Abstract
Selenium (Se) plays an important role in geochemistry and is an essential trace element for humans and animals. This review summarizes the transformation and accumulation of Se in the plant-soil-microbe system. As one of the important reservoirs of Se, soil is an important material basis of its entry into the food chain through plants. Soil with an appropriate amount of Se is beneficial for plant growth and plays a valuable role in a stress-resistant environment. Among the many migration and transformation pathways, the transformation of Se by microorganisms is particularly important and is the main form of Se transformation in the soil environment. In this review, the role and form transformation of Se in plants, soil, and microorganisms; the role of Se in plants; the form, input, and output of Se in soil; the absorption and transformation of Se by plants; and the role of microorganisms in Se transformation are presented. In addition to describing the migration and transformation laws of Se in the environment, this review expounds on the main directions and trends of Se research in the agricultural field as well as current gaps and difficulties in Se-related research. Overall, this reviews aims to provide necessary information and theoretical references for the development of Se-rich agriculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Selenium (Se) is a necessary trace element for human and animals that participates in many biological metabolic processes in the human body. Se deficiency can cause or induce many diseases, such as Keshan disease, skeletal muscle necrosis, and cardio-cerebrovascular disease (Vinceti et al. 2018). Although Se is not necessarily a necessary nutrient for plant growth, plants are the main source of Se intake for humans and animals (Natasha et al. 2018). Dietary Se supplement is the most common and important route of Se entry into the body. Se content and its existing form in crops determine the amount of Se absorbed by humans through food (Ekumah et al. 2021). The Se absorption and transformation abilities of plants and the transfer and enrichment abilities of the plant itself to its edible portion determine the Se enrichment effect of the plant (Guignardi and Schiavon 2017). According to the standards of the World Health Organization (WHO) and the International Food and Agriculture Organization (FAO), the global population with severe Se deficiency (daily intake of 7–11 μg) reached 0.5–1 billion, and the population with Se deficiency is markedly more than the population with excessive Se (Winkel et al. 2012). In China alone, nearly 2/3 of the planting soil is Se-deficient, posing health risks to people that live in these areas owing to insufficient Se intake (Zhang et al. 2014b). As in-depth research continues to be carried out, the important role of Se in humans has been discovered. Se can participate in the development of selenoprotein followed by glutathione peroxidase (GSH-Px), thioredoxin reductase (TrxR), iodothyroninedeiodinases, and a series of enzyme systems that display functions, such as anti-disease, anti-cancer, and inhibition of HIV development and other related immune system disorders (Ekumah et al. 2021).
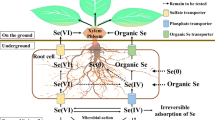
Se can be found in different geographical features, such as the atmosphere, lithosphere, hydrosphere, and biosphere, and its basic fractions are affected by chemical processes (pH, redox properties, and organic matter content), physical processes (adsorption and deposition effects), and biological processes (microbial effects) (Sharma et al. 2015). The form of Se found in the soil of natural Se-rich areas is mainly antimonselite, supplemented by selenate. The form of Se in ores cannot be directly absorbed and utilized by plants. Further, Se salts are produced by long-term leaching and weathering of Se ores, which are highly toxic and unevenly distributed (Deng et al. 2018). The level of Se in plants is determined by the status of Se in the soil (Favorito et al. 2020). The form of Se, especially its effective content, is key to determining its mobility and toxicity (Galić et al. 2021). A series of environmental chemical reactions, such as (biological) oxidation–reduction, precipitation-dissolution, and adsorption–desorption, among others, occur between Se in the soil and soil components, such as iron and manganese oxidation, clay minerals, and organic matter (Wang et al. 2019). An appropriate amount of Se can increase the photosynthetic pigment content in plant leaves under stress, increase the activity of antioxidant enzymes in leaves and fruits, improve the osmotic adjustment ability, increase the organic acid content, and assist in plant growth under stress (Morales-Espinoza et al. 2019; Zahedi et al. 2019). Se is cycled in soil mainly through the microbial pathway (Mehdi et al. 2013). Microorganisms serve as the decisive factor of Se transformation (especially Se reduction) in soil as they participate in the formation and transformation of Se in various valence states (Gómez-Gómez et al. 2019; Fischer et al. 2020). The circulation of Se in the crop growth environment is shown in Fig. 1.
Understanding the migration and role of Se in plant-soil-microorganisms and clarifying the absorption of Se by plants are of particular importance. Moreover, understanding the process, influencing factors, and physiological functions of Se absorption and transformation by plants based on agroecology as well as the law of microbial transformation of Se and the migration of Se in soil can better guide production practices. Based on current studies on crop ecology in the development of Se-rich functional agricultural systems, the migration law and role of Se in plants, soil, and microorganisms are summarized in this review. The information presented herein is expected to serve as basic information for future research on the absorption and transformation of Se in agro-ecology and its physiological mechanism as well as a reference and basis for the development of Se-rich functional agricultural systems.
Se has various effects in plants, including promoting plant growth, enhancing plant resistance, and improving crop quality, among others (Table 1). An appropriate concentration of Se can promote the accumulation of starch in plant chloroplasts, which is beneficial to plant growth, as demonstrated in plant studies with lettuce (Rios et al. 2010), chicory (Germ et al. 2007), and potato (Turakainen et al. 2004). An appropriate amount of Se can increase the chlorophyll content in plants owing to the ability of Se to restore enzyme activity in the photoresponse and the electron transport chain of stressed plants (Diao et al. 2014). The addition of suitable concentration of Se could reduce the damage of chloroplast to some extent and increase the content of chlorophyll (Chu et al. 2010; Yao et al. 2011; Malik et al. 2012). Excessive Se application aggravates the damage to chloroplasts, which is not conducive to plant photosynthesis (Wang et al. 2012a). Zhang et al. (2014b) revealed that the application of Se (< 50 g hm−2) increased the photosynthetic rate (Pn), intercellular CO2 concentration (Ci), electron transport rate (ETR), and chlorophyll fluorescence parameters, such as Fv, Fo, Fv/Fo, and Fv/Fo, in rice; however, the photosynthesis index decreased when 100 g hm−2 Se was applied. The photosynthetic physiology of plants is particularly sensitive to environmental stress. Further, the mechanistic effect of Se on plant photosynthesis may be similar to that of the antioxidant system; when electron transport is blocked during photosynthesis, reactive oxygen species (ROS) accumulation will be induced (Zhang et al. 2007). Se affects plant photosynthesis by inhibiting or inducing ROS accumulation and the photosynthesis-related enzyme system. Se may also affect electron transport and photosynthetic energy conversion by affecting Fe-S protein synthesis (Van Hoewyk et al. 2007; Feng et al. 2013). Freeman et al. (2010) compared the ETR between Se-hyperaccumulating plants and non-Se-hyperaccumulating plants under Se treatment. The ETR of Se-hyperaccumulating plants was significantly increased after treatment with 20 μmol selenate, whereas that of non-Se-hyperaccumulating plants decreased significantly under the same conditions. At present, only few studies have assessed the effects of Se on plant photosynthetic physiology, which must be further examined at the physiological level. Further, the molecular mechanism should be detected.
An appropriate amount of Se can promote plant growth and increase the yield of crops, such as rice (Zhang et al. 2014b), wheat (Nawaz et al. 2015), and lentils (Ekanayake et al. 2015), which is related to an increase in chlorophyll content, promotion of photosynthesis, enhanced antioxidant capacity of crops, and improved stress resistance of crops (Broadley et al. 2010; Jiang et al. 2015).
Drought stress can lead to abnormal accumulation of ROS in plant cells, resulting in different degrees of oxidative damage to biofilm, protein, and DNA, which in turn affects plant growth, respiration, and photosynthesis, and can even lead to death in severe cases (Mittler 2002). Feng et al. (2013) suggested that Se may control the ROS level of plants under stress via three ways: by mediating the disproportionation of superoxide anion (O2−·) to H2O2; through the direct involvement of Se compounds in the scavenging activities of O2−· and hydroxyl radicals (·OH); and by regulating the antioxidant enzyme system. Se metabolism in plant cells controls the balance of ROS concentration by regulating the concentration of free metal ions (Fe2+, Cu+). Se can directly or indirectly regulate the formation of antioxidant enzymes in plants, especially GSH-Px (Feng et al. 2013). Akladious (2012) indicated that exogenous Se can increase the content of proline and reduce plant stress injury. Se plays an important role in increasing the growth rate of crops (Cartes et al. 2010), reducing ultraviolet radiation oxidative damage (Yao et al. 2013), increasing chlorophyll and carotenoid contents in plant leaves (Dong et al. 2013), increasing the activity of antioxidant enzymes, and regulating the content of osmotic substances under heavy metal stress (Kumar et al. 2012). In addition, Se alleviates the adverse effects of drought stress on wheat (Nawaz et al. 2015), barley (Habibi 2013), rape (Hasanuzzaman and Fujita 2011), and other crops by improving plant photosynthetic capacity, enhancing antioxidant capacity, and increasing the content of osmotic adjustment substances.
Heavy metals in the environment cannot be degraded, and the various forms of these metals will transform into each other as environmental factors change (Nagajyoti et al. 2010). The toxicity of different forms of heavy metals is quite different, which poses a great challenge to the treatment of heavy metal pollution (Yao et al. 2012). An appropriate amount of Se can inhibit the absorption and transport of heavy metals by crop roots (Zhang et al. 2012). Further, Se can directly react with heavy metals in the rhizosphere soil to inhibit its toxicity (Zeng et al. 2005). Se can affect the root surface iron plaque, soil solid phase, pH, microorganisms, root exudates and other possible indirect action pathways, convert heavy metals from a more toxic form to a less toxic form, complex/chelate with heavy metals, and reduce the mobility of heavy metals (Huang et al. 2018, 2015; Cai et al. 2019; Chen et al. 2019), to ultimately reduce the availability of heavy metals in the soil and the accumulation of heavy metals in crops. An appropriate addition of Se could significantly reduce the accumulation of heavy metals in crops. Se could inhibit the transport of As from underground to the shoot (Hu et al. 2014) and limit the transport of Hg from the root to shoot of garlic (Allium sativum), thereby reducing the accumulation of Hg in the shoot (Zhao et al. 2013). Selenate could repair damage caused by Cd to the chloroplast membrane structure (Filek et al. 2010). The antagonistic effect of Se on the accumulation of heavy metals is affected by many factors, including the concentration and form of Se and heavy metals. The inhibitory effect of Se on plant Cr (III) absorption was found to be significantly stronger than that on Cr (VI); however, the antagonistic effects of different fractions of Se on plant Cr absorption were similar (Srivastava et al. 1998). Se may also increase the heavy metal content in plants. For example, Se treatment increased As accumulation in Thunbergia alata (Bluemlein et al. 2009) and increased Cd and Cu contents in wheat shoots (Landberg and Greger 1994), which may be due to unreasonable Se addition or different crop varieties (Feng and Wei 2012). Se can alleviate the toxic and side effects of heavy metals in plants by: directly inhibiting or forming complexes with heavy metals to inhibit the absorption and transport of heavy metals in plants (Feng et al. 2011; Malik et al. 2012) or participating in the regulation of the plant antioxidant system to alleviate heavy metal poisoning (Belzile et al. 2006; Filek et al. 2010).
At present, only few reports have been published on the effect of Se on plants under temperature stress and salt stress. Both high and low temperature induce plant oxidative stress, affect the stability of many types of enzyme activities in plants, and interfere with the normal growth and development of plants (Chiang et al. 2015). After soaking in a suitable concentration of Se solution, the contents of auxin, chlorophyll, anthocyanin, proline, and the activities of related antioxidant enzymes under temperature stress increase in wheat seeds to reduce cell membrane damage (Akladious 2012). The application of exogenous Se can enhance the antioxidant system of crops, reduce the concentration of malondialdehyde (MDA), increase the content of proline, strengthen the protection of the mitochondrial electron transport chain, induce an increase in the protective substances in vivo, and alleviate the effects of temperature stress on plants (Djanaguiraman et al. 2018).
High salinity will destroy the structure of cytoplasmic membrane, hinder the absorption of mineral elements beneficial for growth, and cause secondary stress effects (Liang et al. 2017). Due to high osmotic pressure, osmotic stress occurs in plants, resulting in ultrastructural damage of chloroplasts, limited or even closure of the respiratory stomata, and a decrease in the rate of photosynthesis and respiration (Hanin et al. 2016). Under salt stress, Se acts as a coenzyme factor of antioxidant enzymes, such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT). The increase in Se concentration increases antioxidant enzyme activity, enhances antioxidant levels, and decreases ROS concentration and MDA content in plants. The main effect of exogenous Se is to scavenge ROS, improve plant antioxidation, and reduce the damage caused by salt stress (Hu et al. 2013). Under salt stress, exogenous Se increases the absorption of N, K, Ca, and induces more metabolites and stress signals in plants (Elkelish et al. 2019). In addition, exogenous Se inhibits the increase in cell membrane permeability of wheat under salt stress, which could effectively alleviate the damage caused by salt stress to plants (Yigit et al. 2012). It is inferred that exogenous Se may reduce the expression of Na+ and K+ transporter gene, thereby maintaining the balance of cell infiltration and improving the salt tolerance of plants. The comprehensive role of Se in plant stress shows that Se can improve the resistance of plants to abiotic stress by affecting ROS and the antioxidant system, interfering with the absorption and transport of heavy metals, changing the transformation process of heavy metals, and repairing the structure of cell membrane and chloroplast as well as the photosynthesis system (Feng et al. 2013). Crops are vulnerable to various diseases and insect pests and stress. Less free radicals and increased proline content can help plants resist stress, whereas Se can directly eliminate excessive free radicals, increase proline accumulation, enhance antioxidant enzyme activity, improve plant immunity, and increase its resistance to biotic and abiotic stresses (Kimani et al. 2013; Priyadarsini et al. 2013; Steinbrenner and Sies 2013; El-Demerdash and Nasr 2014).
Se inhibits the growth of pathogenic microorganisms in vitro. Further, it has a strong inhibitory effect on Aspergillus funiculosus isolated from banana and Alternaria tenuis and Fusarium sp. isolated from tomato; the growth of these pathogenic fungi was terminated in 10 mg kg−1 Na2SeO3 solution (Razak et al. 1991). The minimum inhibitory concentration of Se is very low, and it serves as a beneficial essential element at low concentration. If Se is used as an antifungal agent, it will certainly reduce the negative effects of similar pollutants on the environment. When treated with Na2SeO3, the level of ROS in the spores of a fungal pathogen, Penicillium expansum, increased, and the antioxidant system was destroyed, thereby weakening the cellular function of the pathogen and directly leading to plasma membrane damage of the pathogen, which inhibits the pathogen growth (Wu et al. 2014). Exogenous Se treatment can not only inhibit the growth of pathogenic microorganisms, but also achieve the effect of Se enrichment in fruits. The preharvest and postharvest treatment of apple and tomato with Na2SeO3 revealed that although Se delayed plant tissue senescence and inhibited blue mold disease occurrence, the Se content in apple and tomato was 6 and 5.5 times higher than that in the control fruits, respectively, and remained within the safe edible range (Wu et al. 2015; Wu et al. 2016). The use of exogenous Se helps the rape rhizosphere soil to accumulate beneficial rhizosphere microorganisms, thereby promoting plant growth (Cheng et al. 2021). Soil Se inhibits Sclerotinia sclerotiorum by affecting the dissolved organic matter in rape straw and upregulating antifungal pathway-related genes (Cheng et al. 2020).
Soil adsorption and fixation, leaching and migration, rice absorption, and gaseous volatilization are the four main processes of Se migration and transformation in the soil–plant system (Fernández-Martínez and Charlet 2009). Soil Se has different fractions, such as soluble state, exchange state and carbonate bound state, iron manganese oxide bound state, organic bound state and residue state (Wang et al. 2012b). The proportion of various fractions of Se in the soil is closely related to the physical and chemical properties of the soil, such as redox potential, pH, organic matter (Sharma et al. 2015). The Se form in the soil is the result of the combined effect of soil acidity and alkalinity and redox conditions (Neal et al. 1987). Changes in water content affect the redox conditions of soil (Tokunaga et al. 1996; Hefting et al. 2004), and changes in soil redox characteristics ultimately affect the form of Se in the soil (Gambrell 1994; Dwire et al. 2006). Soil drought, pH, clay content, and plant transpiration also play an important role in changing the soil's ability to fix Se (Jones et al. 2017).
There are five relatively stable valence states of Se in the environment: − 2, − 1, 0, + 4, and + 6 (Fernández-Martínez and Charlet 2009). In soil, the most common fractions of Se are inorganic, SeO42− and SeO32−, and the proportion of the two in the soil is controlled by the soil redox potential. When soil has alkaline and oxidizing conditions, SeO42− is dominant; however, when soil has reducing conditions, SeO32− is the main Se compound in the soil (Jacobs 1990). The aerobic soil mainly contains Se in the form of selenite; however, in anoxic soil (such as flooded paddy soil), Se mainly exists in the form of selenite (Zhu et al. 2009). The other organic fractions of Se in the soil depend on Se transformation by plants and microorganisms (Martens and Suarez 1996). The concentration of Se in most soils is 0.01–2 mg kg−1, with an average value of 0.4 mg kg−1 (Fordyce 2013).
The Se in solid materials, such as rocks and soil, only accounts for 30–60% of the total Se in the environment, whereas a large proportion of Se exists in water systems, such as oceans (Zhang et al. 2004; Winkel et al. 2012). Therefore, Se in the atmosphere is mainly derived from the volatilization of marine substances (Blazina et al. 2017). Volatile Se is mainly dimethyl Se (DMSe) and dimethyl diselenide (DMDSe), which are easily soluble in water and can enter the soil via rainfall. Volatile Se is an important source of Se in soil (Amouroux et al. 2001; Wen and Carignan 2009). There is a significant positive correlation between Se content in soil and precipitation (Sun et al. 2016). When the total Se in soil is 0.08–0.12 mg kg−1, water soluble Se accounts for 1–2%. According to the calculation of 100% loss of water-soluble Se in the rainy season each year, the amount of Se loss per hectare can reach 2–8 g a−1 (Wang and Gao 2001). Therefore, a large amount of rainfall can accelerate the loss of soluble Se in soil (Jones et al. 2017). Human farming activities also have an important influence on the migration cycle of Se on the surface (Bailey 2017). However, the aging process of exogenous Se fertilizer in soil markedly varies according to different soil types (Wang et al. 2017). In general, only 5–30% of Se fertilizers that are directly inputted into the soil can be absorbed and utilized by plants; 70–90% Se remains in the soil or is leached into the surrounding water (Sager 2006).
More studies have found that the distribution of Se in soil is not consistent with that of the parent material, and the distribution of Se in soil cannot be explained by soil parent material alone (Blazina et al. 2014; Sun et al. 2016). Climate and soil properties are the two most important factors affecting soil Se concentration globally (Winkel et al. 2012; Jones et al. 2017). The shading effect of plants on the soil, and surface vegetation can effectively reduce soil erosion by rainwater (Ravi et al. 2010), thereby reducing Se loss from surface soil. However, plants can also indirectly affect the Se content and the form of soil by changing the physical and chemical properties of the soil. Jones et al. (2017) found remarkable differences in soil organic carbon content under different vegetation types; the soil organic carbon content of farmland, woodland, and grassland increased, and the increase in organic carbon could fix soil Se to a certain extent, reduced Se loss, and indirectly increased soil Se retention. Therefore, there may be great differences in soil Se under different land use types (Pilon-Smits et al. 2017). Among different plants, Se-hyperaccumulating plants have a greater effect on soil Se, and they can effectively change the distribution of soil Se and soil Se fractions (Pilon-Smits et al. 2017). This is mainly achieved through the decomposition of litter and the action of root exudates of Se-hyperaccumulating plants (El Mehdawi and Pilon-Smits 2011). Soil and plants are thus an inseparable whole, and good vegetation coverage can play a positive role in the fixation of soil and its nutrients and the reduction of loss induced by leaching.
In the process of regulating the adsorption and desorption of Se and Fe, Al, Ca, Mg ions in the soil, the pH of soil affects the Se form (Goh and Lim 2004). When Se chelates with the iron in soil to form an insoluble selenite-iron complex, it is not easily used by plants and reduces the amount of Se migration (Peak and Sparks 2002). Compared with clay minerals, iron oxide has strong adsorption capacity, and its oxides can simultaneously adsorb Se (+ 4 and + 6), of which, Se (+ 4) is the main type adsorbed. However, under alkaline conditions, the negative salts formed by iron oxide and Se converts selenite into soluble selenate, increasing the migration amount of Se, and form a more stable complex under acidic reduction conditions (Gustafsson and Johnsson 1992). After Se is applied to the soil, Se can be adsorbed or fixed in a short time, thereby transforming it into a form that is difficult to be absorbed by crops. In terms of the ability to fix Se, oxides are the strongest in all components of soil, followed by organic matter (Hawkes and Kutnink 1996).
Organic Se in soil is mainly recognized through the process of biodegradation and biosynthesis; however, the content of organically-bound Se in the soil is closely related to the content of soil organic matter. When the soil organic matter content is high, the content of organically-bound Se in the soil increases (Gustafsson and Johnsson 1992). Some related data show that organically-bound Se is related to its molecular weight. When the combination of soil organic matter and Se exists in the form of low molecular weight mixture, organically-bound Se can be directly absorbed and utilized by plants, whereas organically-bound Se with a larger molecular weight cannot be directly absorbed and utilized by plants (Zayed et al. 1998). Thus, the blind application of organic Se fertilizer and the pursuit of a high-Se-rich state in the soil to increase the Se content in plants are not desirable. At the same time, organic selenides in soil can be decomposed by microorganisms to form methylation fractions and cause gaseous volatilization. The products are mainly alkyl selenides, such as DMDSe and DMSe complexes, which reflect the loss pathway and quantity of soil Se (Gammelgaard et al. 2011). Inorganic selenides can also be methylated under the action of molds, and the methylation of Se may be related to the pH of soil (Zawislanski and Zavarin 1996).
Phosphorus has a similar structure to Se. Phosphate fertilizer regulates the migration of Se, which proves that the amount of phosphate fertilizer in the soil significantly changes the adsorption of Se in the soil (Altansuvd et al. 2014). When the phosphorus content is high, Se adsorption by soil is inhibited, thereby enhancing Se absorption by plants and further increasing Se mobility (Nakamaru et al. 2006; Altansuvd et al. 2014). The Se content in plants is related to the content of S amino acids in plants, where S in the structure of amino acids is often replaced by Se, resulting in an increase in Se content in plants (Ip and Ganther 1994).
The structure and chemical properties of Se are similar to those of S. Most plants are unable to distinguish between Se and S, resulting in the absorption of sulfate. Selenate in soil is transported by the S transport pathway through the transporters, SULTR1;1 and SULTR1;2, in plants (Barberon et al. 2008). SULTR2;1 and SULTR2;2 are involved in the selenate transfer process between roots, stems, and leaves, whereas SULTR4;1 and SULTR4;2 are mainly responsible for the phase transfer of Se between roots and stems (Zuber et al. 2010; Schiavon et al. 2015). SULTR3;1 transporters are mainly present in chloroplasts and are responsible for the transport of selenate across the membrane to chloroplasts (Cao et al. 2013). In summary, the pathway of plant absorption of selenate is highly related to the absorption of sulfate. Plant species and nutrition levels in the growing environment affect selenate transport efficiency in roots (White et al. 2004). Some plant species growing in Se-rich soil display Se accumulation, and their Se absorption capacity is more than 100 times higher than that of ordinary plants, up to 1–15 g kg−1 (dry matter); these plants are called Se-hyperaccumulating plants (Beath et al. 1939). Besides ordinary plants, there may be specific selenate transporters responsible for the transport and migration of Se in plants (Schiavon et al. 2015). The absorption of Se by plants is an active process, which occurs via two main routes: Se in soil and Se in the atmosphere. Selenate, selenite, and organic Se are the main uptake forms of Se by plants. When selenate is absorbed by plants, the valence state of Se does not change during transport. After being transported to leaves, it is reduced to + 4 valence state, converted into organic Se compounds, and finally distributed to other organs and tissues of plants (Li et al. 2010). After selenite is absorbed by plants, Se is converted into organic Se compounds in the roots, and most of the transformed organic Se compounds are retained in the roots, whereas a small portion is transported to the aboveground parts of plants (Keskinen et al. 2010).
Unlike plant selenate absorption, the mechanism of plant selenite absorption is not clear (Zhu et al. 2009). The transfer ability of plants to selenite is relatively poor, as selenite is easily converted into organic selenides after being absorbed by roots (Zhang et al. 2014a). Huang et al. (2016) found that the content of organic Se in wheat treated with selenite for 3 days was close to 90% of the total Se, and most of the substances produced were directly accumulated in the roots and could not be easily transferred to the aboveground parts of plants. The absorption of selenite by wheat is a metabolism-dependent active absorption process, which may be partially mediated by phosphorus transporters (Li et al. 2010). The silicon transport carrier, OsNIP2;1, in rice is also suggested to be related to the absorption of selenite. Selenite can enter the plant through OsNIP2;1, but this is regulated by environmental pH (Zhao et al. 2010). Some studies suggest that the absorption of selenite by plants is a passive diffusion process (Shrift and Ulrich 1969; Arvy 1989, 1993). In particular, Terry et al. (2000) reported that there is no evidence of membrane-mediated selenite uptake by plants. The studies on the absorption mechanism of selenite in plants are far less than that of selenate, which needs to be explored from the point of view of physiology and molecular biology.
Excessive Se has a toxic effect on plants, and there are remarkable differences in the symptoms of Se poisoning among different plants. When non-Se-hyperaccumulating crops are grown in high Se medium, normal growth and development of plants are inhibited. Further, withering and shedding of plant leaves, a decrease in protein synthesis, and dwarfism of plants occur (Trelease and Beath 1951; Mengel et al. 1982). Excessive Se leads to general inhibition in crops and reduces crop yield; however, phosphorus has a detoxification effect in a certain range (Singh and Singh 1978). Se poisoning has not been reported to occur in Se-hyperaccumulating crops after the absorption of a large amount of Se.
The accumulation of Se in plants will affect plant pollen development and fertilization. The accumulation of a large amount of non-toxic selenomethylcysteine (MeSeCys) in plant flowers inhibits pollen formation. Further, the maternal parents who accumulate a large amount of Se in flowers will have an important effect on their reproductive fertilization (Prins et al. 2011; Quinn et al. 2011).
Under the interaction of appropriate concentrations of S and Se, Se can inhibit or stimulate the absorption of S by plants (Cheng et al. 2016). When Se and S are combined, the resistance to cadmium stress is stronger than that of a single element. The ability of plants treated with S and Se to absorb Se was significantly higher than that of plants treated with Se alone (Golob et al. 2016). Si and Se have a strong synergistic effect on reducing Cd toxicity (Huang et al. 2020). Si-Se interaction increases the content of glutathione (GSH) and plant chelate (PC), causes more Cd to be distributed in the cell wall and organelles, and decreases the transport coefficient of Cd and accumulation in buds (Tang et al. 2015; Pereira et al. 2018).
The process of using plants to transfer Se from the Se-contaminated environment to alleviate soil Se pollution is called Se phytoremediation (Pilon-Smits 2005). There are more than 30 species of Se-hyperaccumulating plants, such as Stanleya, Astragalus, and Symphyotrichum (Cappa and Pilon-Smits 2014). Because of the Se enrichment characteristics of the above plants, they are often used in the phytoremediation of Se-contaminated soil (Salt et al. 1998; Pilon-Smits 2005). Phytoremediation has the advantage of ecological balance, but the prerequisite for phytoremediation of high Se soil is to screen plants that meet the tolerance conditions. Further, the area to be repaired should have the basic conditions of light, temperature, water, and heat needed for plant growth (Wu et al. 2015).
Microorganisms are the decisive factor of Se transformation (especially Se reduction) in soil (Yanke et al. 1995; Martens and Suarez 1996; Kessi and Hanselmann 2004). Therefore, understanding the role of soil microorganisms in Se transformation is particularly important to further clarify Se absorption by plants. The process of microbial transformation of Se generally involves dissimilatory reduction, assimilation reduction, oxidation, methylation, and demethylation (Dungan and Frankenberger 1999). Among them, dissimilatory reduction can reduce the toxic oxidized Se (SeO32−, SeO42−) to non-toxic Se0 (Wen and Carignan 2007). As the synthesis method of the reduced product nano-Se is green, environmentally friendly, safe, and less toxic, nano-Se is widely used in electrochemical sensing and anticancer (Mehdi et al. 2013).
The reduction of selenate to selenite and the reduction of selenite to elemental Se are two separate processes (Kuroda et al. 2011). The mechanism of reduction of selenate to selenite has been thoroughly studied. In this process, there is a significant difference between the selenate reductase complex of gram-negative bacteria and gram-positive bacteria, which leads to a significant difference in the reduction process (Fig. 2). Membrane-bound molybdate plays an important role in the reduction of selenate to selenite. In Gram-positive bacteria, SrdBCA is a membrane-bound molybdenum enzyme. SrdA contains one [4Fe-4S] cluster, SrdB contains four [4Fe-4S] clusters, and SrdC contains two transmembrane domains, indicating that the protein is located on the cell membrane. The SeO42− reduction process is related to the oxidation of hydroquinone. Hydroquinone combined with SrdC is oxidized to quinones, releasing 2 protons to the outside of the membrane and providing 2 electrons for SrdB (Nancharaiah and Lens 2015). Yee et al. (2007) inferred that the process of selenate reduction may be related to anaerobic respiration. In Gram-negative bacteria, SerABC is a soluble periplasmic molybdenum enzyme and SerA combines a molybdenum cofactor with catalytic activity and a subunit containing one [4Fe-4S] cluster. SerB contains three [4Fe-4S] clusters and one [3Fe-4S] cluster, and heme b (Nancharaiah and Lens 2015) is in SerC. SerABC accepts electrons from cytochrome c4, combines with hydroquinone, and undergoes reduction under the catalysis of quinol-cytochrome c oxidoreductase (Lowe et al. 2010).
Schematic of selenate reduction by gram-positive bacteria (a) and gram-negative bacteria (b). MoCo, molybdenum cofactor; SerABC, selenate reductase; SrdBCA, selenate reductase; cytc4, cytochrome c4; QCR, quinol-cytochrome c oxidoreductase; Q, quinones; QH2, quinols.Adapted from Nancharaiah and Lens (2015) and Kuroda et al. (2011)
The mechanism of reduction of selenite to elemental Se has not been clarified. There are three well-recognized hypotheses regarding the SeO32− reduction mechanism: (1) the role of selenite reductase in the periplasmic space (Li et al. 2014); (2) sulfide-mediated selenite reduction (Nelson et al. 1996); and (3) GSH-mediated selenite reduction (Kessi and Hanselmann 2004). Because GSH exists widely in a variety of microorganisms, this process is recognized by most people.
Compared with the dissimilation reduction of Se by microorganisms, there are few reports on other Se transformation methods. Based on microorganism-based Se methylation studies, some microorganisms can use selenate to form DMSe in the process of photoautotrophy as well as selenides to form DMSe (McCarthy et al. 1993). The Se methylating microorganisms isolated from soil and sediment include fungi and bacteria, whereas the Se methylating microorganisms in water are mainly bacteria (Swift 2002). The bacterial thiopurine methyltransferase (bTPMT) encoded by the tpm gene and the novel methylase encoded by the mmtA gene can convert selenite into DMSe and DMDSe (Ranjard et al. 2003, 2004, 2002). In anoxic sediments or anaerobic conditions, methylSe and dimethyl sulfides can undergo demethylation under the action of microorganisms; however, the current number of demethylated isolates is relatively small, and the types of reaction products have not been identified (Francis et al. 1974). In the study of Se assimilation by microorganisms, Se combines with amino acids through covalent bonds to form selenomethionine (Se-Met) and selenocysteine (Sec, U); Se-Met can non-specifically replace Met to participate in protein synthesis, and U specifically participates in protein synthesis (Böck et al. 2006). At present, few bacterial strains are known to be able to oxidize Se. As Se and S have similar chemical properties, the oxidation methods of Se and S are very similar (Blau 1961). Studies have shown that the oxidation of Se0 to SeO42− and SeO32 is mainly a biological process, and the speed is relatively slow (Torma and Habashi 1972; Dowdle and Oremland 1998). However, the oxidation mechanism of Se by microorganisms is still unclear and thus should be further examined.
Plant endophytes can also transform Se. Staicu et al. (2015) isolated the Se-tolerant endophytic strain, Pseudomonas moraviensis, from the Se hyperaccumulating plant, Stanleya pinnata, which can reduce the Se4+ of 790 mg L−1 to nanoscale Se0 below the detection limit within 48 h, indicating that the endophytic strain has a strong ability to metabolize Se. Sura-de Jong et al. (2015) isolated a variety of Se-tolerant endophytes from the roots, stems, and leaves of Stanleya pinnata and Astragalus bisulcatus in Se-rich areas of California, which can reduce 15.8 g L−1 selenate and selenite to elemental Se. These endophytes can still grow in high Se medium containing 10 mg L−1 Se, whereas rhizosphere fungi isolated from non-Se hyperaccumulating plants in the same area cannot grow in high Se medium. Altogether, microorganisms participate in the transformation of Se in various valence states and the formation of organic Se. Further, the transformation of Se in nature cannot be separated from microorganisms.
In recent years, the physiological level and molecular mechanism of Se absorption and transformation in plants have been discovered. A certain basis exists for the study of plant absorption and transformation of selenate; however, the absorption and transformation of selenite and organic Se and the synergistic effect of environmental factors on Se absorption still need to be discussed. Se uptake by plants can induce a series of complex physiological responses in plants. Owing to many factors, such as environmental factors and Se levels, the accumulation of Se in plants can affect their own reproduction and development, and may also affect the habitat of plants. Therefore, it is of great significance to study the ecological effects of the interaction between Se and plants in the environment.
The absorption and metabolism of Se in plants are complex, but are closely related to the physical and chemical properties of soil. However, many related mechanisms are still unclear. From the viewpoint of human nutrition and health, how to improve the Se content in the edible part of crops must be urgently established. Se is unevenly distributed in various organs of plants and is mainly distributed in the unharvested parts of crops (Carvalho et al. 2003). With the development of molecular biology and by studying the mechanism of plant metabolism, it is possible to achieve the overexpression of target genes related to Se enrichment in specific plant tissues, such as grains, to increase the Se content of specific fractions in specific tissues of crops.
Although some progress has been made in the study of the interaction between Se and heavy metals, the mechanism of the interaction between Se and heavy metals is not clear. At present, research on the appropriate amount of Se that can alleviate the toxicity of heavy metals is mainly focused on the antioxidation of Se. However, the relationship between the plant antioxidant system and Se form and concentration under heavy metal stress and the relationship between the plant antioxidant system and heavy metal species must be clarified. In addition to activating the antioxidant system in plants, Se may also antagonize the toxic effects of heavy metals through other mechanisms; however, more experiments are needed to prove this hypothesis.
Further investigations are still needed to elucidate the mechanism of Se metabolism by microorganisms. Furthermore, more microbial resources involved in Se metabolism, especially the discovery of Se oxidizing bacteria, should be explored. However, the process of oxidation of elemental Se is markedly slower than the reduction process of Se. For the reduction and methylation of Se, much room still exists to explore the microbial resources. In addition, the reduction of Se is the most studied at present. However, the general law of selenate to selenite has not been fully clarified. The role of microorganisms in the transformation of soil Se is yet to be understood. The change in mode of microbial transformation of soil Se and how it affects the way plants absorb Se also need to be demonstrated. These questions need to be simultaneously resolved by researchers. The relationship between the existing fractions of Se in soil and the role of microorganisms must be understood to determine the status and ability of plants to absorb Se.
In this review, we discussed the migration and accumulation of Se in the plant-soil-microbe system. The physical and chemical properties of soil, microorganisms, and vegetation affect the migration of Se. Microbes play an important role in the transformation of Se. Se promotes plant growth and development and enhances plant resistance to adversity stress. Its metabolism in plants is an important physiological process. The circulation route of Se in the environment is relatively clear; however, there are still many aspects to be studied in depth. Understanding the distribution characteristics and migration rules of Se in the environment provides a reference and scientific basis for the development and utilization of Se resources and the development of Se-enriched agricultural systems.
References
Akladious SA (2012) Influence of different soaking times with selenium on growth, metabolic activities of wheat seedlings under low temperature stress. Afr J Biotech 11:14792–14804. https://doi.org/10.5897/AJB12.2140
Alla MMN, Badran EG, Mohammed FA, Hassan NM, Abdelhamid MA (2020) Overexpression of Na+-manipulating genes in wheat by selenium is associated with antioxidant enforcement for enhancement of salinity tolerance. Rendiconti Lincei Scienze Fisiche e Naturali 31:177–187. https://doi.org/10.1007/s12210-019-00868-8
Altansuvd J, Nakamaru YM, Kasajima S, Ito H, Yoshida H (2014) Effect of long-term phosphorus fertilization on soil Se and transfer of soil Se to crops in northern Japan. Chemosphere 107:7–12. https://doi.org/10.1016/j.chemosphere.2014.02.056
Amouroux D, Liss PS, Tessier E, Hamren-Larsson M, Donard OFX (2001) Role of oceans as biogenic sources of selenium. Earth Planet Sci Lett 189:277–283. https://doi.org/10.1016/S0012-821X(01)00370-3
Arvy MP (1989) Some factors influencing the uptake and distribution of selenite in the bean plant (Phaseolus vulgaris). Plant Soil 117:129–133. https://doi.org/10.1007/BF02206265
Arvy MP (1993) Selenate and selenite uptake and translocation in bean plants (Phaseolus vulgaris). J Exp Bot 44:1083–1087. https://doi.org/10.1093/jxb/44.6.1083
Bailey RT (2017) Review: selenium contamination, fate, and reactive transport in groundwater in relation to human health. Hydrogeol J 25:1191–1217. https://doi.org/10.1007/s10040-016-1506-8
Barberon M, Berthomieu P, Clairotte M, Shibagaki N, Davidian JC, Gosti F (2008) Unequal functional redundancy between the two Arabidopsis thaliana high-affinity sulphate transporters SULTR1;1 and SULTR1;2. New Phytol 180:608–619. https://doi.org/10.1111/j.1469-8137.2008.02604.x
Beath OA, Gilbert CS, Eppson HF (1939) The use of indicator plants in locating seleniferous areas in Western United States. I. General. Am J Bot 26:257–269. https://doi.org/10.1002/j.1537-2197.1939.tb12900.x
Belzile N, Wu GJ, Chen YW, Appanna VD (2006) Detoxification of selenite and mercury by reduction and mutual protection in the assimilation of both elements by Pseudomonas fluorescens. Sci Total Environ 367:704–714. https://doi.org/10.1016/j.scitotenv.2006.03.008
Blau M (1961) Biosynthesis of [75Se]selenomethionine and [75Se] selenocystine. Biochem Biophys Acta 49:389–390. https://doi.org/10.1016/0006-3002(61)90140-8
Blazina T, Sun Y, Voegelin A, Lenz M, Berg M, Winkel LHE (2014) Terrestrial selenium distribution in China is potentially linked to monsoonal climate. Nat Commun 5:4717. https://doi.org/10.1038/ncomms5717
Blazina T, Läderach A, Jones GD, Sodemann H, Wernli H, Kirchner JW, Winkel LHE (2017) Marine primary productivity as a potential indirect source of selenium and other trace elements in atmospheric deposition. Environ Sci Technol 51:108–118. https://doi.org/10.1021/acs.est.6b03063
Bluemlein K, Klimm E, Raab A, Feldmann J (2009) Selenite enhances arsenate toxicity in Thunbergia alata. Environ Chem 6:486–494. https://doi.org/10.1071/EN09101
Böck A, Rother M, Leibundgut M, Ban N (2006) Selenium metabolism in prokaryotes. In: Hatfield DL, Berry MJ, Gladyshev VN (eds) Selenium. Springer, Boston, MA, pp 9–28. https://doi.org/10.1007/0-387-33827-6_2
Broadley MR et al (2010) Selenium biofortification of high-yielding winter wheat (Triticum aestivum L.) by liquid or granular Se fertilisation. Plant Soil 332:5–18. https://doi.org/10.1007/s11104-009-0234-4
Cai M et al (2019) Selenium induces changes of rhizosphere bacterial characteristics and enzyme activities affecting chromium/selenium uptake by pak choi (Brassica campestris L. ssp. Chinensis Makino) in chromium contaminated soil. Environ Pollut 249:716–727. https://doi.org/10.1016/j.envpol.2019.03.079
Cao MJ, Wang Z, Wirtz M, Hell R, Oliver DJ, Xiang CB (2013) SULTR3;1 is a chloroplast-localized sulfate transporter in Arabidopsis thaliana. Plant J 73:607–616. https://doi.org/10.1111/tpj.12059
Cappa JJ, Pilon-Smits EAH (2014) Evolutionary aspects of elemental hyperaccumulation. Planta 239:267–275. https://doi.org/10.1007/s00425-013-1983-0
Cartes P, Jara AA, Pinilla L, Rosas A, Mora ML (2010) Selenium improves the antioxidant ability against aluminium-induced oxidative stress in ryegrass roots. Ann Appl Biol 156:297–307. https://doi.org/10.1111/j.1744-7348.2010.00387.x
Carvalho KM, Gallardo-Williams MT, Benson RF, Martin DF (2003) Effects of selenium supplementation on four agricultural crops. J Agric Food Chem 51:704–709. https://doi.org/10.1021/jf0258555
Chen Y, Zhu Q, Dong X, Huang W, Du C, Lu D (2019) How Serratia marcescens HB-4 absorbs cadmium and its implication on phytoremediation. Ecotoxicol Environ Saf 185:109723. https://doi.org/10.1016/j.ecoenv.2019.109723
Cheng B et al (2016) Effects of selenium and sulfur on antioxidants and physiological parameters of garlic plants during senescence. J Integr Agric 15:566–572. https://doi.org/10.1016/s2095-3119(15)61201-1
Cheng Q et al (2020) Enhancement and improvement of selenium in soil to the resistance of rape stem against Sclerotinia sclerotiorum and the inhibition of dissolved organic matter derived from rape straw on mycelium. Environ Pollut 265:114827. https://doi.org/10.1016/j.envpol.2020.114827
Cheng Q, Hu C, Ming J, Cai M, Liu K, Tang Y, Zhao X (2021) Effects of selenium on microorganisms in the rhizosphere soil of oilseed rape. J Agric Res Environ 38:104–110. https://doi.org/10.13254/j.jare.2020.0061
Chiang CM, Chien HL, Chen LFO, Hsiung TC, Chiang MC, Chen SP, Lin KH (2015) Overexpression of the genes coding ascorbate peroxidase from Brassica campestris enhances heat tolerance in transgenic Arabidopsis thaliana. Biol Plant 59:305–315. https://doi.org/10.1007/s10535-015-0489-y
Chu J, Yao X, Zhang Z (2010) Responses of wheat seedlings to exogenous selenium supply under cold stress. Biol Trace Elem Res 136:355–363. https://doi.org/10.1007/s12011-009-8542-3
de Lima Lessa JH et al (2019) Agronomic biofortification of rice (Oryza sativa L.) with selenium and its effect on element distributions in biofortified grains. Plant Soil 444:331–342. https://doi.org/10.1007/s11104-019-04275-8
Deng X, Zhao Z, Zhou JJ, Chen JZ, Lv C, Liu X (2018) Compositional analysis of typical selenium ore from Enshi and its effect on selenium enrichment in wetland and dryland crops. Plant Soil 433:55–64. https://doi.org/10.1007/s11104-018-3822-3
Diao M, Ma L, Wang J, Cui J, Fu A, Liu H-y (2014) Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J Plant Growth Regul 33:671–682. https://doi.org/10.1007/s00344-014-9416-2
Djanaguiraman M, Belliraj N, Bossmann SH, Prasad PVV (2018) High-temperature stress alleviation by selenium nanoparticle treatment in grain sorghum. ACS Omega 3:2479–2491. https://doi.org/10.1021/acsomega.7b01934
Dong JZ et al (2013) Selenium increases chlorogenic acid, chlorophyll and carotenoids of Lycium chinense leaves. J Sci Food Agric 93:310–315. https://doi.org/10.1002/jsfa.5758
Dowdle PR, Oremland RS (1998) Microbial oxidation of elemental selenium in soil slurries and bacterial cultures. Environ Sci Technol 32:3749–3755. https://doi.org/10.1021/es970940s
Dungan RS, Frankenberger WT (1999) Microbial transformations of selenium and the bioremediation of seleniferous environments. Bioremediat J 3:171–188. https://doi.org/10.1080/10889869991219299
Dwire KA, Kauffman JB, Baham JE (2006) Plant species distribution in relation to water-table depth and soil redox potential in montane riparian meadows. Wetlands 26:131–146. https://doi.org/10.1672/0277-5212(2006)26[131:PSDIRT]2.0.CO;2
Ekanayake LJ, Vial E, Schatz B, McGee R, Thavarajah P (2015) Selenium fertilization on lentil (Lens culinaris Medikus) grain yield, seed selenium concentration, and antioxidant activity. Field Crop Res 177:9–14. https://doi.org/10.1016/j.fcr.2015.03.002Get
Ekumah J-N, Ma Y, Akpali-Tsigbe NDK, Kwaw E, Ma S, Jie H (2021) Global soil distribution, dietary access routes, bioconversion mechanisms and the human health significance of selenium: a review. Food Biosci 41:100960. https://doi.org/10.1016/j.fbio.2021.100960
El Mehdawi AF, Pilon-Smits EAH (2011) Ecological aspects of plant selenium hyperaccumulation. Plant Biol 14:1–10. https://doi.org/10.1111/j.1438-8677.2011.00535.x
El-Demerdash FM, Nasr HM (2014) Antioxidant effect of selenium on lipid peroxidation, hyperlipidemia and biochemical parameters in rats exposed to diazinon. J Trace Elem Med Biol 28:89–93. https://doi.org/10.1016/j.jtemb.2013.10.001
Elkelish AA, Soliman MH, Alhaithloul HA, El-Esawi MA (2019) Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol Biochem 137:144–153. https://doi.org/10.1016/j.plaphy.2019.02.004
Favorito JE, Grossl PR, Davis TZ, Eick MJ, Hankes N (2020) Soil-plant-animal relationships and geochemistry of selenium in the Western Phosphate Resource Area (United States): a review. Chemosphere 266:128959. https://doi.org/10.1016/j.chemosphere.2020.128959
Feng RW, Wei CY (2012) Antioxidative mechanisms on selenium accumulation in Pteris vittata L., a potential selenium phytoremediation plant. Plant Soil Environ 58:105–110. https://doi.org/10.17221/162/2011-PSE
Feng R, Wei C, Tu S, Tang S, Wu F (2011) Detoxification of antimony by selenium and their interaction in paddy rice under hydroponic conditions. Microchem J 97:57–61. https://doi.org/10.1016/j.microc.2010.06.003
Feng R, Wei C, Tu S (2013) The roles of selenium in protecting plants against abiotic stresses. Environ Exp Bot 87:58–68. https://doi.org/10.1016/j.envexpbot.2012.09.002
Fernández-Martínez A, Charlet L (2009) Selenium environmental cycling and bioavailability: a structural chemist point of view. Rev Environ Sci Bio/Technol 8:81–110. https://doi.org/10.1007/s11157-009-9145-3
Filek M, Gzyl-Malcher B, Zembala M, Bednarska E, Laggner P, Kriechbaum M (2010) Effect of selenium on characteristics of rape chloroplasts modified by cadmium. J Plant Physiol 167:28–33. https://doi.org/10.1016/j.jplph.2009.07.003
Fischer S et al (2020) Bacillus safensis JG-B5T affects the fate of selenium by extracellular production of colloidally less stable selenium nanoparticles. J Hazard Mater 384:121146. https://doi.org/10.1016/j.jhazmat.2019.121146
Fordyce FM (2013) Selenium deficiency and toxicity in the environment. In: Selinus O (ed) Essentials of medical geology. Springer, Dordrecht, pp 375–416. https://doi.org/10.1007/978-94-007-4375-5_16
Francis AJ, Duxbury JM, Alexander M (1974) Evolution of dimethylselenide from soils. Appl Microbiol 28:248–250. https://doi.org/10.1128/aem.28.2.248-250.1974
Freeman JL et al (2010) Molecular mechanisms of selenium tolerance and hyperaccumulation in Stanleya pinnata. Plant Physiol 153:1630–1652. https://doi.org/10.1104/pp.110.156570
Galić L, Vinković T, Ravnjak B, Lončarić Z (2021) Agronomic biofortification of significant cereal crops with selenium—A review. Agronomy 11:1015. https://doi.org/10.3390/agronomy11051015
Gambrell RP (1994) Trace and toxic metals in wetlands—a review. J Environ Qual 23:883–891. https://doi.org/10.2134/jeq1994.00472425002300050005x
Gammelgaard B, Jackson MI, Gabel-Jensen C (2011) Surveying selenium speciation from soil to cell—forms and transformations. Anal Bioanal Chem 399:1743–1763. https://doi.org/10.1007/s00216-010-4212-8
Germ M, Stibilj V, Osvald J, Kreft I (2007) Effect of selenium foliar application on chicory (Cichorium intybus L.). J Agric Food Chem 55:795–798. https://doi.org/10.1021/jf0629888
Goh K-H, Lim T-T (2004) Geochemistry of inorganic arsenic and selenium in a tropical soil: effect of reaction time, pH, and competitive anions on arsenic and selenium adsorption. Chemosphere 55:849–859. https://doi.org/10.1016/j.chemosphere.2003.11.041
Golob A, Gadžo D, Stibilj V, Djikić M, Gavrić T, Kreft I, Germ M (2016) Sulphur interferes with selenium accumulation in Tartary buckwheat plants. Plant Physiol Biochem 108:32–36. https://doi.org/10.1016/j.plaphy.2016.07.001
Gómez-Gómez B, Pérez-Corona T, Mozzi F, Pescuma M, Madrid Y (2019) Silac-based quantitative proteomic analysis of Lactobacillus reuteri CRL 1101 response to the presence of selenite and selenium nanoparticles. J Proteomics 195:53–65. https://doi.org/10.1016/j.jprot.2018.12.025
Guignardi Z, Schiavon M (2017) Biochemistry of Plant Selenium Uptake and Metabolism. In: Pilon-Smits E, Winkel L, Lin ZQ (eds) Selenium in plants, vol 11. Springer, Cham, Switzerland. https://doi.org/10.1007/978-3-319-56249-0_2
Gustafsson JP, Johnsson L (1992) Selenium retention in the organic matter of Swedish forest soils. Eur J Soil Sci 43:461–472. https://doi.org/10.1111/j.1365-2389.1992.tb00152.x
Habibi G (2013) Effect of drought stress and selenium spraying on photosynthesis and antioxidant activity of spring barley. Acta Agriculturae Slovenica 101:31–39. https://doi.org/10.2478/acas-2013-0004
Hanin M, Ebel C, Ngom M, Laplaze L, Masmoudi K (2016) New insights on plant salt tolerance mechanisms and their potential use for breeding. Front Plant Sci 7:01787. https://doi.org/10.3389/fpls.2016.01787
Hasanuzzaman M, Fujita M (2011) Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol Trace Elem Res 143:1758–1776. https://doi.org/10.1007/s12011-011-8998-9
Hawkes WC, Kutnink MA (1996) High-performance liquid chromatographic-fluorescence determination of traces of selenium in biological materials. Anal Biochem 241:206–211. https://doi.org/10.1006/abio.1996.0401
Hefting M et al (2004) Water table elevation controls on soil nitrogen cycling in riparian wetlands along a European climatic gradient. Biogeochemistry 67:113–134. https://doi.org/10.1023/B:BIOG.0000015320.69868.33
Hu KL, Zhang L, Wang JT, You Y (2013) Influence of selenium on growth, lipid peroxidation and antioxidative enzyme activity in melon (Cucumis melo L.) seedlings under salt stress. Acta Soc Bot Pol 82:193–197. https://doi.org/10.5586/asbp.2013.023
Hu Y, Duan G-L, Huang Y-Z, Liu Y-X, Sun G-X (2014) Interactive effects of different inorganic As and Se species on their uptake and translocation by rice (Oryza sativa L.) seedlings. Environ Sci Pollut Res 21:3955–3962. https://doi.org/10.1007/s11356-013-2321-6
Huang Q, Yu Y, Wang Q, Luo Z, Jiang R, Li H (2015) Uptake kinetics and translocation of selenite and selenate as affected by iron plaque on root surfaces of rice seedlings. Planta 241:907–916. https://doi.org/10.1007/s00425-014-2227-7
Huang QQ, Wang Q, Wan YN, Yu Y, Li HF (2016) Application of X-ray absorption near edge spectroscopy to the study of the effect of sulphur on selenium uptake and assimilation in wheat seedlings. Biol Plant 61:726–732. https://doi.org/10.1007/s10535-016-0698-z
Huang Q, Xu Y, Liu Y, Qin X, Huang R, Liang X (2018) Selenium application alters soil cadmium bioavailability and reduces its accumulation in rice grown in Cd-contaminated soil. Environ Sci Pollut Res 25:31175–31182. https://doi.org/10.1007/s11356-018-3068-x
Huang H et al (2020) Synergistic effect of silicon and selenium on the alleviation of cadmium toxicity in rice plants. J Hazard Mater 401:123393. https://doi.org/10.1016/j.jhazmat.2020.123393
Ip C, Ganther HE (1994) Novel strategies in selenium cancer chemoprevention research. In: Burk RF (ed) Selenium in biology and human health. Springer, New York, NY, pp 169–180. https://doi.org/10.1007/978-1-4612-2592-8_10
Jacobs LW (1990) Selenium in Agriculture and the Environment. Soil Sci 149:121. https://doi.org/10.1097/00010694-199002000-00008
Jiang C, Zu C, Shen J, Shao F, Li T (2015) Effects of selenium on the growth and photosynthetic characteristics of flue-cured tobacco (Nicotiana tabacum L.). Acta Soc Bot Pol 84:71–77. https://doi.org/10.5586/asbp.2015.006
Jiang C, Zu C, Lu D, Zheng Q, Shen J, Wang H, Li D (2017) Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci Rep 7:42039. https://doi.org/10.1038/srep42039
Jones GD et al (2017) Selenium deficiency risk predicted to increase under future climate change. Proc Natl Acad Sci USA 114:2848–2853. https://doi.org/10.1073/pnas.1611576114
Keskinen R, Turakainen M, Hartikainen H (2010) Plant availability of soil selenate additions and selenium distribution within wheat and ryegrass. Plant Soil 333:301–313. https://doi.org/10.1007/s11104-010-0345-y
Kessi J, Hanselmann KW (2004) Similarities between the abiotic reduction of selenite with glutathione and the dissimilatory reaction mediated by Rhodospirillum rubrum and Escherichia coli. J Biol Chem 279:50662–50669. https://doi.org/10.1074/jbc.M405887200
Kimani MM, Bayse CA, Stadelman BS, Brumaghim JL (2013) Oxidation of biologically relevant chalcogenones and their Cu(I) complexes: insight into selenium and sulfur antioxidant activity. Inorg Chem 52:11685–11687. https://doi.org/10.1021/ic401366c
Kumar M, Bijo AJ, Baghel RS, Reddy CRK, Jha B (2012) Selenium and spermine alleviate cadmium induced toxicity in the red seaweed Gracilaria dura by regulating antioxidants and DNA methylation. Plant Physiol Biochem 51:129–138. https://doi.org/10.1016/j.plaphy.2011.10.016
Kuroda M et al (2011) Molecular cloning and characterization of the srdBCA operon, encoding the respiratory selenate reductase complex, from the selenate-reducing bacterium Bacillus selenatarsenatis SF-1. J Bacteriol 193:2141–2148. https://doi.org/10.1128/JB.01197-10
Landberg T, Greger M (1994) Influence of selenium on uptake and toxicity of copper and cadmium in pea (Pisum sativum) and wheat (Triticum aestivum). Physiol Plant 90:637–644. https://doi.org/10.1111/j.1399-3054.1994.tb02518.x
Li HF, McGrath SP, Zhao FJ (2010) Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol 178:92–102. https://doi.org/10.1111/j.1469-8137.2007.02343.x
Li D-B et al (2014) Selenite reduction by Shewanella oneidensis MR-1 is mediated by fumarate reductase in periplasm. Sci Rep 4:3735. https://doi.org/10.1038/srep03735
Liang W, Ma X, Wan P, Liu L (2017) Plant salt-tolerance mechanism: a review. Biochem Biophys Res Commun 495:286–291. https://doi.org/10.1016/j.bbrc.2017.11.043
Lowe EC et al (2010) Quinol-cytochrome c oxidoreductase and cytochrome c4 mediate electron transfer during selenate respiration in Thauera selenatis. J Biol Chem 285:18433–18442. https://doi.org/10.1074/jbc.M110.115873
Malheiros RS, Gonçalves FC, Brito FA, Zsögön A, Ribeiro DM (2020) Selenomethionine induces oxidative stress and modifies growth in rice (Oryza sativa L.) seedlings through effects on hormone biosynthesis and primary metabolism. Ecotoxicol Environm saf 189:109942. https://doi.org/10.1016/j.ecoenv.2019.109942
Malik JA, Goel S, Kaur N, Singh I, Nayyar H (2012) Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ Exp Bot 77:242–248. https://doi.org/10.1016/j.envexpbot.2011.12.001
Martens DA, Suarez DL (1996) Selenium speciation of soil/sediment determined with sequential extractions and hydride generation atomic absorption spectrophotometry. Environ Sci Technol 31:133–139. https://doi.org/10.1021/es960214+
McCarthy S, Chasteen T, Marshall M, Fall R, Bachofen R (1993) Phototrophic bacteria produce volatile, methylated sulfur and selenium compounds. FEMS Microbiol Lett 112:93–97. https://doi.org/10.1111/j.1574-6968.1993.tb06429.x
Mehdi Y, Hornick J-L, Istasse L, Dufrasne I (2013) Selenium in the environment, metabolism and involvement in body functions. Molecules 18:3292–3311. https://doi.org/10.3390/molecules18033292
Mengel K, Kirkby EA, Kosegarten H, Appel T (1982) Principles of plant nutrition. Springer, Dordrecht, Netherlands. https://doi.org/10.1007/978-94-010-1009-2
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410. https://doi.org/10.1016/S1360-1385(02)02312-9
Morales-Espinoza MC et al (2019) Se nanoparticles induce changes in the growth, antioxidant responses, and fruit quality of tomato developed under NaCl Stress. Molecules 24:3030. https://doi.org/10.3390/molecules24173030
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216. https://doi.org/10.1007/s10311-010-0297-8
Nakamaru Y, Tagami K, Uchida S (2006) Effect of phosphate addition on the sorption-desorption reaction of selenium in Japanese agricultural soils. Chemosphere 63:109–115. https://doi.org/10.1016/j.chemosphere.2005.07.046
Nancharaiah YV, Lens PNL (2015) Selenium biomineralization for biotechnological applications. Trends Biotechnol 33:323–330. https://doi.org/10.1016/j.tibtech.2015.03.004
Natasha SM, Niazi NK, Khalid S, Murtaza B, Bibi I, Rashid MI (2018) A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ Pollut 234:915–934. https://doi.org/10.1016/j.envpol.2017.12.019
Nawaz F, Ahmad R, Ashraf MY, Waraich EA, Khan SZ (2015) Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotoxicol Environ Saf 113:191–200. https://doi.org/10.1016/j.ecoenv.2014.12.003
Neal RH, Sposito G, Holtzclaw KM, Traina SJ (1987) Selenite adsorption on alluvial soils: I. Soil composition and pH effects. Soil Sci Soc Am J 51:1161–1165. https://doi.org/10.2136/sssaj1987.03615995005100050012x
Nelson DC, Casey WH, Sison JD, Mack EE, Ahmad A, Pollack JS (1996) Selenium uptake by sulfur-accumulating bacteria. Geochim Cosmochim Acta 60:3531–3539. https://doi.org/10.1016/0016-7037(96)00221-9
Peak D, Sparks DL (2002) Mechanisms of selenate adsorption on iron oxides and hydroxides. Environ Sci Technol 36:1460–1466. https://doi.org/10.1021/es0156643
Pereira AS et al (2018) Selenium and silicon reduce cadmium uptake and mitigate cadmium toxicity in Pfaffia glomerata (Spreng.) Pedersen plants by activation antioxidant enzyme system. Environ Sci Pollut Res 25:18548–18558. https://doi.org/10.1007/s11356-018-2005-3
Pilon-Smits E (2005) Phytoremediation. Annu Rev Plant Biol 56:15–39. https://doi.org/10.1146/annurev.arplant.56.032604.144214
Pilon-Smits EAH, Winkel LHE, Lin Z-Q (2017) Selenium in Plants. Springer, Cham, Switzerland. https://doi.org/10.1007/978-3-319-56249-0
Prins CN, Hantzis LJ, Quinn CF, Pilon-Smits EAH (2011) Effects of selenium accumulation on reproductive functions in Brassica juncea and Stanleya pinnata. J Exp Bot 62:5633–5640. https://doi.org/10.1093/jxb/err247
Priyadarsini KI, Singh BG, Kunwar A, Prabhu P, Jain VK Selenium compounds as antioxidants and radioprotectors. In: Bañuelos GS, Lin Z-Q, Yin X (eds) The 3rd International Conference on Selenium in the Environment and Human Health, Hefei, China, 2013. Taylor & Francis Group, p 37
Quinn CF et al (2011) Selenium accumulation in flowers and its effects on pollination. New Phytol 192:727–737. https://doi.org/10.1111/j.1469-8137.2011.03832.x
Ranjard L, Prigent-Combaret C, Nazaret S, Cournoyer B (2002) Methylation of inorganic and organic selenium by the bacterial thiopurine methyltransferase. J Bacteriol 184:3146–3149. https://doi.org/10.1128/jb.184.11.3146-3149.2002
Ranjard L, Nazaret S, Cournoyer B (2003) Freshwater bacteria can methylate selenium through the thiopurine methyltransferase pathway. Appl Environ Microbiol 69:3784–3790. https://doi.org/10.1128/aem.69.7.3784-3790.2003
Ranjard L, Prigent-Combaret C, Favre-Bonté S, Monnez C, Nazaret S, Cournoyer B (2004) Characterization of a novel selenium methyltransferase from freshwater bacteria showing strong similarities with the calicheamicin methyltransferase. BBA - Gene Str Express 1679:80–85. https://doi.org/10.1016/j.bbaexp.2004.05.001
Ravi S, Breshears DD, Huxman TE, D’Odorico P (2010) Land degradation in drylands: interactions among hydrologic–aeolian erosion and vegetation dynamics. Geomorphology 116:236–245. https://doi.org/10.1016/j.geomorph.2009.11.023
Razak AA, El-Tantawy H, El-Sheikh HH, Gharieb MM (1991) Influence of selenium on the efficiency of fungicide action against certain fungi. Biol Trace Elem Res 28:47–56. https://doi.org/10.1007/BF02990462
Rios JJ et al (2010) Response of nitrogen metabolism in lettuce plants subjected to different doses and forms of selenium. J Sci Food Agric 90:1914–1919. https://doi.org/10.1002/jsfa.4032
Sager M (2006) Selenium in agriculture, food, and nutrition. Pure Appl Chem 78:111–133. https://doi.org/10.1351/pac200678010111
Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol 49:643–668. https://doi.org/10.1146/annurev.arplant.49.1.643
Schiavon M, Pilon M, Malagoli M, Pilon-Smits EAH (2015) Exploring the importance of sulfate transporters and ATP sulphurylases for selenium hyperaccumulation-a comparison of Stanleya pinnata and Brassica juncea (Brassicaceae). Front Plant Sci 6:2. https://doi.org/10.3389/fpls.2015.00002
Sharma VK, Mcdonald TJ, Sohn M, Anquandah GAK, Pettine M, Zboril R (2015) Biogeochemistry of selenium. A review. Environ Chem Lett 13:49–58. https://doi.org/10.1007/s10311-014-0487-x
Shrift A, Ulrich JM (1969) Transport of Selenate and Selenite into Astragalus Roots. Plant Physiol 44:893–896. https://doi.org/10.1104/pp.44.6.893
Singh M, Singh N (1978) Selenium toxicity in plants and its detoxication by phosphorus. Soil Sci 126:255–262. https://doi.org/10.1097/00010694-197811000-00001
Srivastava S, Shanker K, Srivastava S, Shrivastav R, Das S, Prakash S, Srivastava MM (1998) Effect of selenium supplementation on the uptake and translocation of chromium by spinach (Spinacea oleracea). Bull Environ Contam Toxicol 60:750–758. https://doi.org/10.1007/s001289900690
Staicu LC et al (2015) Pseudomonas moraviensis subsp. stanleyae, a bacterial endophyte of hyperaccumulator Stanleya pinnata, is capable of efficient selenite reduction to elemental selenium under aerobic conditions. J Appl Microbiol 119:400–410. https://doi.org/10.1111/jam.12842
Steinbrenner H, Sies H (2013) Selenium homeostasis and antioxidant selenoproteins in brain: Implications for disorders in the central nervous system. Arch Biochem Biophys 536:152–157. https://doi.org/10.1016/j.abb.2013.02.021
Subramanyam K, Du Laing G, Van Damme EJ (2019) Sodium selenate treatment using a combination of seed priming and foliar spray alleviates salinity stress in rice. Front Plant Sci 10:116. https://doi.org/10.3389/fpls.2019.00116
Sun G-X, Meharg AA, Li G, Chen Z, Yang L, Chen S-C, Zhu Y-G (2016) Distribution of soil selenium in China is potentially controlled by deposition and volatilization? Sci Rep 6:20953. https://doi.org/10.1038/srep20953
Sura-de Jong M et al (2015) Selenium hyperaccumulators harbor a diverse endophytic bacterial community characterized by high selenium resistance and plant growth promoting properties. Front Plant Sci 6:113. https://doi.org/10.3389/fpls.2015.00113
Swift MC (2002) Stream ecosystem response to, and recovery from, experimental exposure to selenium. J Aquat Ecosyst Stress Recover 9:159–184. https://doi.org/10.1023/A:1021299003516
Tang H et al (2015) Effects of selenium and silicon on enhancing antioxidative capacity in ramie (Boehmeria nivea (L.) Gaud.) under cadmium stress. Environ Sci Pollut Res 22:9999–10008. https://doi.org/10.1007/s11356-015-4187-2
Terry N, Zayed AM, de Souza MP, Tarun AS (2000) Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol 51:401–432. https://doi.org/10.1146/annurev.arplant.51.1.401
Tokunaga TK, Pickering IJ, Brown GE (1996) Selenium transformations in ponded sediments. Soil Sci Soc Am J 60:781–790. https://doi.org/10.2136/sssaj1996.03615995006000030015x
Torma AE, Habashi F (1972) Oxidation of copper (II) selenide by Thiobacillus ferrooxidans. Can J Microbiol 18:1780–1781. https://doi.org/10.1139/m72-278
Trelease SF, Beath OA (1951) Selenium: its geological occurrence and its biological effects in relation to botany, chemistry, agriculture, nutrition and medicine. J Geol 59:181. https://doi.org/10.1086/625840
Turakainen M, Hartikainen H, Seppänen MM (2004) Effects of selenium treatments on potato (Solanum tuberosum L.) growth and concentrations of soluble sugars and starch. J Agric Food Chem 52:5378–5382. https://doi.org/10.1021/jf040077x
Van Hoewyk D, Abdel-Ghany SE, Cohu CM, Herbert SK, Kugrens P, Pilon M, Pilon-Smits EAH (2007) Chloroplast iron-sulfur cluster protein maturation requires the essential cysteine desulfurase CpNifS. Proc Natl Acad Sci USA 104:5686–5691. https://doi.org/10.1073/pnas.0700774104
Vinceti M, Filippini T, Wise LA (2018) Environmental selenium and human health: an update. Curr Environ Health Rep 5:464–485. https://doi.org/10.1007/s40572-018-0213-0
Wang Z, Gao Y (2001) Biogeochemical cycling of selenium in Chinese environments. Appl Geochem 16:1345–1351. https://doi.org/10.1016/S0883-2927(01)00046-4
Wang S, Liang D, Wang D, Wei W, Fu D, Lin Z (2012a) Selenium fractionation and speciation in agriculture soils and accumulation in corn (Zea mays L.) under field conditions in Shaanxi Province, China. Sci Total Environ 427–428:159–164. https://doi.org/10.1016/j.scitotenv.2012.03.091
Wang Y-D, Wang X, Wong Y-S (2012b) Proteomics analysis reveals multiple regulatory mechanisms in response to selenium in rice. J Proteomics 75:1849–1866. https://doi.org/10.1016/j.jprot.2011.12.030
Wang D, Zhou F, Yang W, Peng Q, Man N, Liang D (2017) Selenate redistribution during aging in different Chinese soils and the dominant influential factors. Chemosphere 182:284–292. https://doi.org/10.1016/j.chemosphere.2017.05.014
Wang D et al (2019) Effects of straw amendment on selenium aging in soils: mechanism and influential factors. Sci Total Environ 657:871–881. https://doi.org/10.1016/j.scitotenv.2018.12.021
Wen H, Carignan J (2007) Reviews on atmospheric selenium: emissions, speciation and fate. Atmos Environ 41:7151–7165. https://doi.org/10.1016/j.atmosenv.2007.07.035
Wen H, Carignan J (2009) Ocean to continent transfer of atmospheric Se as revealed by epiphytic lichens. Environ Pollut 157:2790–2797. https://doi.org/10.1016/j.envpol.2009.04.021
White PJ et al (2004) Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J Exp Bot 55:1927–1937. https://doi.org/10.1093/jxb/erh192
Winkel LHE, Johnson CA, Lenz M, Grundl T, Leupin OX, Amini M, Charlet L (2012) Environmental selenium research: from microscopic processes to global understanding. Environ Sci Technol 46:571–579. https://doi.org/10.1021/es203434d
Wu Z-L, Yin X-B, Lin Z-Q, Bañuelos GS, Yuan L-X, Liu Y, Li M (2014) Inhibitory effect of selenium against Penicillium expansum and its possible mechanisms of action. Curr Microbiol 69:192–201. https://doi.org/10.1007/s00284-014-0573-0
Wu Z, Bañuelos GS, Lin Z, Liu Y, Yuan L, Yin X, Li M (2015) Biofortification and phytoremediation of selenium in China. Front Plant Sci 6:136. https://doi.org/10.3389/fpls.2015.00136
Wu Z et al (2016) Effect of selenium on control of postharvest gray mold of tomato fruit and the possible mechanisms involved. Front Microbiol 6:1441. https://doi.org/10.3389/fmicb.2015.01441
Wu Z et al (2018) Comparison of foliar silicon and selenium on cadmium absorption, compartmentation, translocation and the antioxidant system in Chinese flowering cabbage. Ecotoxicol Environ Saf 166:157–164. https://doi.org/10.1016/j.ecoenv.2018.09.085
Xia Q et al (2020) Methods of selenium application differentially modulate plant growth, selenium accumulation and speciation, protein, anthocyanins and concentrations of mineral elements in purple-grained wheat. Front Plant Sci 11:1114
Yanke LJ, Bryant RD, Laishley EJ (1995) Hydrogenase I of Clostridium pasteurianum functions as a novel selenite reductase. Anaerobe 1:61–67. https://doi.org/10.1016/S1075-9964(95)80457-9
Yao X, Chu J, He X, Ba C (2011) Protective role of selenium in wheat seedlings subjected to enhanced UV-B radiation. Russ J Plant Physiol 58:283–289. https://doi.org/10.1134/S1021443711020257
Yao Z, Li J, Xie H, Yu C (2012) Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ Sci 16:722–729. https://doi.org/10.1016/j.proenv.2012.10.099
Yao X, Chu J, He X, Liu B, Li J, Yue Z (2013) Effects of selenium on agronomical characters of winter wheat exposed to enhanced ultraviolet-B. Ecotoxicol Environ Saf 92:320–326. https://doi.org/10.1016/j.ecoenv.2013.03.024
Yee N, Ma J, Dalia A, Boonfueng T, Kobayashi DY (2007) Se(VI) Reduction and the precipitation of Se(0) by the facultative bacterium Enterobacter cloacae SLD1a-1 are regulated by FNR. Appl Environ Microbiol 73:1914–1920. https://doi.org/10.1128/AEM.02542-06
Yigit E, Akbulut GB, Gok Y, Bayram D (2012) The effects of organic selenium on some physiological and biochemical parameters in Hordeum Vulgare L. and Triticum Aestivum L. exposed to salt stress. Fresenius Environ Bull 21:743–747
Zahedi SM, Abdelrahman M, Hosseini MS, Hoveizeh NF, Tran L-SP (2019) Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ Pollut 253:246–258. https://doi.org/10.1016/j.envpol.2019.04.078
Zawislanski PT, Zavarin M (1996) Nature and rates of selenium transformations: a laboratory study of kesterson reservoir soils. Soil Sci Soc Am J 60:791–800. https://doi.org/10.2136/sssaj1996.03615995006000030016x
Zayed A, Lytle CM, Terry N (1998) Accumulation and volatilization of different chemical species of selenium by plants. Planta 206:284–292. https://doi.org/10.1007/s004250050402
Zeng H, Uthus EO, Combs GF Jr (2005) Mechanistic aspects of the interaction between selenium and arsenic. J Inorg Biochem 99:1269–1274. https://doi.org/10.1016/j.jinorgbio.2005.03.006
Zhang Y, Zahir ZA, Frankenberger WT (2004) Fate of colloidal-particulate elemental selenium in aquatic systems. J Environ Qual 33:559–564. https://doi.org/10.2134/jeq2004.5590
Zhang L, Ackley AR, Pilon-Smits EAH (2007) Variation in selenium tolerance and accumulation among 19 Arabidopsis thaliana accessions. J Plant Physiol 164:327–336. https://doi.org/10.1016/j.jplph.2006.01.008
Zhang H et al (2012) Selenium in soil inhibits mercury uptake and translocation in rice (Oryza sativa L.). Environ Sci Technol 46:10040–10046. https://doi.org/10.1021/es302245r
Zhang L et al (2014a) OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol 201:1183–1191. https://doi.org/10.1111/nph.12596
Zhang M, Tang S, Huang X, Zhang F, Pang Y, Huang Q, Yi Q (2014b) Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ Exp Bot 107:39–45. https://doi.org/10.1016/j.envexpbot.2014.05.005
Zhao XQ, Mitani N, Yamaji N, Shen RF, Ma JF (2010) Involvement of silicon influx transporter OsNIP2;1 in selenite uptake in rice. Plant Physiol 153:1871–1877. https://doi.org/10.1104/pp.110.157867
Zhao J et al (2013) Selenium inhibits the phytotoxicity of mercury in garlic (Allium sativum). Environ Res 125:75–81. https://doi.org/10.1016/j.envres.2013.01.010
Zhong Y, Cheng JJ (2017) Effects of selenite on unicellular green microalga Chlorella pyrenoidosa: bioaccumulation of selenium, enhancement of photosynthetic pigments, and amino acid production. J Agric Food Chem 65:10875–10883. https://doi.org/10.1021/acs.jafc.7b04246
Zhu Y-G, Pilon-Smits EAH, Zhao F-J, Williams PN, Meharg AA (2009) Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 14:436–442. https://doi.org/10.1016/j.tplants.2009.06.006
Zuber H, Davidian J-C, Wirtz M, Hell R, Belghazi M, Thompson R, Gallardo K (2010) Sultr4;1 mutant seeds of Arabidopsis have an enhanced sulphate content and modified proteome suggesting metabolic adaptations to altered sulphate compartmentalization. BMC Plant Biol 10:78. https://doi.org/10.1186/1471-2229-10-78
Funding
This work received support from Scientific Startup Foundation for Doctors of Yulin Normal University (CN) (Grant No. G2020ZK13), Scientific Research and Technology Development Program of Guangxi (Grant Nos. GuiKe AA17202037, GuiKe AD19245169, GuiKe AD18281072), Yulin Science and Technology Program Project (Grant Nos. Yushikeneng 20194301, yushike20204038, yushikete202020001).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Z., Huang, W. & Pang, F. Selenium in Soil–Plant-Microbe: A Review. Bull Environ Contam Toxicol 108, 167–181 (2022). https://doi.org/10.1007/s00128-021-03386-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-021-03386-2