Abstract
Selenium (Se) is a vital micronutrient for human beings, and the global population facing Se deficiency is estimated to be around one billion individuals. To tackle this issue, the enrichment of staple crops with Se has emerged as a potential solution. However, it is important to note that Se can also be detrimental in excessive amounts, and contamination of the environment due to Se from agricultural and industrial sources has resulted in catastrophic ecological disasters over the past half-century. Consequently, the utilization of Se-enriched plants for both human supplementation and phytoremediation purposes has become an invaluable approach towards pollution control. An in-depth comprehension of how plants absorb and metabolize Se is pivotal in the realms of biofortification and phytoremediation. This comprehensive review concisely outlines the origins, mechanisms of absorption, conversion, and metabolism of Se in plants, while also elucidating the various factors that influence its uptake and accumulation. These influential factors encompass soil moisture, organic matter, pH levels, soil texture, microorganisms, and unique plant species characteristics. Furthermore, a thorough analysis of the potential mechanisms that underlie such influences is conducted. It is evident that both biofortification and phytoremediation possess substantial promise in confronting the challenges pertaining to Se, thereby fostering advancements in environmental sustainability. Building upon the current progress in research, this review provides suggestions for future directions aimed at establishing a theoretical framework for Se supplementation in human nutrition and the mitigation of Se-induced pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is a non-metallic element, classified as a group 16 element (chalcogen), and was originally discovered by the renowned Swedish chemist Berzelius in the early 18th century. While historically recognized as a toxin substance (Brown and Arthur 2001; Lu and Holmgren 2009; Reich and Hondal 2016), Se has since emerged as a crucial contributor to the well-being of plants, serving as an essential nutrient for both humans and animals and intricately linked to their overall health. At the core of Se’s biological significance lies selenocysteine (SeCys), which is considered the “21st amino acid”. Within the vast expanse of the human genome, there exist 25 genes encoding a diverse range of selenoproteins (Gladyshev et al. 2016; Labunskyy et al. 2014; Santesmasses et al. 2020). In the context of human health, Se confers numerous beneficial attributes, encompassing robust antioxidant action, fortification of the immune system, and a diminished susceptibility to afflictions such as cancer, cardiomyopathy, and muscular disorders (Hatfield et al. 2014; Ying and Zhang 2019; Zhao et al. 2020). Nonetheless, caution must be exercised, as exceeding the threshold of Se intake may prove detrimental to these organisms. The demarcation between deficiency and toxicity in the context of Se is a narrow precipice indeed, particularly in the realm of human physiology (Wang et al. 2022). This fine line of demarcation, outlining the essentiality versus the precarious precipice of toxicity, varies across organisms, species, and hinges upon the specific levels and chemical speciation of Se in question (Hawrylak-Nowak et al. 2015). Though Se claims the status of an indispensable trace element for humans and animals, its prevalence within human physiology is not always optimal. Global distribution of Se resources remains far from uniform, and while an excessive Se content can provoke environmental oxidative stress and toxicity (Dai et al. 2019; Espinosa-Ortiz et al. 2017), deficiencies in Se continue to present a substantial global environmental challenge, imperiling the well-being of approximately one billion individuals worldwide, with an estimated 700 million residing in China. Regional diseases attributed to Se deficiency, such as Kashin-Beck disease and Keshan disease, have been documented in Europe, the United States, and China (Lima et al. 2018; Chen et al. 2020).
Se plays a crucial role in a wide range of vital biological metabolic processes within the human body. Insufficient Se levels, known as Se deficiency, can have significant detrimental effects on the immune system’s function and can contribute to various health conditions, including heart disease, cancer, Kaschin-Beck disease, Keshan disease, cretinism, and cardio-cerebrovascular diseases (Vinceti et al. 2018). Unfortunately, the human body lacks the inherent capability to synthesize Se on its own, necessitating external supplementation to fulfill its requirements. The primary and most significant source of Se intake is through dietary means. The National Institutes of Health in the United States recommend a daily Se intake of 55 µg for humans (Schiavon et al. 2017), whereas the China Nutrition Society put forth a proposed dietary Se intake of 50 µg for adults in the year 2000. In terms of the source of this indispensable element, are primarily responsible for Se uptake in the human body, with organic Se from plants emerging as the current epitome of safety and efficiency (Finley et al. 2001). The distribution of Se resources within China exhibits striking disparities. While regions like Ziyang in Shaanxi and Enshi in Hubei boast abundant Se reserves, a noticeable belt of Se deficiency extends from the northeast to the southwest (Wang et al. 2001). Approximately 66% of agricultural soil in China suffers from Se deficiency, posing health risks to residents in these areas due to inadequate Se intake (Zhang et al. 2014). Soil plays a critical role as the primary source of Se, with plants absorbing, transforming, and accumulating Se from the soil. Through the food chain, plants act as carriers, facilitating the transfer of Se to higher trophic levels, including humans. Therefore, plants play a vital role in bridging the gap between Se-deficient animals and humans through consumption. The significance of plants in the transport and allocation of Se resources is evident.
Research has demonstrated that the Se content and its various forms within plants play a crucial role in determining the uptake of Se by humans and animals through their dietary intake (Ekumah et al. 2021). Consequently, in order to enhance the efficacy of plants as carriers of Se, this study undertakes a comprehensive and systematic review of recent investigations concerning the intricate relationship between Se-soil (the primary source of Se) and plants (the conduits for Se transmission). The objective is to lay a solid foundation for the cultivation of Se-enriched plants and to address the prevalent Se deficiency affecting billions of individuals in China and worldwide.
Sources of Se enrichment in plants
Soil and water are the primary reservoirs for Se accumulation in plants, with soil being the predominant source of Se uptake. The Se concentration in soil is the key factor to determine Se content in plants, and there is usually a positive correlation between them. The spatial distribution of Se in soil manifests intricate patterns, characterized by regional variation and spatial heterogeneity (White et al. 2004). On a global scale, geological terrain emerges as one of the most influential factors impacting soil Se content, the Se element in soil mainly originates from geological activities such as volcanism and magmatism in the Earth’s crust. Regions with active geological structures, such as the Himalayas, the Andes, and the Alps, experience substantial release of Se due to dynamic rock formations, resulting in elevated Se levels in the soil. High-selenium areas on Earth are primarily distributed in a plate-like pattern, while low-selenium areas are primarily found in the mid-to-high latitude regions above 30° and exhibit a strip-like distribution. In China, a strip-like selenium-deficient zone stretches from the northeast to the southwest, with the selenium-rich areas concentrated primarily in the northwest and a few regions in the southwest.
In addition, climate and environmental conditions have a significant impact on soil Se content. Soil in arid regions usually contains higher Se levels than soil in humid areas. Moreover, soil Se concentrations in certain areas display discernible spatial trends, revealing a decrease from mountainous to lowland regions or from southeastern to northwestern areas (Sors et al. 2005a; Zhong et al. 2021). Additionally, soil Se content is influenced by various factors, such as soil pH, organic matter content, redox potential, and soil type (Wang et al. 2022). The pH value of the soil significantly influences the Se levels. Higher soil Se concentrations are typically found in soils with a pH range of 6.5 to 7.5, which enhances plant absorption. Conversely, excessively high or low soil pH values result in reduced Se content. Under conditions of low pH, high levels of iron and aluminum in the soil contribute to the formation of Se compounds that are less available for plant absorption, leading to decreased Se levels in the plants. Generally, an optimal soil pH of 6.5–7.5, supplemented by ample organic matter and appropriate redox potential, promotes effective Se assimilation by plants. Moreover, different soil types exhibit substantial variations in Se content, with sandy soils generally presenting lower Se concentrations compared to clay-rich soils. The relative order of Se content among various zonal soils is as follows: red soil > yellow soil > black soil > dark brown soil (Gupta and Gupta 2017; Wu et al. 2015).
Water is also an important source for plants to accumulate Se. When the water source contains an optimal concentration of Se, it can be conveyed to the soil through irrigation and other means, consequently augmenting the Se content within plants. However, different plants have varying abilities to absorb and tolerate Se (Fleming 1962). Studying the uptake and tolerance abilities of different plants towards Se is crucial in determining the optimal concentration of Se solution for biofortification of plants with exogenous Se, as excessive Se can be toxic to them. Thus, achieving an appropriate balance in Se content is imperative. The intricate distribution pattern of Se in soil stems from a multitude of interconnected factors. A comprehensive comprehension of this distribution pattern holds significant importance when endeavoring to devise approaches and methodologies for enhancing Se enrichment in plants.
Mechanism of Se enrichment in plants
Se absorption and transport in plants
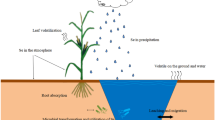
Se is indeed a vital trace element necessary for plant growth and development, even though it is often limited in soil. Therefore, plants have developed specific mechanisms to absorb and transport Se in order to support their normal physiological processes. The absorption and transportation of Se within plants involve complex processes that occur in multiple stages, including uptake, transport, and distribution (Fig. 1). The chemical form of Se in soil is influenced by the natural Se cycle, and its presence within the soil is a crucial determinant of Se’s bioavailability. Selenate (Se6+), selenite (Se4+), elemental Se (Se0), selenide (Se2−), and organic Se compounds represent the primary forms of Se existing in soil. Among them, selenate (Se6+), selenite (Se4+), and organic Se compounds are among the primary forms of Se that exist in soil. Of these forms, selenate, selenite, and organic Se compounds, including selenomethionine (SeMet) and selenocysteine (SeCys), are the primary ones that plants can absorb and utilize for their growth and development (Wu et al. 2015). Selenate and selenite stand out as the predominant inorganic forms of Se in soil, primarily stemming from Se deposition, biological metabolism, and the oxidation of Se minerals. Presently, extensive research has been conducted on the absorption and utilization of selenate and selenite by plants in soil. Notably, it has been observed that selenite displays a propensity for adsorption by soil clay minerals and organic matter, thereby diminishing its effectiveness. In contrast, selenate demonstrates remarkable mobility within the soil and is readily taken up by plants, highlighting its superior bioavailability.
Selenate permeates plant tissues through the roots, remaining unchanged during its journey. Upon reaching the leaves, selenate undergoes reduction to selenite, subsequently transforming into organic Se compounds for absorption and utilization. Se shares intriguing similarities with sulfur (S), both belonging to the same elemental group, thereby exhibiting akin chemical structures and properties. Seminal findings by Wu et al. (2003) revealed the influence of S on selenate transport, wherein Se metabolism intricately intertwines with S metabolism. The transportation of selenate employs sulfate transport proteins while higher plants assimilate Se6+ in a manner analogous to sulfate ions, traversing a path akin to the journey of sulfate ions. Once taken up by plant roots, selenite readily undergoes metamorphosis, transmuting into diverse forms such as SeMet, their oxides, and methylselenocysteine (MeSeCys), amongst others (Li et al. 2008). Nevertheless, the transport of both selenate and selenite within plants is limited, with only a small fraction of these substances being able to translocate throughout the plant, while the majority accumulates in the roots. In parallel with selenate, selenite does not possess a specific transport pathway. However, recent evidence suggests that selenite absorption may not solely rely on simple passive diffusion but instead involves active transport mechanisms. Studies indicate a potential association between selenite absorption and phosphate transport proteins. Interestingly, Hopper et al. (1999) made a remarkable discovery regarding the relationship between phosphate concentration and selenite absorption. They found that an increase in phosphate concentration hinders the absorption of selenite, while a decrease in phosphate concentration facilitates its uptake. This observed trend is similar to the effect of sulfate on selenate absorption. It is important to note that the migration proportion of selenate exceeds that of selenite. This has been supported by the findings of Asher et al. (1977), who used the 75Se tracer atom method and chromatography to demonstrate that selenate is the primary form of Se transferred within the plant.
Compared to metals like lead, cadmium, nickel, and chromium, Se tends to accumulate more in the above-ground parts of plants rather than the roots (Cabannes et al. 2011). However, the extent of Se transfer from roots to above-ground parts largely depend on the plant species and the type and form of Se present in the soil or growing medium (Cartes et al. 2005). In comparison to Se(IV), less selenite accumulates in the above-ground parts, with most Se transferred as Se(VI). Research has demonstrated that over half of the applied selenate to plant roots is transferred to above-ground tissues, while selenite remains primarily concentrated in the root tissues. Hawrylak-Nowak et al. (2015) reported that the total Se concentration in the above-ground part of cucumber supplemented with exogenous selenate was nearly double that of cucumber supplemented with exogenous selenite. In a study focusing on rice (Oryza sativa), researchers employed synchrotron-based X-ray fluorescence microtomography to investigate the transport mechanisms of organic and inorganic forms of Se. The results revealed that organic Se forms such as SeMet and MeSeCys were exclusively transported through the phloem pathway, while inorganic Se forms (Se(IV) and Se(VI) were transported through both the phloem and xylem pathways (Carey et al. 2012). Selenate can be transported from roots to above-ground parts via the xylem and subsequently reaches the reproductive organs through the endodermis (Winkel et al. 2015; Zhu et al. 2009). Selenate ions are efficiently taken up by the xylem and transported through the xylem sap (Saha et al. 2017). Se(VI) is directly transferred as an ion through the xylem, whereas Se(IV) undergoes a conversion process into an organic form before being transported to the xylem for translocation. The primary form of Se in plants is in the form of ions, which can be bound by various protein and non-enzymatic molecules. The transportation of Se within plants is facilitated by a series of protein and transport carriers, including Se transporters (SeT), Se amino acid transporters (SAT), and selenoproteins. SeTs are a group of membrane proteins that exhibit high conservation and selectively bind Se, enabling its transportation across cell membranes. In plants, SeTs are typically classified as belonging to the ATP-binding cassette (ABC) superfamily or the ion transport (IRT) superfamily (Byrne et al. 2010). SAT, another membrane protein involved in Se transport, plays a crucial role in facilitating the uptake of specific Se amino acids into cells, such as selenoprotein amino acids like MeSeCys, MeSeCys, which is required for the synthesis of selenoproteins (Chao et al. 2022). Selenoproteins (proteins that bind Se), widely distributed in plant tissues, are pivotal in Se uptake, transport, and metabolism. They function by absorbing Se from the soil through plant roots and distributing it throughout the plant, including leaves and fruits, through a dynamic balance process. Moreover, selenoproteins can store Se within plants and release it when needed, as elucidated by Trippe et al. (2021). Therefore, selenoproteins are crucial for both the absorption and utilization of Se in plants. It should be noted that plants can absorb Se not only in the form of water-soluble compounds but also other forms present in the soil, such as selenide and organic Se compounds, although the absorption efficiency of these forms is relatively low. Overall, the absorption and transport of Se in plants are complex processes influenced by various factors, including the biological availability and form of Se, soil conditions, and the presence of specific proteins and transporters.
Metabolism of Se in plants
The assimilation process of Se in plants encompasses a series of enzymatic reactions and metabolic pathways. Inorganic Se, such as Se(IV), necessitate conversion into organic forms before becoming accessible for plant utilization, primarily occurring within the chloroplasts (Fig. 2). The initial stage of Se metabolism involves the sequential enzymatic reduction of Se(VI) to Se(IV). This reduction is facilitated by two enzymes, namely ATP sulfurylase (APS) and APS reductase (APR) (Gupta and Gupta 2017). APS catalyzes the combination of ATP with Se(VI) to yield adenosine phosphoselenate (APSe), subsequently reduced to Se(IV) by APR (White 2018). The subsequent assimilation process is mediated by sulfite reductase (SiR), which catalyzes the reduction of Se(IV) to selenide. Selenide can also be generated from the non-enzymatic reaction of the reducing agent glutathione (GSH) with Se(IV), where glutathione plays a pivotal role in the reduction process. Subsequently, selenide reacts with O-acetylserine (OAS) under the catalysis of cysteine synthase (CSase), leading to the formation of SeCys (Van Hoewyk et al. 2008). Subsequently, the metabolism of SeCys can proceed in different directions. Firstly, under the catalysis of selenocysteine lyase (SL), SeCys can be cleaved to produce elemental Se. Secondly, a portion of Se undergoes methylation by the enzyme selenocysteine methyltransferase (SMT), resulting in the formation of MSeCys and eventually volatile dimethyl diselenide (DMDSe), which is released outside the plant (Philip 2018). These metabolic steps are typically observed in Se-accumulating plants (El-Ramady et al. 2016). Thirdly, through a three-step conversion, SeCys is transformed into selenomethionine (SeMet). Selenocysteine γ-synthase (CγS) generates selenocystathionine, which is then converted by cystathionine β-lyase (CβL) to produce selenohomocysteine (SeHCys). Finally, methionine synthase (MTR) catalyzes the formation of SeMet (Wang et al. 2018). The first two reactions occur in chloroplasts, while the final step takes place in the cytoplasm (Sors et al. 2005b). Additionally, in Se-accumulating plants, SeMet can be transformed into methylselenomethionine (SeMSeMet) by methionine methyltransferase (MMT), followed by the further generation of volatile dimethyl selenides (DMSe), which is a volatile and non-toxic Se compound that can be volatilized in the atmosphere (Gupta and Gupta 2017). This phenomenon of efflux serves as a self-protective mechanism in plants (Philip 2018).
Factors influencing the accumulation of Se in plants
The growth milieu of plants exhibits a multifaceted and assorted nature, wherein the accrual of Se in plants is subject to the intricate interplay of numerous determinants. The primary source of Se accumulation in plants primarily emanates from the Se in plants. Through the agency of plant root tissues, Se is assimilated from the soil, subsequently undergoing a sequence of conversions and transfers before ultimately amassing within distinct plant tissues. Ergo, the factors that exert influence on Se accumulation in plants can predominantly be categorized into two domains: internal factors, pertaining to the plants themselves, and external factors, encompassing the environmental constituents of the soil (Fig. 3). The subsequent elucidation will expound upon these factors with meticulous detail.
Physiological and biochemical properties of soil affect Se accumulation
Soil, as a crucial reservoir of Se, plays a critical role in facilitating the entry of Se into the food chain via plants (Wang et al. 2022). To date, the focus of numerous studies has primarily revolved around the total Se content in soil, rather than the bioavailable Se content, which represents the forms of Se that plants can absorb and utilize. The effectiveness of Se in soil is primarily determined by its various chemical forms in the soil. Se can be categorized into six distinct forms based on its chemical speciation: Se(0), Se(VI), Se(IV), selenide, organic Se, and volatile Se (Fernández-Martínez and Charlet 2009). Se(0) is present in soil in minimal quantities and is formed through the reduction of Se(VI) and Se(IV) by microorganisms. Se(0) is characterized by its insolubility in water, which makes it inaccessible for direct uptake and utilization by plants. However, under specific conditions, it can undergo transformations into plant-usable forms of Se, including Se(VI) and Se(IV). The presence of Se(VI) is more prominent in alkaline soil, where it exhibits high water solubility and shows low susceptibility to oxidation. Consequently, the proportion of total soil Se in this form tends to be relatively small. Conversely, Se(IV) prevails as the primary form of Se in acidic or neutral soil. It possesses solubility in water and represents the highest concentration among the various forms of Se in soil. Notably, Se(IV) serves as the primary form that plants can absorb and utilize to support their growth (Winkel et al. 2012). Except for alkali metal selenides, most Se compounds have limited solubility in water, rendering them inaccessible to plants. However, organic Se compounds play a crucial role in the availability of Se in soil. They are primarily bound to humic acids, such as fulvic acid and humic acid. Some organic Se can undergo mineralization processes, converting into selenates or selenites, which are more easily absorbed and utilized by plants (Zhang et al., 2022). Research on the absorption efficiency of different inorganic Se forms by plants indicates that Se6+ demonstrates the highest efficacy, while the utilization rate of organically bound Se is comparatively lower (Keskinen et al. 2013). Consequently, the uptake of Se by plants is intricately linked to its various forms in the soil, and the relative proportions of these forms are influenced by the physical and chemical properties of the soil itself (Sharma et al. 2015).
The uptake of Se by plants is influenced by several soil factors, including Se content, texture, pH, redox potential, moisture content, and salinity. Wang et al. (2022) observed significant variations in soil organic carbon levels among different vegetation types. Increased organic carbon content in agricultural, forest, and grassland soils can partially capture and retain Se in the soil, leading to a reduction in the loss of Se (Jones et al. 2017). The accumulation of Se in plants generally increases with higher available Se levels in the soil, provided that the soil Se concentration falls within an appropriate range. Soil pH affects Se speciation, as higher pH levels lead to reduced Se adsorption in the soil. This reduction is attributed to the decrease in positive charges on clay minerals and oxide edges, enhancing the mobility of inorganic Se in the environment and, consequently, increasing Se bioavailability (Eich-Greatorex et al. 2007). According to the study conducted by Haygarth et al. (1995), it was observed that grass leaves could absorb approximately 50% of the available Se at a soil pH of 6.0. However, at a slightly higher pH of 7.0, the absorption rate increased to over 50%. This suggests that soil pH plays a crucial role in the uptake of Se by plants. Additionally, the influence of soil texture on Se availability is mainly associated with the rapid adsorption of Se(VI) and Se(IV) by clay minerals and iron oxides. This phenomenon reduces the bioavailability of Se in the soil. Notably, Se(IV) chemical forms exhibit a tendency to form irreversible adsorption complexes with clay minerals, soil organic matter, and iron compounds. This characteristic has implications for the utilization efficiency of Se within the soil system, affecting its overall availability to plants.
Se uptake refers to the absorption and accumulation of Se by plants from the soil. According to Johnsson (1991), sandy soils tend to have the highest Se uptake, while it decreases as the clay content in the soil increases. This can be attributed to the differences in soil properties and the ability of different soil types to retain and release Se, especially in relation to the rapid uptake of SeO32− facilitated by clay minerals and iron oxides. In the presence of SeO32− within the soil, it tends to become immobilized on the surface of soil colloids, thereby diminishing its efficacy and availability for plant absorption. Organic matter in the soil plays a crucial role in the quantity of organic Se present. Generally, there is a positive correlation between soil organic matter and organic Se content. Organic matter plays a significant role in the availability of Se in the soil, but its impact is contingent upon the extent of organic matter decomposition. Insufficient decomposition of organic matter can have a detrimental effect on the bioavailability of soil Se, as it may impede the accessibility of Se to plants. This relationship between soil organic matter and Se content has been established in various studies, including that of Yamada et al. (2009) in Japan. Therefore, to enhance the bioavailability of Se in soils, it is crucial to ensure adequate organic matter decomposition. They found a clear positive correlation between these two variables in Se-enriched soils. This correlation further supports the notion that soil organic matter is closely related to the quantity of organic Se present in the soil. The redox conditions of soil, which are significantly influenced by changes in soil moisture content, also play a critical role in determining Se speciation in soil. Specifically, the availability of oxygen and the presence of oxidizing or reducing agents in the soil environment affect the various chemical forms that Se can exist in soil. As such, it is important to consider the impact of soil moisture content on redox conditions when studying Se speciation in soil. In environments with limited oxygen availability, known as reducing conditions, Se tends to exist primarily in the form of less soluble compounds such as iron selenides (e.g., iron selenide). These conditions promote the formation of chemical species that are tightly bound to the soil matrix, making it more challenging for plant roots to absorb Se effectively. As a result, plants face difficulties in taking up Se from these compounds. Conversely, under oxidizing conditions, the predominant forms of available Se in the soil are selenate and selenite. Compared to the Se compounds found under reducing conditions, selenate and selenite exhibit higher solubility and bioavailability. This means that they are more easily dissolved in soil solution and are more accessible to plant roots for uptake. Consequently, the presence of selenate or selenite allows plants to efficiently absorb and utilize Se. Altering the oxidation-reduction conditions of the soil can significantly impact the availability and usability of Se. Improving drainage, increasing oxygen supply, or applying oxidizing agents can promote the conversion of reducing conditions to oxidizing conditions. This shift can enhance the solubility and bioavailability of Se, thereby increasing the uptake and accumulation of Se by plants (Dwire et al. 2006). Soil drought and plant transpiration also influence the soil’s ability to retain Se (Jones et al. 2017). During periods of drought, the concentration of solutes in the soil solution increases, potentially affecting the mobility and availability of Se. Additionally, plant transpiration, which involves the movement of water through the plant, can alter the soil’s water content and influence the distribution and behavior of Se in the rhizosphere. Taken together, the absorption and accumulation of Se by plants are influenced by various factors, including soil redox conditions, the presence of specific Se compounds, soil moisture content, and plant physiological processes. Understanding these complex interactions is crucial for optimizing plant Se uptake and ensuring efficient utilization of Se in agricultural and environmental contexts.
Microorganisms in soil affect Se accumulation
Soil microorganisms are integral to the process of Se enrichment in plants. Through symbiotic relationships with plant roots or metabolic conversion processes, they can enhance the bioavailability of Se in the soil and promote its uptake and accumulation in plants. Microorganisms facilitate Se transformation through a range of processes such as oxidation-reduction and demethylation (Dungan et al. 2003). Among these microorganisms, arbuscular mycorrhizal fungi (AMF) are particularly noteworthy due to their widespread distribution and prevalence in soils. AMF can form symbiotic relationships with a large proportion of terrestrial plant species, including many major crop species worldwide (Kobae et al. 2018). Microorganisms, such as AMF, play a crucial role in mediating communication between plant roots and the soil (Natasha Shahid et al. 2018). The beneficial effects of AMF symbiosis on host plants for acquiring both macro and micronutrients have gained significant attention in the agricultural industry, with AMF being considered as a promising biofertilizer (Pellegrino and Bedini 2014; Dehghanian et al. 2018; Ryan and Graham 2018). The primary function of AMF is to enhance plant growth and promote the uptake of essential mineral nutrients, particularly phosphates (P) and zinc (Zn). It is worth noting that up to 24% of Zn found in plant stems can be transported through the mycorrhizal pathway (Watts-Williams et al. 2015). Furthermore, certain strains of AMF have demonstrated tolerance to heavy metals such as arsenic, lead, and Se in contaminated soils (Giasson et al. 2006). Other studies have shown that AMF can influence the structure of bacterial communities in the rhizosphere soil (Nuccio et al. 2013) and alter the bioavailability of metals in the soil, thereby affecting plant uptake of metals such as Zn, Se, and iron (Koshila Ravi et al. 2019). In rice, the combined application of AMF (Funneliformis mosseae and Glomus versiforme) with Se fertilizer was more effective in increasing the organic Se content in rice grains compared to the sole application of inorganic Se. This was mainly because AMF could change the bacterial community structure by increasing the relative abundance of thick-walled bacteria, AMF and associated rhizobacteria could stimulate Se transformation into more bioavailable forms in the soil, as well as facilitated Se absorption and transportation to various plant tissues (Chen et al. 2020). Other studies have also indicated that bacteria such as Bacillus and Paenibacillus species can stimulate Se conversion and uptake in crops (Acuña et al. 2013). AMF have been shown to enhance the absorption area of the roots by increasing external fungal hyphae, as well as improve the uptake of various elements by promoting the expression of element transport proteins in the epidermis and root hairs (Smith and Read 2010). In numerous studies involving garlic (Larsen et al. 2006), wheat (Wu et al. 2022), and green asparagus spears (Conversa et al. 2019), inoculation with AMF has been observed to expedite the uptake of Se by host plants. Although the effects of AMF inoculation on Se accumulation in plants have been quantified in several studies, the final results vary depending on the exogenous Se levels in the soil and the specific plant species used (Goicoechea et al. 2015). In addition to AMF, other microorganisms such as Actinobacteria, Firmicutes, and Acidobacteria have also been implicated in Se metabolism (Nancharaiah and Lens 2015; Natasha Shahid et al. 2018; Patel et al. 2018). Pseudomonas moraviensis, a Se-tolerant endophytic bacterium isolated from Se hyperaccumulating plant Stanleya pinnata, can rapidly reduce Se(IV) to Se(0), demonstrating its strong Se metabolism capability (Staicu et al. 2015). Additionally, a variety of Se-tolerant endophytes were isolated from S. pinnata and Astragalus bisulcatus plants growing in Se-enriched areas, which could transform high concentrations of Se(IV) and Se(VI) into Se(0), while rhizosphere fungi from non-Se-enriched plants in the same area could not grow in Se-enriched medium (Sura-de et al. 2015). Microorganisms play a crucial role in mediating the transformation of various forms of selenium with different valence states and the subsequent formation of organic-Se compounds. This microbial activity significantly influences the absorption and accumulation of selenium by plants. Understanding the intricate interactions between microorganisms and selenium can provide valuable insights into optimizing selenium bioavailability and uptake in plant systems. These findings underscore the importance of considering the microbial component when studying the dynamics of selenium in soil-plant systems.
Interaction between elements affect Se accumulation
In addition to its role in Se bioavailability through adsorption and fixation processes, soil composition also exerts influence on Se uptake by plants and its binding sites through competitive interactions with specific ions. Notably, Phosphorus (P) and S are two ions that significantly impact the bioavailability of Se in the soil. Because S and Se belong to the same group in the periodic table, they have similar chemical properties and can compete for the adsorption sites in the soil. This competitive relationship underscores the intricate interplay between these elements and highlights the importance of considering their interactions when studying Se dynamics in soil systems. Elevated levels of S in the soil can potentially diminish the efficacy of Se and subsequently reduce Se uptake by plants. Furthermore, S plays a crucial role in various metabolic processes and enzyme systems within plant organisms, including the biosynthesis of essential amino acids like cysteine and methionine, vitamin production, and Se metabolism. Insufficient supply of S can adversely affect the utilization and metabolism of Se by plants, leading to a decrease in Se uptake capacity. Moreover, S can form compounds with Se, such as Se-S (selenosulfate), which can impede the absorption and transport of Se. The formation of Se-S can limit the availability of Se in plant tissues and hinder its distribution and transportation within plants (Boldrin et al. 2016). To ensure optimal uptake of Se by plants, it is crucial to consider the S content and supply in the soil. Elevated levels of S in the soil can potentially impede the effectiveness of Se, while inadequate S supply can impact the utilization of Se by plants. Thus, maintaining a balanced relationship between S and Se in the soil is essential for the absorption and utilization of Se by plants. In hydroponic studies involving barley and rice, researchers have observed a synergistic interaction between Se-S and low concentrations of SO42− (sulfate). As the concentration of Se6+ increases, there is a corresponding increase in the aboveground plant tissue content of S. However, when exposed to high concentrations of SO42−, the synergistic effect between Se and S becomes negligible (Mikkelsen et al. 1990), suggesting that the presence and concentration of sulfate in the growth medium can modulate the relationship between Se and S. Under low-concentration S conditions, as the application of S increases, the accumulation of Se in aboveground parts of the plant also increases, indicating a synergistic effect. However, under high-concentration S conditions, the accumulation of Se decreases with increasing S application, indicating an antagonistic effect (Milchunas et al. 1983). By adding Se/S to agar medium, the content of Se and S elements in Se-non-accumulator plant Arabidopsis thaliana was studied. It was found that increasing the lower concentration of S (0–2 mM) in agar medium resulted in increased shoot fresh weight, shoot S concentration, and shoot Se concentration. However, when the concentration exceeded approximately 2 mM, there was a decreasing trend in shoot Se concentration (White et al. 2004). Different plant species exhibit varying abilities to compete for Se and S. The Se hyperaccumulator Stanleya pinnata and non-hyperaccumulators Stanleya elata and Brassica juncea were treated with a range of selenate concentrations in the presence of two sulfate concentrations (El Mehdawi et al. 2018). The results showed that at low sulfate concentrations (0.5 mM), S. pinnata and B. juncea had similar Se concentrations in their roots, while S. elata had concentrations two-fold lower than the other two plant species. When subjected to high sulfate concentration (5 mM), the root Se accumulation of all three plant species decreased, but the inhibitory effect was more pronounced in B. juncea and S. elata (El Mehdawi et al. 2018). These findings suggest that adjusting the levels of sulfur and phosphorus in the soil can facilitate Se uptake by plants, but the effect may depend on the specific plant species and growth conditions.
In the presence of SeO32− within the soil, it tends to become immobilized on the surface of soil colloids, thereby diminishing its efficacy and availability for plant absorption. However, when PO43− (phosphate) is simultaneously present in the soil, the effectiveness of SeO32− can be enhanced. This is primarily because the coexistence of PO43− and SeO32− gives rise to a competition for adsorption sites on the soil colloid surface. Since cations on the soil colloid surface exhibit a predilection for adsorbing PO43−, they have a tendency to reduce the number of sites accessible for SeO32− adsorption. This competitive interaction serves to liberate and augment the mobility of SeO32− in the soil, ultimately heightening the efficiency by which plants absorb SeO32− (Liu et al. 2004). Therefore, it can be inferred that PO43− indirectly affects the bioavailability of SeO32− in clayey soils.
Different plants have different the ability of Se accumulation
The absorption and accumulation of Se in plants vary depending on the plant species. Plants are generally classified into three categories based on their Se content: Se-hyperaccumulators, Se-accumulators, and Se-non-accumulators. Se-hyperaccumulator plants thrive in Se-rich soil and possess the unique ability to accumulate exceptionally high levels of Se in their tissues, often exceeding several thousand milligrams per kilogram (mg·kg− 1). However, Se-non-accumulator plants have relatively low Se content in their tissues, typically lower than 30 mg·kg− 1. This category includes most edible plants, some weeds, and Gramineae plants. Fleming (1962) conducted a study on the Se absorption capacity of different crops and observed variations in the ability of various plants, such as cabbage, rapeseed, radish, onion, pea, lettuce, and some Gramineae plants to absorb Se. Lettuce and specific Gramineae plants exhibited relatively weaker Se absorption capacity compared to others. Se-hyperaccumulator plants can accumulate substantial amounts of Se due to their robust Se metabolism. In the case of Se-hyperaccumulators like S. pinnata, A. bisulcatus, Asparagus racemosus, and Cardamine hupingshanensis, they can effectively convert SeCys into other non-protein amino acids with lower toxicity. This enables a significant accumulation of Se within the plant (Rao et al. 2021; Freeman et al. 2006; Yuan et al. 2013). Such specific metabolic pathways are primarily found in Se-hyperaccumulator plants. Through these pathways, SeCys can be competitively converted into MeSeCys, which cannot bind with proteins. Consequently, this conversion helps to avoid the toxic effects of Se on plants (Neuhierl et al. 1996; Hoewyk 2013).
The Se absorption capacity of various plant components also exhibits variability. Wan et al. (1988) conducted a study on Se metabolism in agricultural crops in California, examining Se absorption and utilization in different segments of three crops: barley, beet, and tomato. It was observed that the Se content in barley stems surpassed that of the grains, while beet leaves demonstrated a greater propensity for Se absorption compared to the roots. Furthermore, the Se content in tomato leaves and stems exceeded that found in the fruits. Likewise, an analysis of the Se content in various segments of 17 vegetable varieties, encompassing both edible and non-edible portions, revealed that non-edible parts possessed higher Se concentrations than their edible counterparts (Hamilton et al. 1963). These investigations substantiate the notion that different plant components possess distinctive capabilities in Se absorption, with non-edible parts generally exhibiting heightened absorption capacities compared to the edible parts.
Conclusion and future perspectives
This review systematically summarized the factors influencing Se enrichment in plants, laying the foundation for cultivating Se-enriched plants and addressing the Se deficiency issue faced by approximately one billion people worldwide. Firstly, the importance and roles of Se were introduced, including its benefits to human health as well as the potential toxicity associated with excessive Se intake. The mechanism of Se accumulation in plants, the source of Se enrichment in plants, and the metabolism of Se in plants were summarized, with emphasis on the absorption and transportation of Se by plants and the metabolic process of Se in plants. Furthermore, the factors influencing Se accumulation in plants were elaborated, including internal factors, that is the genetic background and species characteristics of plants; the external factors, that is the soil physiological and biochemical properties, microbial environment, etc. focus on how different factors affect plant Se enrichment.
Despite the progress made in understanding the influencing factors of Se enrichment in plants, several research gaps remain to be addressed: (1) there is a need to gain a deeper understanding of the absorption and transport mechanisms of Se by plants under different soil types and environmental conditions, in order to optimize soil management and fertilization strategies and enhance plant Se accumulation. (2) the effects of soil microorganisms on Se enrichment through their interactions with plants need to be further explored, with the goal of developing targeted microbial products to promote Se absorption and accumulation in plants. (3) a better understanding of the competition and synergistic effects between Se and other elements is essential to elucidate the mechanisms of Se enrichment in plants and devise more effective regulatory measures. (4) further exploration of the differences in Se accumulation abilities among different plants can be achieved by studying their genetic backgrounds and species characteristics, leading to the identification and utilization of more plants with high Se accumulation capacities. This approach will facilitate the development of Se-enriched crops and plant varieties, providing additional sources of Se nutrition for human consumption.
References
Acuña JJ, Jorquera MA, Barra PJ, Crowley DE, De la Luz Mora M (2013) Selenobacteria selected from the rhizosphere as a potential tool for Se biofortification of wheat crops. Biol Fertil Soils 49:175–185. https://doi.org/10.1007/s00374-012-0705-2
Asher CJ, Butler GW, Peterson PJ (1977) Selenium transport in root systems of tomato. J Exp Bot 28:279–291. https://doi.org/10.1093/jxb/28.2.279
Boldrin PF, de Figueiredo MA, Yang Y, Luo H, Giri S, Hart JJ, Faquin V, Guilherme LR, Thannhauser TW, Li L (2016) Selenium promotes sulfur accumulation and plant growth in wheat (Triticum aestivum). Physiol Plant 158:80–91. https://doi.org/10.1111/ppl.12465
Brown K, Arthur J (2001) Selenium, selenoproteins and human health: a review. Public Health Nutr 4:593–599. https://doi.org/10.1079/phn2001143
Byrne SL, Durandeau K, Nagy I, Barth S (2010) Identification of ABC transporters from Lolium perenne L. that are regulated by toxic levels of selenium. Planta 231:901–911. https://doi.org/10.1007/s00425-009-1096-y
Cabannes E, Buchner P, Broadley MR, Hawkesford MJ (2011) A comparison of sulfate and selenium accumulation in relation to the expression of sulfate transporter genes in Astragalus species. Plant Physiol 157:2227e2239. https://doi.org/10.1104/pp.111.183897
Carey AM, Scheckel KG, Lombi E, Newville M, Choi Y, Norton GJ, Price AH, Meharg AA (2012) Grain accumulation of selenium species in rice (Oryza sativa L). Environ Sci Technol 46:5557–5564. https://doi.org/10.1021/es203871j
Cartes P, Gianfreda L, Mora ML (2005) Uptake of selenium and its antioxidant activity in ryegrass when applied as selenate and selenite forms. Plant Soil 276:359–367. https://doi.org/10.1007/s11104-005-5691-9
Chao W, Rao S, Chen Q, Zhang W, Liao Y, Ye J, Cheng S, Yang X, Xu F (2022) Advances in research on the involvement of selenium in regulating plant ecosystems. Plants-Basel 11:2712. https://doi.org/10.3390/plants11202712
Chen X, Zhang Z, Gu M, Li H, Shohag MJI, Shen F, Wang X, Wei Y (2020) Combined use of arbuscular mycorrhizal fungus and selenium fertilizer shapes microbial community structure and enhances organic selenium accumulation in rice grain. Sci Total Environ 748:141166. https://doi.org/10.1016/j.scitotenv.2020.141166
Conversa G, Lazzizera C, Chiaravalle AE, Miedico O, Bonasia A, La Rotonda P, Elia A (2019) Selenium fern application and arbuscular mycorrhizal fungi soil inoculation n enhance Se content and antioxidant properties of green asparagus (Asparagus officinalis L.) spears. Sci Hortic 252:176–191. https://doi.org/10.1016/j.scienta.2019.03.056
Dai ZH, Imtiaz M, Rizwan M, Yuan YA, Huang HL, Tu SX (2019) Dynamics of Selenium uptake, speciation, and antioxidant response in rice at different panicle initiation stages. Sci Total Environ 691:827–834. https://doi.org/10.1016/j.scitotenv.2019.07.186
Dehghanian H, Halajnia A, Lakzian A, Astaraei A (2018) The effect of earthworm and arbuscular mycorrhizal fungi on availability and chemical distribution of Zn, Fe and Mn in a calcareous soil. Appl Soil Ecol 130:98–103. https://doi.org/10.1016/j.apsoil.2018.06.002
Dungan RS, Yates SR, Frankenberger WT (2003) Transformations of selenate and selenite by Stenotrophomonas maltophilia isolated from a seleniferous agricultural drainage pond sediment. Environ Microbiol 5:287–295. https://doi.org/10.1046/j.1462-2920.2003.00410.x
Dwire KA, Kaufman JB, Baham JE (2006) Plant species distribution in relation to water-table depth and soil redox potential in montane riparian meadows. Wetlands 26:131–146. https://doi.org/10.1672/0277-5212(2006)26[131:PSDIRT]2.0.CO;2
Eich-Greatorex S, Sogn TA, Øgaard AF, Aasen I (2007) Plant availability of inorganic and organic selenium fertiliser as influenced by soil organic matter content and pH. Nutr Cycl Agroecosy 79:221–231. https://doi.org/10.1007/s10705-007-9109-3
Ekumah JN, Ma Y, Akpali-Tsigbe NDK, Kwaw E, Jie H (2021) Global soil distribution, dietary access routes, bioconversion mechanisms and the human health significance of selenium: a review. Food Biosci 41:100960. https://doi.org/10.1016/j.fbio.2021.100960
El Mehdawi AF, Jiang Y, Guignardi ZS, Esmat A, Pilon M, Pilon-Smits EAH, Schiavon M (2018) Influence of sulfate supply on selenium uptake dynamics and expression of sulfate/selenate transporters in selenium hyperaccumulator and nonhyperaccumulator Brassicaceae. New Phytol 217:194–205. https://doi.org/10.1111/nph.14838
El-Ramady H, Abdalla N, Taha HS, Alshaal T, El-Henawy A, Faizy SEDA, Schnug E (2016) Selenium and nano-selenium in plant nutrition. Environ Chem Lett 14:123–147. https://doi.org/10.1007/s10311-015-0535-1
Espinosa-Ortiz EJ, Rene ER, Guyot F, van Hullebusch ED, Lens PNL (2017) Biomineralization of tellurium and selenium-tellurium nanoparticles by the white-rot fungus phanerochaete chrysosporium. Int Biodeter Biodegr 124:258–266. https://doi.org/10.1016/j.ibiod.2017.05.009
Fernández-Martínez A, Charlet L (2009) Selenium environmental cycling and bioavailability: a structural chemist point of view. Rev Environ Sci Bio 8:81–110. https://doi.org/10.1007/s11157-009-9145-3
Finley JW, Davis CD (2001) Selenium (Se) from high-selenium broccoli is utilized differently than selenite, selenate and selenomethionine, but is more effective in inhibiting colon carcinogenesis. BioFactors 14:191–196. https://doi.org/10.1002/biof.5520140124
Fleming GA (1962) Selenium in Irish soils and plants. Soil Sci 94:28–35. https://doi.org/10.1097/00010694-196207000-00005
Freeman JL, Zhang LH, Marcus MA, Fakra S, McGrath SP, Pilon-Smits EA (2006) Spatial imaging, speciation, and quantification of selenium in the hyperaccumulator plants Astragalus bisulcatus and Stanleya pinnata. Plant Physiol 142:124–134. https://doi.org/10.1104/pp.106.081158
Giasson P, Jaouich A, Cayer P, Gagné S, Moutoglis P, Massicotte L (2006) Enhanced phytoremediation: a study of mycorrhizoremediation of heavy metal–contaminated soil. Remediation 17:97–110. https://doi.org/10.1002/rem.20115
Gladyshev VN, Regina BF, Arn´er ES, Berry MJ, Bruford EA, Burk RF, Carlson BA, Castellano S, Chavatte L, Conrad M, Copeland PR, Diamond AM, Driscoll DM, Ferreiro A, Floh´e L, Green FR, Guigo´ R, Handy DE, Hatfeld DL, Zhang Y (2016) Selenoprotein gene nomenclature. J Biol Chem 291:24036–24040. https://doi.org/10.1074/jbc.M116.756155
Goicoechea N, Garmendia I, Fabbrinc E, Bettonic M, Palopd J, Sanmartín C (2015) Selenium fertilization and mycorrhizal technology may interfere in enhancing bioactive compounds in edible tissues of lettuces. Sci Hortic 195:163–172. https://doi.org/10.1016/j.scienta.2015.09.007
Gupta M, Gupta S (2017) An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci 7:2074. https://doi.org/10.3389/fpls.2016.02074
Hamilton JW, Beath OA (1963) Selenium uptake and conversion by certain crop plants. Agron J 55:528–531. https://doi.org/10.2134/agronj1963.00021962005500060008x
Hatfield DL, Tsuji PA, Carlson BA, Gladyshev VN (2014) Selenium and selenocysteine: roles in cancer, health, and development. Trends Biochem Sci 39:112–120. https://doi.org/10.1016/j.tibs.2013.12.007
Hawrylak-Nowak B, Matraszek R, Pogorzelec M (2015) The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiol Plant 37:1e13. https://doi.org/10.1007/s11738-015-1788-9
Haygarth PM, Harrison AF, Jones KC (1995) Plant selenium from soil and the atmosphere. J Environ Qual 24:768–771. https://doi.org/10.2134/jeq1995.00472425002400040030x
Hoewyk DV (2013) A tale of two toxicities: malformed selenoproteins and oxidative stress both contribute to selenium stress in plants. Ann Bot 112:965–972. https://doi.org/10.1093/aob/mct163
Hopper JL, Parker DR (1999) Plant availability of selenite and selenate as influenced by the competing ions phosphate and sulfate. Plant Soil 210:199–207. https://doi.org/10.1023/A:1004639906245
Johnsson L (1991) Selenium uptake by plants as a function of soil type, organic matter content and pH. Plant Soil 133:57–64. https://doi.org/10.1007/BF00011899
Jones GD, Droz B, Greve P, Gottschalk P, Poffet D, McGrath SP, Seneviratne SI, Smith P, Winkel LH (2017) Selenium deficiency risk predicted to increase under future climate change. Proc Natl Acad Sci USA 114:2848–2853. https://doi.org/10.1073/pnas.1611576114
Keskinen R, Yli-Halla M, Hartikainen H (2013) Retention and uptake by plants of added selenium in peat soils. Commun Soil Sci Plant Anal 44:3465–3482. https://doi.org/10.1080/00103624.2013.847955
Kobae Y, Kameoka H, Sugimura Y, Saito K, Ohtomo R, Fujiwara T, Kyozuka J (2018) Strigolactone biosynthesis genes of rice are required for the punctual entry of arbuscular mycorrhizal fungi into the roots. Plant Cell Physiol 59:544–553. https://doi.org/10.1093/pcp/pcy001
Koshila Ravi R, Anusuy S, Balachandar M, Muthukumar T (2019) Microbial interactions in soil formation and nutrient Cycling. In: Varma A, Choudhary D (eds) Mycorrhizosphere and Pedogenesis. Springer, Singapore, pp 363–382
Labunskyy VM, Hatfeld DL, Gladyshev VN (2014) Selenoproteins: molecular pathways and physiological roles. Physiol Rev 94:739–777. https://doi.org/10.1152/physrev.00039.2013
Larsen EH, Lobinski R, Burger-Meã K, Hansen M, Ruzik R, Mazurowska L, Rasmussen PH, Sloth JJ, Scholten O, Kik C (2006) Uptake and speciation of selenium in garlic cultivated in soil amended with symbiotic fungi (mycorrhiza) and selenate. Anal Bioanal Chem 385:1098–1108. https://doi.org/10.1007/s00216-006-0535-x
Li HF, McGrath SP, Zhao FJ (2008) Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol 178:92–102. https://doi.org/10.1111/j.1469-8137.2007.02343.x
Lima LW, Pilon-Smits EAH, Schiavon M (2018) Mechanisms of selenium hyperaccumulation in plants: a survey of molecular, biochemical and ecological cues. BBA-Gen Subj 1862:2343–2353. https://doi.org/10.1016/j.bbagen.2018.03.028
Liu Q, Wang DJ, Jiang XJ, Cao ZH (2004) Effects of the interactions between selenium and phosphorus on the growth and selenium accumulation in rice (Oryza sativa). Environ Geochem Health 26:325–330. https://doi.org/10.1023/b:egah.0000039597.75201.57
Lu J, Holmgren A (2009) Selenoproteins. J Biol Chem 284:723–727. https://doi.org/10.1074/jbc.R800045200
Mikkelsen RL, Wan HF (1990) The effect of selenium on sulfur uptake by barley and rice. Plant Soil 12l:151–153. https://doi.org/10.1007/BF00013109
Milchunas DG, Lavenroth WK, Dodd JL (1983) The interaction of atmospheric and soil sulfur on the sulfur and selenium concentration of range plants. Plant Soil 72:117–125. https://doi.org/10.1007/BF02185101
Nancharaiah YV, Lens PNL (2015) Ecology and biotechnology of selenium-respiring bacteria. Microbiol Mol Biol Rev 79:61–80. https://doi.org/10.1128/MMBR.00037-14
Natasha Shahid M, Niazi NK, Khalid S, Murtaza B, Bibi I, Rashid MI (2018) A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ Pollut 234:915–934. https://doi.org/10.1016/j.envpol.2017.12.019
Neuhierl B, Böck A (1996) On the mechanism of selenium tolerance in selenium-accumulating plants: purification and characterization of a specific selenocysteine methyltransferase from cultured cells of Astragalus Bisculatus. Eur J Biochem 239:235–238. https://doi.org/10.1111/j.1432-1033.1996.0235u.x
Nuccio EE, Hodge A, Pett-Ridge J, Herman DJ, Weber PK, Firestone MK (2013) An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environ Microbiol 15:1870–1881. https://doi.org/10.1111/1462-2920.12081
Patel PJ, Trivedi GR, Shah RK, Saraf M (2018) Selenorhizobacteria: as biofortification tool in sustainable agriculture. Biocatal Agric Biotechnol 14:198–203. https://doi.org/10.1016/j.bcab.2018.03.013
Pellegrino E, Bedini S (2014) Enhancing ecosystem services in sustainable agriculture: biofertilization and biofortification of chickpea (Cicer arietinum L.) by arbuscular mycorrhizal fungi. Soil Biol Biochem 68:429–439. https://doi.org/10.1016/j.soilbio.2013.09.030
Philip JW (2018) Selenium metabolism in plants. BBA-Gen Subj 1862:2333–2342. https://doi.org/10.1016/j.bbagen.2018.05.006
Rao S, Yu T, Cong X, Zhang W, Xu F (2021) Effects of selenate applied at two growth stages on the nutrient quality of cardamine violifolia. Sci Hortic 288:110352. https://doi.org/10.1016/j.scienta.2021.110352
Reich HJ, Hondal RJ (2016) Why nature chose selenium. ACS Chem Biol 11:821–841. https://doi.org/10.1021/acschembio.6b00031
Ryan MH, Graham JH (2018) Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol 220:1092–1107. https://doi.org/10.1111/nph.15308
Saha U, Fayiga A, Sonon L (2017) Selenium in the soil-plant environment: a review. Int J Appl Agr Sci 3:1–18. https://doi.org/10.11648/j.ijaas.20170301.11
Santesmasses D, Mariotti M, Gladyshev VN (2020) Bioinformatics of selenoproteins. Antioxid Redox Signal 33:525–536. https://doi.org/10.1089/ars.2020.8044
Schiavon M, Pilon-Smits EA (2017) The fascinating facets of plant selenium accumulation - biochemistry, physiology, evolution and ecology. New Phytol 213:1582–1596. https://doi.org/10.1111/nph.14378
Sharma VK, Mcdonald TJ, Sohn M, Anquandah GAK, Pettine M, Zboril R (2015) Biogeochemistry of selenium. A review. Environ Chem Lett 13:49–58. https://doi.org/10.1007/s10311-014-0487-x
Smith SE, Read DJ (2010) Mycorrhizal symbiosis. For Sci 32:547–548. https://doi.org/10.1093/forestscience/32.2.547
Sors TG, Ellis DR, Na GN, Lahner B, Lee S, Leustek T, Leustek T, Pickering IJ, Salt DE (2005a) Analysis of sulfur and selenium assimilation in Astragalus plants with varying capacities to accumulate selenium. Plant J 42:785–797. https://doi.org/10.1111/j.1365-313X.2005.02413.x
Sors TG, Elli DR, Salt DE (2005b) Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth Res 86:373–389. https://doi.org/10.1007/s11120-005-5222-9
Staicu LC, Ackerson CJ, Cornelis P, Ye L, Berendsen RL, Hunter WJ, Noblitt SD, Henry CS, Cappa JJ, Montenieri RL, Wong AO, Musilova L, Sura-de Jong M, van Hullebusch ED, Lens PN, Reynolds RJ, Pilon-Smits EA (2015) Pseudomonas moraviensis subsp. stanleyae, a bacterial endophyte of hyperaccumulator Stanleya pinnata, is capable of efficient selenite reduction to elemental selenium under aerobic conditions. J Appl Microbiol 119:400–410. https://doi.org/10.1111/jam.12842
Sura-de Jong M, Reynolds RJ, Richterova K, Musilova L, Staicu LC, Chocholata I, Cappa JJ, Taghavi S, van der Lelie D, Franti T, Dolinova I, Strejcek M, Cochran AT, Lovecka P, Pilon-Smits EA (2015) Selenium hyperaccumulators harbor a diverse endophytic bacterial community characterized by high selenium resistance and plant growth promoting properties. Front Plant Sci 6:113. https://doi.org/10.3389/fpls.2015.00113
Trippe RC 3rd, Pilon-Smits EAH (2021) Selenium transport and metabolism in plants: phytoremediation and biofortification implications. J Hazard Mater 404:124178. https://doi.org/10.1016/j.jhazmat.2020.124178
Van Hoewyk D, Takahashi H, Inoue E, Hess A, Tamaoki M, Pilon-Smits EA (2008) Transcriptome analyses give insights into selenium-stress responses and selenium tolerance mechanisms in Arabidopsis. Physiol Plant 132:236–253. https://doi.org/10.1111/j.1399-3054.2007.01002.x
Vinceti M, Filippini T, Wise LA (2018) Environmental selenium and human health: an update. Curr Environ Health Rep 5:464–485. https://doi.org/10.1007/s40572-018-0213-0
Wan HF, Mikkelsen RL, Page AL (1988) Selenium uptake by some agricultural crops from central California soil. J Environ Qual 17:269–272. https://doi.org/10.2134/jeq1988.00472425001700020018x
Wang Z, Gao Y (2001) Biogeochemical cycling of selenium in Chinese environments. Appl Geochem 16:1345–1351. https://doi.org/10.1016/S0883-2927(01)00046-4
Wang JM, Cappa JJ, Harris JP, Edger PP, Zhou W, Pires JC, Adair M, Unruh SA, Simmons MP, Schiavon M, Pilon-Smits EAH (2018) Transcriptome-wide comparison of selenium hyperaccumulator and non-accumulator Stanleya species provides new insight into key processes mediating the hyperaccumulation syndrome. Plant Biotechnol J 16:1582–1594. https://doi.org/10.1111/pbi.12897
Wang Z, Huang W, Pang F (2022) Selenium in Soil-Plant-Microbe: a review. Bull Environ Contam Toxicol 108:167–181. https://doi.org/10.1007/s00128-021-03386-2
Watts-Williams SJ, Smith FA, McLaughlin MJ, Patti AF, Cavagnaro TR (2015) How important is the mycorrhizal pathway for plant zn uptake? Plant Soil 390:157–166. https://doi.org/10.1007/s11104-014-2374-4
White PJ (2018) Selenium metabolism in plants. BBA-Gen Subj 1862:2333–2342. https://doi.org/10.1016/j.bbagen.2018.05.006
White PJ, Bowen HC, Parmaguru P, Fritz M, Spracklen WP, Spiby RE, Meacham MC, Mead A, Harriman M, Trueman LJ, Smith BM, Thomas B, Broadley MR (2004) Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J Exp Bot 55:1927–1937. https://doi.org/10.1093/jxb/erh192
Winkel LHE, Johnson CA, Lenz M, Grundl T, Leupin OX, Amini M, Charlet L (2012) Environmental selenium research: from microscopic processes to global understanding. Environ Sci Technol 46:571–579. https://doi.org/10.1021/es203434d
Winkel LH, Vriens B, Jones GD, Schneider LS, Pilon-Smits E, Banuelos GS (2015) Selenium cycling across soil-plant-atmosphere interfaces: a critical review. Nutrients 7:4199–4239. https://doi.org/10.3390/nu7064199
Wu L, Guo X, Bañuelos GS (2003) Selenium and sulfur accumulation and soil selenium dissipation in planting of four herbaceous plant species in soil contaminated with drainage sediment rich in both selenium and sulfur. Int J Phytoremediation 5:25–40. https://doi.org/10.1080/16226510390856457
Wu Z, Bañuelos GS, Lin ZQ, Liu Y, Yuan L, Yin X, Li M (2015) Biofortification and phytoremediation of selenium in China. Front Plant Sci 6:136. https://doi.org/10.3389/fpls.2015.00136
Wu F, Luo W, Li J, Xing W, Lyu L, Yang J, Liu R, Shi Z (2022) Effects of arbuscular mycorrhizal fungi on accumulation and translocation of selenium in winter wheat. J Sci Food Agric 102:6481–6490. https://doi.org/10.1002/jsfa.12015
Yamada H, Kamada A, Usuki M, Yanai J (2009) Total selenium content of agricultural soils in Japan. Soil Sci Plant Nutr 55:616–622. https://doi.org/10.1111/j.1747-0765.2009.00397.x
Ying H, Zhang Y (2019) Systems biology of selenium and complex Disease. Biol Trace Elem Res 192:38–50. https://doi.org/10.1007/s12011-019-01781-9
Yuan L, Zhu Y, Lin ZQ, Banuelos G, Li W, Yin X (2013) A novel selenocystine-accumulating plant in selenium-mine drainage area in Enshi, China. PLoS ONE 8:e65615. https://doi.org/10.1371/journal.pone.0065615
Zhang L, Chu C (2022) Selenium uptake, transport, metabolism, reutilization, and biofortification in rice. Rice 15:30. https://doi.org/10.1186/s12284-022-00572-6
Zhang M, Tang S, Huang X, Zhang F, Pang Y, Huang Q, Yi Q (2014) Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fuorescence in rice (Oryza sativa L.). Environ Exp Bot 107:39–45. https://doi.org/10. 1016/j. envex pbot. 2014. 05. 005
Zhao R, Guo JB, Song YY, Chen Z, Lu CC, Han Y, Li HB, Hou YA, He Y (2020) Mediated electron transfer efficiencies of Se(IV) bioreduction facilitated by meso-tetrakis (4-sulfonatophenyl) porphyrin. Int Biodeter Biodegr 147:104838. https://doi.org/10.1016/j.ibiod.2019.104838
Zhong X, Gan Y, Deng Y (2021) Distribution, origin and speciation of soil selenium in the black soil region of Northeast China. Environ Geochem Health 43:1257–1271. https://doi.org/10.1007/s10653-020-00691-3
Zhu YG, Pilon-Smits EA, Zhao FJ, Williams PN, Meharg AA (2009) Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 14:436–442. https://doi.org/10.1016/j.tplants.2009.06.006
Funding
This work was funded by the key R&D Program of Hubei Province [Grant no. 2023BBB065]; the Dawning Plan Project of Knowledge Innovation Special Project of Wuhan City [Grant no. 2023020201020456]; the Doctoral Research Funding Project of Wuhan Polytechnic University [Grant no. 2023RZ014].
Author information
Authors and Affiliations
Contributions
XM Liu: Conceptualization, Writing, Software. H Cheng: Visualization, Investigation. SY Cheng: Resources, Writing-review & editing. F Xu: Resources, Writing-review & editing, Supervision. S Rao: Writing-reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial or commercial conflict of interest.
Additional information
Communicated by Mohsin Tanveer.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Cheng, H., Cheng, S. et al. Advances in research on influencing factors of selenium enrichment in plants. Plant Growth Regul 103, 243–255 (2024). https://doi.org/10.1007/s10725-023-01107-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-023-01107-9