Abstract
Microalgae are an effective soil biostimulant. However, pathways for the enhancement of plant growth are still unclear. In this study, the effects of Chlorella vulgaris (C. vulgaris) on wheat growth promotion and its direct and indirect mechanisms were investigated under hydroponic experiment condition in pots in a constant temperature indoor laboratory. Living C. vulgaris showed significant promoting effect on wheat growth in terms of root length (52.41%), shoot length (44.44%) and dry weight (13.86%). Besides the function of supplying inorganic nutrient, the organic molecules in the culture supernatant and cell extract of C. vulgaris promoted wheat growth directly through interaction with the plant roots. The culture supernatant fraction increased root length, shoot length and dry weight of wheat by 27.59%. 11.84%, 16.53%, respectively. The cell extract fraction had a larger effect with the increase in root length, shoot length and dry weight by 33.10%, 20.86% and 27.10%, respectively. Changes in the bacterial community in the rhizosphere under co-culturing of bacteria and microalgae was also investigated to determine indirect mechanisms on plant growth promotion. The results showed living C. vulgaris and rhizosphere bacteria had a synergistic interaction. Compared with initial rhizosphere bacterial community at genus level, the number of beneficial rhizosphere bacteria such as Sphingobacterium, Comamonas, Acetobacter and Mucilaginibacter significantly increased when co-cultured with the supernatant of C. vulgaris. In conclusion, considering the presence of bacteria in the soil environment, it is important to maintain the activity of microalgal cells to release extracellular polymer substances sustainably to promote plant growth.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In order to promote the development of agriculture and meet the needs of China's growing population, the use of fertilizers is still playing an important role in agriculture (Bello et al. 2021). Chemical fertilizers consist of abundant nitrogen, phosphorus and potassium. While increasing crop yields, chemical fertilizers can cause a number of problems such as groundwater pollution and soil structure damage (Zou et al. 2020). Innovative technologies based on biological resources (e.g., biological stimulation or biostimulation) are an effective way to improve crop production while reducing chemical fertilizer application (Lv et al. 2019; Zou et al. 2020). Biostimulants not only alter physiological processes to optimize crop yields, but also improve nutrient uptake (Alvarez et al. 2021; Ortiz-Moreno et al. 2020).
Microalgae can be used as biostimulants and soil conditioners in agricultural systems (Sharma et al. 2021; Suleiman et al. 2020). Microalgae release a variety of active substances into the surrounding environment (Battacharyya et al. 2015; Mógor et al. 2017; Sunarpi et al. 2021), including plant hormones (cytokinins, gibberellins, etc.), polysaccharides, amino acids and other substances, which can promote the growth of plants by improving soil fertility, promoting nutrient cycling, and reducing the loss of nutrients to the environment (Alvarez et al. 2021).
For example, Chlorella spp. extracts promoted the growth of maize and increased the content of nitrogen, phosphorus and potassium in the plant (Dineshkumar et al. 2017). The carotenoid, chlorophyll a, b content were higher in leaves treated with Chlorella vulgaris (Hajnal-Jafari et al. 2020). The similar result occurred in Medicago truncatula treated with Chlorella sp, leading to a higher rate of photosynthesis, growth and final yield (Gitau et al. 2021). The effects of crude extracts of 18 strains of microalgae and cyanobacteria significantly enhanced plant growth, chlorophyll content and nutrient absorption in tomato (Chanda Mutale-Joan et al. 2020). The composition of microalgae extracellular polymeric substance in supernatant and extracts are different and influence plant growth via different mechanisms.
The interaction between microalgae and rhizosphere bacteria plays an important role in plant growth (Anwar et al. 2019; Kang et al. 2021; Munees & Kibret 2014). Microalgae release O2 and inorganic substances to promote microbial growth and metabolism. Rhizosphere bacteria transform inorganic nitrogen and phosphorus, produce CO2, growth-promoting factors and other substances which are beneficial to microalgae (Mu et al. 2021) (Fig. 1). For example, the growth of microalgae can be stimulated by the secretion of indole-3-acetic acid by symbiotic bacteria (Dao et al. 2018). Microalgae-bacteria consortium play an important role in promoting plant growth through synergistic action (Kang et al. 2021). How living microalgae and their released/extracted chemicals influence bacterial communities and promote plant growth needs further study.
While current studies have focused on the plant growth-promoting effects of microalgae, there are only a few studies investigating the mechanism by which microalgae affect plants. Algae cultures contained substances that were secreted into the medium by the algal cells which could promote plant growth (Wake et al. 1992). Samples composed of partially fragmented cells had higher protein release compared to intact microalgal cells (Martini et al. 2021). The fragmentation of microalgal cells increased the release of cellular contents, among which polysaccharides, amino acids and other substances were effective for plant growth (Kholssi et al. 2018). When living microalgae are added to plants, their extracellular polymer substances (EPS) and intracellular polymer substances (IPS) act on plants simultaneously. The aim of this study was to explore the effect and mechanisms of microalgae on plant growth promotion. The direct function of supernatant and extract of microalgae on plants, and the indirect function of regulating bacterial community were both studied to guide the further application of microalgae-based stimulant.
Materials and Methods
Microalgae Preparation
Chlorella vulgaris FACHB-415 (C. vulgaris) was obtained from the Freshwater Algae Culture Collection at the Institute of Hydrobiology in Wuhan Province, China. It was cultured in 500 mL BG11 medium in a biochemical incubator (LRH-100-4B; YIHENG, CHINA) at 25 ℃ and 12 h: 12 h light:dark period. When C. vulgaris reached a cell density of 107 cells/mL, the supernatant (labelled Supernatant, abbreviated to “Sup” in Figures and Tables) was separated by centrifugation (5430 R; Eppendorf, Germany) for 10 min at 17,217 g (Lv et al. 2020). The C. vulgaris biomass was washed three times with distilled water and resuspended with distilled water (labelled Living Biomass, abbreviated to “Liv” in Figures and Tables). C. vulgaris suspension (107 cells/mL, 50 mL) was placed in ultrasonic cell breaker (Skorupskaite et al. 2019) for 1 min to break the microalgal cells to obtain cell extracts (labelled Cell Extract, abbreviated to “Ext” in Figures and Tables).
Bacteria Preparation
Rhizosphere soil was collected from the campus of Shandong University in Qingdao, Shandong Province (39.91°N, 116.41°E). The soil was freeze-dried (SJIA-10N; SJ, China) and sieved (≤ 2 mm). Soil (10 g) and sterile water (90 mL) were placed in a conical bottle, sealed, and oscillated on a 130 r oscillator for 30 min. After standing for 10 min, the supernatant was collected. The bacteria in the supernatant were inoculated in Luria–Bertani liquid medium at 30 ℃, and the inoculation was placed in a shaker at 200 rpm (Smith 1993). The number of bacteria was determined by the plate counting method. Bacteria were diluted to 108 cells/mL with distilled water for the experiment.
Plant Material
The wheat (Triticum aestivum L.) cv. 'Lumai 15' cultivar ((TAL Yangmai No. 1 BI/757318) FI//104–14) was obtained from Shandong Province. Wheat seeds with similar size, shape and full grains were selected for the study. The wheat seeds were washed and disinfected by soaking in a 75% alcohol solution for 8 min and then rinsed three times with sterile distilled water. Then, the seeds were placed in 100 mm diameter Petri dishes with filter paper and kept moist with 8 mL distilled water. They were placed in a cool, ventilated area to germinate. The experiments were initiated when the seedlings were 1 cm in length (Saddozai et al. 2022).
Hydroponic Setup
Germinated wheat seeds were placed in a tube (15 mm × 150 mm) with small stones (0.50–0.70 mm) at the bottom. There was one seed per tube and 5 tubes per treatment. Culture medium (13 mL) were added to each tube. The tubes were placed in an incubator at 25 °C at 18 \(\upmu\) mol/m2/s light intensity for 7 days. A magnesium lamp was located above the device. The light/ dark period was 12 h: 12 h. Each experiment ran for seven days and was repeated three times. Experimental settings are shown in Table 1.
Experiment 1: Effect of Intact C. vulgaris Biomass on Wheat Growth
The water quality of the culture medium was simulated as Nansi Lake (labelled Nansi Lake solution, abbreviated to “lake water” in Tables) with 1.00 mg/L total nitrogen (TN) and 0.05 mg/L total phosphorus (TP). Treatments were: (1) Wheat plants irrigated with Nansi Lake solution containing C. vulgaris biomass (107 cells/mL, 15 mL). (2) Wheat plants irrigated with Nansi Lake solution only. (3) Wheat plants irrigated with distilled water without nitrogen (N) and phosphorus (P) (Control).
Experiment 2: Mechanism of C. vulgaris on Promoting Plant Growth Directly
To determine the functions of different fractions on plant growth, three solutions containing living C. vulgaris biomass, supernatant and extract were prepared, respectively. A solution with the same concentration of N and P as the supernatant of C. vulgaris (TN = 62.85 mg/L, TP = 1.68 mg/L, labelled ANP) was also prepared to explain the function of other organic fractions in the algal supernatant. These solutions were added to the wheat plants in the hydroponic system as in the previous experiment.
Experiment 3: Mechanism of Algae-Bacteria Co-Culture on Promoting Plant Growth Indirectly
The cultured rhizosphere bacteria were centrifuged and washed with distilled water. At the beginning of the experiment, rhizosphere bacteria were combined with Living biomass solution, Supernatant solution, and Extract solution with 108 cells/mL. No extra rhizosphere bacteria were added during the experiment. The control group was treated with rhizosphere bacteria in distilled water without microalgae or their fractions. The experiment was conducted with the same hydroponic set up as described above.
Determination of Wheat Plant Growth Parameters
Length and Dry Weight
The root and shoot lengths of 7-day old seedlings were measured manually with a ruler. The harvested wheat plants were then placed in a drying oven at 105℃ for 20 min (Saddozai et al. 2022), then dried at 80℃ to a constant weight for the dry weight measurement. All determinations were performed in triplicate.
Plant Hormones
Fresh leaves (1 g FW) were quickly frozen and ground with liquid nitrogen and then dissolved in 10 mL dimethyl sulfoxide. After centrifugation for 15 min at 10,000 g at 4 ℃, supernatant was taken for plant hormone determination. Indole-3-acetic acid (IAA), gibberellin (GA), cytokinin (CTK), indole-3-propionic acid (IPA) and abscisic acid (ABA) were measured using enzyme-linked immunosorbent assay (ELISA). The kits were provided by Jiangsu Jingmei Biotechnology Co., Ltd., Jiangsu China. All determinations were performed in triplicate.
Chlorophyll Fluorescence Parameters
PSII maximum photochemical quantum yield (Fv/Fm) and actual light energy conversion efficiency (Y(II)) were determined by basic harmonic fluorimeter (JUNIOR-PAM; WALZ, Germany). Wheat seedlings were dark adapted for 30 min prior to the measurements. All determinations were performed in triplicate.
Nitrogen Content of Wheat Leaves
Wheat leaves (1 g FW) were dehydrated, carbonized and oxidized with 5 mL concentrated sulfuric acid and then digested with 4 mL hydrogen peroxide. Total nitrogen was determined by potassium persulfate oxidation absorbance spectrophotometry (Felix-Cuencas et al. 2021; Hu et al. 2021). Under the alkaline medium condition at 120–124 ℃, the oxygen produced by decomposition of potassium persulfate oxidized ammonia nitrogen into nitrate in the test solution. The OD210 of the solution was determined by ultraviolet spectrophotometer (UV-2600i; SHIMADZU, Japan). The total nitrogen content was quantified according to the standard curve. All determinations were performed in triplicate.
Microalgae Cell Number
In order to investigate the activity of microalgae at the end of the experiment, the plate counting method was used to count the living microalgae. The microalgae were diluted 104,105,106 times, inoculated into BG11 solid medium and cultured in an incubator. The number of colonies per milliliter was calculated using Eq. (1):
Where C denotes the average number of colonies growing on the plate at a certain dilution, V denotes the volume (mL) of diluent used when coating the plate, and M denotes the dilution ratio.
Determination of the Characteristic Peak of C. vulgaris
The characteristic peaks of the supernatant and extract of C. vulgaris (20 mL) were determined by infrared spectrometer (Nicolet iS50; Thermo, USA) after freeze-drying. All determinations were performed in triplicate.
Structure of Rhizosphere Microbial Community
Microbial community was measured to determine the indirect effects of C. vulgaris on wheat growth. Beijing Nuohe Zhiyuan Co., Ltd. was commissioned to detect the rhizosphere bacterial community. Follow-up analysis was based on the original data (Supplementary Material). The amplification region of 16S rDNA PCR was 16Sv4. High-throughput sequencing was performed using Illumina NovaSeq sequencing technology, followed by OTUs (Operational Taxonomic Units) clustering and species classification analysis based on available data (97% agreement).
Statistical Analysis
Statistical analyses were performed with SPSS. Descriptive statistics and statistically significant differences between the mean values from control and treated plant samples were determined using One-way ANOVA and Tukey via SPSS (SPSS 19.0, IBM, USA). Canoco (Canoco 5; Microcomputer Power, USA) software was used for redundancy analysis (RDA) to find the correlation between wheat growth indicators and experimental variables.
Results
Growth Promoting Effect of Intact C. vulgaris on Wheat Plants
After the addition of C. vulgaris, root length, shoot length and dry weight of wheat plants increased significantly by 52.41%, 44.44% and 13.86% compared to those without C. vulgaris (P < 0.05) (Table 2). Fv/FM and the content of GA and CTK were significantly increased with C. vulgaris compared to control treatment (P < 0.05) (Table 2). Thus the addition of C. vulgaris significantly promoted the growth of wheat and enhanced the elongation of plant cells and biomass accumulation.
Direct Promotion Mechanism of Culture Supernatant and Cell Extract of C. vulgaris on Wheat Growth
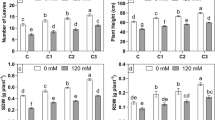
The growth of wheat cultured with supernatant of C. vulgaris was significantly better than that cultured with only N and P solution. Root length, shoot length and dry weight increased by 27.58%, 12.55% and 16.53%, respectively (P < 0.05) (Figs. 2a, b; Table 2). The Fv/Fm and Y(II) were also significantly improved (P < 0.05) (Fig. 2, d). The contents of IAA, GA and CTK were significantly increased by 12.31%, 19.79% and 36.09%, respectively (P < 0.05) (Table 3).
Root length, shoot length (a), dry weight (b), Fv/Fm (c) and Y(II) (d) of wheat under direct action of living Chlorella vulgaris / supernatant / cell extract without bacteria addition (ASup: Supernatant of C. vulgaris; ANP: Only N and P solutions with the same concentration as ASup; AExt: Extract of C. vulgaris; ALiv: Living biomass; Control: Distilled water) *Different letters indicate significant difference (p < 0.05), according to Tukey, One-way ANOVA
Root length, shoot length and dry weight of wheat treated with extract of C. vulgaris were significantly higher than those treated with living biomass (P < 0.05) (Fig. 2a, b), which were 33.10%, 20.86% and 37.17%, respectively. Fv/Fm and Y(II) of wheat were increased by 8.9% and 26.91%. Compared with wheat supplemented with living biomass, endogenous IAA, GA and CTK were 17.75%, 150.88% and 69.79% higher than those treated with extract of C. vulgaris (P < 0.05) (Fig. 2c and Table 3).
Characteristics of C. vulgaris Supernatant and Extract Components
In order to explain the difference of plant growth promotion between supernatant and extract of C. vulgaris, infrared characterization was used to characterize these two components. The absorption peaks of culture supernatant of C. vulgaris indicated the characteristics of polysaccharide: The absorption peak at 3369 cm−1 was the stretching vibration peak of − OH. The peak at 2921 cm−1 was caused by the stretching vibration of saccharide C–H bond. The peak at 1344 cm−1 was saccharide C–H variable angle vibration, which could determine the presence of polysaccharide in C. vulgaris culture supernatant. The characteristic absorption peak at 834 cm−1 showed α-glycoside bond, indicating α-polysaccharide. The characteristic peak at 1600–1700 cm−1 was characteristic of amide I band, which contained abundant secondary structure information of protein (Fig. S1). The infrared spectra showed that the main peak positions of the supernatant and the extract were similar, but the intensities of the each peak were different (Fig. S1 and S2).
Mechanism of Algae- Bacteria Co-system on Wheat Growth Promotion Indirectly
Root length, shoot length and dry weight of wheat plants treated by bacterial and the supernatant of C. vulgaris were significantly increased by 42.21%, 22.07% and 24.78% (P < 0.05) (Fig. 3a, b) compared with the group with only N and P solution added. Y(II) increased by 57.56%. Fv/Fm also increased slightly (P < 0.05) (Fig. 3c, d). By analyzing the bacterial community structure after the cultivation, the number of operational taxonomic units (OTU) in the supernatant of C. vulgaris was 7 fewer than that in N and P solution. However, there were more functional bacteria (such as nitrogen fixation and phosphorus solubilization) found in the top 30 genus level bacteria C. vulgaris supernatant treatment (Figs. 4 and 5). For example, Comamonas, Sphingobacterium and Flavobacterium increased by 1.5%, 17.97%, 4.32%, respectively.
The root length, shoot length (a), dry weight (b), Fv/Fm (c) and Y(II) (d) of wheat under combined action of living Chlorella vulgaris / supernatant / cell extract with the addition of rhizosphere bacteria. (Cultured rhizosphere bacteria “B” were added into ASup, ANP, ALiv and AExt, denoted as A’Sup, A’NP, A’Liv and A’Ext. Control’: Distilled water with bacteria) *Different letters indicate significant difference (p < 0.05), according to Tukey, One-way ANOVA
Wheat growth under the living microalgae-bacteria system was better than the treatment under the microalgae extract-bacteria system. Root length, shoot length and dry weight increased by 20.99%, 27.97% and 35.18%, respectively (P < 0.05) (Fig. 3a, b). The contents of ABA, GA and CTK also increased significantly (P < 0.05) (Table 4). By analyzing the changes of microbial community structure (Fig. 5b), the number of nitrogen-fixing Acetobacter increased 3.60%. The mass balance analysis showed that the sum of nitrogen in the whole system (including 3.317 ± 0.060 mg in wheat biomass and 0.66 ± 0.168 mg in culture solution) at the end of experiment was greater than the nitrogen amount added during the experiment (3.44 ± 1.015 mg), which proved the function of nitrogen fixation bacteria. Mucilaginibacter and Leuconostoc with high EPS production were 8.91% and 1.72% higher in treatments with living biomass compared to those treated with extract of C. vulgaris.
Discussion
Mechanisms of Direct Action of C. vulgaris on Wheat Plants
Microalgae slowly release nitrogen, phosphorus, polysaccharides, phytohormone and other bioactive substances that can be absorbed and used by plants to promote their own growth (Schreiber et al. 2018; Friml and Palme 2002; Hedden and Thomas, 2012; Sun 2010; Meng et al. 2017). In the process of microalgae being used as biostimulants, living microalgae continuously released EPS. The cell walls of dead microalgae were broken down so that IPS is released. In the present study, there were differences between the composition of culture supernatant (mainly containing EPS) and cell extract (mainly consisting of IPS) of C. vulgaris (Figs. S1 and S2), which influenced their functions. Polysaccharides account for 40%-95% of the total culture supernatant in the metabolically active substances of microalgae (H.-C. et al. 2001). This may explain why supernatant and extract of C. vulgaris showed different effects of plant growth promotion.
Although the amount of EPS secreted by microalgae is small (César et al. 2019), the comparison of N and P solution applied alone and the supernatant revealed that the organic fractions in the supernatant of C. vulgaris significantly stimulated the accumulation of various metabolic activities and plant hormones in wheat. The supernatant of C. vulgaris contained polysaccharides (Fig. 2 and Table 2), thus playing an important role on plant growth-promoting effect. C. vulgaris culture supernatant also contained plant growth regulators such as IAA, GA, or CTK (Ordog et al. 2004; Stirk et al. 2002; Tarakhovskaya et al. 2007), which could be absorbed and utilized by plants as exogenous hormones to promote plant growth.
In the absence of rhizosphere bacteria, the extract of C. vulgaris was better at promoting wheat seedling growth compared to adding living biomass. In the microalgae extract, the contents of sonicated microalgal cells were released one-time in larger quantities after breaking the cell wall, whereas C. vulgaris releases active substances slowly and consistently provided C. vulgaris remained active over the experimental period. These results indicated that the bioactive substances contained in microalgae can stimulate plants to promote their growth and metabolism. The cell wall breaking treatment can significant enhance the biostimulatory effect.
Mechanisms of Combined of Algae-Bacteria on Wheat Plants
Indirect Promotion Mechanisms of Algae-Bacteria Co-culture on Wheat Growth
The addition of rhizosphere bacteria significantly improved the growth of wheat (root length increased by 15.53% and shoot length increased by 16.38%), indicating that rhizosphere bacteria had a promoting effect on plant growth. Regardless of whether rhizosphere bacteria were added, C. vulgaris culture supernatant had a more beneficial effect than the corresponding control group, which further clarified that culture supernatant of C. vulgaris had an important effect on wheat in both direct and indirect effects.
Small molecule substances in C. vulgaris culture supernatant can be absorbed and utilized by bacteria to promote their own growth, thus generating plant hormones and other active substances to promote plant growth and development (Alvarez et al. 2021). Under the action of C. vulgaris, the number of functional bacteria with nitrogen fixation (Comamonas), phosphorus-solubilizing (Flavobacterium) and excessive secretion of extracellular polymers (Sphingobacterium) increased significantly (Wu et al. 2018; Nafees et al. 2022; Dutta et al. 2022). Sphingobacterium can produce exopolysaccharides (Nafees et al. 2022), which have high metabolic capacity and multifunctional physiological characteristics. Sphingobacteria can interact with plants, improve the activity of enzymes in plants and enhance the resistance of plants to heavy metals (Markovska et al. 2009; Wang et al. 2020; Yan et al. 2018). Flavobacterium was a phosphorus-solubilizing bacterium, which promoted phosphorus uptake by plants. It can promote the absorption of plant root nutrients and enhance plant disease resistance (Dutta et al. 2022). Therefore, microalgae released chemicals could promote plant growth indirectly by adjusting bacterial community and functions.
Synergistic Functions of C. vulgaris and Rhizosphere Bacteria
Under co-culture conditions, bacteria could significantly promote the growth of microalgae. In this experiment, the number of living microalgae reached 107 cells/mL in the hydroponic solution at the end of the culture period, which was a similar density as the initial phase of experiment. Thus C. vulgaris could maintain good activity in the co-culture system of algae and bacteria. Both living microalgae and rhizosphere bacteria can continuously release active substances into the supernatant (Dao et al. 2018) and benefit for plant growth, such as plant hormones and polysaccharides. They played a synergistic role and significantly improved the wheat growth.
Under algae-bacteria synergistic interaction, the number of beneficial rhizosphere functional bacteria increased such as nitrogen-fixing bacteria (Acetobacter) (Urquiaga et al. 1992) and plentiful EPS producing bacteria (Mucilaginibacter and Leuconostoc). Leuconostoc produced EPS (such as dextran, alternating glucan, fructan and inulin) and inhibited the growth of pathogenic microorganisms which can promote plant growth (Kim et al. 2008; Zikmanis et al. 2020). In a previous study, the plant growth-promoting bacteria (A. brasilense) significantly increased the growth of Chlorella sorokiniana (UTEX 2714), driven in part by the secretion of the auxin hormone indole-3-acetic acid (IAA) (Hai et al., 2020). In this study, rhizosphere bacteria and living microalgae grew in coordination. Compared with only adding C. vulgaris, wheat growth showed a better performance under the co-culture system of algae and bacteria. This experiment was a hydroponics experiment conducted in the laboratory, and it will be necessary to further verify whether the effect will be consistent in field. Combined with the results above, it was deduced that keeping microalgae alive is better for its growth-promoting function. Though intercellular molecules stimulate plants directly, living microalgae continuously release EPS and adjust the bacterial community, which showed better plant growth promotion.
Correlationship Between Enhanced Wheat Growth and Microalgae/bacteria Addition
The growth of wheat was the result of various hormone interactions. The root length, shoot length and dry weight were positively correlated with IAA, GA, CTK and IPA, but negatively correlated with ABA. Shoot length of wheat was closely related to dry matter accumulation. IAA and GA played an important role in shoot elongation of wheat; CTK had a greater effect on root elongation (Fig. 6).
Microalgae produce plant hormones mainly including IAA and CTK (Graziani et al. 2020). In the presence of bacteria, the living biomass and the supernatant had better treatment effect. CTK in both groups had a greater influence on plants. Therefore, it is hypothesized that in the presence of rhizosphere bacteria, the active substances released by microalgae may play a major role in the influence of plants.
The contents of IAA, GA, CTK and IPA in plants were significantly increased after the addition of bacteria. Rhizosphere bacteria have a synergistic relationship with plants, directly promoting plant growth by promoting resource acquisition and/or regulating plant metabolic level (Bumandalai and Tserennadmid 2019; Zhang et al. 2018). Rhizosphere bacteria can secrete plant hormones such as CTK, IAA, GA, indole acetic acid and ethylene, which can indirectly regulate the content of endogenous hormones in plant (An et al. 2022; Xie et al. 2022). In groups A’sup and A’liv, plant showed higher hormone levels, which indicated that rhizosphere bacteria had a great effect on plant growth promotion with the occurrence of microalgal EPS.
Conclusion
Wheat after microalgae application showed a significant increase in terms of plant height, nutrient content, phytohormone content and other indicators. Besides nitrogen and phosphorus provided by the algae, C. vulgaris culture supernatant and cell extract containing various organic molecules have great growth-promoting effects on plants. The cell extract had a more prominent growth-promoting effect on the wheat seedlings due to its organic composition. Combined with the rhizosphere bacteria, the close interaction between microalgae and bacteria produced more complex effects on the plants. Besides the direct function of microalgae on the growth of wheat, C. vulgaris and the culture supernatant regulated the bacteria community, and enhanced plant hormone release. Thereby, wheat cultured under algae-bacteria co-culture conditions showed the best growth under laboratory conditions. In practical agricultural applications, it is particularly important to keep microalgae active for continuous EPS release into the soil to enhance the bacterial community and promote plant growth.
References
Alvarez AL, Weyers SL, Goemann HM, Peyton BM, Gardner RD (2021) Microalgae, soil and plants: a critical review of microalgae as renewable resources for agriculture. Algal Res 54:102200
An XC, Wang ZF, Teng XM, Zhou RR, Wang XX, Xu M, Lian B (2022) Rhizosphere bacterial diversity and environmental function prediction of wild salt-tolerant plants in coastal silt soil. Ecol Ind 134:108503
Anwar MS, Paliwal A, Firdous N, Verma A, Kumar A, Pande V (2019) Co-culture development and bioformulation efficacy of psychrotrophic PGPRs to promote growth and development of Pea (Pisum sativum) plant. J Gen Appl Microbiol 65(2):88–95
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Rev Plant Physiol Plant Mol Biol 50(1):601–639
Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B (2015) Seaweed extracts as biostimulants in horticulture. Sci Hortic 196:39–48
Bello AS, Saadaoui I, Ben-Hamadou R (2021) “Beyond the source of bioenergy”: microalgae in modern agriculture as a biostimulant, biofertilizer, and anti-abiotic stress. Agronomy 11(8):1610
Brasil, B.D.S.A.F., Siqueira, F.G.d., Salum, T.F.C., Zanette, C.M., Spier, M.R (2017) Microalgae and cyanobacteria as enzyme biofactories. Algal Res 25: 76-89
Bumandalai O, Tserennadmid R (2019) Effect of Chlorella vulgaris as a biofertilizer on germination of tomato and cucumber seeds. Int J Aquatic Biol-IJAB 7(2):95–99
Burns DL, Barbieri JT, Iglewski BH, Rappuoli R (2014) Emerging infectious diseases. Bacterial Toxins B(3): 917–929.
César C, Marisa F, Natacha N, Artur F, Nereida C (2019) Marine vs freshwater microalgae exopolymers as biosolutions to microplastics pollution. Environ Pollut 249:372–380
Chentir I, Hamdi M, Doumandji A, Hadjsadok A, Ouada HB, Nasri M, Jridi M (2017) Enhancement of extracellular polymeric substances (EPS) production in Spirulina (Arthrospira sp.) by two-step cultivation process and partial characterization of their polysaccharidic moiety. Int J Biol Macromol 105(2): 1412
Chew W, Ling K, Chuan T, Juan C, Yap J, Ying J, Suan HN (2017) Microalgae biorefinery: High value products perspectives. Bioresource Technol 229: 53-62
Dao GH, Wu GX, Wang XX, Zhang TY, Zhan XM, Hu HY (2018) Enhanced microalgae growth through stimulated secretion of indole acetic acid by symbiotic bacteria. Algal Res 33:345–351
Dineshkumar R, Subramanian J, Gopalsamy J, Jayasingam P, Arumugam A, Kannadasan S, Sampathkumar P (2017) The Impact of Using Microalgae as Biofertilizer in Maize (Zea mays L.). Waste Biomass Valorization 10(5): 1101–1110.
Dutta P, Muthukrishnan G, Gopalasubramaiam SK, Dharmaraj R, Karuppaiah A, Loganathan K, Periyasamy K, Pillai MA, Upamanya GK, Boruah S, Deb L, Kumari A, Mahanta M, Heisnam P, Mishra AK (2022) Plant growth-promoting rhizobacteria (PGPR) and its mechanisms against plant diseases for sustainable agriculture and better productivity. Biocell 46(8):1843–1859
Felix-Cuencas L, Garcia-Trejo JF, Lopez-Tejeida S, de Leon-Ramirez JJ, Gutierrez-Antonio C, Feregrino-Perez AA (2021) Nitrogen and phosphorus flux in wastewater from three productive stages in a hyperintensive tilapia culture. Water Reuse 11(3):520–530
Friml J, Palme K (2002) Polar auxin transport—old questions and new concepts? Plant Mol Biol 49(3–4):273–284
Gitau MM, Farkas A, Balla B, Ordog V, Futo Z, Maroti G (2021) Strain-specific biostimulant Effects of Chlorella and Chlamydomonas Green Microalgae on Medicago truncatula. Plants-Basel 10(6):1060
Graziani G, Ritieni A, Cirillo A, Cice D, Di Vaio C (2020) Effects of biostimulants on annurca fruit quality and potential nutraceutical compounds at harvest and during storage. Plants-Basel 9(6):3096
Hajnal-Jafari T, Seman V, Stamenov D, Duric S (2020) Effect of Chlorella vulgaris on growth and photosynthetic pigment content in swiss chard (Beta vulgaris L. subsp. cicla). Pol J Microbiol 69:1–4
Hedden P, Thomas S, Stephen G (2012) Gibberellin biosynthesis and its regulation. Biochem J 444(1):11–25
Hu CY, Ren SC, Lin YL, Zhang JC, Zhu YY, Xiong C, Wang QB (2021) Kinetics of diatrizoate degradation by ozone and the formation of disinfection by-products in the sequential chlorination. Water Reuse 11(4):560–571
Kang Y, Kim M, Shim C, Bae S, Jang S (2021) Potential of algae-bacteria synergistic effects on vegetable production. Front Plant Sci 12:656662
Kholssi R, Marks EAN, Montero O, Mate AP, Debdoubi A, Rad C (2018) The growth of filamentous microalgae is increased on biochar solid supports. Biocatal Agric Biotechnol 13:182–185
Kim JF, Jeong H, Lee JS, Choi SH, Ha M, Hur CG, Kim JS, Lee S, Park HS, Park YH, Oh TK (2008) Complete genome sequence of Leuconostoc citreum KM20. J Bacteriol 190(8):3093–3094
Liu XY, Hong Y, Gu WP (2021) Influence of light quality on Chlorella growth, photosynthetic pigments and high-valued products accumulation in coastal saline-alkali leachate. Water Reuse 11(2):301–311
Lv J, Zhao F, Feng J, Liu Q, Nan F, Liu X, Xie S (2020) The impact of particulate and soluble organic matter on physicochemical properties of extracellular polymeric substances in a microalga Neocystis mucosa SX. Algal Res 51:102064
Lv J, LS, Feng J, Liu Q, Guo J, Wang L, Jiao S, Xie S (2019) Effects of microalgal biomass as biofertilizer on the growth of cucumber and microbial communities in the cucumber rhizosphere. Turk J Bot 44(2): 167-+.
Markovska YK, Gorinova NI, Nedkovska MP, Miteva KM (2009) Cadmium-induced oxidative damage and antioxidant responses in Brassica juncea plants. Biol Plant 53(1):151–154
Martini F, Beghini G, Zanin L, Varanini Z, Zamboni A, Ballottari M (2021) The potential use of Chlamydomonas reinhardtii and Chlorella sorokiniana as biostimulants on maize plants. Algal Res 60:102515
Meng WJ, Cheng ZJ, Sang YL, Zhang MM, Rong XF, Wang ZW, Tang YY, Zhang XS (2017) Type-B arabidopsis response regulators specify the shoot stem cell niche by dual regulation of Wuschel. Plant Cell 29(6):1357
Mógor ÁF, Ördög V, Lima GPP, Molnár Z, Mógor G (2017) Biostimulant properties of cyanobacterial hydrolysate related to polyamines. J Appl Phycol 30(1):453–460
Mu RM, Jia YT, Ma GX, Liu LR, Hao KX, Qi F, Shao YY (2021) Advances in the use of microalgal-bacterial consortia for wastewater treatment: Community structures, interactions, economic resource reclamation, and study techniques. Water Environ Res 93(8):1217–1230
Munees A, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ Sci 26(1):1–20
Mutale-Joan C, Redouane B, Najib E, Yassine K, Lyamlouli K, Laila S, Zeroual Y, Hicham EA (2020) Screening of microalgae liquid extracts for their bio stimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L. Sci Rep 10(1):2820
Nafees M, Ullah S, Ahmed I (2022) Modulation of drought adversities in Vicia faba by the application of plant growth promoting rhizobacteria and biochar. Microsc Res Tech 85(5):1856–1869
Ordog V, Stirk WA, van Staden J, Novak O, Strnad M (2004) Endogenous cytokinins in three genera of microalgae from the chlorophyta. J Phycol 40(1):88–95
Ortiz-Moreno ML, Solarte-Murillo LV, Sandoval-Parra KX (2020) Biofertilization with chlorophyta and cyanophyta: an alternative for organic food production. Acta Biológica Colombiana 25(2):303–313
Saddozai UK, Baloch MS, Alizai AA, Khakwani AA (2022) Wheat genotypes potential with longest coleoptile length sown at different sowing depths. Pak J Bot 54(4):1349–1358
Schreiber C, Schiedung H, Harrison L, Briese C, Ackermann B, Kant J, Schrey SD, Hofmann D, Singh D, Ebenhoh O, Amelung W, Schurr U, Mettler-Altmann T, Huber G, Jablonowski ND, Nedbal L (2018) Evaluating potential of green alga Chlorella vulgaris to accumulate phosphorus and to fertilize nutrient-poor soil substrates for crop plants. J Appl Phycol 30(5):2827–2836
Sharma GK, Khan SA, Shrivastava M, Bhattacharyya R, Sharma A, Gupta DK, Kishore P, Gupta N (2021) Circular economy fertilization: Phycoremediated algal biomass as biofertilizers for sustainable crop production. J Environ Manage 287:112295
Skorupskaite V, Makareviciene V, Sendzikiene E, Gumbyte M (2019) Microalgae Chlorella sp. cell disruption efficiency utilising ultrasonication and ultrahomogenisation methods. J Appl Phycol 31(4): 2349–2354.
Smith D (1993) Long-term preservation of test strains (Fungus). Int Biodeterior Biodegradation 31(3):227–230
Steinrücken P, Erga SR, Mjos SA, Kleivdal H, Prestegard SK (2017) Bioprospecting North Atlantic microalgae with fast growth and high polyunsaturated fatty acid (PUFA) content for microalgae-based technologies. Algal Res 26:392–401
Stirk WA, Ördög V, Jäger J (2002) Cytokinin- and auxin-like activity in Cyanophyta and microalgae. J Appl Phycol 14(3):215–221
Suleiman AKA, Lourenço KS, Clark C, Luz RL, da Silva GHR, Vet LEM, Cantarella H, Fernandes TV, Kuramae EE (2020) From toilet to agriculture: fertilization with microalgal biomass from wastewater impacts the soil and rhizosphere active microbiomes, greenhouse gas emissions and plant growth. Resour Conserv Recycl 161:104924
Sun TP (2010) Gibberellin-GID1-DELLA: a pivotal regulatory module for plant growth and development. Plant Physiol 154(2):567–570
Sun Y, Wang H, Guo G, Pu Y, Yan B (2014) The isolation and antioxidant activity of polysaccharides from the marine microalgae Isochrysis galbana. Carbohyd Polym 113:22–31
Sunarpi H, Nikmatullah A, Ambana Y, Ilhami BTK, Abidin AS, Ardiana N, Kirana IAP, Kurniawan NSH, Rinaldi R, Jihadi A, Prasedya ES (2021) Phytohormone content in brown macroalgae Sargassum from Lombok coast, Indonesia. IOP Conf Ser 712(1):012042
Tarakhovskaya ER, Maslov YI, Shishova MF (2007) Phytohormones in algae. Russ J Plant Physiol 54(2):163–170
Urquiaga S, Cruz KHS, Boddey RM (1992) Contribution of Nitrogen-Fixation to Sugar-Cane-N-15 And Nitrogen-Balance Estimates. Soil Sci Soc Am J 56(1):105–114
Viegas C, Gouveia L, Goncalves M (2021) Aquaculture wastewater treatment through microalgal. Biomass potential applications on animal feed, agriculture, and energy. J Environ Manag 286: 112187.
Wake H, Akasaka A, Umetsu H, Ozeki Y, Shimomura K, Matsunaga T (1992) Enhanced germination of artificial seeds by marine cyanobacterial extract. Appl Microbiol Biotechnol 36(5):684–688
Wang Q, Ge C, Xu SA, Wu Y, Yang X (2020) The endophytic bacterium Sphingomonas SaMR12 alleviates Cd stress in oilseed rape through regulation of the GSH-AsA cycle and antioxidative enzymes. BMC Plant Biol 20(1): 63.
Wu Y, Zaiden N, Cao B (2018) The core- and pan-genomic analyses of the genus comamonas: from environmental adaptation to potential virulence. Front Microbiol 9:3096
Wu H, Haig T, Pratley J, Lemerle D, An M (2001) Allelochemicals in wheat (Triticum aestivum L.): cultivar difference in the exudation of phenolic acids. J Agricult Food Chem 49(8): 3742–3745.
Xie Y, Wang Z, Cheng X, Qiu R, Hamoud YA, Hong C, Zong X, Wang Y, Agathokleous E, Guo X (2022) Dissecting the combined effects of cultivar, fertilization, and irrigation on rhizosphere bacterial communities and nitrogen productivity in rice. Sci Total Environ 835:155534
Yan Hu, Lingli Lu, Shengke T, Senman Li, Xiaoxia L (2018) Cadmium-induced nitric oxide burst enhances Cd tolerance at early stage in roots of a hyperaccumulator Sedum alfredii partially by altering glutathione metabolism. Sci Total Environ 650(2):2761–2770
Zhang Z, Tang L, Zhang Y (2018) Algae-bacteria interactions and their ecological functions in the ocean. Microbiol China 45(9):2043–2053
Zikmanis P, Brants K, Kolesovs S, Semjonovs P (2020) Extracellular polysaccharides produced by bacteria of the Leuconostocgenus. World J Microbiol Biotechnol (formerly MIRCEN J Appl Microbiol Biotechnol) 36(11):161
Zou Y, Zeng Q, Li H, Liu H, Lu Q (2020) Emerging technologies of algae-based wastewater remediation for bio-fertilizer production: a promising pathway to sustainable agriculture. J Chem Technol Biotechnol 96(3):551–563
Funding
This study was supported by China National Funds For Distinguished Young Scientists (Grant numbers- 51925803), and Natural Science Foundation of China (Grant numbers- 52000122; 51878388).
Author information
Authors and Affiliations
Contributions
S-NW: formal analysis, investigation, data curation, writing—original draft. S-HG: writing—review & editing. L-LZ: conceptualization, methodology, funding acquisition, writing—review & editing. JZ: funding acquisition, writing—review & editing
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Wendy Stirk.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, SN., Ge, SH., Zhuang, LL. et al. Multiple Pathways for the Enhancement of Wheat Growth by Chlorella vulgaris. J Plant Growth Regul 43, 550–562 (2024). https://doi.org/10.1007/s00344-023-11113-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-11113-w