Abstract
Dose-dependent effects of selenium on growth and physiological trait of wheat seedlings (Triticum aestivum L. cv Han NO.7086) exposed to cold stress are reported. Responses of seedlings were different depending on the Se concentration. The treatments with 0.5 and 1.0 mg Se kg−1 significantly increased biomass and chlorophyll content of seedlings. However, the treatments at 2.0 and 3.0 mg Se kg−1 only induced an evident increase in chlorophyll content and did not promote biomass accumulation of seedlings. Antioxidant compounds content (anthocyanins, flavonoids, and phenolic compounds) and antioxidant enzymes’ activities (peroxidase and catalase) increased by different Se treatments, while only the treatment with 1.0 mg Se kg−1 induced a significant reduce in malondialdehyde content and the rate of superoxide radical production of wheat seedlings. The results of this study demonstrated that Se supply could increase antioxidant capacity of seedlings, and optimal Se supply reduced production of free radicals, membrane lipid peroxidation, and promoted biomass accumulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cold injury is a worldwide problem that affects the growth and development of crops. Many crops often encounter cold stress in growing periods, which brings larger loss to agricultural production. In recent years, it often brings up against cold invasion in early spring in China, and the sudden falling of temperature brings serious effects on overwintering plant species. In addition, warm winter caused by global warming leads to faster growth of overwintering plants in winter such as winter wheat, which makes plant more prone to cold weather in spring. Researches have shown that cold stress could induce over production of free radicals and result in oxidative stress eventually [1–3].
Recent researches have identified that Se could not only promote growth and development of plants but also increase resistance and antioxidant capacity of plants, although Se is not considered to be required by higher plants [4–9]. In the previous studies on effects of Se on wheat seedlings subjected to drought and enhanced UV-B radiation, we observed that extra Se supply increased antioxidant enzymes’ activities and reduced malondialdehyde (MDA) content, suggesting that Se can reduce oxidative stress in seedlings subjected to drought and enhanced UV-B stress. However, to our knowledge, there has been no effort to understand the role of Se in plants under cold stress.
Wheat is one of the most important crops in the world, and cold stress in spring has seriously influenced the growth and development of wheat seedlings in many regions. The paper studied the dose-dependent effects of Se supply on growth and some physiological traits of wheat seedlings exposed to cold stress. We hypothesize that Se supply will regulate the responses of wheat seedlings to cold stress, in order to better understand the roles of Se in plants subjected to stressful environment.
Materials and Methods
Plant Material and Experimental Design
The selected seeds of wheat (Triticum aestivum L. cv Han NO. 7086) from Baoding agricultural market were disinfected by immersion in a 2.5% solution of sodium hypochlorite for 5 min and washed five times with distilled water [10]. Seeds were sowed in plastic pots (25 × 20 cm), 50 seeds per pot. The substrate used for growing the seedlings was sieved topsoil from farmland. Thirty seedlings of similar size were saved in each pot after germination, and each treatment had five replicates. Extra Se was added as sodium selenite (Na2SeO3) from 0.5 to 3 mg Se kg−1 soil, and sodium selenite was dissolved in water and mixed with the soil before sowing. The experiment was conducted in the growth chamber with 12/12-h photoperiod, 25°C/18°C (day/night) temperature, and 300-µmol m−2 s−1 light intensity. Cold treatment was performed in the growth chamber at 4°C with a 12/12-h photoperiod and 250-µmol m−2 s−1 light intensity, when seedlings grown with three fully expanded leaves. Leaf samples were collected at 72 h after cold treatment for measurements.
Measurements
Samples from five replicates (pots) were harvested. Roots were rinsed free of soil. The fresh weight was measured immediately after harvest. Values were calculated in gram per pot.
Leaves tissue was ground in 80% acetone for chlorophyll and carotenoids determination. Total chlorophyll and carotenoids contents were determined according to Lichtenthaler [11].
The degree of lipid peroxidation in leaf tissue was assessed by MDA content. MDA content was determined by the thiobarbituric acid (TBA) reaction. Leaves (0.5 g) were homogenized with 5 ml of 20% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 3,500×g for 20 min. To 2 ml of the aliquot of the supernatant, 2 ml of 20% TCA containing 0.5% (w/v) TBA and 100 μl 4% (w/v) butylated hydroxytoluene in ethanol were added. The mixture was heated at 95°C for 30 min and then quickly cooled on ice. The contents were centrifuged at 10,000×g for 15 min, and the absorbance was measured at 532 nm. The value for nonspecific absorption at 600 nm was subtracted. The concentration of MDA was calculated using an extinction coefficient of 155 mM−1 cm−1. Results were expressed as micromoles per gram FW.
The rate of superoxide radical production (O −2 ) was measured as described by Ke et al. [12], by monitoring the nitrite formation from hydroxylamine in the presence of O −2 . Leaves (0.5 g) were homogenized with 1.5 ml of 65 mM potassium phosphate (pH 7.8) and centrifuged at 5,000×g for 10 min. The incubation mixture contained 0.45 ml of 65 mM phosphate buffer (pH 7.8), 0.5 ml of 10 mM hydroxylamine hydrochloride, and 0.5 ml of the supernatant. After incubation at 25°C for 20 min, 8.5 mM sulfanilamide and 3.5 mM α-naphthylamine were added to the incubation mixture. After reaction at 25°C for 20 min, the absorbance in the aqueous solution was read at 530 nm. A standard curve with NO −2 was used to calculate the production rate of O −2 from the chemical reaction of O −2 and hydroxylamine.
Anthocyanins, flavonoids, and phenolic compounds were extracted from leaves in acidified methanol (HCl/methanol, 1:99, v/v). Anthocyanins, flavonoids, and phenolic content were estimated from absorbances at 530,300 and 280 nm, respectively [13, 14]. Results were expressed as absorbance per gram FW (A g−1 FW).
The free proline content was determined according to the method described by Bates et al. [15]. Leaf samples (0.5 g) were homogenized using a pestle and mortar with 5 ml of sulfosalicylic acid (3% w/v). After centrifugation (5 min at 20,000×g), 0.5 ml of the supernatant was incubated at 100°C for 60 min with 0.5 ml of glacial acetic acid and 0.5 ml of ninhydrin reagent. After cooling, 1 ml of toluene was added to the mixture, and the absorbance of the chromophore containing toluene was recorded at 520 nm.
Catalase activity (CAT; EC 1.11.1.6) was determined in the homogenates by measuring the decrease in absorption at 240 nm in a reaction medium containing 50 mM potassium phosphate buffer (pH 7.2), 10 mM H2O2, and 50 μl enzyme extract. The activity was calculated using the extinction coefficient (40 mM−1 cm−1) for H2O2.
Peroxidase activity (POD; EC 1.11.1.7) was based on the determination of guaiacol oxidation (extinction coefficient 26.6 mM−1 cm−1) at 470 nm by H2O2. The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 20.1 mM guaiacol, 12.3 mM H2O2, and enzyme extract in a 3-ml volume.
Soluble protein contents were determined as described by Bradford [16], using bovine serum albumin as a calibration standard.
All data were subjected to an analysis of variance using the Software Statistical Package for the Social Science version 11.0. Individual treatment means were compared with Duncan’s test to determine whether they were significantly different at the 0.05 probability level.
Results
Effects of Se Supply on Biomass Accumulation and Chlorophyll Content
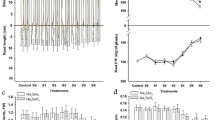
Under cold stress, biomass and chlorophyll content of seedlings were different depending on the Se concentration (Fig. 1). Compared with the control, the treatments at 0.5 and 1.0 mg Se kg−1 significantly promoted biomass accumulation and increased chlorophyll content of seedlings. In addition, the treatments with 2.0 and 3.0 mg Se kg−1 only increased chlorophyll content, and chlorophyll content was the most in seedlings treated with 1.0 mg Se kg−1.
Effects of Se Supply on MDA Content and the Rate of O −2 Production
The treatment at 1.0 mg Se kg−1 induced an evident reduction in MDA content and the rate of O −2 production of seedlings compared with the control (Fig. 2). The lower Se (0.5 mg kg−1) treatment also significantly reduced the rate of O −2 production over the control but did not significantly affect on MDA content.
Effects of Se Supply on Antioxidant Compounds Content
Compared with the control, Se treatments significantly increased anthocyanins, flavonoids, and phenolic compounds content of seedlings subjected to cold stress (Fig. 3). Proline content only increased by the treatment with 1.0 mg Se kg−1. However, Se treatments did not significantly affect on carotenoids content.
Effects of Se Supply on Antioxidant Enzyme Activities
The effects of different Se treatments on POD and CAT activities in seedlings exposed to cold stress were showed in Fig. 4. The activities exhibited similar changes in response to Se supply. Significant increase in POD and CAT activities was observed by different Se treatments over the control.
Discussion
Some reports showed that Se was beneficial for some plants [8, 17, 18] and could increase the tolerance of plants to stressful environment [19–22]. The results of this study demonstrated that the effects of Se on growth of wheat seedlings subjected to cold stress depended on the Se concentration (Fig. 1). Compared with the control, treatments with 0.5 and 1.0 mg Se kg−1 promoted the biomass accumulation of seedlings, and all Se treatments induced an increase in chlorophyll content, and the increased amount in chlorophyll content was the most at 1.0 mg Se kg−1 treatment. The increase in chlorophyll content is beneficial for biomass accumulation of seedlings.
The production of reactive oxygen species (ROS) is the important cause of damage to plants when exposed to environmental stress, which resulted in the growth inhibition, the breakdown of lipid membrane, and oxidative stress [20, 23, 24]. Liang et al. [1] reported that cold damage significantly increased oxidative stress in wheat seedlings, and Liu et al. [25] also reported that low temperature induced an increase in MDA content in wheat seedlings. Our study demonstrated that Se treatments with 1.0 mg kg−1 significantly reduced MDA content and the rate of O −2 production in wheat seedlings grown under cold stress, which indicated that suitable Se treatment reduced membrane lipid peroxidation and oxidative stress in seedlings subjected to stressful condition.
Anthocyanins, flavonoids, phenolic compounds, proline, and carotenoids are some important low-molecular antioxidant compounds in plants [2], which play an important role in stress defense. Flavonoids, anthocyanins, and phenolic compounds have been proven to have the ability to scavenge free radicals and inhibit membrane lipid peroxidation of seedlings [26–28]. In our study, Se treatments significantly increased anthocyanins, flavonoids, and phenolic compounds content (Fig. 3). Proline metabolism is a typical mechanism of biochemical adaptation in living organisms subjected to stress conditions [29], and stress-related alterations in proline metabolism may impinge on systems of redox control of plant gene expression [30], which may protect plant cells against peroxidative processes [31]. Yao et al. [22] and Hawrylak-Nowak [32] reported that Se supply increased proline content in wheat seedlings subjected to drought stress and in cucumber seedlings subjected to salt stress, respectively. From the present results, proline accumulation in seedlings subjected to cold stress depended on Se concentration (Fig. 3). Treatment with 1.0 mg Se kg−1 significantly increased proline content in seedlings. In addition, carotenoids also play a major role in the protection of plants against oxidative stress [33], but carotenoids content did not evidently affect by Se treatments in here. By analysis, we know that the responses of different antioxidant compounds in plants to Se are different depending on plant species and stressful environment.
POD and CAT are important enzymes in plants that protect plants against oxidative damage caused by stressful environment. Kong et al. [20] reported that Se modulates the activities of antioxidant enzymes and promotes the growth of sorrel seedlings under salt stress. In our study, a significant increase in activities of POD and CAT was observed in Se-treated wheat seedlings under cold stress (Fig. 4). The increase in antioxidant enzymes’ activities and antioxidant compounds content probably decreased the toxicity of ROS (Fig. 2). The results of the study indicated that Se could provide an ecological adaptation for young seedlings subjected to stress conditions by the increase in antioxidant compounds content and antioxidant enzymes’ activities.
In conclusions, suitable Se promoted biomass accumulation of seedlings exposed to cold stress. The reasons are probably related to the increase in chlorophyll content and antioxidant capacity and the decrease in production of free radicals and membrane lipid peroxidation.
References
Liang YC, Zhu J, Li ZJ et al (2008) Role of silicon in enhancing resistance to freezing stress in two contrasting winter wheat cultivars. Environ Exper Bot 64:286–294
Radyuk MS, Domanskaya IN, Shcherbakov RA et al (2009) Effect of low above-zero temperature on the content of low-molecular antioxidants and activities of antioxidant enzymes in green barley leaves. Russ J Plant Physiol 56:175–180
Wang Y, Yang ZM, Zhang QF et al (2009) Enhanced chilling tolerance in Zoysia matrella by pre-treatment with salicylic acid, calcium chloride, hydrogen peroxide or 6-benzylaminopurine. Biol Plantarum 53:179–182
Hartikainen H, Xue T (1999) The promotive effect of selenium on plant growth as triggered by ultraviolet radiation. J Environ Qual 28:1372–1375
Hartikainen H, Xue T, Piironen V (2000) Selenium as an antioxidant and pro-oxidant in ryegrass. Plant Soil 225:193–200
Terry N, Zayed AM, de Souza MP et al (2000) Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol 51:401–423
Seppänen M, Turakainen M, Hartikainen H (2003) Selenium effects on oxidative stress in potato. Plant Sci 165:311–319
Djanaguiraman M, Durga D, Shanker AK et al (2005) Selenium—an antioxidative protectant in soybean during senescence. Plant Soil 272:77–86
Yao XQ, Chu JZ, Wang GY (2009) Effects of selenium on wheat seedlings under drought stress. Biol Trace Elem Res 130:283–290
Bakke IA, de Oliveira Freire AL, Bakke OA et al (2006) Water and sodium chloride effects on Minosa Tenuiflora (Willd.) poiret seed germination. Caatinga (Mossoró, Brasil) 19:261–267
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Ke D, Wang A, Sun G et al (2002) The effect of active oxygen on the activity of ACC synthase induced by exogenous IAA. Acta Bot Sin 44:551–556 (in Chinese)
Nogués S, Baker NR (2000) Effects of drought on photosynthesis in Mediterranean plants grown under enhanced UV-B radiation. J Exp Bot 51:1309–1317
Peng CL, Lin ZF, Lin GZ et al (2006) The anti-photooxidation of anthocyanins-rich leaves of a purple rice cultivar. Sci China (C: Life Sci) 36:209–216
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–208
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Xue TL, Hartikainen H, Piironen V et al (2001) Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil 237:555–561
Du ZY, Shi YX, Wang QH (2004) Effects of selenium application on the selenium absorption and transformation of eggplant and its qualities. Plant Nut Fert Sci 10:298–301 (in Chinese with English abstract)
Valkama E, Kivimäenpää M, Hartikainen H et al (2003) The combined effects of enhanced UV-B radiation and selenium on growth, chlorophyll fluorescence and ultrastructure in strawberry (Fragaria × ananassa) and barley (Hordeum vulgare) treated in the field. Agr Forest Meteorol 120:267–278
Kong LG, Wang M, Bi DL (2005) Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress. Plant Growth Regul 45:155–163
Tadina N, Germ M, Kreft I et al (2007) Effects of water deficit and selenium on common buckwheat (Fagopyrum esculentum Moench.) plants. Photosynthetica 45:472–476
Yao XQ, Chu JZ, Wang GY (2009) Effects of drought stress and selenium supply on growth and physiological characteristics of wheat seedlings. Acta Physiol Plant 31:1031–1036
Yao XQ, Liu Q (2007) Changes in photosynthesis and antioxidant defenses of Picea asperata seedlings to enhanced ultraviolet-B and to nitrogen supply. Physiol Plant 129:364–374
Xu H, Biswas DK, Li WD et al (2007) Photosynthesis and yield responses of ozone-polluted winter wheat to drought. Photosynthetica 45:582–588
Liu YY, Li JZ, Chen L et al (2006) Effects of low temperature stress on peroxidation product of membrane lipids and activity of related enzymes in wheat seedling leaves. J Triticeae Crops 26:70–73 (in Chinese with English abstract)
Tusda T, Shiga K, Ohshima K et al (1996) Inhibition of lipid peroxidation and the active oxygen radical scavenging effect of anthocyanin pigments isolated from Phaseolus vulgaris L. Biochem Pharmacol 52:1033–1039
Steyn WJ, Wand SJE, Holcroft DM et al (2002) Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New Phytol 155:349–361
Peng Q, Zhou Q (2009) Antioxidant capacity of flavonoid in soybean seedlings under the joint actions of rare earth element La(III) and ultraviolet-B stress. Biol Trace Elem Res 127:69–80
Delauney AJ, Verma DPS (1993) Proline biosynthesis and osmo-regulation in plants. Plant J 4:215–223
Hare PD, Cress WA (1997) Metabolic implications of stress-induced proline accumulation in plant. Plant Growth Regul 21:79–102
Saradhi PP, Alia SA, Prasad KV (1995) Proline accumulates in plants exposed to UV radiation and protects them against UV induced peroxidation. Biochem Biophys Res Commun 209:1–5
Hawrylak-Nowak B (2009) Beneficial effects of exogenous selenium in cucumber seedlings subjected to salt stress. Biol Trace Element Res. doi:10.1007/s12011-009-8402-1
Singh A (1996) Growth, physiological, and biochemical responses of three tropical legumes to enhanced UV-B radiation. Can J Bot 74:135–139
Acknowledgments
This study was supported by Young Fund, Hebei University (2008Q46), the Open Fund of Key Laboratory of Crop Nutrition and Nutrient Cycling, Ministry of Agriculture of China (2008-1) and Dr. Fund, Hebei University (2007-102), China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chu, J., Yao, X. & Zhang, Z. Responses of Wheat Seedlings to Exogenous Selenium Supply Under Cold Stress. Biol Trace Elem Res 136, 355–363 (2010). https://doi.org/10.1007/s12011-009-8542-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-009-8542-3