Abstract
The present study aimed at alleviating the impacts of salinity on wheat growth, antioxidants and ROS homeostasis by selenium (Se). Seeds were soaked in water or 15 µM Se and sown in plastic pots supplemented with long Ashton (100%) for 2 days then treated with NaCl at 75, 150, and 225 mM. Samples were harvested on the 21st day after sowing. The results show that NaCl significantly decreased growth of wheat seedlings, K+, K/Na ratio, soluble sugars, ascorbic acid and glutathione. Also, activities of catalase, peroxidase, ascorbate peroxidase, and the reduced glutathione reductase, as well as ribulose-1.5-bisphosphate carboxylase/oxygenase (Rubisco) were inhibited. Similar decreases were detected in the expression of the alternative oxidase (AOX), salt overly sensitive (SOS1) and sodium hydrogen antiporter (NHX1) genes. On the contrary, there were elevations in Na+, H2O2, lipid peroxides, phenolics, and proline. The effect of NaCl was dose-dependent. Nonetheless, soaking wheat grains in Se (added as sodium selenite, Na2SeO3 solution) mitigated the injury of salinity; the amelioration in growth parameters coincided with elevation in K+ concentrations, K/Na ratio, antioxidants, Rubisco and the expression of AOX, SOS1, and NHX1 genes. Concomitantly, there was retraction in the accumulated Na, lipid peroxides and H2O2. These findings conclude that Se enhances wheat tolerance to NaCl stress through improving the antioxidants and over expression of Na-manipulating genes to cope with harsh habitats.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Salinity affects the physiology and biochemistry of plants by inducing an osmotic stress, ion toxicity, membrane damage, oxidative stress, nutrient imbalance, inhibition in enzymes, and alteration in growth regulators (Nemat Alla et al. 2014a, b; Badran et al. 2015; Kong et al. 2017). The generated oxidative stress could be overcome by the production of antioxidants either enzymatically or non-enzymatically. The non-enzymatic antioxidants such as reduced glutathione (GSH) and ascorbate (AsA) are associated with the cellular redox balance which may function as signals for the regulation of antioxidant mechanisms (Nemat Alla et al. 2007; Badran et al. 2015; Chakraborty et al. 2019) while the antioxidant enzymes such as catalase (CAT), peroxidase (POX), ascorbate peroxidase (APX), and glutathione reductase (GR) are important indicators of plant stress resistance (Borella et al. 2019). Phenolic compounds have high reactivity as electron or hydrogen donors and possess antioxidant properties by chelating transition metal ions as well as stabilizing and delocalizing the unpaired electrons (Arora et al. 2000). Plant tolerance to salinity can be accomplished by effectors of stress adaptation that mediate scavenging of reactive oxygen species (ROS) which disturb redox homeostasis and enhance membrane lipid peroxidation, protein oxidation, enzyme inhibition and DNA and RNA damage (Hassan et al. 2008; Khedr et al. 2011; Chakraborty et al. 2019). Of the Na+-manipulating genes are AOX which is involved in the antioxidant defense mechanism and plays a role in the protection of protein due to its ability to maintain mitochondrial function (Badran et al. 2015), SOS1 which has a vital role in plant sodium homeostasis by extruding Na+ coupled by the influx of H+ (Feki et al. 2017) and NHX1 which improves salt tolerance via ion homeostasis due to downhill movement of H+ to the uphill movement of Na+ (Shi et al. 2003; Brini et al. 2005).
Plants possess endogenous mechanisms to cope with stresses; however, these mechanisms are often not enough; therefore, exogenous supporter such as osmoprotectants, antioxidants, polyamines, and trace elements are effective in mitigating the effect of salinity and increase the capability for enhancing plant growth and stress tolerance to salinity (Nemat Alla et al. 2011; Perveen et al. 2018). Moreover, tolerance was associated with high levels of the antioxidant GSH and the activities of CAT, POX, APX and GR.
Selenium affects plant growth and development through enhancement of the enzymatic antioxidant activities (Diao et al. 2014). It has a role in regulation and alleviation of many biotic and abiotic stresses (Hawrylak-Nowak 2009; Diao et al. 2014). Although the protective role of Se has been reported under conditions of abiotic stress, its physiological role still needs elucidation. Wheat is the first strategic crop in the world; it is the staple food for billions of people (Hassan et al. 2015). Due to its paramount importance, studying its tolerance to salinity is very important for using reclaimed, salt-affected lands in cultivation to narrow the gap between production and consumption. Therefore, the present work aims at providing an overview for the role of Se in the regulation of some responses in wheat for enhancing tolerance to NaCl salinity stress by soaking grains in Se to cope with harsh habitats probably through its action on the antioxidant system, Na+, K+, Rubisco, and proline and to emphasize the relation with the expression of Na-manipulating genes (AOX, SOS1, and NHX1).
2 Materials and methods
2.1 Conditions of growth
Surface sterilization of the grains of wheat (Triticum aestivum L., Gemmiza-12) was performed using sodium hypochlorite (3%) for 10 min, followed by washing with distilled water and soaking for 8 h either in distilled water or 15 µM selenium (as sodium selenite, Na2SeO3) and then air-dried. Fifty pots (13-cm in diameter) were used for the experiment, which is repeated twice (100 pots for 2 independent experiments). For each pot, 10 seeds were sown in perlite in trays of 40 × 60 cm dimensions and irrigated with full strength Long Ashton nutrient solution for 2 days, then the pots of each experiment were divided into 4 groups, one served as the control (no salinity) and the other three groups received NaCl at 75, 150 and 225 mM. The pots of each experiment were distributed randomly into trays under controlled conditions (60% RH, 25 ± 2/10 ± 2 °C day/night regime, 10 h photoperiod, and irradiance of 300 µmol m−2 s−1). On the 21st day of sowing, the seedlings were removed, washed with deionized water and the shoots were separated from roots, washed with distilled water, blotted gently and weighed. Dry weights were recorded after drying at 80 °C for 2 days and used for subsequent analysis. The oldest second leaves were frozen in liquid nitrogen for enzyme assays and gene expression or used fresh for the other analyses.
2.2 Determination of lipid peroxidation and H2O2
Lipid peroxides and H2O2 were extracted in chilled 0.1% trichloroacetic acid (TCA) and centrifuged at 12,000×g for 15 min. Lipid peroxides were assayed as malondialdehyde (MDA) (Heath and Packer 1968). An aliquot of the supernatant was reacted with 20% TCA containing 0.5% thiobarbituric acid at 95 °C for 30 min, cooled in an ice bath, centrifuged at 12,000×g for 10 min and the absorbance of the supernatant was measured at 532 nm. The concentration of MDA was calculated using an extinction coefficient of 155 mM−1 cm−1. H2O2 was assayed in the reaction mixture containing potassium phosphate buffer (10 mM, pH 7.0) and 1 M potassium iodide (Alexieva et al. 2001). The reaction was developed for 1 h in darkness and the absorbance was measured at 390 nm.
2.3 Determination of K+ and Na+
K and Na were extracted in ultrahigh purity water heated at 95 °C for 1 h and centrifuged for 20 min at 5000×g. K and Na contents were determined in diluted supernatants using a Jenway PFP7 flame photometer (Hansen and Munns 1988). The concentrations of K and Na were measured using standard curves in the range of 20–100 ppm.
2.4 Determination of soluble sugars and proline
Ethanol (80%) was used for the extraction of soluble sugars. Aliquots of the extract were mixed with anthrone reagent (8.6 mM anthrone in 80% H2SO4) then heated for 10 min. After cooling in ice bath, the absorbance was measured at 623 nm (Schlüter and Crawford 2001). Sulfosalicylic acid (3%) was used for the extraction of proline. It was assayed by reaction of aliquots with glacial acetic acid and acid ninhydrin (1.25 g ninhydrin warmed in 30 ml of glacial acetic acid) and 6 M phosphoric acid for 1 h in a water bath at 100 °C. The red color was extracted in toluene and the chromophore-containing toluene was warmed to room temperature then absorbance at 520 nm was read (Bates et al. 1973).
2.5 Determination of phenolics, GSH and AsA
Phenolic compounds were extracted in 80% methanol then the homogenates were centrifuged at 10,000×g for 20 min. Phenolic compounds were determined according to Singleton and Rossi (1956) using gallic acid as a standard for the calibration curve, using 0.25 N Folin–Ciocalteu reagent, then after 3 min 0.2 M sodium carbonate solution was added and incubated for 60 min and absorbance was read at 765 nm. GSH was extracted in TCA (5%) containing EDTA (10 mM) and centrifuged at 12,000×g for 15 min. GSH was assayed in 100 mM phosphate buffer, pH 6.8 containing 10 mM EDTA, 1 mM 1-chloro-2,4-dinitrobenzene and 1.0 U equine glutathione-S-transferase and incubated at 35 °C for 30 min. The absorbance at 340 nm was recorded before commencing the reaction and after the reaction had run to completion (Anderson and Gronwalds 1991). AsA was extracted in 62.5 mM phosphoric acid and centrifuged at 12,000×g for 20 min. AsA was determined in sodium molybdate (0.66%) containing 0.2 ml H2SO4 (0.05 N) and 0.1 ml sodium phosphate (0.025 mM) and centrifuged at 4000 × g for 5 min then absorbance was read at 660 nm (Ahn et al. 1999).
2.6 Assay of activities of antioxidant enzymes
The extraction of enzymes was performed in Na phosphate buffer (50 mM) containing EDTA (2 mM) and β-mercaptoethanol (5 mM) then centrifuged for 10 min at 12,000×g at 4 °C. Protein was estimated by the procedure of Bradford (1976). The activity of CAT was measured by the method of Aebi (1984) by determining the rate of change in the absorbance at 240 nm in potassium phosphate (pH 6.9, 50 mM) containing 11.6 mM H2O2 and 10 mM dithiotretol at 25 °C, and the decreased absorbance of H2O2 (ε = 397.4 mM−1 cm−1) at 240 nm was recorded 1 min later. The activity of POX was measured according to Chance and Maehly (1955) in potassium phosphate (50 mM, pH 6.4) containing 0.3 mM guaiacol, 0.14 mM H2O2 at 470 nm by its ability to convert guaiacol to tetraguaiacol (ε = 26.6 mM−1 cm−1). GR activity was measured by following the oxidation of NADPH at 340 nm for 3 min as described by Schaedle and Bassham (1977). NADPH was determined using an extinction coefficient of 6.22 mM−1 cm−1. APX activity was determined using the spectrophotometric method of Nakano and Asada (1981) by monitoring the decrease in ascorbate at 290 nm in potassium phosphate buffer (50 mM, pH 7.0) containing 0.5 mM ascorbate, 0.1 mM H2O2 and 0.5 mM EDTA (ε = 2.8 mM−1 cm−1). The catalytic activity of these enzymes was expressed as katal (kat, mol s−1) that is the amount of the enzyme which converts one mole of the substrate in 1 s).
2.7 Rubisco quantification
For SDS-PAGE of Rubisco, Bio-Rad Mini Protein 3 kit (Bio-Rad Laboratories, Hercules, CA, USA) was used (Laemmli 1970). Acrylamide concentration in the resolving and the stacking gel was 11 and 5%, respectively. The gels were stained in brilliant blue R-250, destained in 20% methanol and scanned. The quantification of Rubisco was calculated from band volumes using Image Studio software.
2.8 The quantification of gene expression by semiquantitative RT-PCR
Extraction of RNA was performed according to Chomczynski and Sacchi (1987) and the protocol of RNA extraction followed Badran et al. (2015). The designed forward and reverse primers were as follows:
Gene | Forward primers | Reverse primers | Cycles |
|---|---|---|---|
AOX | GTGGAAGTGGTCGCTTCAGG | GAGGAACTCGGTGTAGGAGTGG | 35 and 40 |
SOS1 | CATTTCTGGGAAATGGTTGC | GCCTCCAATGCCTTGTTTAG | 32 and 35 |
NHX1 | CCGCTGGGTCAATGAGTC | CATTATGCCAAGTGTAATG | 32 and 35 |
Initial denaturation was performed at 95 °C for 3 min, annealing at 52 °C for 30 s, extension at 72 °C for 45 s and final extension at 72 °C for 4 min. Ten µl was collected for semiquantitative analysis after 35 and 40 cycles for AOX; however, analyses for either SOS1 or NHX1 were performed after 32 and 35 cycles before reaching the plateau phase. RNA was extracted and primers were designed as follows:
Gene | Forward primers | Reverse primers |
|---|---|---|
AOX | CGAGTGGAAGTGGTCTTG | GTTGAAGAAGACGCCCTG |
SOS1 | CATTTCTGGGAAATGGTTGC | GCCTCCAATGCCTTGTTTAG |
NHX1 | CCGCTGGGTCAATGAGTC | CATTATGCCAAGTGTAATG |
18S rRNA | CCACCCATAGAATCAAGAAAGAG | GCAAATTACCCAATCCTGAC |
2.9 Statistical analysis
The design was completely randomized and repeated twice comprises 160 pots [NaCl treatments (4 sets) × selenium treatments (2 sets) × replications (10) × repetitions (2)]. The mean values (± SD) were determined (n = 3 for Rubisco and gene expression or = 6 for all other parameters). The experiment is factorial with two factors; Se with two levels and NaCl with four levels. So a two-way ANOVA was performed and LSD was calculated at 0.05.
3 Results
3.1 Growth, K+ and Na+
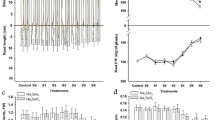
Treatments with NaCl at concentrations of 75, 150, and 225 mM markedly reduced fresh weight, dry weight, water content and height of wheat shoot (Fig. 1). The magnitude of decrease was most pronounced in fresh weight and shoot height but least evident in dry weight and water content. Increasing salinity from 0 to 225 mM NaCl reduced fresh weight and shoot height by 58% and 28%, respectively, versus 10% and 15% reductions in dry weight and water content, respectively.
Influence of NaCl at 75, 150, and 225 mM on fresh weight, dry weight, water content and height of shoots of 21-day-old wheat seedlings grown from grains soaked either in water or in 15 µM selenium (Se). Values are mean ± SD of six determinations from two independent experiments. LSD (entire, without Se; dotted, with Se) was calculated at P ≤ 0.05. The letter s at the column top means significant difference from the untreated control
Nevertheless, soaking wheat grains in 15 µM Se resulted in great alleviation of the effect of NaCl on growth parameters. Shoot fresh weight derived from soaked grains in Se was greater than treated samples by all NaCl concentrations by about 51–81% whereas the decreases relative to untreated control were only about 2–23%. Soaking of grains in Se was advantageous in the overall, particularly under salt stress. Also, shoot dry weight was lower in salinity-treated than in the untreated control by about 10–16% but there was no more than 5% decreases relative to control in response to Se. Meanwhile, Se increased water content of NaCl-treated shoots, with a negligible effect on the non-salinized plants by about 5–15% but only slightly affected when compared to control (only 2% decrease). Similarly, Se application led to elevations in shoot height such that 9–27% increases were detected relative to NaCl treatment although there were about 4–9% decreases when compared to the untreated control.
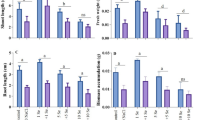
In absence of Se presoaking, increasing salinity from 0 to 225 mM NaCl decreased shoot K+ concentration by 80% but increased Na+ concentration by about 1.6 folds (166%), with a concomitant reduction of 93% in the K/Na ratio (Fig. 2). By contrast, in Se-treated grains, increasing salinity from 0 to 225 mM NaCl resulted in 23% increase in shoot K+ concentration and 128% increase in Na+ concentration with 66% reduction in the K/Na ratio.
Influence of NaCl at 75, 150, and 225 mM on K, Na, and K/Na ratio of shoots of 21-day-old wheat seedlings grown from grains soaked either in water or in 15 µM selenium (Se). Values are mean ± SD of six determinations from two independent experiments. LSD (entire, without Se; dotted, with Se) was calculated at P ≤ 0.05. The letter s at the column top means significant difference from the untreated control
3.2 Osmoprotectants, antioxidants and ROS
NaCl resulted in significant accumulations of lipid peroxides and H2O2 in wheat shoots (Fig. 3). The magnitude of accumulation of lipid peroxides and H2O2 at 225 mM NaCl amounted to 106% and 32%, respectively, above the control in absence of Se. Nonetheless, soaking grains in Se partially alleviated the effects of all concentrations of NaCl on lipid peroxides and H2O2. Lipid peroxides were lower than NaCl-treated samples by about 32–37; however, there were still increases relative to untreated control (6–40%). In the same pattern, Se lowered H2O2 by about 23–17% as compared to NaCl-treated samples; however, there were only about 2% increases relative to untreated control.
Influence of NaCl at 75, 150, and 225 mM on lipid peroxides and H2O2 of shoots of 21-day-old wheat seedlings grown from grains soaked either in water or in 15 µM selenium (Se). Values are mean ± SD of six determinations from two independent experiments. LSD (entire, without Se; dotted, with Se) was calculated at P ≤ 0.05. The letter s at the column top means significant difference from the untreated control
Also, soluble sugar contents were significantly elevated by NaCl; the magnitude of the elevation augmented with increasing NaCl concentrations to reach about 22–35% of the control values (Fig. 4). Similarly, NaCl provoked significant rises in proline contents by 38–55% in relation to control. However, soaking grains in Se resulted in great retractions in soluble sugar and proline contents. In spite of these retractions that reached 6–17% in soluble sugars and 9–29% in proline, the contents still significantly higher than the control values by about 7–15% in sugars and by about 11–28% in proline.
Influence of NaCl at 75, 150, and 225 mM on soluble sugars and proline contents of shoots of 21-day-old wheat seedlings grown from grains soaked either in water or in 15 µM selenium (Se). Values are mean ± SD of six determinations from two independent experiments. LSD (entire, without Se; dotted, with Se) was calculated at P ≤ 0.05. The letter s at the column top means significant difference from the untreated control
Meanwhile, there was a significant elevation in phenolic contents in seedlings due to treatment with NaCl at all concentrations by about 26–49% in relation to control. On the contrary, NaCl significantly decreased the contents of GSH and AsA, the higher the concentration, the greater the drop was resulting in decreases in GSH by 43–74% and in AsA by 47–66% (Fig. 5). Nevertheless, soaking grains in Se led to partial alleviation of NaCl effects on the accumulation of phenolics or on the drop of GSH and AsA. Phenolic contents were lowered by Se under salinity treatment relative to values detected in plants treated with NaCl alone by about 10–26%; however, there were about 7–19% increases relative to untreated control. Unlike in the context, Se led to increases in GSH under salinity treatment by about 18–127% respecting the treated samples, moreover, Se rendered the level 75 mM NaCl ineffective (4% decreases in GSH) with respect to control but 150 and 225 mM NaCl still inducing 15 and 41% reductions, respectively. Also, contents of AsA became close to control upon soaking in Se, these levels were greatly higher than the levels of the treated samples by about 86–144%.
Influence of NaCl at 75, 150, and 225 mM on reduced glutathione (GSH), ascorbic acid (AsA), and phenolics contents of shoots of 21-day-old wheat seedlings grown from grains soaked either in water or in 15 µM selenium (Se). Values are mean ± SD of six determinations from two independent experiments. LSD (entire, without Se; dotted, with Se) was calculated at P ≤ 0.05. The letter s at the column top means significant difference from the untreated control
Regarding the enzymatic antioxidants, NaCl provoked significant inhibitions in CAT, POX, and APX activities; however, the effect on GR was non-significant. The magnitude of decrease was greatest in CAT and POX particularly with the highest NaCl concentration (Fig. 6). NaCl at all concentrations led to inhibitions, relative to the control, of 51–70% in CAT, 44–64% in POX, 19–48% in APX and 2–5% in GR. Soaking grains in Se mitigated most of NaCl-induced inhibitions. The inhibitions of the enzyme activities by all concentrations of NaCl were retracted by Se to become 8–36% in CAT, 14–25% in POX and less than 4% in APX and GR relative to the respective control. Nonetheless, Se led to stimulation in the enzyme activities relative to the treated samples by about 86–114% in CAT, 54-107% in POX, 28–84% in APX and 5% in GR in spite of the slight decreases respecting control by 8–36% and 14–25% in CAT and POX, respectively, and almost no effect in APX and GR.
Influence of NaCl at 75, 150, and 225 mM on activities of catalase (CAT), peroxidase (POX), ascorbate peroxidase (APX), and glutathione reductase (GR) of shoots of 21-day-old wheat seedlings grown from grains soaked either in water or in 15 µM selenium (Se). Values are mean ± SD of six determinations from two independent experiments. LSD (entire, without Se; dotted, with Se) was calculated at P ≤ 0.05. The letter s at the column top means significant difference from the untreated control
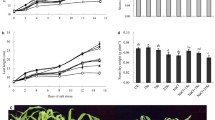
3.3 Rubisco and Na+-manipulating genes
Figure 7 presents the changes in Rubisco content and also in the expression of AOX, SOS1 and NHX1 genes as a result of treatment of NaCl with or without Se. Increasing NaCl concentration from 0 to 225 mM led to 58% inhibition of Rubisco activity in absence of Se; but in the presence of Se, the inhibition was moderate (non-significant) and amounted to only 15%. Moreover, Se resulted in great elevation in Rubisco by about 76–116% relative to the treated samples. The expression of AOX was decreased by NaCl; in absence of Se, increasing salinity from 0 to 75 mM NaCl and then to 150 mM led to 33 and 34% reduction in AOX expression with almost no further depression at higher salinity, 225 mM (38%) lower than control (Fig. 7). However, soaking gains in Se markedly upregulated the expression of AOX by 59–66% in comparison with NaCl-treated samples with alleviation of the effect of salinity such that values became very close to control (no more than 6% decreases). The transcript level of SOS1 was most likely affected by all concentrations of NaCl resulting in downregulation by a range of 10–13% relative to control (Fig. 7). On the contrary, soaking seeds in Se induced a great upregulation of about 29–33% relative to that detected in the NaCl-treated samples and values became higher than control by 11–16%. On the other hand, the transcript level of expression of NHX1 was also downregulated by NaCl treatment at all concentrations by about 36–45% in relation to the control values (Fig. 7). Nonetheless, soaking seeds in Se led to great upregulation in NHX1 expression levels by about 41–64% as compared to the NaCl-only-treated plants although there was a decrease of about 10–20% relative to control.
Influence of NaCl at 75, 150, and 225 mM on ribulose-1.5-bisphosphate carboxylase/oxygenase (Rubisco) content and the expression of alternative oxidase (AOX), salt overly sensitive (SOS1), and sodium hydrogen antiporter (NHX1) of shoots of 21-day-old wheat seedlings grown from grains soaked either in water or in 15 µM selenium (Se). Values are mean ± SD of three determinations (n = 3). All data were subjected to ANOVA and LSD (entire, without Se; dotted, with Se) was calculated at P ≤ 0.05. Rubisco content was expressed in arbitrary units. The quantification of AOX, NHX1, and SOS1 gene was performed using semiquantitative RT-PCR after normalization with the internal control 18S rRNA. The letter s at the column top means significant difference from the untreated control at P ≤ 0.05
4 Discussion
4.1 Growth, K+ and Na+
Salinity affects plant growth by various effects, including restriction of photosynthesis due to partial stomatal closure, reduced carbon fixation because of ion toxicity, growth limitations because of nutritional imbalances, and waste of energy due to ion exclusion and osmotic adaptation (Grattan et al. 2006; Kong et al. 2017). Due to salinity conditions, salts may build up in the chloroplast and exert a direct toxic effect on photosynthetic processes, they may build up in the cytoplasm and inhibit enzymes, or they can build up to excessive levels in the apoplast and dehydrate the cell (Munns and Tester 2008). This might occur in crops because they have low tolerance/resistance to salinity stress. The present work suggests that the salinity-induced reduction in wheat growth may be due to decreases in water availability, ion toxicity, and/or overproduction of ROS, which cause damage to lipids, proteins, and DNA. In accordance, Na accumulation was increased by NaCl but decreases were detected in K+ and K/Na ratio confirming the impairment of ionic balance in the salinity-treated samples. These findings confirm the induction of ion imbalance following NaCl treatment. Meanwhile, NaCl enhanced accumulation of lipid peroxides and H2O2 in plant tissue, concomitant with decreases in AsA and GSH as well as lowering the activities of CAT, POX and APX indicating the establishment of an oxidative stress. In this context, many reports indicated that plants exposed to abiotic stress, e.g., salinity, exhibited rises in the concentrations of lipid peroxides and H2O2 (Gill and Tuteja 2010; Kong et al. 2017). These reports are in consistence with the increases in lipid peroxidation and H2O2 in wheat in the present results. These increases might be signals for stress and could enforce the plant to activate its defense system, through the production of antioxidants. Consequently, exogenous supporters would be needed for the plant to withstand the harsh conditions.
4.2 Osmoprotectants, antioxidants and ROS
In response to salinity stress, proline and soluble sugars were accumulated in wheat by NaCl. They are nontoxic small metabolites compatible solutes that act as osmoprotectants and osmoregulators to perform protection of the plant from osmotic potential via contributions in osmotic adjustment, ROS scavenging systems, and protein stabilization (Hasegawa et al. 2013). In plants, proline is involved in the response to numerous environmental stresses and in different developmental processes. The accumulation of proline in response to different stresses is a well-established fact, and different roles for proline as an osmolyte, as an energy source and/or as ROS scavenger have been proposed (Trovato et al. 2008). Proline accumulation increased under stress and is correlated with osmotic adjustment to improve plant tolerance to salinity (Nemat Alla et al. 2011, 2016; Perveen et al. 2018). Soluble sugars and proline can reduce the inhibitory effects of ions on enzyme activity, increase the thermal stability of enzymes and prevent the dissociation of enzyme complexes (Hayat et al. 2012). Moreover, they counterbalance the increased osmotic potential from NaCl and protect membranes and metabolites by scavenging ROS, thus protecting cells from further damage. So the increased proline and soluble sugars in the present study by NaCl could confirm their importance as osmoprotectants and osmoregulators which confer salt tolerance to the plant. Moreover, carbohydrates could supply precursors for proline synthesis.
Due to over production of ROS which disturbs redox homeostasis and enhances protein oxidation, membrane lipid peroxidation, enzyme inhibition and DNA and RNA damage (Chakraborty et al. 2019), plants face a challenge between production and detoxification of ROS for balancing the cellular ROS load. This balancing is controlled by antioxidants that have the capability of scavenging ROS. Phenolics are known to scavenge ROS and thereby prevent lipid peroxidation by virtue of their role as substrates for the antioxidative enzymes (Chu et al. 2010; Perveen et al. 2018). In the present results, phenolic compounds were accumulated in stressed seedlings revealing that wheat tried to overcome oxidative stress imposed by salinity, yet the stress and its indices still present indicating inefficient antioxidant system towards ROS generation. In confirmation, the important non-enzymatic antioxidant members (GSH and AsA) were greatly suppressed. Both are very important antioxidants participating in H2O2 scavenging via the AsA–GSH cycle. This suppression could indicate impairment in the antioxidant system. In this account, Song et al. (2005) concluded that AsA biosynthesis is impaired under stress. So, the decreases detected in the non-enzymatic antioxidants (GSH and AsA), in the present results, confirm the dysfunction of the endogenous antioxidant system particularly with the inhibitions found in the enzymatic antioxidants (CAT, POX, and APX) which are very important in ROS scavenging. In this respect, Gill and Tuteja (2010) indicated that CAT, POX, and APX are very important in scavenging of H2O2. CAT catalyzes the conversion of H2O2 to O2 and H2O while POX uses H2O2 as an oxidizing agent with a consequent reduction of H2O2 whereas APX decomposes H2O2 in the GSH–AsA cycle. Therefore, the inhibition in the activity of these enzymes by NaCl would lead to accumulation of H2O2. These enzymes protect plants from the damage of oxidative stress. Moreover, GR is an enzyme related to the AsA–GSH cycle, which acts against ROS buildup and also for maintenance of GSH. Nonetheless, GR activity was not affected by NaCl probably due to the efficient roles of other enzymes in regulating the AsA–GSH cycle and in defense against ROS. So, the inhibitions in the antioxidant enzymes concomitant with the decreased non-enzymatic antioxidants would provide conditions for the oxidative stress to be established.
4.3 Rubisco and Na+-manipulating genes
This oxidative stress status seems to affect all aspect in plant metabolism and could lead to degradation of Rubisco. In this account, Feller et al. (2008) observed Rubisco degradation during abiotic stress. Rubisco is the most abundant protein and is considered as the key enzyme for carbohydrate formation (Nemat Alla and Hassan 2012). Beside their role as proximate energy source and building block provider, carbohydrates can aid in mitigation of the impact of salinity stress through participation in osmotic adjustment. The decrease in the content of Rubisco by NaCl might point to protein changes and could confirm the induced malfunction in plant cell due to salinity (Badran et al. 2015). To emphasize the malfunction in plant cell induced by NaCl, the expression of AOX, SOS1 and NHX1 was related to the antioxidant system and ROS homeostasis. The results reported herein indicated dropping in the expression of these genes and the antioxidant system under salinity conditions. As AOX plays a role in ROS metabolism, its expression would have impacts on ROS homeostasis. With decreasing AOX transcription, lipid peroxides would accumulate thus aggravating the severity of oxidative stress. On the other hand, SOS1 plays its role in salinity resistance through catalyzing Na+/H+ exchange and Na+ efflux (Munns and Tester 2008). SOS1 contributes to Na/H exchange, so plays a role in plant tolerance to salinity. Therefore, the observed downregulation of SOS1 expression by NaCl, synchronized with reduction in growth, K+, K/Na ratio and antioxidants with concomitant increases in Na+ and decreases in free radicals, could support the role of SOS1 in salinity tolerance. In confirmation, Shi et al. (2003) showed that Na was accumulated in the SOS1 mutant transgenic Arabidopsis to a greater extent than in the normal. Also, the depression of NHX1 gene hinders the sequestration of Na+ in the vacuole. The accumulation of Na+ in the cytosol due to less efficient sequestration into vacuoles or exclusion to the apoplast would impose inhibitory effects on various metabolic processes with a subsequent inhibition of growth.
Therefore, increased lipid peroxidation and H2O2 contents in the present findings coincided with the drop in the levels of antioxidants, meanwhile, the accumulation of Na+ as a consequence of depressed transcription of Na+-manipulating genes reveal that the plant is suffering from salinity stress and that the internal defense system is insufficient. So, the use of Se as an external supporter before treatment with NaCl alleviated to great extent the adverse effects of NaCl on plant performance. In accordance, Hawrylak-Nowak (2009) indicated that treatment with Se led to improvement in cucumber growth and enhanced the content of proline under salinity. Shekari et al. (2019) reported that Se has a positive effect on control of stress conditions and improvement of flowering indices and total yield in cucumber plant under stress. In confirmation, the present findings indicate that the efficiency of Se in minimizing the accumulation of lipid peroxides and H2O2 in wheat can participate in alleviation of salinity stress via restriction of ROS production. Moreover, the elevation in K+ concentration, concomitant with the lowering in Na+ concentration with the consequent rise in the K/Na ratio indicate that Se could mediate mitigation of salinity stress by adjusting the mineral balance. In conformity, Se application to sorrel plants under salt stress enhanced K+ accumulation in the leaves (Kong et al. 2005). Hawrylak-Nowak (2009) confirmed that enhancing the plant antioxidant capacity by Se might lead to suppression of MDA formation and protection from abiotic stress.
In addition to its role in mineral balance, Se also affected the production of osmoprotectants; it regulated proline, soluble sugars, and phenolics concomitant with amelioration of growth characteristics. These findings could support that phenolics, proline, and sugars are implicated as mediators in wheat tolerance mechanisms to salinity and hence can account for Se efficiency in enforcing the plant to withstand the harsh conditions. Se improved protection against damage due to oxidative stress, as indicated from lowering H2O2 and lipid peroxidation via supporting the antioxidant defense system that would allow plants to cope with stress conditions. So, an efficient antioxidant system would mediate ROS homeostasis during NaCl-salt stress resulting in reduction of the oxidative damage and consequently enhances the plant tolerance to salinity. Se application counterbalanced NaCl-induced drops of AsA and GSH. Upon soaking, AsA and GSH contents were increased indicating that Se can boost their synthesis (Mozafariyan et al. 2016). Moreover, the enhanced CAT activity indicates more protection by Se for plants grown under salt stress through degrading H2O2. Also, there were stimulations in APX activity by Se which augment scavenging of ROS. Moreover, Jiang et al. (2019) reported that the induced tolerance by Se was related to the enhanced GSH content and activities of CAT, APX and GR. In the present results, the stimulations of the antioxidant enzymes coincided with decreases in the levels of lipid peroxides and H2O2 as well as enhancement in the contents of GSH and AsA indicating that the antioxidant system became more efficient upon soaking the seeds in Se. Also, Se overcame the hazards of NaCl on Rubisco suggesting counterbalancing the impact of salinity on Rubisco degradation.
On the other hand, the expression of AOX, SOS1 and NHX1 was upregulated by Se concurrently with retardation of the accumulated Na+, lipid peroxides and H2O2 but increased K+, antioxidant system and phenolics content. This could suggest that these responses represent a mechanism to overcome the osmotic and oxidative stresses and this relieving effect is mediated by the role of Se in the upregulation of the transcription levels of AOX, SOS1, and NHX1. The sequestration of Na+ into vacuoles or the exclusion to the apoplast is performed efficiently by SOS1 and NHX1 with a consequent alleviation of Na+ toxicity and improvement of salt tolerance (Shi et al. 2003; Feki et al. 2017). In particular, the detected gene upregulations synchronous with the decrease in Na+ content coincided with increases in K+ and K/Na ratio as well as growth parameters could indicate the efficacy of this route in adaptation of wheat to salinity tolerance. In this account, Feki et al. (2017) suggested that SOS1 plays a crucial role in response to oxidative stress of durum wheat to oxidative damage arising from adding H2O2 and Shi et al. (2003) indicated accumulation of Na+ in the SOS1 mutant Arabidopsis thaliana confirming the improvement in tolerance of wheat to NaCl by Se since SOS1 and also NHX1 were upregulated for more Na+ efflux. Hence, the upregulation of some essential genes related to NaCl stress in wheat by Se could contribute to improvement of plant resistance to salinity stress.
5 Conclusions
Salinity would cause hyperosmotic and hyperionic effects on plants leading to increase in ROS levels, disorganization of membranes, and metabolic impairment. NaCl salinity reduced wheat growth probably through accumulation of lipid peroxides and H2O2, i.e., enhanced ROS production. In addition, NaCl increased plant Na+ content but decreased K+ content and the K/Na ratio concurrently with the drop in the expression of AOX, NHX1, and SOS1. To cope with these oxidative stress conditions, the plant would activate its antioxidant system either on its own or via external supporter. Proline and soluble sugars were increased for osmoregulation or osmoprotection; however, the antioxidant system was inhibited. So an external supporter is essential for the plant to withstand this stress. Se retracted the effects of NaCl on growth, Na+, K+, K/Na ratio, Rubisco, as well as AOX, SOS1, and NHX1 expression. Lowering Na+ by Se coincided with mitigation of the decreases in K+ and K/Na ratio in synchronization with growth improvement, suggesting that the role of Se in relief of salinity stress is accomplished by stimulating AOX, SOS1, and NHX1 expression in addition to regeneration of AsA and GSH. This could support the role of Se in counterbalancing the harmful effects of salinity by stimulating efficient antioxidant system, ROS homeostasis and Na+-manipulating genes to restrict the entry of Na+ to the cytoplasm
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahn YO, Kwon SY, Lee HS, Park IH, Wak SS (1999) Biosynthesis and metabolism of vitamin C in suspension cultures Scutellaria baicalensis. J Biochem Mol Biol 32:451–455
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant, Cell Environ 24:1337–1344
Anderson MP, Gronwalds JW (1991) Atrazine resistance in velvetleaf (Abutilon theophrasti) biotype due to enhanced glutathione S-transferase activity. Plant Physiol 96:107–109
Arora A, Byrem TM, Nair MG, Strasburg GM (2000) Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch Biochem Biophys 373:102–109
Badran EG, Abogadallah GM, Nada RM, Nemat Alla MM (2015) Role of glycine in improving the ionic and ROS homeostasis during NaCl stress in wheat. Protoplasma 252:835–844
Bates LE, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Borella J, Becker R, Lima MC, de Oliveira DS, Braga EJ, de Oliveira AC, do Amarante L (2019) Nitrogen source influences the antioxidative system of soybean plants under hypoxia and re-oxygenation. Sci Agric 76:51–62
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brini F, Gaxiola RA, Berkowitz GA, Masmoudi K (2005) Cloning and characterization of a wheat vacuolar cation/proton antiporter and pyrophosphatase proton pump. Plant Physiol Bioch 43:347–354
Chakraborty K, Bishi SK, Goswami N, Singh AL, Bhaduri D, Zala PV (2019) Salinity-induced changes in seed germination and the expression profile of antioxidant enzymes in peanut as early and late responses in emerging radicles. Acta Physiol Plant 41:134–149
Chance B, Maehly AC (1955) Assay of catalase and peroxidases. Method Enzymol 2:764–775
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Chu J, Yao X, Zhang Z (2010) Responses of wheat seedlings to exogenous selenium supply under cold stress. Biol Trace Elem Res 136:355–363
Diao M, Ma L, Wang J, Cui J, Fu A, Liu H (2014) Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J Plant Growth Regul 33:671–682
Feki K, Tounsi S, Masmoudi K, Brini F (2017) The durum wheat plasma membrane Na+/H+ antiporter SOS1 is involved in oxidative stress response. Protoplasma 254:1725–1734
Feller U, Anders I, Mae T (2008) Rubiscolytics: fate of Rubisco after its enzymatic function in a cell is terminated. J Exp Bot 59:1615–1624
Grattan SR, Grieve CM, Smith TE, Lauchli A, Poss JA, Suarez DL (2006) Can broccoli tolerate higher concentrations of boron under saline conditions? 18th World Congress of Soil Science, Philadelphia, 75
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerancein crop plants. Plant Physiol Biochem 48:909–930
Hansen EH, Munns DN (1988) Effects of CaSO4 and NaCl or growth and nitrogen fixation of Leucaena leucocephala. Plant Soil 107:95–99
Hasegawa T, Sakai H, Tokida T, Nakamura H, Zhu CW, Usui Y, Yoshimoto M, Fukuoka M, Wakatsuki H, Katayanagi N, Matsunami T, Kaneta Y, Sato T, Takakai F, Sameshima R, Okada M, Mae T, Makino A (2013) Rice cultivar responses to elevated CO2 at two free-air CO2 enrichment (FACE) sites in Japan. Funct Plant Biol 40:148–159
Hassan NM, Serag MS, El-Feky FM, Nemat Alla MM (2008) In vitro selection of mung bean and tomato for improving tolerance to NaCl. Ann Appl Biol 152:319–330
Hassan NM, El-Bastawisy ZM, El-Sayed AK, Ebeed HT, Nemat Alla MM (2015) Role of defense enzymes, proteins, solutes and Δ1-pyrroline-5-carboxylate synthase in wheat tolerance to drought. Rend Fis Acc Lincei 26:281–291
Hawrylak-Nowak B (2009) Beneficial effects of exogenous selenium in cucumber seedlings subjected to salt stress. Biol-Trace Elem Res 132:259–269
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments. Plant Signal Behav 7:1456–1466
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. 1. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Jiang L, Liu C, Cao H, Chen Z, Yang J, Cao S, Wei Z (2019) The role of cytokinin in selenium stress response in Arabidopsis. Plant Sci 281:122–132
Khedr A, Serag MS, Nemat-Alla MM, El-Naga AZ, Nada RM, Quick PW, Abogadallah GM (2011) Growth stimulation and inhibition by salt in relation to Na manipulating genes in xero-halophyte Atriplex halimus L. Acta Physiol Plant 33:1769–1784
Kong L, Wang M, Bi D (2005) Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress. Plant Growth Regul 45:155–163
Kong X, Luo Z, Dong H, Li W, Chen Y (2017) Non-uniform salinity in the root zone alleviates salt damage by increasing sodium, water and nutrient transport genes expression in cotton. Sci Rep-UK 7:2879
Laemmli EK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Mozafariyan M, Kamelmanesh MM, Hawrylak-Nowak B (2016) Ameliorative effect of selenium on tomato plants grown under salinity stress. Arch Agron Soil Sci 62:1368–1380
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Biol 59:651–681
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxide in spinach chloroplast. Plant Cell Physiol 22:867–880
Nemat Alla MM, Hassan NM (2012) A possible role for C4 photosynthetic enzymes in tolerance of Zea mays to NaCl. Protoplasma 249:1109–1117
Nemat Alla MM, Badawi AM, Hassan NM, El-Bastawisy ZM, Badran EG (2007) Induction of glutathione and glutathione-associated enzymes in butachlor-tolerant plant species. Am J Plant Physiol 2:195–205
Nemat Alla MM, Khedr AA, Serag MM, Abu-Alnaga AZ, Nada RM (2011) Physiological aspects of tolerance in Atriplex halimus L. to NaCl and drought. Acta Physiol Plant 33:547–557
Nemat Alla MM, Abogadallah GM, Badran EG, Nada RM (2014a) Differential tolerance of two wheat cultivars to NaCl is related to antioxidant potentialities. Braz J Bot 37:207–215
Nemat Alla MM, Abogadallah GM, Badran EG, Nada RM, Hassan NM (2014b) Supplementary CaCl2 ameliorates wheat tolerance to NaCl. Acta Physiol Plant 36:2103–2112
Nemat Alla MM, Abogadallah GM, Badran EG, Nada RM, Hassan NM (2016) Role of CaCl2 in osmoregulation and up-regulation of the salt stress related genes, NHX1 and SOS1 in wheat during NaCl stress. Agrochimica 60:29–42
Perveen S, Iqbal N, Saeed M, Zafar S, Arshad Z (2018) Role of foliar application of sulfur-containing compounds on maize (Zea mays L. var. Malka and hybrid DTC) under salt stress. Braz J Bot 41:805–815
Schaedle M, Bassham JA (1977) Chloroplast glutathione reductase. Plant Physiol 59:1011–1012
Schlüter U, Crawford RM (2001) Long-term anoxia tolerance in leaves of Acorus calamus L. and Iris pseudacorus L. J Exp Bot 52:2213–2225
Shekari L, Aroiee H, Mirshekari A, Nemati H (2019) Protective role of selenium on cucumber (Cucumis sativus L.) exposed to cadmium and lead stress during reproductive stage role of selenium on heavy metals stress. J Plant Nutr 42:529–542
Shi HZ, Lee BH, Wu SJ, Zhu JK (2003) Overexpression of a plasma membrane Na/H antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat Biotechnol 21:81–85
Singleton VL, Rossi JA (1956) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult 16:144–158
Song XS, Hu WH, Mao WH, Ogweno JO, Zhou YH, Yu JQ (2005) Response of ascorbate peroxidase isoenzymes and ascorbate regeneration system to abiotic stresses in Cucumis sativus L. Plant Physiol Biochem 43:1082–1088
Trovato M, Mattioli R, Costantino P (2008) Multiple roles of proline in plant stress tolerance and development. Rend Fis Acc Lincei 19:325–346
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nemat Alla, M.M., Badran, E.G., Mohammed, F.A. et al. Overexpression of Na+-manipulating genes in wheat by selenium is associated with antioxidant enforcement for enhancement of salinity tolerance. Rend. Fis. Acc. Lincei 31, 177–187 (2020). https://doi.org/10.1007/s12210-019-00868-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-019-00868-8