Abstract
Selenium (Se) alleviates cadmium (Cd) accumulation in several plants. Nevertheless, it is still unclear why it has such effect. Thus, this study aimed to investigate the effects of Se on soil Cd bioavailability, and Cd accumulation in flooded rice plants, and to determine the mechanisms underlying these effects. Concentration of Cd and Se in different rice tissues was determined along Cd and Se concentrations in the soil solution and soil Cd fractions. Results showed that exogenous selenite and selenate treatments significantly increased rice grain Se by 4.25- and 2.39-fold and decreased Cd by 36.5% and 25.3% relative to control treatment, respectively. The addition of Se to Cd-contaminated soil significantly decreased total Cd concentration in the soil solution by 11.2–13.0%, increased soil pH by 0.06–0.32 units, and enhanced soil Cd immobilization in relation to control. Exogenous Se also reduced diethylenetriaminepentaacetic acid-Cd, exchangeable, and residual Cd but increased the levels of Cd bound to carbonate and iron and manganese oxides. Thus, amending Cd-contaminated soil with Se may help decrease Cd content as well as increase Se levels in rice grain, as Se may mitigate Cd accumulation in rice plants by increasing soil pH, reducing Cd bioavailability, and inhibiting Cd translocation from roots to shoots.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal contamination of agricultural soils has become a serious environmental issue worldwide over the past three decades. In particular, cadmium (Cd) is generally regarded as one of the most widespread and harmful farmland pollutants. It is not an essential nutrient for living organisms and long-term Cd intake can cause chronic toxicity diseases in livestock and humans (Chaney et al. 2004). Among food crops, paddy rice (Oryza sativa) is the most widely consumed grain on earth and it is the primary dietary staple for more than half of the world’s population. Moreover, rice tends to accumulate more Cd than other cereals and is the major source of dietary Cd intake in rice-consuming populations; thus, Cd-tainted rice had been a serious food security threat for people relying on this crop as a dietary staple (Meharg et al. 2013). Effective measures are, therefore, needed to decrease Cd accumulation in rice and reduce consumer health risk for the agro-environmental sustainability of rice production and food safety.
Selenium (Se) is a trace element required by both humans and animals. Although it is not an essential plant nutrient, it enhances plant growth (Tamaoki and Maruyama-Nakashita 2017). Studies have demonstrated that Se may promote antioxidant capacity and enhance plant tolerance to abiotic stresses, such as heavy metal exposure (Feng et al. 2013), UV-induced oxidation (Yao et al. 2013), drought (Proietti et al. 2013), cold (Chu et al. 2010), and heat (Djanaguiraman et al. 2010). For most populations, cereals such as rice and wheat are the main dietary sources of Se (Lyons et al. 2004, 2005), albeit having generally low Se levels (Williams et al. 2009; Zhu et al. 2009). A global rice survey showed that 75% of the samples had insufficient Se concentrations to meet human requirements (Williams et al. 2009). Insufficient dietary Se intakes are associated with health disorders, including oxidative stress-related conditions, reduced fertility and immune function, and an increased risk of cancers (Rayman 2000; Whanger 2004). Food crops can be biofortified by foliar or root Se applications, which are safe and effective methods of improving human dietary Se intake (Broadley et al. 2010; Pezzarossa et al. 2012).
Selenium has gained considerable attention for its ability to counteract the deleterious effects of heavy metals (Lin et al. 2012; Mroczek-Zdyrska and Wójcik 2012; Feng et al. 2013; Wang et al. 2016). It has been reported to regulate ROS metabolism, by scavenging excessive oxygen free radicals and decreasing lipid peroxidation and enhance activity of antioxidant enzymes, which, in turn, alleviate heavy metal-induced oxidative stress (Lin et al. 2012; Saidi et al. 2014; Wu et al. 2016). Previous studies have shown that exogenous Se may reduce heavy metal [e.g., arsenic (As), Cd, mercury (Hg), and lead (Pb)] accumulation in plants. Lin et al. (2012) reported that Se significantly reduced the Cd content in hydroponically grown rice. Liao et al. (2016) indicated that root selenite application significantly decreased As and Cd levels in the grains of rice grown on Cd-contaminated soil. Unfortunately, few studies have attempted to elucidate the biochemical mechanism underlying Cd decontamination by Se. Nevertheless, most of the aforementioned studies focused on the interaction between Se and Cd uptake in hydroponically grown plants. In soil and rhizospheres, however, Se might alter the chemical form of Cd, which, in turn, might influence plant Cd accumulation. To date, few studies have analyzed this aspect of soil chemistry, and the effects of Se on Cd bioavailability in soil-rice systems are poorly understood. Therefore, a pot experiment using Cd-contaminated soil was conducted in the present study to investigate the effects of Se treatments on the dynamics of Se concentrations in soil solution, soil Cd bioavailability, and Cd accumulation in rice plants, and if Se amendment increases Se and decreases Cd levels in rice grown on Cd-contaminated paddy fields, determining the mechanisms underlying such effects.
Materials and methods
Materials
Soil used in the pot experiment was collected from the plow layer (0–20-cm depth) of a paddy field in Guangxi county, Hunan province, China. The soil was air-dried, passed through a 2-mm sieve, and mixed with solid fertilizers [phosphorous (P) as CaH2PO4·H2O at 0.15 g P2O5 kg−1, potassium (K) as KCl at 0.2 g K2O kg−1, and nitrogen (N) as CO(NH2)2 at 0.2 g N kg−1]. Soil characteristics were determined following the methods of Bao (2000). The main soil characteristics were as follows: total Cd 1.75 mg kg−1, total Se 0.85 mg kg−1, pH (H2O) 6.71; organic matter 62.00 g kg−1, total N 2.40 g kg−1, total P 0.54 g kg−1, available N 98.0 mg kg−1, available P 19.56 mg kg−1, and available K 59.87 mg kg−1. According to the China Soil Environmental Quality Standards (GB 15618-1995, pH within a range of 6.5–7.5 for a paddy field, 0.30 mg kg−1 for Cd), the soil Cd concentration was 5.83 times higher than the quality standard.
Rice seeds (Oryza sativa L., variety Chuanxiang 8) were surface-sterilized in 30% v/v H2O2 for 15 min, rinsed with deionized water, and soaked in a saturated CaSO4 solution overnight, in darkness, at 25 °C. The seeds were then germinated in a greenhouse on a sterile plastic net floating in deionized water at 25 °C. After 1 week, uniformly grown seedlings were transferred to soil-filled pots. The growing conditions were as follows: 14-h natural sunlight period per day supplemented with sodium vapor lamps to maintain light intensity > 350 μmol m−2 s−1; 28 °C and 20 °C daytime and nighttime temperatures, respectively; and 60–70% relative humidity (RH).
Experimental design
A compartmented rhizo-bag culture system was used. Each pot (height 25 cm; diameter 20 cm) contained 6 kg of soil; 1.2 kg of which was placed in a nylon mesh bag (height 12 cm; diameter 8 cm) set in the center of the pot to create a rhizosphere. The 4.8 kg of soil outside the bag was treated as bulk (non-rhizosphere) soil. The mesh was 30 μm, which allowed the movement of water and dissolved nutrients but not root penetration. Selenium, in the form of Na2SeO4 (selenate) or Na2SeO3 (selenite), was blended into the soil at the rate of 1.0 mg kg−1. A blank base treatment containing no Se was also included (CK). Each treatment was replicated three times. After a 2-week equilibration period, the pots were flooded to 2–3 cm above soil level with deionized water, and two rice seedlings were then transplanted into each rhizosphere bag. Flooding conditions were maintained throughout the rice-growing period using deionized water. After transplantation, the pots were randomly arranged on a bench inside a greenhouse.
Plant sampling and analysis
After 135 days, rice plants grown in each mesh bag were harvested by cutting their stems 2 cm above the soil surface. Shoots were rinsed with deionized water, oven-dried at 75 °C to a constant weight, and separated into brown grain, husk, and straw. Root samples within the nylon mesh bags were carefully washed with tap water to remove soil, rinsed with deionized water, and oven-dried at 75 °C to a constant weight. All dried rice samples were threshed, and then ground in a stainless steel mill.
To determine Cd and Se concentrations in plant tissues, milled subsamples, each weighing 0.2500 g (dry weight), were digested with 8-mL HNO3 (Guaranteed Reagent Grade) using a DigiBlock ED54 digestion system (LabTech, Beijing, China). Total Cd and Se concentrations in the digest solution were determined using inductively coupled plasma mass spectrometry (ICP-MS) (iCAP Q, Thermo Fisher Scientific, Waltham, MA, USA). A certified reference material (GSB-23, rice flour) and blanks were included for quality assurance purposes. The recovery rate of GSB-23 was 85–105%.
Soil sampling and analysis
Two sampling devices (Rhizon MOM; length 10 cm, o.d. 2.5 mm, Rhizosphere Research Products, Wageningen, The Netherlands) were buried diagonally in the soil, to sample bulk soil solution, and in the rhizo-bag of each pot, to sample rhizosphere soil solution. Samples were collected 1, 4, 8, 21, 70, and 135 days after planting. Five milliliters of 5% HNO3 (v/v) was added to 5 mL of soil solution immediately after collection, to prevent iron oxide/hydroxide precipitation, and Cd and Se concentrations in soil solutions were determined with ICP-MS. The pH value of each soil solution (before HNO3 treatment) was measured using a pH electrode.
Rhizosphere and bulk soil were sampled from each pot when mature rice plants were harvested. Rhizosphere soil was defined as the soil present inside the nylon bag and closely adhering to rice roots. Soil samples were air-dried at room temperature and sieved to 1 mm for determining pH and available Cd. Some soil samples were also ground to < 0.149 mm to analyze the sequential extraction of soil Cd. Soil pH was measured in a 1:5 solid-to-liquid ratio suspension of soil in deionized water. Available Cd and Se were determined using diethylenetriaminepentaacetic acid (DTPA) extraction [0.005-M DTPA + 0.01-M CaCl2 + 0.1-M triethanolamine (TEA), pH = 7.3]. Soil samples weighing 5.0 g were dispersed into 25-mL DTPA solution, shaken for 2 h, and analyzed by ICP-MS. Sequential Cd extraction was performed according to the methods of Tessier et al. (1979), in which Cd is partitioned into exchangeable fraction (Exc-Cd), carbonate-bound fraction (CB-Cd), iron and manganese oxide-bound fraction (OX-Cd), organic matter-bound fraction (OM-Cd), and residual fraction (Res-Cd). Cadmium concentrations in each fraction and in the digests of all soil samples were determined by ICP-MS.
Statistical analyses
All data were subjected to analysis of variance (ANOVA). Significantly different means between treatments were separated by the least significant difference (LSD) method at the 0.05 level. The data were processed using SAS v. 9.1 and are presented as means ± standard deviation (SD; n = 3).
Results
Plant growth and Cd and Se in rice tissues
The application of selenite or selenate significantly altered the biomass of rice grain, husk, straw, and root at maturity (Table 1), when compared to the control treatment. As shown in Table 1, the addition of selenate increased the dry weights of the rice grain, husk, straw, and root by approximately 39.8%, 10.1%, 16.2%, and 16.3%, respectively, when compared to the control treatment (CK) (P < 0.05). In contrast, selenite addition decreased the dry weights of the rice husk, straw, and root by approximately 9.67%, 24.20%, and 10.3%, respectively, and increased that of rice grain by 21.6%, relative to the control treatment.
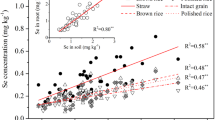
Cd and Se concentrations in plant tissues followed this order: root > straw > husk ≈ brown grain (Fig. 1). The application of selenite or selenate significantly affected Cd and Se concentrations (P < 0.05). Cadmium concentrations in the rice root, straw, husk, and brown grain significantly decreased with exogenous Se application (P < 0.05) (Fig. 1a), particularly for the selenite treatment. Compared to the control treatment, selenite reduced Cd levels in the rice root, straw, husk, and brown grain by 28.9%, 33.8%, 35.4%, and 36.5%, respectively. The effect of selenate was only slightly lower than that of selenite. Moreover, to eliminate the biomass dilution effects on Cd and Se uptake by rice roots, Cd and Se concentrations expressed on the basis of per unit root weight are shown in Fig. 2. Similar to the effect of exogenous Se on Cd concentration in different rice tissues (Fig. 1a), Cd levels expressed as per unit root mass were significantly reduced with exogenous Se application as compared to the control, by 34.8% in the selenite treatment and 24.6% in the selenate treatment which might suggest that Cd uptake by rice roots could be decreased by the exogenous Se application. Unlike Cd concentrations in rice tissues, exogenous Se application might significantly increase Se uptake (Fig. 2b). Se concentrations in rice root, straw, husk, and brown grain significantly increased with selenite or selenate application relative to the control treatment (P < 0.05). This increase was much greater after selenite than selenate application (Fig. 2b). In the selenite treatment, the Se concentrations in rice root, straw, husk, and brown grain were 1.52, 3.36, 2.19, and 4.25-fold that in the control treatment, respectively, while the same concentrations were 1.10, 1.67, 1.39, and 2.39 times higher in the selenate than in the control treatment, respectively.
Figure 3 shows the effects of selenite or selenate addition on the distributions of Cd and Se in different rice tissues at maturity. In the control treatment, Cd concentrated mainly in rice root (47.5%) and straw (43.2%). Only 4.15% and 5.11% of the uptake Cd was transported to rice husk and brown grain, respectively. The application of selenite significantly increased Cd in rice root by 13.0% but decreased it in rice straw, husk, and brown grain by 9.44%, 1.82%, and 1.72%, respectively, in relation to the control treatment. On the other hand, selenate application did not significantly affect Cd distribution pattern in rice tissues, although it slightly increased the Cd in the rice root and decreased it in the shoot, relative to that observed in the control (Fig. 3a). Under the selenate treatment, Se was also mainly distributed in the root (59.5%) and straw (35.7%), and only approximately 2.02% and 2.69% was present in the husk and brown grain, respectively. When compared to the control, the addition of selenite or selenate decreased Se in the root but increased it in the shoot (Fig. 3b).
Changes in pH, Cd, and Se levels in the soil solution
During the entire growth season, the pH in all soil solution samples ranged from 6.94 to 7.62. Exogenous selenite addition increased the pH of both rhizosphere and bulk soil solutions by 0.07–0.30 units, while the pH of these solutions in the selenate treatment were similar to those in the control treatment. The pH of rhizosphere soil solutions tended to be slightly higher than that of bulk soil solutions in the first four sampling times (Fig. 4).
Effects of exogenous Se addition on soil solution pH at different sampling times. Data are means ± SD (n = 3). CK-B, bulk soil in the control treatment (no Se addition); CK-R, rhizosphere soil in the control treatment; SeIV-B, bulk soil in the selenite treatment; SeIV-R, rhizosphere soil in the selenite treatment; SeVI-B, bulk soil in the selenate treatment; and SeVI-R, rhizosphere soil in the selenate treatment
The effects of selenite or selenate addition on total Cd and Se concentrations in soil solutions are shown in Fig. 5. As indicated in Fig. 5a, irrespective of the treatment, total Cd in bulk and rhizosphere soil solutions significantly increased in the first 8 days after planting and decreased to a relatively stable level thereafter. Regardless of the sampling time, total Cd levels in rhizosphere soil solutions were, on average, 16.8%, 14.2%, and 8.10% lower than in bulk soil solutions under control, selenite, and selenate treatments, respectively. Exogenous Se addition also decreased total Cd in the rhizosphere and bulk soil solutions by 13.0% and 11.2%, on average, under the selenite and selenate treatments, respectively, in relation to the control treatment.
Effects of exogenous Se addition on the dynamics of a total cadmium (Cd) and b Se concentrations in rhizosphere and bulk soil solutions. Data are means ± SD (n = 3). CK-B, bulk soil in the control treatment (no Se addition); CK-R, rhizosphere soil in the control treatment; SeIV-B, bulk soil in the selenite treatment; SeIV-R, rhizosphere soil in the selenite treatment; SeVI-B, bulk soil in the selenate treatment; and SeVI-R, rhizosphere soil in the selenate treatment
The application of selenite or selenate significantly increased total Se in both bulk and rhizosphere soil solutions, and this increase was initially higher under the selenate than under the selenite treatment (Fig. 5b). In both bulk and rhizosphere soil solutions, total Se decreased rapidly in the first 21 days after application, but it remained relatively steady thereafter. Regardless of sampling time, total Se in the rhizosphere soil solutions was 5.10%, 8.60%, and 9.70% higher, on average, than in bulk soil solutions under the control, selenite, and selenate treatments, respectively.
Changes in pH, Cd, and Se contents in the soil
The application of selenite or selenate significantly affected soil pH, and DTPA-extractable Cd and Se in bulk and rhizosphere soils (P < 0.05). As shown in Table 2, selenite significantly increased soil pH of rhizosphere and bulk soils, by about 0.32 and 0.33 pH units, respectively, with respect to the control. Selenate, however, had no significant effect on soil pH. Rhizosphere soil pH values were 0.38, 0.47, and 0.34 units higher than those of bulk soil under control, selenite, and selenate treatments, respectively.
The application of selenite or selenate slightly decreased soil DTPA-extractable Cd. Compared with the control treatment, exogenous Se addition decreased the rhizosphere and bulk soil DTPA-extractable Cd by 5.40–7.90% and 2.10–8.90%, respectively. The level of DTPA-extractable Cd in the rhizosphere soil was lower than that in the bulk soil under all treatments. On the other hand, selenite and selenate significantly increased soil DTPA-extractable Se (P < 0.05). Relative to the control treatment, selenite significantly increased bulk and rhizosphere soil DTPA-extractable Se by 33.3% and 23.2%, respectively, and selenate increased them by 21.8% and 4.00%, respectively (Table 2).
Cd fractions in soil at rice maturity
The Cd content in the soil samples collected at rice maturity was divided into five fractions. The effect of exogenous Se addition on the Cd fractions was expressed as percentages of total Cd in the soil and is shown in Table 3. In general, Cd occurred mainly in the Exc-Cd, OX-Cd, and Res-Cd fractions. These comprised 84–92% of the total Cd in bulk and rhizosphere soils under all treatments. The percentages of Cd in the fractions followed this order: Res-Cd (37–45%) > Exc-Cd (28–34%) > OX-Cd (13–20%) > CB-Cd (6–13%) > OM-Cd (2%). As shown in Table 3, in both bulk and rhizosphere soils, selenite and selenate significantly increased the relative amounts of CB-Cd and OX-Cd, but slightly decreased Cd levels in Exc-Cd and Res-Cd fractions. Selenite and selenate addition had no effect on OM-Cd levels. Irrespective of the treatment, Cd levels in the Exc-Cd and Res-Cd fractions were lower in rhizosphere than in bulk soil, while Cd levels in CB-Cd and OX-Cd fractions showed the opposite trend.
Discussion
In the present study, exogenous Se significantly reduced Cd concentrations in various tissues as well as Cd translocation from roots to shoots at maturity (Figs. 1a, 2a, and 3a). Similar results were obtained in previous studies (Lin et al. 2012; Hu et al. 2014; Liao et al. 2016). Furthermore, Se-Cd antagonism has been observed in many different plants (He et al. 2004; Wan et al. 2016; Wu et al. 2016). The mechanisms underlying this antagonism might be attributed to the inhibition of Cd uptake and xylem translocation to the shoot (Uraguchi et al. 2009; Lin et al. 2012; Saidi et al. 2014). However, it has been demonstrated that Cd and Se are taken up by plant roots through different channels or transporters; while Cd is taken up by plant roots via zinc importer proteins (ZIP) or via cation channels (Lux et al. 2011), selenate is taken up by plant roots via sulfate transporters, and selenite might be incorporated by phosphate transporters (Li et al. 2008; Zhang et al. 2013). Moreover, Wu et al. (2016) showed that the addition of Se had no effect on Cd concentration or proportion in root saps, and Wan et al. (2016) showed that selenite only slightly promoted Cd influx into rice root whereas selenate had no effect on Cd influx. These results imply that Se addition might have no significant effect on Cd uptake at the root surface, and that other mechanisms might be involved. In fact, the present study showed that, irrespective of sampling time, exogenous Se significantly decreased total Cd concentrations in soil solutions by 11.2–13.0% on average (Fig. 5a). Moreover, exogenous Se significantly increased Cd levels in CB-Cd and OX-Cd and slightly decreased Cd levels in Exc-Cd fractions (Table 4). Thus, the decline in Cd contents in rice tissues under selenite or selenate treatment might be attributed to the decreased bioavailability of Cd in soil. Two possible reasons might explain how Se exerts this effect. First, selenite, selenate, and their products in soil (e.g., selenide, elemental Se, organic Se) might thermodynamically react with Cd to form Cd-Se complexes that are unavailable to the plant root. Wang et al. (2016) showed that nanoparticles containing Hg–Se at a molar ratio of 1:1 were present in Se-amended soils. Thus, further research is required to identify the formation of Cd-Se complexes in Cd-contaminated soil amended with Se. Second, exogenous Se application might affect the rhizosphere microenvironment (Dong et al. 2007). Our results showed that the rhizosphere immobilizes Cd in the soil by increasing soil pH and restricting Cd diffusion. Therefore, Se addition might change the rhizosphere environment and alter the form on which Cd is present, thereby reducing the amount of Cd released into the soil solution and eventually affecting Cd uptake. After root absorption, Cd is transported from the root to the shoot by xylem translocation (Uraguchi et al. 2009; Li et al. 2017). In the present study, selenite significantly decreased Cd distribution in shoots (Fig. 3a), similar to the results obtained by Wan et al. (2016). Selenite absorbed by plant root is rapidly assimilated into organic forms and selenide, and its metabolic products tend to accumulate in the root, with limited translocation to the shoot (Li et al. 2008). Thus, Se might restrict Cd root to shoot transportation due to the formation and retention of Cd-Se compounds in the root. This mechanism should be further investigated.
Exogenous Se addition significantly reduced Cd concentration (Figs. 1a and 2a) and increased Se content in rice tissues, which is in agreement with previous studies (Li et al. 2010; Hu et al. 2014; Liao et al. 2016). A comparison of the selenite and selenate treatments showed that the former was more effective at increasing Se and decreasing Cd in rice than the latter. Variations in bioavailable Se levels in the soil and selenite/selenate uptake mechanisms may account for differences in rice Se accumulation (Li et al. 2008). In the present study, selenate was far more soluble than selenite in the soil solution for the first 21 days, but this pattern was reversed thereafter (Fig. 5b). Moreover, under the selenite treatment, the DTPA-extractable Se levels were 9.40% and 18.5% higher than those under the selenate treatment in rhizosphere and bulk soil, respectively, and these differences were significant (Table 2). Moreover, our previous study showed that rice roots absorb selenite faster than selenate (Huang et al. 2014). Additionally, iron plaque on rice root surfaces facilitates Se uptake during rice growth and absorbs selenite from the substrate more effectively than selenate (Huang et al. 2015a, b). Therefore, rice plants absorb more Se from selenite than selenate treatment (Fig. 3b). In the present study, the Cd levels in different rice tissues were negatively correlated with those of Se (data not shown), indicating Se antagonism for Cd bioaccumulation in rice plants. More specifically, selenite reduces rice plant Cd accumulation more effectively than selenate (Figs. 1a and 2a). Accordingly, selenite addition was more effective at increasing Se in rice tissues than selenate, suggesting that the differences in rice Cd accumulation may be related to variations in selenite or selenate bioavailability in soil and to their capacity to react with soil Cd forming stable Cd-Se complexes.
Conclusion
In conclusion, the present study shows that adding selenite or selenate to Cd-contaminated soil increased Se and decreased Cd levels in rice tissues. Results also indicate that selenite more effectively reduces Cd accumulation and enhances Se content in rice plants than selenate. Exogenous Se also significantly decreased Cd content in both rhizosphere and the bulk soil solutions, and reduced Cd bioavailability in the soil by increasing pH, decreasing soil DTPA-extractable and Exc-Cd fractions, and increasing CB-Cd and OX-Cd fractions in the soil. Overall, the present results improve our understanding on the mechanism by which Se mitigates Cd accumulation in soil-rice systems.
References
Bao SD (2000) Soil agro-chemistrical analysis. China Agriculture Press, Beijing (in Chinese)
Broadley MR, Alcock J, Alford J, Cartwright P, Foot I, Fairweather-Tait SJ, Hart DJ, Hurst R, Knott P, McGrath SP, Meacham MC, Norman K, Mowat H, Scott P, Stroud JL, Tovey M, Tucker M, White PJ, Young SD, Zhao FJ (2010) Selenium biofortification of high-yielding winter wheat (Triticum aestivum L.) by liquid or granular Se fertilisation. Plant Soil 332:5–18
Chaney RL, Reeves PG, Ryan JA, Simmons RW, Welch RM, Angle JS (2004) An improved understanding of soil Cd risk to humans and low cost methods to phytoextract Cd from contaminated soils to prevent soil Cd risks. Biometals 17:549–553
Chu JZ, Yao XQ, Zhang ZN (2010) Responses of wheat seedlings to exogenous selenium supply under cold stress. Biol Trace Elem Res 136:355–363
Djanaguiraman M, Prasad PVV, Seppanen M (2010) Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Biochem 48:999–1007
Dong J, Mao WH, Zhang GP, Wu FB, Cai Y (2007) Root excretion and plant tolerance to cadmium toxicity-a review. Plant Soil Environ 53:193–200
Feng RW, Wei CY, Tu SX, Ding YZ, Song ZG (2013) A dual role of Se on Cd toxicity: evidences from the uptake of Cd and some essential elements and the growth responses in paddy rice. Biol Trace Elem Res 151:113–121
He PP, Lv XZ, Wang GY (2004) Effects of Se and Zn supplementation on the antagonism against Pb and Cd in vegetables. Environ Int 30:167–172
Hu Y, Norton GJ, Duan GL, Huang YC, Liu YX (2014) Effect of selenium fertilization on the accumulation of cadmium and lead in rice plants. Plant Soil 384:131–140
Huang QQ, Chen SY, Wang Q, Qiao YH, Li HF (2014) Effects of selenite/selenate and their coexistence in plant on selenium uptake and translocation in rice. J Agro Environ Sci 33:2098–2103 (In Chinese)
Huang QQ, Wang Q, Luo Z, Yu Y, Jiang RF, Li HF (2015a) Effects of root iron plaque on selenite and selenate dynamics in rhizosphere and uptake by rice (Oryza sativa). Plant Soil 388:255–266
Huang QQ, Yu Y, Wang Q, Luo Z, Jiang RF, Li HF (2015b) Uptake kinetics and translocation of selenite and selenate as affected by iron plaque on root surfaces of rice seedlings. Planta 241:907–916
Li HF, McGrath SP, Zhao FJ (2008) Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol 178:92–102
Li HF, Lombi E, Stroud JL, McGrath SP, Zhao FJ (2010) Selenium speciation in soil and rice: influence of water management and Se fertilization. J Agric Food Chem 58:11837–11843
Li H, Luo N, Li YW, Cai QY, Li HY, Mo CH, Wong MH (2017) Cadmium in rice: transport mechanisms, influencing factors, and minimizing measures. Environ Pollut 224:622–630
Liao GJ, Xu Y, Chen C, Wu QH, Feng RW, Guo JK, Wang RG, Ding YZ, SunY XYM, Xia W, Fan ZL, Mo LY (2016) Root application of selenite can simultaneously reduce arsenic and cadmium accumulation and maintain grain yields, but show negative effects on the grain quality of paddy rice. J Environ Manag 183:733–741
Lin L, Zhou W, Dai H, Cao F, Zhang G, Wu F (2012) Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J Hazard Mater 235:343–351
Lux A, Martinka M, Vaculík M, White PJ (2011) Root responses to cadmium in the rhizosphere: a review. J Exp Bot 62:21–37
Lyons GH, Lewis J, Lorimer MF, Holloway RE, Brace DM, Stangoulis JC, Graham RD (2004) High-selenium wheat: agronomic biofortification strategies to improve human nutrition. J Food Agric Environ 2:171–178
Lyons GH, Ortiz-Monasterio I, Stangoulis J, Graham R (2005) Selenium concentration in wheat grain: is there sufficient genotypic variation to use in breeding? Plant Soil 269:369–380
Meharg AA, Norton G, Deacon C, Williams P, Adomako EE, Price A, Zhu YG, Li G, Zhao FJ, McGrath SP, Villada A, Sommella A, Mangala P, De Silva CS, Brammer H, Dasgupta T, Islam MR (2013) Variation in rice cadmium related to human exposure. Environ Sci Technol 47:5613–5618
Mroczek-Zdyrska M, Wójcik M (2012) The influence of selenium on root growth and oxidative stress induced by lead in Vicia faba L. minor plants. Biol Trace Elem Res 147:320–328
Pezzarossa B, Remorini D, Gentile ML, Massai R (2012) Effects of foliar and fruit addition of sodium selenate on selenium accumulation and fruit quality. J Sci Food Agric 92:781–786
Proietti P, Nasini L, Del Buono D, D’Amato R, Tedeschini E, Businelli D (2013) Selenium protects olive (Olea europaea L.) from drought stress. Sci Hortic-Amsterdam 164:165–171
Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241
Saidi I, Chtourou Y, Djebali W (2014) Selenium alleviates cadmium toxicity by preventing oxidative stress in sunflower (Helianthus annuus) seedlings. J Plant Physiol 171:85–91
Tamaoki M, Maruyama-Nakashita A (2017) Molecular mechanisms of selenium responses and resistance in plants. In: Selenium in plants. Springer International Publishing, pp 35–51
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S (2009) Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot 60:2677–2688
Wan YN, Yu Y, Wang Q, Qiao YH, Li HF (2016) Cadmium uptake dynamics and translocation in rice seedling: influence of different forms of selenium. Ecotoxicol Environ Saf 133:127–134
Wang YJ, Dang F, Evans RD, Zhong H, Zhao J, Zhou DM (2016) Mechanistic understanding of MeHg-Se antagonism in soil-rice systems: the key role of antagonism in soil. Sci Rep 6:19477
Whanger PD (2004) Selenium and its relationship to cancer: an update. Br J Nutr 91:11–28
Williams PN, Lombi E, Sun GX, Scheckel K, Zhu YG, Feng XB, Zhu JM, Carey AM, Adomako E, Lawgali Y, Deacon C, Meharg AA (2009) Selenium characterization in the global rice supply chain. Environ Sci Technol 43:6024–6030
Wu ZC, Wang FH, Liu S, Du YQ, Li FR, Du RY, Wen D, Zhao J (2016) Comparative responses to silicon and selenium in relation to cadmium uptake, compartmentation in roots, and xylem transport in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ Exp Bot 131:173–180
Yao XQ, Chu JZ, He XL, Liu BB, Li JM, Yue ZW (2013) Effects of selenium on agronomical characters of winter wheat exposed to enhanced ultraviolet-B. Ecotoxicol Environ Saf 92:320–326
Zhang LH, Hu B, Li W, Che RH, Deng K, Li H, Yu FY, Ling HQ, Li YJ, Chu CC (2013) OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol 201:1183–1191
Zhu YG, Pilon-Smits EAH, Zhao FJ, Williams PN, Meharg AA (2009) Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 14:436–442
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 41601343), Central Public Research Institute Basic Fund for Research and Development (No.2016-szjj-HQQ), and the Funds for Science and Technology Innovation Project from the Chinese Academy of Agricultural Sciences (No. CAAS-XTCX-2016018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Huang, Q., Xu, Y., Liu, Y. et al. Selenium application alters soil cadmium bioavailability and reduces its accumulation in rice grown in Cd-contaminated soil. Environ Sci Pollut Res 25, 31175–31182 (2018). https://doi.org/10.1007/s11356-018-3068-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3068-x