Abstract
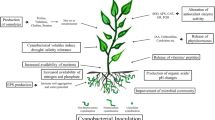
Cyanobacteria are commonly used for the phytostimulation and biofertilization of agriculture crops due to their nitrogen-fixing ability. However, the contribution by their phytohormones has been neglected. This study focuses on the screening of rhizospheric and free-living cyanobacteria for in vitro phytohormones production and growth stimulation in wheat. Selected isolates were shown to release cytokinin and indole-3-acetic acid (IAA) by using UPLC coupled with MS/MS via an electrospray interface. The maximum cytokinin and IAA concentration was 22.7 pmol mg−1 ch-a and 38 pmol mg−1 ch-a, respectively, in the culture medium of Chroococcidiopsis sp. Ck4 and Anabaena sp. Ck1. The growth of wheat inoculated with cyanobacterial strains was stimulated under axenic as well as field conditions. Seed germination, shoot length, tillering, number of lateral roots, spike length, and grain weight were significantly enhanced in inoculated plants. The maximum increase in grain weight (43%) was demonstrated in wheat plants inoculated with Chroococcidiopsis sp. Ck4 under natural conditions. Positive linear correlation of cyanobacterial cytokinin with shoot length (r = 0.608; P = 0.01), spike length (r = 0.682; P = 0.01), and grain weight (r = 0.0.869; P = 0.01) was recorded. Similarly, cyanobacterial IAA was correlated with the root growth parameters shoot length (r = 0.588; P = 0.01), spike length (r = 0.0.689; P = 0.01), and weight of seeds (r = 0.480; P = 0.05). The endogenous phytohormones pool of the plant was enhanced significantly as a result of the plant–cyanobacteria association in the rhizosphere. It was concluded that cyanobacterial phytohormones are a major tool for improved growth and yield in wheat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biofertilizers are attracting considerable attention for replacing chemical fertilizers, which are of environmental concern. Rhizosphere bacteria having the ability to synthesize indole-3-acetic acid (IAA), mobilize phosphate, and fix atmospheric nitrogen are already suggested for the biofertilization of crop plants, including wheat, maize, rice, and sugarcane [2, 15, 22, 24, 28]. In addition to the bacterial biofertilizers, nitrogen-fixing cyanobacteria have been used to inoculate rice [8, 10, 31]. However, other crops, including wheat, have attracted very little attention [1, 18]. The agronomic potential of cyanobacteria has long been recognized. Besides several other ways, cyanobacteria may use phytohormonal signaling (direct mechanism of phytostimulation) as a tool for plant growth promotion. The biosynthesis of cytokinins and IAA is considered to be crucial for plant growth and development [25]. Cyanobacteria have the ability to release phytohormones in the rhizosphere from where plant roots may absorb these hormones [3]. Therefore, such isolates may be of interest to be used as biofertilizers. The aim of the current study was to explore the phytostimulation and biofertilization potential of locally isolated phytohormones producing cyanobacteria in wheat crop.
Materials and methods
Isolation and growth conditions of cyanobacterial strains
Cyanobacterial strains were isolated from the rice fields and fresh water ponds located at the University of the Punjab, Lahore, Pakistan. Rice plants were uprooted and the roots along with rhizosphere were shifted to the lab in sterile containers to isolate cyanobacteria. In the lab, 1 g of rhizospheric soil from the roots was shaken to suspend the sediments and aliquots of 10 μl were transferred to sterile distilled water, which was then filtered aseptically through a 47-mm membrane. Water samples were directly filtered. The filters were incubated on plates containing BG11 medium supplied with cyclohexamide (400 mg l−1) for 3 weeks. Cyanobacterial colonies (mixed cultures) growing on the surface of the filter were picked and transferred to 20 ml of BG11 media in flasks in order to obtain sufficient biomass. Cells or filaments were washed after being incubated for three days in light (18 μmol photons m−2s−1) and 24 h in the dark at 25 ± 1°C under cyclohexamide selection. Cyanobacterial suspensions were made in BG11 medium and the suspensions were then spread on plates of BG11 containing cyclohexamide. Purified colonies or filaments were picked and transferred to a new BG11 plate. Contamination and cyanobacterial growth was monitored on a weekly basis under a dissecting microscope. Purified colonies or filaments were transferred to BG11 broth without cyclohexamide, monitored for contamination regularly and maintained for further experiments. Axenic cyanobacterial cultures were screened for cytokinin-like activity by cucumber cotyledon bioassay as described previously [9].
Identification
The strains were identified by molecular techniques based on 16S rDNA sequencing and BLAST homology. To extract genomic DNA, 5 ml of pure cyanobacterial cultures incubated for 15 days were processed with UltraClean Soil DNA Isolation Kit™ (Mo Bio Laboratories, Carlsbad, CA, USA) following the manufacturer’s instructions. A 16S rDNA fragment was amplified by using forward primer PB36 (5-AGRGTTTGATCMTGGCTCAG-3) and reverse primer PB38 (5-GKTACCTTGTTACGACTT-3) under optimized conditions [33]. The amplified fragment was cloned in pGEMT vector after the vector:insert molar ratio was adjusted to 3:1 by using Rapid Ligation Buffer (Promega, Madison, WI, USA) and 1 U Ligase (Promega). After overnight incubation, the plasmids were transformed into maximum efficiency competent cells (Life Technologies, Rockville, MD, USA) and plated onto LB Amp (60 μg ml−1) agar, followed by another round of overnight incubation at 37°C. The cloned fragment was amplified by pGEMF and pGEMR primers and the polymerase chain reaction (PCR) product was digested with the endonuclease HaeIII (Life Technologies, Rockville, MD, USA) at 37°C for 3 h. Sequencing was done by using an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
Phytohormones determination (UPLC-ESI–MS/MS)
Cyanobacterial strains were grown in a 250 ml conical flask containing BG11 media supplemented with 500 μg ml−1 of tryptophan (precursor of IAA) and 10 μM adenine (cytokinins precursor) at 25°C under continuous light (18 μmol photons m−2s−1) for 4 weeks (inoculum density adjusted to 0.1 μg ml−1 ch-a). Each strain was grown in three sets of four flasks labeled as 1, 2, 3, and 4, incubated for 1, 2, 3, and 4 weeks, respectively (three replicates for each week). Control flasks were without inoculation. Phytohormones released in the cultures were determined from these flasks at the end of the incubation period. Biomass was separated from culture media by centrifugation at 4°C and 1,1000 rpm, and filtered through filters of 22 μm pore size. To extract phytohormones from the culture media, 250 ml of supernatant was concentrated under reduced pressure and extracted three times with Bieleski buffer (60% methanol, 25% CHCl3, 10% HCOOH and 5% H2O) containing 1 pmol [2H5] tZ and [2H3] DHZR and 10 pmol [2H5] IAA to monitor recovery and quantify phytohormones. The pooled extract was evaporated to dryness and reconstituted in 5 ml of acidified water adjusted to pH 3. The reconstituted extract was passed through an SPE column (CHROMABOND® HR-XC, 3 ml, 200 mg) following the manufacturer’s instructions. The eluent was dried, re-dissolved in 15 mM ammonium formate (pH 4.0), and analyzed via UPLC-ESI–MS/MS as described earlier [9]. Cytokinins and IAA were analyzed in positive and negative modes, respectively.
ch-a determination in cyanobacteria
Pellets of cyanobacterial biomass collected by centrifugation were ground in liquid nitrogen and the resulting homogenate was dissolved in 80% methanol. Supernatant was obtained by removing the cell debris through centrifugation after the homogenate was incubated in darkness for 2 h. ch-a (mg l−1) was quantified by measuring the absorbance of the supernatant at 665 nm against 80% methanol (blank), calculated as OD 665 nm × 13.9 [30].
Endogenous phytohormones in wheat seedlings
Surface-sterilized seeds of wheat were soaked in cyanobacterial suspension (0.1 μg ml−1 ch-a) and grown in test tubes containing autoclaved calcinated sand. Seedlings were harvested after 2 weeks. A quantity of 200 mg of frozen plant material was ground in liquid nitrogen and extracted essentially as described [17], with few modifications. The crude extract was purified by an SPE column (MCX) as outlined for cyanobacteria. Cytokinin and IAA were determined by UPLC-ESI–MS/MS under similar conditions as described for cyanobacteria.
Phytostimulation experiments
Seeds of Triticum aestivum var. Uqab 2000 procured from NARC Islamabad, Pakistan, were surface-sterilized with 0.1% HgCl2 for 5–10 min with constant shaking. The seeds were then rinsed five times with sterile water. Two types of plant growth experiments were conducted (lab and wire house). Firstly, surface-sterilized seeds were grown under axenic conditions. Before sowing, the seeds were dipped in cyanobacterial suspension (cultures were incubated for 2 weeks) adjusted to 0.1 μg ml−1 ch-a. Unicellular strains were directly suspended in sterile water, while in the case of filamentous strains, the filaments were homogenized to distribute them equally in the suspension. The pots, trays, and covers were sterilized by dipping them in 5% sodium hypochlorite solution for 20 min while the soil was autoclaved. Seeds treated with the strains were sown and, after germination, their growth was followed for 2 weeks. The plants were kept at 22 ± 1°C, 60% relative humidity, 12 h photoperiod, and the light intensity was adjusted to 180 μmol m−2 s−1. For wire house experiments, the seeds were surface-sterilized and soaked in inoculum as mentioned. Pots (30 × 30 cm) containing 10 kg of unfertilized garden soil (pH 7.4; EC 40 ds m−1; organic content 0.60%) were prepared. The experiment was started with 15 seeds in each pot (replicated ten times), which was reduced to ten seeds per pot after germination within 10–12 days. The pots were laid down in a completely randomized design. The third round of thinning was accomplished after a growth period of 6 weeks by retaining five seedlings per pot, which were grown till maturity. During the growth period (December 2007 to April 2008), plants were kept in the wire house in the Botanical Garden of the Department of Botany, University of the Punjab, Lahore, Pakistan, under ambient light and temperature. After reaching physiological maturity, five plants were harvested from each pot. Growth, biomass, and yield parameters (shoot length, number of tillers, spike’s length, and weight of 100 seeds) were measured.
Statistical analysis
The data obtained in the experiments were subjected to statistical analysis using the software package SPSS 12 (SPSS Inc., Chicago, IL, USA). The statistical tests performed were standard error of the mean, analysis of variance (ANOVA; P = 0.05), Duncan’s multiple-range test (P = 0.05), and Pearson’s correlation (bivariate).
Results
Isolation and identification of cyanobacteria
Five cyanobacterial strains, including Anabaena sp. Ck1, Oscillatoria sp. Ck2, Phormidium sp. Ck3, Chroococcidiopsis sp. Ck4, and Synechocystis sp. Ck5, showing cytokinin-like activity in cucumber cotyledon bioassay were selected (Table 1). Organisms isolated from the rice field habitat included three filamentous and one unicellular strain. The dominant cyanobacterium was a free-floating, filamentous species, Oscillatoria sp. Ck2. Dominant flora in the rice rhizosphere was represented by a mixed population of Anabaena sp. Ck1 (filamentous) and Chroococcidiopsis sp. Ck4 (unicellular). Pond water, on the other hand, was dominated by a free-living unicellular organism identified as Synechocystis sp. Ck5 under a light microscope. Under laboratory conditions, Synechocystis sp. Ck5 showed the most intensive growth, which produced 5.65 mg l−1 ch-a (chlorophyll-a) after an incubation period of 15 days. Over the same duration, Anabaena sp. Ck1, Oscillatoria sp. Ck2, Phormidium sp. Ck3, and Chroococcidiopsis sp. Ck4 accumulated 3.83, 3.15, 2.93, and 2.22 mg l−1 ch-a, respectively (Table 1). Initially, selected cyanobacterial strains were identified by studying their morphological features a under light microscope and their identity was confirmed on the basis of homology between rRNA gene partial sequences from the strains with the sequences present in the GenBank database. The isolates Ck1, Ck2, Ck3, Ck4, and Ck5 were assigned to cyanobacterial genera Anabaena, Oscillatoria, Phormidium, Chroococcidiopsis, and Synechocystis, respectively. The GenBank accession numbers of the strains are presented in Table 1.
Phytohormones quantification
Cyanobacteria were cultured for 4 weeks and a sample was drawn for phytohormones analysis via UPLC-ESI–MS/MS every week. Phytohormones in the culture media of cyanobacteria were quantified as pmol mg−1 ch-a. Cyanobacterial growth was determined simultaneously by measuring the ch-a concentration in their culture. The most prolific growth was shown by Synechocystis sp. Ck5. Its growth peaked by the end of 2 weeks of incubation (5.65 mg ch-a l−1), followed by a gradual decrease during the third and fourth weeks of incubation. The same pattern of growth was observed in Chroococcidiopsis sp. Ck4, where the maximum growth was recorded after 2 weeks of incubation (2.721 mg l−1 ch-a). The highest concentration of ch-a in Anabaena sp. Ck1 (3.83 mg ch-a l−1), Oscillatoria sp. Ck2 (3.15 mg ch-a l−1), and Phormidium sp. Ck3 (2.93 mg ch-a l−1) was recorded after 3 weeks of incubation followed by a decline in its accumulation during the fourth week of growth. The selected strains were able to release five different cytokinins species, including trans zeatin (tZ), cis zeatin (cZ), zeatin riboside (ZR), dihydrozeatin riboside (DHZR), and zeatin-o-glucoside (ZOG), in their cultures. Unicellular strains, Chroococcidiopsis sp. Ck4, was the most efficient cytokinins producer among the selected strains, whereas Oscillatoria sp. Ck2 (filamentous) released the least amount of this class of hormones (Fig. 1). Among the filamentous strains, Anabaena sp. Ck1 released the highest amount of cytokinins. Chroococcidiopsis sp. Ck4 culture had a greater variety of cytokinins as demonstrated by the presence of tZ, cZ, ZR, DHZR, and ZOG. The rest of the isolates released two or three species of cytokinins. For instance, tZ, ZR, and DHZR were detected and quantified in the culture of Anabaena sp. Ck1, while Oscillatoria sp. Ck2 and Phormidium sp. Ck3 released two isomers of zeatin (cZ and tZ), along with ZR. Similarly, Synechocystis sp. Ck5 synthesized only the zeatin type of cytokinin. All cyanobacterial strains were able to release indole-3-acetic acid (IAA) in the presence of tryptophan. The strain Anabaena sp. Ck1 released IAA most efficiently among the selected strains. Oscillatoria sp. Ck2 and Synechocystis sp. Ck5 showed almost similar potential for IAA synthesis. While Chroococcidiopsis sp. Ck4 released a relatively smaller amount of this hormone, the least concentration was recorded in the culture of Phormidium sp. Ck3 (Fig. 1). When the difference of hormonal concentration in the culture media was calculated between two adjacent weeks, there was a clear dependence of their release on the cyanobacterial growth cycle in spite of continuous secretion during 4 weeks of incubation. The concentration of cytokinins ranged between 0.25 and 2.21 pmol in the culture media of different cyanobacteria after 1 week of growth. Continuous addition to the cytokinins concentration was recorded in the following weeks, with some variations in their synthesis during different periods of growth cycle. While unicellular strains released the maximum amount of cytokinins during the second and third week of incubation, the cultures of filamentous strains secreted a greater amount of the hormone during the third and fourth weeks of growth. Oscillatoria sp. Ck2 (filamentous strain) was an exception to this general rule, as this strain secreted a uniform amount of cytokinins during the 3-week growth period; however, in the last week, the release of the hormones was sharply increased. IAA concentration in the culture of cyanobacteria was continuously increased during the 4-week growth period. However, the amount released during a particular week varies greatly among cyanobacterial strains. Of the selected strains, Anabaena sp. Ck1 and Synechocystis sp. Ck5 released the greatest amount of IAA during the first week (17.4 and 12.3 pmol, respectively) of growth and a gradual reduction was recorded in the following weeks (Table 2). In the case of Oscillatoria sp. Ck2 and Phormidium sp. Ck3, the secretion of IAA was continuously increased during the 4-week growth cycle. The release of IAA peaked during the second week (7.1 pmol), followed by a lower increase in the amount of this hormone in the culture of Chroococcidiopsis sp. Ck4 during the third and fourth weeks (4.7 and 2.8 pmol, respectively).
Phytostimulation under axenic conditions
Cyanobacterial inoculations stimulated vegetative growth parameters significantly compared to uninoculated (control) seedlings. Seedlings inoculated with cyanobacteria under axenic conditions showed significant increase in shoot height (Table 3). The significant increases in shoot height relative to the control were recorded with Phormidium sp. Ck3 (52%), Chroococcidiopsis sp. Ck4 (49%), and Anabaena sp. Ck1 (40%). Unlike the shoot height, a decline in root length was observed as a result of cyanobacterial inoculation, with the exception of Phormidium sp. Ck3, where a 24% increase over the control was observed (Table 3). However, the decline in root length was significant only in seedlings colonized with Anabaena Ck1 (17.8%). In the majority of treatments, a decline in primary root length was associated with an increase in the number of lateral roots as the typical auxins response (Table 3). The maximum increase in the number of lateral roots was 52% over the control with Anabaena sp. Ck1 inoculation, the most prolific IAA secretor in the culture media. Phormidium had no significant effect on the growth of lateral roots (some decrease was observed) as compared to the control. Biomass parameters of wheat seedlings were also significantly improved by cyanobacterial inoculation (Table 3). Green biomass was maximally enhanced with Chroococcidiopsis sp. Ck4 (40%) inoculation over the control. The content of dry matter was elevated to the highest level (64% over the control) with Phormidium sp. Ck3 among the different inoculations.
Phytostimulation under natural conditions
Wheat plants were harvested at their full maturity and different parameters, including shoot length, number of tillers, spike length, and weight of seeds, were recorded (Table 3). Cyanobacterial inoculations significantly enhanced growth as well as the yield parameters of wheat crop under natural conditions. Among the growth parameters, the shoot length was increased between 15 and 31% with cyanobacterial inoculations over control plants. A significant increase in the yield parameters was recorded with the selected inoculations. Spike length, number of tillers, and weight of seeds were stimulated significantly (although not significantly in all cases) by cyanobacterial inoculations (Table 3). Maximum improvement in the three yield parameters was caused by Chroococcidiopsis sp. Ck4 (26, 52, and 43% respectively) as compared to the control.
Endogenous phytohormones
The majority of the cyanobacterial inoculations enhanced the phytohormones content of T. aestivum var. Uqab 2000 under in vitro conditions. The effect of cyanobacterial inoculation on the phytohormone content of the root was more pronounced as compared to the shoot. While alteration in the IAA content in the shoots in most of the cases was not significant, accumulation of this hormone was more striking in the root (Fig. 2). A four-folds increase in IAA accumulation was recorded in the roots of the seedlings inoculated with Anabaena sp. Ck1, the most prolific producer of this hormone. Synechocystis sp. Ck5, on the other hand, caused more than a three-fold increase in the endogenously accumulated IAA in the root. In the shoot, while IAA accumulation was increased by Anabaena sp. Ck1 and Synechocystis sp. Ck5, the remaining strains caused only a marginal increase in the amount of this hormone. There was a positive linear correlation of cyanobacterial IAA with endogenously accumulated IAA in the shoot (r = 0.987; P = 0.01) as well as the root (r = 0.984; P = 0.01) of the seedlings. Accumulation of cytokinins (Z + ZR) by the end of 14 days incubation was almost equal in roots and shoots (Fig. 2). The maximum increase in cytokinins content of the root and shoot (three-fold over the control) was caused by Chroococcidiopsis sp. Ck4 as compared to the control. The rest of the strains also enhanced the cytokinin content of both root and shoot significantly. Phytohormones in cyanobacterial cultures were positively correlated to their endogenous level in the seedlings. A positive linear correlation (r = 0.902; P = 0.05) was observed between cytokinins released to the cyanobacterial culture media (in vitro) and endogenous plant cytokinins level in the shoot. Cytokinins in cyanobacterial cultures were also positively correlated (r = 0.841; P = 0.05) with their endogenous level in the seedlings’ roots.
Discussion
The present work reports on the phytostimulation and biofertilization of wheat by cyanobacteria capable of releasing phytohormones (cytokinins and IAA) in their culture media. The isolated cyanobacteria were shown to secrete a number of cytokinins (tZ, cZ, ZR, DHZR, and ZOG) along with IAA in the culture media. The release of cytokinins and IAA was clearly dependent on the growth cycle of the selected strains. This observation was in agreement with Sergeeva et al. [26], who demonstrated the growth-dependent release of IAA in free-living and symbiotic Nostoc strains. Cyanobacteria undergo intimate associations with the roots of wheat and stimulate its growth [12]. While most of the work regarding phytostimulation by cyanobacteria is focused on rice and other plants have attracted very little attention, the impact of cyanobacteria on plant growth under fully axenic conditions is limited to a few publications [19]. Several cyanobacteria, including Anabaena, Oscillatoria, and Phormidium, grow naturally in wheat and rice fields [20, 21]. Here, we provide the first evidence that cyanobacteria improve a number of growth parameters in inoculated wheat plants by modifying their endogenous phytohormones. Inoculation with cyanobacteria stimulated the accumulation of endogenous cytokinins and IAA in wheat seedlings under in vitro conditions. A linear correlation of cyanobacterial cytokinins and IAA was recorded with endogenous hormones of the seedlings. There are previous reports that rhizobacteria can have marked effects on plant growth by contributing an exogenous source of phytohormones [3, 5]. The co-cultivation of wheat seedlings with cyanobacteria under axenic conditions, as well as natural conditions, caused a considerable enhancement in growth, biomass, and yield parameters. Contrary to previous reports that root growth is positively affected by cyanobacteria [18], we recorded a significant reduction in root length by some strains, for instance, Anabaena sp. Ck1 and Synechocystis sp. Ck5 associated with an increase in lateral roots initiation, as the typical IAA response. These strains had low cytokinin to IAA ratios in their culture. These results are in line with Barazani and Friedman [4], who reported that high concentrations of L-tryptophan inhibited the root elongation of lettuce seedlings because of excessive secretion of IAA. Significant negative correlation of IAA with root length (r = −0.641; P = 0.01) and positive correlation of this hormone with lateral root (r = 0.877; P = 0.01) demonstrated the involvement of IAA in such responses. However, some strains, including Phormidium sp. Ck3, enhanced root growth by 24% over the control. Contrary to root growth, other parameters, for instance, the number of tillers, weight of 100 seeds, and fresh weight increased linearly with the increase in cytokinins secretion by cyanobacterial strains, as evident from the positive correlation of cytokinin content with these parameters (Table 3). The shoot length and spike length, on the other hand, responded equally to IAA and cytokinins, as evident from the correlation study. The relative amounts of cytokinin and IAA seemed to be an important factor for controlling the dry biomass of seedlings instead of their actual concentration (Table 3). The phytostimulatory potential of cyanobacteria was attributed to the atmospheric nitrogen fixation, making it available to the associated plants [11, 26]. Anabaena sp. associated with wheat plants were shown to enhance grain yield, dry weight, and nitrogen content in the plants [27]. Reversal of the salinity effect on rice by extracellular extract of a cyanobacterium Scytonema hofmanni was attributed to gibberellins-like activity in the extract [23]. Cyanobacteria-like Anabaena have the ability to fix nitrogen and add nitrates to the soil, which are absorbed by seedlings for their growth requirements [11]. Similarly, vitamins produced by certain cyanobacterial isolates may also enhance the growth of the seedlings [14]. Oscillatoria and Synechocystis improved the growth in wheat under heavy metal stress [7]. While these in vivo experiments have shown the involvement of several metabolites other than cytokinins and IAA, in a number of in vitro plant growth experiments, the role of phytohormones in cyanobacterial interaction with plants have been demonstrated [13, 32]. Although phytohormones in cyanobacterial culture were positively correlated with their endogenous level in seedlings, interestingly, some strains which were characterized by high in vitro phytohormones production could influence the seedlings’ cytokinins and IAA pools relatively less than expected. For instance, Anabaena sp. Ck1 was able to release 153% greater cytokinins relative to Synechocystis sp. Ck5; nevertheless, the difference in the endogenous cytokinins level was only 3% in the case of shoots, while roots were influenced by the former strain to accumulate only 8% more cytokinins as compared to the later strain. In all such cases, the role of IAA seemed to be important. Strains which produced high amounts of IAA (Anabaena sp. Ck1) influenced seedlings’ endogenous cytokinins less than the expected increase based on in vitro cytokinins production. The reason for this may be the antagonistic role of IAA and cytokinins in seedlings. Auxins (IAA) were shown to suppress the ipt gene and control the local synthesis of cytokinins in the nodal stem of Pisum sativum. The same results were reported for pea roots and even Arabidopsis roots [29]. On the other hand, the induction of cytokinins oxidase (CkX) by auxins was also reported in tobacco pith explants where the oxidative breakdown of ZR was increased after the application of naphthaleneacetic acid (NAA), which is an auxin. Similarly, the in vitro conversion of zeatin-type cytokinins to adenine derivatives (inactive conjugates) was demonstrated after NAA treatment [16]. The expression of bacterial auxin biosynthesis genes down-regulated ZR and ZRMP levels in transgenic tobacco plant [6]. The study provides evidence that cyanobacteria may use phytohormones as a tool for plant growth promotion by enhancing endogenous levels of cytokinin and IAA.
References
Abd-Alla MH, Mahmoud ALE, Issa AA (1994) Cyanobacterial biofertilizer improved growth of wheat. Phyton Ann Rei Bot A 34:11–18
Ali B, Sabri AN, Ljung K, Hasnain S (2009) Auxin production by plant associated bacteria: impact on endogenous IAA content and growth of Triticum aestivum L. Lett Appl Microbiol 48:542–547
Arkhipova TN, Veselov SU, Melentiev AI, Martynenko EV, Kudoyarova GR (2005) Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 272:201–209
Barazani OZ, Friedman J (2000) Effect of exogenously applied L-tryptophan on allelochemical activity of plant-growth-promoting rhizobacteria (PGPR). J Chem Ecol 26:343–349
Cohen AC, Bottini R, Piccoli PN (2008) Azospirillum brasilense Sp 245 produces ABA in chemically-defined culture medium and increases ABA content in Arabidopsis plants. Plant Growth Regul 54:97–103
Eklöf S, Åstot C, Sitbon F, Moritz T, Olsson O, Sandberg G (2000) Transgenic tobacco plants co-expressing Agrobacterium iaa and ipt genes have wild-type hormone levels but display both auxin- and cytokinin-overproducing phenotypes. Plant J 23:279–284
Faisal M, Hameed A, Hasnain S (2005) Chromium-resistant bacteria and cyanobacteria: impact on Cr(VI) reduction potential and plant growth. J Ind Microbiol Biotechnol 32:615–621
Hashem MA (2001) Problems and prospects of cyanobacterial biofertilizer for rice cultivation. Aust J Plant Physiol 28:881–888
Hussain H, Krishke M, Roitsch T, Hasanin S (2010) Rapid determination of cytokinins and auxin in cyanobacteria. Curr Microbiol (in press). doi:10.1007/s00284-010-9620-7
Kannaiyan S, Aruna SJ, Merina Prem Kumari S, Hall DO (1997) Immobilized cyanobacteria as a biofertilizer for rice crops. J Appl Phycol 9:167–174
Karthikeyan N, Prasanna R, Nain L, Kaushik BD (2007) Evaluating the potential of plant growth promoting cyanobacteria as inoculants for wheat. Eur J Soil Biol 43:23–30
Karthikeyan N, Prasanna R, Sood A, Jaiswal P, Nayak S, Kaushik BD (2009) Physiological characterization and electron microscopic investigation of cyanobacteria associated with wheat rhizosphere. Folia Microbiol (Praha) 54:43–51
Manickavelu A, Nadarajan N, Ganesh SK, Ramalingam R, Raguraman S, Gnanamalar RP (2006) Organogenesis induction in rice callus by cyanobacterial extracellular product. Afr J Biotech 5:437–439
Misra S, Kaushik BD (1989) Growth promoting substances of cyanobacteria. I: vitamins and their influence on rice plant. Proc Indian Natl Sci Acad B 55:295–300
Naiman AD, Latrónico A, de Salamone IEG (2009) Inoculation of wheat with Azospirillum brasilense and Pseudomonas fluorescens: impact on the production and culturable rhizosphere microflora. Eur J Soil Biol 45:44–51
Nordström A, Tarkowski P, Tarkowska D, Norbaek R, Åstot C, Dolezal K, Sandberg G (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin–cytokinin-regulated development. Proc Natl Acad Sci USA 101:8039–8044
Novák O, Hauserová E, Amakorová P, Dolezal K, Strnad M (2008) Cytokinin profiling in plant tissues using ultra-performance liquid chromatography-electrospray tandem mass spectrometry. Phytochemistry 69:2214–2224
Obreht Z, Kerby NW, Gantar M, Rowell P (1993) Effects of root-associated N2-fixing cyanobacteria on the growth and nitrogen content of wheat (Triticum vulgare L.) seedlings. Biol Fertil Soils 15:68–72
Pedurand P, Reynaud PA (1987) Do cyanobacteria enhance germination and growth of rice? Plant Soil 101:235–240
Prasanna R, Jaiswal P, Nayak S, Sood A, Kaushik BD (2009) Cyanobacterial diversity in the rhizosphere of rice and its ecological significance. Indian J Microbiol 49:89–97
Prasanna R, Jaiswal P, Singh YV, Singh PK (2008) Influence of biofertilizers and organic amendments on nitrogenase activity and phototrophic biomass of soil under wheat. Acta Agron Hung 56:149–159
Rodrigues EP, Rodrigues LS, de Oliveira ALM, Baldani VLD, Teixeira KRdS, Urquiaga S, Reis VM (2008) Azospirillum amazonense inoculation: effects on growth, yield and N2 fixation of rice (Oryza sativa L.). Plant Soil 302:249–261
Rodríguez AA, Stella AM, Storni MM, Zulpa G, Zaccaro MC (2006) Effects of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Saline Systems 2:7
Rosas SB, Avanzini G, Carlier E, Pasluosta C, Pastor N, Rovera M (2009) Root colonization and growth promotion of wheat and maize by Pseudomonas aurantiaca SR1. Soil Biol Biochem 41:1802–1806
Santner A, Calderon-Villalobos LIA, Estelle M (2009) Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol 5:301–307
Sergeeva E, Liaimer A, Bergman B (2002) Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 215:229–238
Spiller H, Gunasekaran M (1990) Ammonia-excreting mutant strain of the cyanobacterium Anabaena variabilis supports growth of wheat. Appl Microbiol Biotechnol 33:477–480
Sundara B, Natarajan V, Hari K (2002) Influence of phosphorus solubilizing bacteria on the changes in soil available phosphorus and sugarcane and sugar yields. Field Crop Res 77:43–49
Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45:1028–1036
Tandeau de Marsac N, Houmard J (1988) Complementary chromatic adaptation: physiological conditions and action spectra. Methods Enzymol 167:318–328
Vaishampayan A, Sinha RP, Hader DP, Dey T, Gupta AK, Bhan U, Rao AL (2001) Cyanobacterial biofertilizers in rice agriculture. Bot Rev 67:453–516
Wake H, Umetsu H, Ozeki Y, Shimomura K, Matsunaga T (1991) Extracts of marine cyanobacteria stimulated somatic embryogenesis of Daucus carota L. Plant Cell Rep 9:655–658
Webb VL, Maas EW (2002) Sequence analysis of 16S rRNA gene of cyanobacteria associated with the marine sponge Mycale (Carmia) hentscheli. FEMS Microbiol Lett 207:43–47
Acknowledgments
The Higher Education Commission (HEC) of Pakistan is acknowledged for providing financial support to Mr. Anwar Hussain to visit Würzburg University, Germany, for the determination of phytohormones. Prof. Dr. Thomas Roitsch, Department of Pharmaceutical Biology, Würzburg University, Germany, is acknowledged for providing the laboratory facilities to conduct part of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the BioMicroWorld 2009 Special Issue.
Rights and permissions
About this article
Cite this article
Hussain, A., Hasnain, S. Phytostimulation and biofertilization in wheat by cyanobacteria. J Ind Microbiol Biotechnol 38, 85–92 (2011). https://doi.org/10.1007/s10295-010-0833-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0833-3