calculated as µg lipid mg−1 of dry wt.
Scanning electron microscopy
Morphological alterations in cyanobacteria exposed to various NaCl concentrations were observed by scanning electron microscopy. A total of 0.5 ml control and NaCl-treated cyanobacterial cells were harvested by centrifugation at 10,000 g for 10 min, following washing of obtained pellet two times with double-distilled water (DDW). Then after 2.5% glutaraldehyde in 1:2 proportion (1 culture: 2 glutaraldehyde) was added to pellet and incubated overnight at 4 °C. Centrifugation at 8000 g for 10 min was performed, and supernatant discarded and washed two times with DDW. Cultures were dehydrated with 30, 50, 70, and 100% ethanol for 15 min at each ethanol concentration. Further, dehydrated pellet was incubated for 1 h at room temperature in 100% ethanol following centrifugation at 10,000 g for 10 min. 0.1% AgNO3 was added to the pellet and incubated at 45 °C in water bath for 1 h. Final centrifugation was done at 10,000 g supernatant discarded and completely dried pellet was used for SEM analysis. SEM observations were recorded on Fei Quanta 200 microscope fitted with LFD detector at a 20-kV working voltage with a working distance of 10 mm and 5000× magnification.
ROS imaging and in vivo detection of ROS
Most widely used fluorometric probe 2′, 7′-dichlorodihydrofluoresceine diacetate (DCFH-DA) (Sigma-Aldrich, USA) was used for the intracellular detection of ROS (He and Hader 2002). A stock solution of 2 mM (w/v) of DCFH-DA was prepared in ethanol and stored in dark at − 20 °C. Approximately 4 µg ml−1 cyanobacterial cells from each treatment was stained with 5 µM (final concentration) DCFH-DA and kept in dark for 1 h. Furthermore, cells were visualized under trinocular inverted fluorescence microscope (Dewinter, Italy). In addition, the fluorescence of samples was also analyzed by spectrofluorometer (Hitachi, Tokyo, Japan) with an excitation wavelength of 485 nm and an emission band between 500 and 600 nm.
Estimation of stress biomarkers
To determine the activity of SOD (superoxide dismutase) (EC 1.15.1.1), 50 mg biomass was homogenized in 100 mM EDTA phosphate buffer (pH 7.8) and centrifuged at 13,000 g for 30 min at 4 °C. SOD activity was determined in the supernatant by inhibition of nitroblue tetrazolium (NBT) using 3 ml reaction mixture containing 1.3 mM riboflavin, 13 mM l-methionine, 0.05 M Na2CO3 (pH 10.2), 63 µM nitroblue tetrazolium chloride and 0.1 ml of crude extract according to the protocol of Giannopolitis and Ries (1977). At the end, riboflavin was added and reaction mixture was illuminated with 78 µmol m−2 s1 for 15 min. Absorbance of the irradiated sample was measured at 560 nm, compared with non-irradiated sample, and SOD activity was calculated by assuming that one unit of SOD activity is the amount of enzyme that causes 50% inhibition of the photochemical reduction of NBT (nitro blue tetrazolium).
To estimate the CAT (catalase) (EC 1.11.1.6) activity, 50 mg biomass was homogenized in the EDTA phosphate buffer (pH 7.8) following centrifugation at 13,000 g. A total of 0.1 ml enzyme extract was added to 3 ml reaction mixture containing phosphate buffer (1.6 ml), 100 µl EDTA (3 mM), 200 µl H2O2 (0.3%), and CAT activity was monitored by measuring the disappearance of H2O2, by measuring a decrease in absorbance at 240 nm against a blank of same sample without 0.3% H2O2 as per the protocol of Chance and Maehly (1995). One unit enzyme activity corresponds to an absorbance change of 0.01 units min−1.
For analysis of APX (ascorbate peroxidase) (EC 1.11.1.11), protocol of Nakano and Asada (1981) was followed. Fifty milligrams of biomass was homogenized in 2 ml phosphate buffer (0.5 M, pH 7.5) following centrifugation at 12,000 g at 4 °C for 30 min. One hundred microliters of crude enzyme extract was added in a reaction mixture containing potassium phosphate buffer, ascorbic acid, and EDTA following addition of 0.3% H2O2 to initiate the reaction. The reaction was followed for 3 min at a wavelength of 290 nm against a blank of same sample without H2O2. Extinction coefficient for ascorbate is 2.8 mM−1 cm−1.
Lipid peroxidation was analyzed in terms of total MDA (malondialdehyde) content as per the protocol of Heath and Packer (1968) under different salt concentration regimes. Exponentially grown cultures were harvested and homogenized in 10 ml of 0.1% TCA following centrifugation at 13,000 g for 15 min. Reaction mixture containing 1 ml supernatant and 4 ml TBA in 20% TCA was incubated at 95 °C in a water bath for 30 min, immediately cooled on ice and again centrifuged at 10,000 g for 10 min. Absorbance of supernatant was recorded at 532 nm, and the non-specific absorbance at 600 nm was subtracted. Lipid peroxidation was expressed in terms of mM mg−1 protein and extinction coefficient for MDA is 155 mM−1 cm−1.
Proline content was estimated as per the protocol of Bates et al. (1975). Cultures were harvested at 10,000 g for 5 min, and obtained pellet was crushed in 2 ml of 40% methanol. 1 ml glacial acetic acid and orthophosphoric acid (6 M, 3:2 v/v) and 25 mg ninhydrin were added to 1 ml of crushed samples, and the reaction mixture was incubated at 100 °C for 1 h and immediately cooled following addition of 5 ml of toluene. Two distinct phases were formed: upper phase was used to measure proline content at 528 nm, and content was expressed as µg ml−1.
Lipid extraction and preparation of FAMEs
Lipid extraction was done following the protocol of Kang and Wang (2005) with slight modification. Exponentially growing cyanobacterial cells were harvested by centrifugation at 10,000 g for 15 min, and pellet was dried, and 1 g of dried biomass was crushed in the chloroform/methanol (2:1) reagent. The crushed biomass was then filtered through Whatman filter paper, and filtrate was collected in 15-ml screw cap tube, and lipid is extracted in chloroform phase by adding 2 ml distilled water and concentrated at room temperature using speed vac (Thermo Fisher Scientific, USA) for 20 min. For saponification, 5 ml of sodium methoxide reagent were added and vortexed. Few drops of boron trifluoride-methanol reagent was added and boiled at 100 °C for 12 min and cooled at room temperature. HPLC-grade hexane (5 ml) was added to each sample and incubated at room temperature for 30 min. Two distinct layers were formed with upper hexane layer containing FAMEs. Two-thirds of the upper layer was transferred to GC vials and was allowed to evaporate until 100 µl od sample remained.
GC/MS (gas chromatography/mass spectrometry) analysis
Changes in FAMEs (Fatty acid methyl esters) profile were analyzed with a Shimadzu GC–MS–QP 2010 plus connected with FID (flame ionization detector) and RTX-5 capillary column (30 m long, 0.25 mm diameter, and 0.25 µm film thick) using helium as carrier gas with the flow rate of 0.7 ml min−1 in splitless mode. Extracted FAMEs (3 µl) were injected in a splitless injection mode. The column temperature was initially held isothermally at 160 °C for 2 min and then ramped to 270 °C at 2 °C min−1, and finally held constant for 75 min. All data were obtained by measuring the full-scan mass spectra within the scan range 40–950 amu. The identification of compounds present in sample was based on direct comparison of the retention time and mass spectral fragmentation pattern with data provided in WILEY8.LIB and NIST11.LIB.
Statistical analysis
Values presented in the text indicate mean value ± SE of three biological replicates. The levels of significance of the data were analyzed by multivariate ANOVA using SPSS software (SPSS Inc., version 16.0).
Results and discussion
Effects of salinity on growth of cyanobacteria
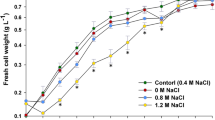
Effects of salinity on the growth of the cyanobacterium Synechococcus sp. PCC 7942 were estimated in terms of both chlorophyll a content and dried biomass (Fig. 1 and supplementary Fig. S1). Results obtained from these experiments showed that higher concentration of NaCl reduces cyanobacterial growth drastically. Maximum growth of Synechococcus sp. PCC 7942 was observed at 10 mM NaCl (approx. 1.5 times) concentration as compared to control but growth showed decreasing trend with increasing salinity above 10 mM and was significantly (approx. 50%) reduced at 500 mM NaCl concentration (Fig. 1). The result of growth experiment clearly demonstrated that 10 mM NaCl was stimulatory for cyanobacterial growth, whereas 500 mM was the LC50 concentration. Growth increment of the test organism at 10 mM NaCl concentration as compared to control may be due to the proper metabolic activities of cyanobacterium. Growth-promoting effect of low level of NaCl on microalgae Chlamydomonas mexicana and Scenedesmus obliquus has been reported by Salama et al. (2013). Similar observation has been made by Becker (1994) who observed some strains of Chlorella grew better in seawater than in normal medium. Salinity-induced reduction in cyanobacterial growth might be because of decreased chlorophyll biosynthesis which is the primary target of salinity (Lu and Zhang 2000). It has been reported in Synechocystis sp. PCC 6803 that salt stress causes damage to PSII activity possibly by inhibiting the repair of photo-damaged PSII (Allakhverdiev et al. 2002). It appears that salinity may be negatively affecting the cyanobacterial metabolism that causes decreased growth at the highest concentration tested (500 mM).
Effects of salinity on carbohydrate and lipid content
Carbohydrate and lipid content of the cyanobacterium showed increasing trend under salinity stress. Approximately fivefold increase in carbohydrate and threefold increase in lipid content of Synechococcus PCC 7942 were noticed at 500 mM NaCl concentration (Fig. 1) as compared to control. Carbohydrate content of the cyanobacterium was found to be positively correlated with salinity level with maximum production at 500 mM NaCl. Many previous studies also reported stress-induced increment in carbohydrate content (Warr et al. 1985; Kirroliaa et al. 2011). Soluble sugars were reported to play important role in osmotic regulation during stress conditions (Gill et al. 2002). The increase in sugar content may be treated as an adaptive measure of cyanobacterium under saline conditions. In addition, Synechococcus sp. also showed increased lipid accumulation with increasing concentration of salt. Cyanobacteria respond to varying salinity levels by changing the lipid synthesis and fatty acid composition. Similar observation has been made by Takagi and Yoshida (2006) in Dunaliella. Such observations simply indicated the positive adaptive relationship between concentration of salinity and accumulation of cellular lipid.
Salinity-induced morphological changes
SEM data (Fig. 2) revealed that there was no significant variation in the cellular appearance of the cyanobacterium at control and 10 mM NaCl, whereas further increment in salt concentration caused cellular alterations such as changes in cell shape and size. Exposure to higher concentrations of NaCl triggered cell elongation and resulted in bending of cells. This distortion was maximum at 500 mM NaCl concentration with almost sickle-shaped appearance of cell. Salt stress induces morphological alterations such as cell elongation and bending in the studied cyanobacterium. These results are in agreement with the observations of Meury (1988) and Piuri et al. (2005) in E. coli and Lactobacillus casei, respectively, where salt stress triggers the cell size elongation.
In vivo ROS imaging and detection
The results of fluorescence microscopy (Fig. 3) showed that increasing concentration of NaCl in the growth medium enhanced ROS production which ultimately resulted in intense green fluorescence under microscope. Generation of green fluorescence is the indicative of ROS production. Most pronounced green fluorescence was observed at 500 mM NaCl followed by 250 mM, and almost negligible green fluorescence was observed in control. Results obtained from this microscopic image analysis were also supported by the G/R ratio (Supplementary Fig. S2). G/R ratio also indicated that maximum generation of ROS took place at 500 mM NaCl. Further, these microscopic observations were verified by spectrofluorometry, and result of this experiment (Fig. 4) showed almost 177% increased fluorescence in 500 mM NaCl-treated culture in comparison with control followed by 141, 122, and 117% under 250, 100, and 50 mM, respectively. Minimum fluorescence (77%) was observed at 10 mM NaCl treatment in comparison with control. Thus, results of both spectrofluorometry and microscopic study supported each other and showed the extent of free radical generation in the cyanobacterial cells under salinity stress. Under unfavorable environmental conditions, several reactive oxygen species (ROS) such as H2O2, superoxide anion, hydroxyl radical are generally produced by cyanobacterial cells. Salt induces osmotic stress which ultimately leads to oxidative stress. To evaluate correlation between increased lipid accumulation and ROS generation, fluorescence microscopy and spectrofluorometry were performed with fluorescent dye DCFH-DA, and results showed that increased lipid accumulation is correlated with increased ROS production. DCFH is a non-fluorescent dye, but it converts to a fluorescent dye DCF by the reaction with cellular ROS. A previous study also reported relation between ROS production and lipid accumulation supporting our data (Yilancioglu et al. 2014). Further, results of ROS generation measurement by fluorescence microscopy and spectrofluorometry were validated by measuring the level of antioxidative enzymes under all salinity-treated condition.
Effect of salinity on stress biomarkers of Synechcococus sp.
Exposure of the cyanobacterium to different concentrations of NaCl enhanced the activity of several antioxidative enzymes. Among all the enzymes studied, SOD showed maximum induction at 500 mM NaCl which was 152% compared to control (Fig. 5a). Almost similar activity of SOD was found at 50 and 100 mM NaCl concentration (82 and 85%, respectively). CAT (Fig. 5a) and APX (Fig. 5a) also showed similar trend with maximum induction of 162 and 121%, respectively, as compared with their respective controls at 500 mM NaCl. Lipid peroxidation estimated in terms of MDA content showed almost tenfold increased peroxidation at highest salinity level of 500 mM (Fig. 5b). Among non-enzymatic antioxidant system, proline content was estimated which showed 255% enhanced accumulation with their respective control at 500 mM NaCl (Fig. 5c). Accumulated ROS under unfavorable condition damages the cellular constituents such as protein, lipid, DNA, and other cellular macromolecules. ROS accumulation in photosynthetic organism is prevented by intrinsic antioxidant system that involved enzymatic defense system including SOD, CAT, APX, and non-enzymatic defense containing proline and ascorbic acid. These antioxidative defense systems were analyzed in order to elucidate the effect of NaCl on ROS generation in cyanobacterial cells which is also supporting the DCFH-DA experiment. SOD constitutes the first line of defense against oxidative stress converting superoxide anion to O2 and H2O2, a relatively more stable product, and catalase is the main enzyme that scavenges hydrogen peroxide (Alscher et al. 2002). Increased SOD activity in the present experiment under saline condition suggests increased ROS generation in the cyanobacterium. This can be concluded that salinity leads to increased superoxide production necessitating increased SOD activity. Similar trend was also observed for CAT and APX. CAT which is a heme-containing enzyme catalyzes the conversion of H2O2 into oxygen and water, whereas APX is involved in the ascorbate glutathione cycle (del Rio et al. 2006; Bhaduri and Fulekar 2012). APX is believed to be the most essential enzyme toward its specificity for H2O2 in comparison with CAT and POX. ROS oxidizes fatty acid which leads to lipid peroxidation and membrane damage (Ali et al. 2013). In this study, lipid peroxidation was estimated in terms of MDA content, and maximum lipid peroxidation at 500 mM NaCl treatment suggest role of salinity toward lipid peroxidation. Similar to the present study, microalgae Scenedesmus was reported to accumulate more MDA at 400 mM NaCl (Pancha et al. 2015) as well as shoot MDA content of Borago officinalis was also reported to increase at 25 mM NaCl (Jaffel et al. 2011). These data strongly suggest that NaCl induces oxidative stress in the cyanobacteria. Apart from enzymatic defense system, non-enzymatic defense involves accumulation of small organic antioxidant compounds like proline and glycine betaine. A dose-dependent increase in free proline content suggests its adaptive role in salinity stress in cyanobacteria. Proline is also known to accumulate under various adverse environmental conditions such as nitrogen starvation (Pancha et al. 2014) and temperature stress (Chokshi et al. 2015). It has a role in maintaining cytosolic pH, stabilization of subcellular structures, also works as a compatible solute and ROS-scavenging agent. Osundeko et al. (2013) reported that in terms of biofuel production potential oxidative stress-tolerant microalgae are more efficient in comparison with non-tolerant microalgae.
Antioxidant enzyme activities under different NaCl concentrations. (a) Spectrophotometric enzymatic assays of SOD, CAT and APX in the cyanobacterium Synechococcus sp. PCC 7942, b Effect of different NaCl concentrations on lipid peroxidation of cyanobacterial cells in terms of MDA content and c NaCl-induced dose-dependent increase in proline content. Data represent the mean values of triplicates
Changes in fatty acids and hydrocarbon profile under salinity stress
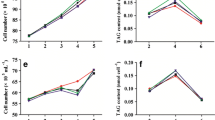
The identified biofuel precursor molecules in the FAMEs of control and NaCl supplemented cyanobacterial cultures are presented in Fig. 6a (Supplementary File S3 includes GC/MS chromatograms and Supplementary Tables S1 and S2 contain the mass spectra with major mass peaks of identified compounds), whereas identified fatty acids and hydrocarbons are presented in Tables 1 and 2, respectively. Of the total compounds identified, fatty acids showed maximum production at 500 mM, hydrocarbons at 10 mM and fatty alcohols at 50 mM NaCl concentration. Short-chain fatty acids were almost equally present in control as well as NaCl supplemented cultures, whereas there was a positive correlation between production of long-chain fatty acid and increased NaCl concentration. Palmitic and palmitoleic acid constitute the major fatty acids of the studied cyanobacteria under control and saline conditions. NaCl-treated cultures showed a significant reduction in production of SFA (saturated fatty acid) almost 82.79% at 10 mM, 12.67% at 50 mM, 5.77% at 100 mM NaCl as well as 74.18% at 250 and 4.58% at 500 mM in comparison with control (Fig. 6b). In all the NaCl-treated culture, significant increase was found in MUFA (mono-unsaturated fatty acid) content with 102.72, 16.05, 7.67, 49.14, and 6.17% increase over control at10, 50, 100, 250, and 500 mM NaCl, respectively. Synthesis of PUFA (polyunsaturated fatty acid) was observed under all the conditions except at 100 and 500 mM NaCl-treated cultures. Very trace amount of odd chain fatty acids such as C13:0, C15:0, and C17:0 were found under both control and saline condition. C17:1 was found only at 250 mM NaCl condition. Hydrocarbons were found in the range of C11 to C20, C15 to C20, C15 to C20, C15 to C20, C12 to C20, and C15 to C20 in control, 10, 50, 100, 250, and 500 mM NaCl concentrations, respectively. There was not much variation in SH (saturated hydrocarbon) production, but production of USH (unsaturated hydrocarbon) and CH (cyclic hydrocarbon) was very much affected by salinity. USH was produced at all the concentrations, whereas CH was produced only at 50 and 100 mM NaCl. Heptadecane was found to be the predominant hydrocarbon at all the tested concentrations. The fatty acid profiling of Synechococcus under saline condition reveals major shift toward production of long-chain and mono-unsaturated fatty acid production with decreased polyunsaturated fatty acid production. Similar observation was also reported by the Pal et al. (2011). Increase in the content of unsaturated fatty acid under salinity stress is an adaptive response of cyanobacterium which is required to maintain membrane fluidity since unsaturated fatty acids have lower melting point than saturated fatty acid (Suutari and Laakso 1992). According to Quintana et al. (2011) the contributions of fatty acids C10:0, C16:1 and C18:1 has been focused for best properties to produce high-quality biodiesel. In NaCl-treated cultures of Synechococcus sp., production of high amount of C16:1 and C18:1 fatty acids can be used to produce improved biodiesel. Linoleic acid is an important fatty acid required for good human health, so its production under salt stress is pharmaceutically beneficial. Maximum MUFA and PUFA production at 10 and 250 mM NaCl concentrations, respectively, suggested that it is not always necessary that increasing salinity will lead increased efficiency for biofuel production potential, so for the industrial application, first it is necessary to optimize the concentration of salt for fatty acid production. Results of the present study demonstrated that salt stress leads to increase in unsaturation of fatty acids. Allakhverdiev et al. (2001) reported that increase in unsaturation of fatty acids in membrane lipids is associated with salt stress tolerance. The observations made in the present study clearly demonstrate that 100 mM NaCl concentration may be used in the production of quality biofuel, while 500 mM NaCl concentration can be taken for quantity production of biofuel. Production of odd-numbered fatty acids under saline stress can be explained as may be these fatty acids play important role in the tolerance of cyanobacterial cells to salt stress. A previous study by Zhu et al. (2007) also reported production of odd-numbered fatty acids under temperature and salt stress. Alkanes of C4–C23 chain length possess higher energy density, hydrophobic property, and compatibility with existing liquid fuel infrastructure, which are the predominant constituents of gasoline, diesel, and jet fuels (Peralta-Yahya and Keasling 2010). Alkanes from nonane to hexadecane form major part of diesel and aviation fuel. This study clearly shows that as the salinity level increased from 10 to 500 mM, proportion of higher alkanes increased making it suitable for production of biodiesel and aviation fuel. Fatty acid is the precursor molecule for alkane production in cyanobacteria which is converted first into an aldehyde and then to alkane by two-step process in cyanobacteria. So, production of maximum alkanes and lower fatty acid at 10 mM concentration may be hypothesized that salinity might be affecting genes involved in alkane production in the present case. Such type of study focusing cyanobacterial morphology and physiology might have strong impact on the development of biotechnological approaches toward biofuel production. Each experiment was performed in triplicate and the standard deviations were denoted as bars in the graphs. Statistical evaluation of experimental data using analysis of variance (ANOVA) showed high degree of significance with p < 0.001 (Table 3).
a Percentage distribution of different groups of compounds under different NaCl concentrations. Fatty acids, hydrocarbons, fatty alcohols, and others showed differential accumulation pattern under different NaCl concentrations. b % changes (as compared to control) in composition of individual fatty acid groups in the cyanobacterium Synechococcus sp. PCC 7942. In comparison with control SFA (saturated fatty acids), MUFA (mono-unsaturated fatty acids), PUFA (polyunsaturated fatty acids) showed differential accumulation pattern under different NaCl concentrations
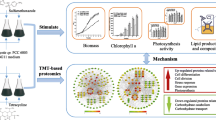
A conceptual model describing the salt stress response in Synechococcus sp. PCC 7942. Salt stress leads to osmotic and ionic stress that ultimately generates ROS production in cyanobacterium. Increased ROS production affects the ultrastructure of cyanobacterium and induces membrane lipid peroxidation. As a protective mechanism against salt-induced ROS production cyanobacteria up regulates the accumulation of osmoprotectants (carbohydrate and proline) and the activity of antioxidative machinery SOD, CAT, APX). Apart from all these aspects, salt stress leads to desaturation of membrane lipid fatty acids and enhanced the fatty acid and hydrocarbon production that could be used to induce the biofuel production potential of cyanobacterium Synechococcus sp. PCC 7942
Conclusion
Although Synechococcus grew well in 10 mM NaCl concentration but the production of carbohydrate, lipid, fatty acid, and hydrocarbon was found to be more in 500 mM NaCl concentration. The present study showed that salt exposure leads to transient generation of ROS which further signal enhanced osmolyte accumulation and lipid synthesis (Fig. 7). This study also supported the previous studies regarding oxidative stress-mediated lipid production. Production of diverse fatty acids, long-chain linear hydrocarbons, and other high-value products such as fatty alcohols under saline condition suggest that Synechococcus sp. PCC 7942 has great potential for petrol-diesel like biofuel production and seems to be a promising natural resource for food and cosmetic industries.
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- CH:
-
Cyclic hydrocarbon
- FAMEs:
-
Fatty acid methyl esters
- GC/MS:
-
Gas chromatography/mass spectrometry
- MDA:
-
Malondialdehyde
- MUFA:
-
Monounsaturated fatty acid
- PUFA:
-
Polyunsaturated fatty acid
- ROS:
-
Reactive oxygen species
- SFA:
-
Saturated fatty acid
- SH:
-
Saturated hydrocarbon
- SOD:
-
Superoxide dismutase
- USH:
-
Unsaturated hydrocarbon
References
Ali S, Huang Z, Li H, Bashir MH, Ren S (2013) Antioxidant enzyme influences germination, stress tolerance, and virulence of Isaria fumosorosea. J Basic Microbiol 53:489–497
Allakhverdiev SI, Kinoshita M, Inaba M, Suzuki I, Murata N (2001) Unsaturated fatty acids in membrane lipids protect the photosynthetic machinery against salt-induced damage in Synechococcus. Plant Physiol 125:1842–1853
Allakhverdiev SI, Nishiyama Y, Miyairi S, Yamamoto H, Inagaki N, Kanesaki Y, Murata N (2002) Salt stress inhibits the repair of photodamaged photosystem II by suppressing the transcription and translation of psbA genes in Synechocystis. Plant Physiol 130:1443–1453
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Bates LS, Waldren RP, Tear ID (1975) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Becker EW (1994) Microalgae biotechnology and microbiology. Cambridge University Press, Great Britain
Bhaduri AM, Fulekar MH (2012) Antioxidant enzyme responses of plants to heavy metal stress. Rev Environ Sci Biotechnol 11:55–69
Chance B, Maehly AC (1995) Assay of catalase and peroxidases. Methods Enzymol 2:764–775
Chokshi K, Pancha I, Trivedi K, George B, Maurya R, Ghosh A, Mishra S (2015) Biofuel potential of the newly isolated microalgae Acutodesmus dimorphus under temperature induced oxidative stress conditions. Bioresour Technol 180:161–171
de Farias Silva CE, Bertucco A (2016) Bioethanol from microalgae and cyanobacteria: a review and technological outlook. Process Biochem. https://doi.org/10.1016/j.procbio.2016.02.016
del Rio LA, Sandalio LM, Corpas FJ, Palma JM, Barroso JB (2006) Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging, and role in cell signaling. Plant Physiol 141:330–335
DuBois M, Gilles K, Hamilton J, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Giannopolitis CN, Ries SK (1977) Superoxide dismutase, I. Occurrence in higher plants. Plant Physiol 59:309–314
Gill PK, Sharma AD, Singh P, Bhullar SS (2002) Osmotic stress induced changes in germination, growth and soluble sugar contents of Sorghum bicolor (L.) Moench seeds under various abiotic stresses. Plant Physiol 128:12–25
Gomaa MA, Al-Haj L, Abed RMM (2016) Metabolic engineering of Cyanobacteria and microalgae for enhanced production of biofuels and high-value products. J Appl Microbiol 121:919–931
Greenwell HC, Laurens LM, Shields RJ, Lovitt RW, Flynn KJ (2010) Placing microalgae on the biofuels priority list: a review of the technological challenges. J R Soc Interface 7:703–726
He YY, Hader DP (2002) UV-B-induced formation of reactive oxygen species and oxidative damage of the cyanobacterium Anabaena sp.: protective effects of ascorbic acid and N acetyl cysteine. J Photochem Photobiol 66:115–124
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Heidorn T, Camsund D, Huang HH, Lindberg P, Oliveira P, Stensjo K, Lindblad P (2011) Synthetic biology in cyanobacteria engineering and analyzing novel functions. Methods Enzymol 497:539–579
Huang HH, Camsund D, Lindblad P, Heidorn T (2010) Design and characterization of molecular tools for a synthetic biology approach towards developing cyanobacterial biotechnology. Nucleic Acids Res 38:2577–2593
Jaffel K, Sai S, Bouraoui NK, Ammar RB, Legendre L, Lachaal M, Marzouk B (2011) Influence of salt stress on growth, lipid peroxidation and antioxidative enzyme activity in borage (Borago officinalis L.). Plant Biosyst 145:362–369
Kang JX, Wang J (2005) A simplified method for analysis of polyunsaturated fatty acids. BMC Biochem 6:5
Kiran B, Pathak K, Kumar R, Deshmukh D, Rani N (2016) Influence of varying nitrogen levels on lipid accumulation in Chlorella sp. Int J Environ Sci Technol 13:1823–1832
Kirroliaa A, Bishnoia NR, Singh N (2011) Salinity as a factor affecting the physiological and biochemical traits of Scenedesmus quadricauda. J Algal Biomass Utln 2:28–34
Koksharova OA, Wolk CP (2002) Genetic tools for cyanobacteria. Appl Microbiol Biotechnol 58:123–137
Lu C, Zhang J (2000) Role of light in the response of PSII photochemistry to salt stress in the cyanobacterium Spirulina platensis. J Exp Bot 51:911–917
Ludwig M, Bryant DA (2012) Synechococcus sp. strain PCC 7002 transcriptome: acclimation to temperature, salinity, oxidative stress, and mixotrophic growth conditions. Front Microbiol 3:354
Machado IMP, Atsumi S (2012) Cyanobacterial biofuel production. J Biotechnol 162:50–56
Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Martínez-Roldán AJ, Perales-Vela HV, Cañizares-Villanueva RO, Torzillo G (2014) Physiological response of Nannochloropsis sp. to saline stress in laboratory batch cultures. J Appl Phycol 26:115–121
Meury J (1988) Glycine-betaine reverses the effects of osmotic stress on DNA replication and cellular division in Escherichia coli. Arch Microbiol 149:232–239
Monshupanee T, Incharoensakdi A (2013) Enhanced accumulation of glycogen, lipids and polyhydroxybutyrate under optimal nutrients and light intensities in the cyanobacterium Synechocystis sp. PCC 6803. J Appl Microbiol 116:830–838
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Osundeko O, Davies H, Pittman JK (2013) Oxidative stress-tolerant microalgae strains are highly efficient for biofuel feedstock production on wastewater. Biomass Bioenergy 56:284–294
Pal D, Khozin-Goldberg I, Cohen Z, Boussiba S (2011) The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl Microbiol Biotechnol 90:1429–1441
Pancha I, Chokshi K, George B, Ghosh T, Paliwal C, Maurya R, Mishra S (2014) Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 156:146–154
Pancha I, Chokshi K, Maurya RK, Trivedi K, Patidar SK, Ghosh A, Mishra S (2015) Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 189:341–348
Peralta-Yahya PP, Keasling JD (2010) Advanced biofuel production in microbes. J Biotechnol 5:147–162
Piuri M, Sanchez-Rivas C, Ruzal SM (2005) Cell wall modifications during osmotic stress in Lactobacillus casei. J Appl Microbiol 98:84–95
Quintana N, Van der Kooy F, Van de Rhee MD, Voshol GP, Verpoorte R (2011) Renewable energy from Cyanobacteria: energy production optimization by metabolic pathway engineering. Appl Microbiol Biotechnol 91:471–490
Rittmann BE (2008) Opportunities for renewable bioenergy using microorganisms. Biotechnol Bioeng 100:203–212
Ruffing AM (2011) Engineered cyanobacteria: teaching an old bug new tricks. Bioeng Bugs 2:136–149
Salama ES, Kim HC, Abou-Shanab RAI, Ji MK, Oh YK, Kim SH, Jeon BH (2013) Biomass, lipid content, and fatty acid composition of freshwater Chlamydomonas mexicana and Scenedesmus obliquus grown under salt stress. Bioprocess Biosyst Eng 36:827–833
Scharlemann JP, Laurance WF (2008) Environmental science. How green are biofuels? Science 319:43–44
Song D, Fu J, Shi D (2008) Exploitation of oil-bearing microalgae for biodiesel. Chin J Biotechnol 24:341–348
Suutari M, Laakso S (1992) Microbial fatty acids and thermal adaptation. Crit Rev Microbiol 20:285–328
Takagi M, Karseno Yoshida T (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101:223–226
Warr SRC, Reed RH, Chudek JA, Foster R, Stewart WDP (1985) Osmotic adjustment in Spirulina platensis. Planta 163:424–429
Yilancioglu K, Cokol M, Pastirmaci I, Erman B, Cetiner S (2014) Oxidative stress is a mediator for increased lipid accumulation in a newly isolated Dunaliella salina strain. PLoS ONE 9:e91957
Zhu L, Zhang X, Ji L, Song X, Kuang C (2007) Changes of lipid content and fatty acid composition of Schizochytrium limacinum in response to different temperatures and salinities. Process Biochem 42:210–214
Acknowledgements
We are thankful to the Head, Department of Botany, Banaras Hindu University, Varanasi, India, for providing laboratory facilities. We thank Prof. Karl Forchhammer, Department of Organismic Interactions (Microbiology), Interfaculty Institute of Microbiology and Infection, Auf der Morgenstelle, 2872076, University of Tübingen, Germany, for providing Synechococcus sp. PCC 7942 strain. Two of us (Ekta Verma and Niveshika) are thankful to the UGC, New Delhi, for financial support in the form of JRF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Rupali Datta.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1.
Growth of the cyanobacterium Synechococcus sp. in terms of dry weight (TIFF 642 kb)
Figure S2
DCF fluorescence-based G/R ratio of Synechococcus sp. obtained from fluorescence microscopic analysis under NaCl stress (TIFF 625 kb)
Figure S3.
GC/MS Chromatograms of control, 10, 50, 100, 250 and 500 mM NaCl-treated cyanobacterial cells (Fig. 1a, 1b, 1c, 1d, 1e, 1f, respectively) (EPS 3134 kb)
Rights and permissions
About this article
Cite this article
Verma, E., Singh, S., Niveshika et al. Salinity-induced oxidative stress-mediated change in fatty acids composition of cyanobacterium Synechococcus sp. PCC7942. Int. J. Environ. Sci. Technol. 16, 875–886 (2019). https://doi.org/10.1007/s13762-018-1720-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-1720-0