Abstract
Cereal grains are the world’s dominant contributors of raw food material and dietary supplements for both humans and livestock. Significant yield losses in cereals are recorded due to abiotic stresses such as extreme temperatures, water availability, ion or physiological pH (salinity and alkalinity), UV radiation, and anoxia/hypoxia in terms of quality and quantity. The fact that abiotic stresses are both multigenic and quantitative complicates understanding how plants respond to them. Plants with improved responses to environmental perturbations are being developed worldwide using physiological, biochemical, and molecular genetic approaches. Further, with the advent of next-generation sequencing technologies, numerous potential abiotic stress-responsive genes in different crop plants have been identified. As a consequence, biologists are focusing their efforts on elucidating the molecular basis of such genes that confer abiotic stress tolerance, as well as investigating the downstream pathways they are involved in. Using genetic engineering technologies including transgenics, functional validation of various target genes involved in different biological processes such as signalling pathways, transcriptional activation/repression, ion homeostasis, and oxidative defence in multiple model systems has been accomplished. Several of these attempts have been made in cereal crops, viz. rice, corn, barley, wheat, sorghum, and millets. The current chapter summarizes the milestones established in the transgenic research unveiling the role of important genes in different abiotic stress responses as well as the transfer of these genes to other cereal crops to help them thrive under such stressful conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Abiotic stress
- Cereals
- Transgenic approach
- Redox
- Ionic balance
- Transcription factor
- Signalling cascade
- Osmotic regulation
9.1 Introduction
The productivity and yield of many agricultural plants, especially grains, are influenced by abiotic stresses. They form the major group of yield-restricting factors for crops (Canter 2018; Zorb et al. 2019). Extreme temperature, drought, salinity, heavy metal stress, anoxia stress, and ultraviolet-B (UV-B) stress, among others, affect the growth of plants and limit crop productivity causing crop failure (Isayenkov and Maathuis 2019; Shikov et al. 2020; Kapoor et al. 2020). Approximately 90% of farmland is subjected to one or more abiotic stress factors (dos Reis et al. 2012). Abiotic stress inflicts 70% of agricultural loss worldwide (Acquaah 2007; Mantri et al. 2012). Studies based on the amalgamation of climate change and crop yield models have anticipated further loss of productivity in major cereals like rice, wheat, and maize causing critical repercussions to total food security (Tigchelaar et al. 2018). Therefore, the development of abiotic stress-resilient crops is important to secure food supply and to establish sustainable agriculture (Fig. 9.1).
Abiotic stresses cause a reduction in biomass production in cereals. For example, high temperature increases the incidence of spikelet sterility and reduces the accumulation of assimilates in rice (Korres et al. 2017). With every 1 °C rise in temperature, there is a 10% loss in wheat yield (Hede et al. 1999). Temperature above 28 °C during anthesis in oat decreases its yield (Hakala et al. 2020). Chilling stress leads to a reduction in germination percentage and emergence efficiency in sorghum, limiting seedling development with stunted root and shoot. It also affects photosynthesis, thereby decreasing dry matter accumulation in sorghum (Ercoli et al. 2004; Chinnusamy et al. 2007; Ortiz et al. 2017). In wheat, drought causes loss of leaf area, kernel abortion, obstruction in the movement of food reserves, and a decrease in the number of amyloplast in grains (Saini and Westgate 1999; Shah and Paulsen 2003). Drought stress during the flowering stage in rice strongly influences physiological characters leading to compromised grain yield (Yang et al. 2019). Salinity causes cell damage and retarded growth in finger millets (Satish et al. 2016). Compared to other cereals, rice is highly susceptible to rhizospheric salinity (Maas and Hoffman 1977; Ashraf 2009; Hussain et al. 2017). Salt stress reduces CO2 fixation, leaf development, leaf cell enlargement, dry mass buildup, and relative growth in rice (Cramer et al. 2001; Khan and Abdullah 2003; Amirjani 2011). Small amounts of heavy metals are required for plant growth and development; however, their excessive amounts can lead to toxicity in plants. For example, arsenic (As) causes a reduction in seed germination, lowering of seedling height, decrease of leaf area, and a reduction of dry matter accumulation in rice (Marin et al. 1993; Abedin et al. 2002). Cadmium (Cd) causes a reduction in seed germination and a decrease in plant nutrient content in wheat (Ahmad et al. 2012; Yourtchi and Bayat 2013). Cd also leads to reduced root and shoot growth in rice and maize (Wang et al. 2007; Ahmad et al. 2012; Roychoudhury et al. 2012; Yourtchi and Bayat 2013). Accumulation of zinc in the leaves of rye causes retardation in growth, decrease in nutrient content, and reduction of photosynthetic energy conversion efficiency (Bonnet et al. 2000). Complete absence of oxygen and UV-B exposure can also affect crop productivity. It was observed that the seeds of barley, wheat, rye, and oat could not synthesize α amylase under anoxia, thus affecting their germination (Perata et al. 1993). Biotechnology provides a plethora of applications in the improvement of cereal crops for abiotic stress tolerance. Although conventional breeding and marker-assisted selection have led to the development of many promising cultivars, transgenic approach has an upper-hand since genes governing important agronomic traits can be sourced from any organism whether plants or microbes and can be introgressed into the plant genome. Conventional breeding of abiotic stress-resistant cereals involves random mixing of thousands of genes together present in both resistant and susceptible varieties, while genetic engineering allows the preferential transfer of desirable genes to susceptible plants and preserve their agronomically important traits. Genetic transformation has been successfully employed to produce many abiotic stress-resilient cereal crops like rice (Sahoo et al. 2012; Ganguly et al. 2020; Shim et al. 2018; El-Esawi and Alayafi 2019), wheat (Zang et al. 2018), and maize (Wei et al. 2011).
9.2 Genetic Engineering Approaches for Plant Abiotic Stress Tolerance
High temperatures, drought, flood, salinity, and heavy metal toxicity are the primary abiotic conditions that hinder growth, culminating in yield declines of up to 70% in vital agricultural crops (Mantri et al. 2012).Traditional breeding, although a strategy of countering such abiotic stress, has several downsides, including the fact that it is a time-consuming procedure that allows for the fertilization of plants of the same or closely related species for hybridization. Genetic engineering technologies to circumvent such barriers are currently in use and one of the significant accomplishments in the generation of biotic/abiotic stress-resilient agricultural crops (Khan et al. 2011). The different genetic transformation techniques are centred on direct and indirect transformation procedures. The gene of interest (GOI) is introduced straightforwardly into the plant genome using direct techniques such as microprojectile bombardment and protoplast transformation or electroporation. In indirect approach, however, either Agrobacterium tumefaciens or Agrobacterium rhizogenes facilitates the transfer of the binary vector harbouring the GOI into the plant genome.
9.2.1 Direct Gene Transfer into Plant Cells
9.2.1.1 Particle Bombardment
Particle bombardment/biolistics, a direct delivery method in which high-velocity microprojectiles are employed to introduce foreign DNA into plant tissues or cells, was developed by John Sandford and co-workers (Sanford et al. 1987). In this technique, the DNA-coated particles (microprojectiles) are accelerated directly into intact cells or tissues. As the microprojectile enters the cells, the transferred DNA dissociates from the particle and integrates into the host genome. Sandford coined the term “biolistic”, which is taken from the words “biological and ballistic”. The most frequently used terms, however, are particle gun or particle bombardment.
In the original gene gun, described by Sanford et al. (1987), a plastic bullet (macrocarrier) loaded with millions of DNA-coated tungsten particles (microcarriers) was placed in front of the 0.22 calibre barrel. When the gunpowder was fired, microcarriers were accelerated towards a stopping disc containing a tiny hole, through which only the microcarriers can pass and reach the target tissue (Sanford 1990; Kikkert 1993). The gunpowder model was effectively utilized in the development of transgenics of different plant species in the majority of genetic transformation protocols. However, the uncontrollable impact of the gunpowder caused physical damage to surrounding cells as well as the target cells, posing a barrier to the generation of stable transformants.
Plant genetic transformation procedure could be outlined briefly as follows: The gene of interest for abiotic stress is isolated from the original source and cloned into a functional construct (plasmid) containing promoters and marker genes. This plasmid is then introduced into the target plant cells using particle bombardment. Transgenic plants are then regenerated from the bombarded cells and further tested for gene expression at the laboratory, greenhouse, and field level. The first stable transformation using biolistics was reported in soybean. Later, maize callus tissues were transformed with the selectable marker gene; neomycin phosphotransferase (NptII) (Klein et al. 1988).
First-generation particle bombardment devices used gunpowder charges; however, improved devices have been developed that include the use of helium gas as a particle propellant for the microparticles through target tissues (Biolistic® PCS-100/He particle delivery system), high-voltage electric discharge to accelerate the gold particles coated with DNA (ACCELLTM particle gun), syringe filter to induce a low-pressure helium burst for acceleration of microcarriers (particle inflow gun), and use of plastic tube that directly accelerates the microcarriers to the target tissues (Helios® gene gun). Helios® gene gun is the most advanced design of particle bombardment (Ibrahim and Kuaybe 2020).
9.2.1.1.1 Biolistic® PDS1000/He Particle Delivery System
Biolistic® gene gun has been commercially available in 1991 (Bio-Rad) which symbolizes technical variants over the gunpowder device. It is an efficient and widely utilized gene transfer method. PDS-1000/He system employs a high-pressure helium (He) gas as a particle propellant for the microparticles through target tissue (Fig. 9.2). The bombardment chamber placed at the top of the device is continuously fed by the gas and held by a “rupture disc”, which is designed to break as microparticles move and pass inside the target cells. The advantage of using this delivery system is that it regulates different bombardment parameters and uniformly distributes microcarriers throughout the target cell, resulting in higher transformation efficiency (Kikkert 1993). In recent years many agriculturally important crops have been transformed using biolistics. Sarangi et al. (2019) bombarded embryogenic calli of a high-yielding scented indica rice variety, Pusa Basmati 1, with AmSOD gene under the control of a ubiquitin promoter against salt stress. Particle bombardment has also been successfully used for plastid transformation, gene silencing, proteolistic, minichromosome delivery, fluorescent dye delivery, and precision genome engineering (Ibrahim and Kuaybe 2020).
9.2.1.2 Protoplast Transformation
Protoplast transformation is based on the use of plant protoplasts (plant cells without a cell wall) in which the naked DNA is treated with polyethylene glycol (PEG) in the presence of divalent cations (usually calcium) that facilitates integration and transformation. The PEG is a polyether compound that has high affinity towards DNA and along with Ca2+ penetrates through the membrane and facilitates transfer of DNA into the target. In this method usually, leaf mesophyll is used to prepare protoplasts. The leaf disc is treated with cellulose and protease enzymes to degrade the cell wall (Sahab et al. 2019). After enzymatic degradation, the protoplast is purified with a mannitol solution. The prepared protoplast is suspended in the solution of PEG/Ca2+ and the DNA solution of concentration 60 μm. The solution of DNA and protoplast is incubated for a brief period of time. The DNA penetrates into the protoplast then enters the nucleus and integrates into the genome. Finally, the protoplast treated with DNA is spread over a suitable medium and the protoplast containing the transferred DNA is isolated by using a marker. Thus, regeneration of the whole plant from the transferred protoplast results in the transgenic (Fig. 9.3). Though the methodology is simple and devoid of costly equipment, it suffers difficulty in the regeneration of plants from a protoplast. The concentration of various chemicals and DNA is standardized based on the target.
9.2.1.3 Electroporation
Electroporation is a transformation technique that applies a high electrical field to facilitate pore formation on the cell membrane as a result of polarity shifts induced by the applied electrical field (alternated or pulsed). When electric field is applied, the hydrophobic lipid bilayer of the cell membrane gets charged which results in the alignment of positive charge on the outer membrane and negative charge towards the inner membrane leading to a pore formation in the cell membrane called hydrophobic pore which mediates insertion of the foreign DNA into the target cells or tissues. The DNA gets aligned to the anode pole and gains entry into the cell by the pore, resulting in the formation of protoplast with the transgene from which transgenic plants can be generated (Fig. 9.4).
An electroporator device is used for electroporation. It is divided into three major components, including (1) a pulse power supply (it houses control units such as electrical pulse settings, field strength, and time), (2) electroporation cuvettes (these are glass cuvettes in which the target cell suspension is transformed and electroporated), and (3) electrodes (electrodes are electrical conductors that facilitate the direct contact between the cell suspension and the electrodes. Upon application of the adjusted electric pulse settings to the cells via electrical conductors and electrodes, a direct contact between cell suspension and electrodes facilitates integration of foreign DNA into the target cells (Bullmann et al. 2015)). The electro-permeabilization of the cell membrane is mediated by different pulse characteristics (including voltage, resistance, and capacitance) and an optimum field strength implicated in transmembrane voltage. During the electro-transformation of nucleic acids, three distinct types of electrical wave pulses (time constant, square wave, and exponential decay) are often applied to electroporators. The two important variables, viz. field strength (kV cm−1) and time constant, distinguish the pulse that is being delivered. These variables can be altered to achieve ideal efficiency in transfection of various cell types (Ibrahim and Kuaybe 2020). The electroporation technique is accurate, generative, swift, extremely effective, and easy to use and results in stable transgenics. Nevertheless, one of its key drawbacks is that the cells may die when subjected to elevated electric voltage.
9.2.2 Indirect Gene Transfer into Plant Cells
9.2.2.1 Agrobacterium-Mediated Genetic Transformation
Agrobacterium tumefaciens is a soil-dwelling gram-negative bacterium that infects plant wound sites. This bacterium triggers crown gall disease in plants by transferring (T)-DNA into target plant cells via the type IV secretion system (T4SS) of bacteria. The oncogenes in the T-DNA region of Ti plasmid can be replaced with the desired gene to perform plant genetic transformation. The Ti plasmid consists of two genetic components—the T-DNA region which is transferred to the host cell and the Vir region that helps in transferring T-DNA into the target host. The border sequences of T-DNA, namely, left border (LB) and right border (RB), are described by the presence of conserved 25 base pair imperfect repeats towards the end of the T-region. The genes that code for auxin, cytokinin, and opine are present in T-DNA. Auxin and cytokinin produce uncontrolled cell division that results in tumour formation and opines act as a nutrient to bacterial growth. The Vir region consists of Vir genes (virulence genes) that code for Vir proteins involved in tumour formation. The Vir protein recognizes the LB and RB sequence and creates nick to produce a single strand of linear T-DNA that gets transferred to the host cell to produce pathogenicity. This natural mechanism was exploited to transfer a gene of interest to the target cell by inserting the sequence to be transferred in place of T-DNA (Christine Desfeux and Bent 2000).
In nature, injured plant tissues exude chemical substances such as high levels of various phenolics, including lignin and flavonoid precursors, which serve as chemotactic agents. These chemicals draw A. tumefaciens to the injured areas and trigger infection in the plants. Plant phenolics directly or indirectly interact with the transmembrane sensory protein VirA/VirG and initiate the signalling pathway which promotes the coding of Vir protein to plant phenols. The peak expression of Vir genes takes place when chromosomal virulence (chvE) gene attaches to monosaccharide released by plants.
The T-DNA is produced by a complex of proteins called the relaxosome protein complex. The protein complex consists of VirD2, VirD1, VirC1, and VirC2 attached to the border sequence of T-DNA. The linear single-strand T-DNA is produced by VirD2 and it remains covalently attached to 5′ end of T-strand (single strand of T-DNA) to produce VirD2-T-DNA nucleoprotein complex. The action of VirD1 is to cleave Vir D2 from superhelical T-border (type I DNA topoisomerase). VirC1 and VirC2 act as T-DNA production enhancer (Meyer et al. 2018). A. tumefaciens adhere to the host cell by two types of binding system—unipolar polysaccharide (UPP)-dependent polar attachment and T pilus-mediated attachment.
For the transport of T-DNA, a pore channel across cell envelope is produced by type IV secretion system (T4SS) (Lacroix and Citovsky 2018) that consists of 11 Vir B proteins and Vir D4 represented as VirB/VirD4 T4SS. The VirD2 T-DNA nucleoprotein complex is transferred via VirB/VirD4 T4SS along with four Vir proteins (VirD5, VirE2, VirE3, and VirF). The VirE2 acts as a single-strand binding protein and protects the complex from host nucleases (Fig. 9.5). The VirD2-T-DNA nucleoprotein complex along with VirE2 coat is called “T-complex” and together with other translocated Vir protein called “super T-complex” (Guo et al. 2019). The integration of T-DNA into the host genome is through the use of the host DNA repair mechanism but it is not proved completely. The T-DNA containing the only exon gets integrated into the actively transcribed region of the host genome. The 3′ end of T-DNA has a promoter of eukaryotes called microhomologies that helps in the expression of T-DNA. The integrated T-DNA shows two types of expression: One is a transient expression in which the gene is transcribed without integration into the genome due to which the gene is not transferred to progeny and the second is a stable transformation, that is, the transferred DNA can be passed to progeny and this is through the integration of the transferred DNA into the host genome. Plant genetic transformation mediated by Agrobacterium tumefaciens is summarized in Fig. 9.6. Transgenic rice with highly tolerant to severe drought stress in both the vegetative and reproductive stages was developed using the AtDREB1A transcription factor through Agrobacterium-mediated transformation (Ravikumar et al. 2014). Recently, success has been achieved in developing in planta transformation methods against abiotic stress (Varalaxmi et al. 2015). An alternative approach, pyramiding of two or more genes through Agrobacterium-mediated transformation, is now in application to enhance the resistance in plants (Ahmad et al. 2010).
9.2.3 RNAi Technology
The RNA interference or RNAi is the mechanism of suppression of gene expression at post-transcriptional or translational level mediated through small non-coding RNAs such as small interfering RNA (siRNA), short hairpin RNA (shRNA), and microRNA (miRNA). This technique has been successfully used for engineering plants for abiotic stress tolerance such as drought, salt, cold, and heat in many important cereal crops. The RNAi technology is not only used for developing abiotic stress tolerance in plants but also to understand the specific function of a gene involved in abiotic stress (Fig. 9.7).
-
(a)
Transgenic approach for siRNA-mediated abiotic stress tolerance
The small non-coding RNAs are the resulting product of double-stranded RNA breaks. The dsRNA is cleaved by the enzyme known as dicer in plants into siRNAs (Kumar et al. 2012).
-
(b)
Transgenic approach for abiotic stress tolerance by regulating stress-responsive miRNAs
miRNAs are small RNAs that regulate gene expression post-transcriptionally. During abiotic stress conditions, the stress-responsive miRNAs genes undergo transcriptions into primiRNAs. The primiRNAs are cleaved into premiRNAs by endonucleases such as Drosha and exported into cytoplasm by Exportin 5 protein. Dicer then cleaves the premiRNAs into miRNAs. The matured miRNAs are then loaded into RISC complex and activate AGO1. The microRNA-RISC complex then complements with target gene mRNA, leading to either degradation or translational repression of the target mRNA.
-
(c)
RNAi-mediated gene regulation for abiotic stress tolerance in major cereals
Overexpression of stress-responsive miRNAs in cereal crops results in abiotic stress tolerance. Many miRNAs have been reported to be abiotic stress responsive. Small RNAs antagonistically work with transcription factors during abiotic stresses. In rice, plants overexpressing miR156 showed negative regulation of SPL9 to increase abiotic stress tolerance such as salinity and drought (Cui et al. 2014). miR166 drives the enlargement of roots in plants overexpressing OsNAC10, resulting in drought tolerance with increased yield (Jeong et al. 2010), whereas the knockdown of miRNA166 in rice conferred drought tolerance by reducing transpiration rate (Zhang et al. 2018). Overexpression of another rice miRNA, Osa-MIR319, resulted in cold tolerance in rice by changing the leaf morphology (Yang et al. 2012). In wheat, nutrition partitioning and grain yield were improved under abiotic stress by decreasing the expression of TaNAM through RNAi (Guttieri et al. 2013). mir169 controlled the expression of transcription factor NUCLEAR FACTOR-Y subunit A coding genes in maize leaves in response to abiotic stress such as drought, abscisic acid, or salt stress (Luan et al. 2015).
Simplified procedure of RNAi in plants against abiotic stress. (a) Plant is susceptible to abiotic stress. (b, c) The RNAi construct is transferred to the plant through direct (gene gun-mediated plant transformation) or indirect (Agrobacterium-mediated transformation) or through the foliar spray of RNA. (d) The transferred RNA is recognized by DICER enzyme and cleaves into 21–25 small nucleotide RNAs known as the siRNA. The siRNA along with the RISC complex degrades the target mRNA. (e, f) The silencing signal is locally and systematically transferred to the entire plant resulting in expected plant tolerance to the said abiotic stress
9.2.4 Genome Editing
Genome editing techniques such as zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats/CRISPR-associated system (CRISPR/Cas) have been utilized for improving various traits such as cold, heat, drought, and salt in cereals. More extensive applications in cereal crop improvement were observed using TALEN and CRISPR/Cas9. Further availability of more advanced variants of CRISPR/Cas such as CRISPR/Cpf1 and Cas9-derived DNA base editors provides possibility of wider application of these techniques.
9.2.4.1 Zinc Finger Nucleases (ZFNs)
ZFNs are sequence-specific nucleases that enable targeted mutation. ZFN is in general zinc finger protein (ZFP) which is fused to the cleavage domain of FokI restriction enzyme (Fig. 9.8a). Target-specific ZFN can be designed by manipulating ZFP. The designed ZFN induces cleavage in the targeted genomic region and repair through either non-homologous end joining (NHEJ) or homology-directed repair (HDR), thereby creating indels or new transgene insertion, respectively. ZFN has not been extensively used for cereal crop improvement against abiotic stress.
9.2.4.2 Transcription Activator-Like Effector Nucleases (TALENs)
TALEN is the next advanced technique of genome editing after ZFN. The basic structural assembly is similar to ZFN where a DNA binding protein domain (TAL) is fused with the cleavage domain of FokI restriction enzyme resulting in targeted double-strand break (Fig. 9.8b). This TAL region is the naturally occurring region unlike ZFN. Manipulating the TAL region of interest which consists of repeat variable diresidues (RVD) enables a target of different sequences for customized nucleases. The application of TALEN technology was not explored for abiotic stress tolerance in cereals.
9.2.4.3 CRISPR-Related Endonuclease Cas9 (CRISPR/Cas9)
Clustered regularly interspaced short palindromic repeats/CRISPR-associated system (CRISPR/Cas) is the most advanced and effective GE technique. Since its discovery, it has been extensively used in various fields including crop improvement due to its ability to edit genes at a precise location. It is an RNA-guided nuclease system and often engineers to modify specific sequences in the target genome (Fig. 9.8). Previously, its use was limited to model plants like Arabidopsis and tobacco; however, it is currently being expanded to cereal crops like as rice, maize, and wheat. Initially, CRISPR/Cas9 system was used to identify the function of a gene by generating mutant lines in cereal crops. Gene-specific targeting of rice genes such as OsPDS, OsMPK2, OsBADH2, OsMPK5, OsMPK2, OsDEP1, OsDERF1, OsPMS3, OsMYB5, OsAOX1a, and OsAnn3 was reported to be involved in various abiotic stresses through CRISPR/Cas technique (Shan et al. 2014; Zhang et al. 2014a, b; Xu et al. 2015; Shen et al. 2017). The mutants of miRNA gene OsMIR528 generated through CRISPR/Cas9 in rice revealed its role as a positive regulator in salt stress (Zhou et al. 2007). CRISPR/Cas9 technique was used for multiple abiotic stress tolerance in rice where the gene drought and salt tolerance (DST) were targeted resulting in moderate tolerance to osmotic stress and salt stress in the seedling stage (Kumar et al. 2020). Another gene, OsRR22, was targeted through CRISPR/Cas9 which conferred salt stress tolerance in rice (Zhang et al. 2019). In maize, drought stress tolerance was achieved with increased crop yield by targeting ARGOS8 through CRISPR/Cas9 (Shi et al. 2017). The guidelines for the GE regulations are still to be designed clearly in India as generation of GE through CRISPR/Cas introduces Cas9 transgene in the target genome. However, Cas9 can be removed in the subsequent generation through self- or back-crossing (Huang et al. 2016).
9.2.5 Cisgenic Approach
The cisgenic approach for gene transfer is genetic manipulation through recombinant DNA technology between the same species or closely related sexually compatible groups of plants. Cisgenic is one of the genetic engineering strategies to increase abiotic stress tolerance in crop plants. The cisgenic approach is considered safer and one of the techniques for food security in future as compared to transgenics crops and conventional breeding. Despite its tremendous potential for crop improvement, GM crops are linked with societal concerns about human health and the environment. Cisgenics overcome the problem such as linkage drag associated with conventional breeding. On the other hand, due to its similar way of selecting gene as classical breeding, cisgenic plants should be treated as classical bred plants. Therefore, cisgenic crops provide a future new generation of genetically modified crops with the possibility of wider acceptance.
9.3 Abiotic Stress Tolerance in Cereal Crops
Plants are incessantly exposed to a range of environmental stressors due to their sessile nature (Suzuki et al. 2014). Nonetheless, there remained a dearth of insights of the mechanisms by which crops uphold production in the face of abiotic stresses until recently. Climate change has a multifaceted impact on the consequences of abiotic stresses, putting agriculture's long-term sustainability and production at risk. Drought, heat, low temperature, and salinity are some of the most significant abiotic stresses that have had a significant impact on agricultural output in recent years (Tardieu and Tuberosa 2010). Numerous techniques have been devised regularly to maximize agricultural output while minimizing pre-harvest and post-harvest losses inflicted by abiotic challenges (Gust et al. 2010). Plants react to multiple stresses through intricate and specialized cellular and molecular mechanisms to prevent loss and increase survival. In response to each stimulus, these mechanisms cause morphological and growth pattern alterations, as well as changes in biochemical and physiological processes.
9.3.1 Drought Stress
Drought, caused by inadequate precipitation or an undersupply of irrigation water, is a significant hazard to plant productivity throughout the globe (Khan et al. 2016). Owing to paucity of water, 33% of the world’s farming land is dry or semi-arid (Rady 2011). Drought stress, in conjunction with other environmental changes, results in a substantial diminution in agricultural production (Huang et al. 2018). Researchers have been studying structural, physiological, biochemical, and molecular underpinnings for more than 20 years in attempt to understand the mechanisms of plants’ drought stress responses. In attempt to discover prospective genes implicated in drought stress resilience, genetic engineering or breeding methodologies are crucial (Mishra et al. 2017; Noman et al. 2017). To uncover potential genes (which might govern a specific trait), several molecular methods such as marker-assisted selection (MAS), genomic selection (GS), and QTL mapping are being used, and CRISPR/Cas9 has been exploited to establish transgenic lines for drought stress tolerance in cereals.
The impacts of drought stress on cereal crops have been discussed in a number of study papers. Drought stress repercussions vary significantly from anatomical to subcellular level and are witnessed at all stages of development, regardless of when the moisture shortage occurs. Drought stress, in general, has a detrimental effect on germination of seeds, resulting in subpar agricultural productivity (Anjum et al. 2011). Drought stress lowers the plant’s cellular water potential and turgor pressure, resulting in higher solute concentration gradient in the cytosol and extracellular matrix. As a consequence of the drop in turgor pressure, cell development is hindered (Lisar et al. 2012). Furthermore, plant wilting is caused by an increased accumulation of abscisic acid (ABA) and compatible osmolytes like proline. At the same time, reactive oxygen species (ROS) such as H2O2 are overproduced. Overproduction of ROS, despite their role as signalling molecules, may inflict significant cellular oxidative damage and photosynthetic slowdown.
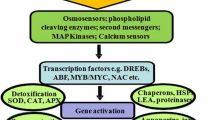
Plant drought stress adaption is a multifaceted phenomenon featuring many genes and signalling networks. In order to adapt to drought conditions, plants have evolved many interwoven networks of signalling pathways to regulate different groups of drought-responsive genes and generate various classes of proteins, such as enzymes, molecular chaperones, TFs, and other functional proteins. These proteins function in a particular manner to aid plants in coping with drought. Hundreds, if not thousands, of genes that modulate plant stress response pathways have been discovered using different functional genomics methods. Single function genes and regulatory genes are two types of genes involved in drought stress responses, based on their biological function (Shinozaki and Yamaguchi-Shinozaki 2007). Osmolyte-accumulating enzymes, reactive oxygen species (ROS) scavengers, ion and water transporters and channels, and lipid biosynthesis enzymes are all coded for by single-function genes (Reguera et al. 2012). Transcription factors (TFs), protein kinases, protein phosphatases, and proteinases are examples of regulatory genes that are involved in signalling pathways and transcriptional or post-transcriptional regulation of gene expression (Shinozaki and Yamaguchi-Shinozaki 2007; Roychoudhury et al. 2008). Additionally, regulatory genes have been shown to play a crucial role in plant drought stress acclimation. As a consequence, changing the transcription of a regulatory gene is more advantageous and is anticipated to be increasingly used in GM crops that can survive abiotic stress (Reguera et al. 2012). Ectopic expression or inhibition of regulatory genes may cause a variety of stress tolerance responses. Furthermore, transcription factors are an important family of proteins that control gene expression at the transcriptional level, enabling plants to endure drought. Multiple transcription factors implicated in drought responses in plants include APETALA2/ethylene-responsive element-binding protein (EREBP), NAM-ATAF1/2-CUC2 (NAC), MYB, basic leucine zipper (bZIP), and zinc finger (Ariel et al. 2007; Ciftci-Yilmaz and Mittler 2008; Fang et al. 2008; Yamaguchi-Shinozaki and Shinozaki 2006; Fang and Xiong 2015). ARBs/ABFs (ABA-responsive element-binding proteins/factors) are bZIP TFs that have been shown to operate in ABA-dependent regulatory pathways during drought stress (Banerjee and Roychoudhury 2017). Employing transgenic strategies such as overexpression and knockdown, many of these genes are being genetically modified (GM) or altered to study their effect on increasing drought resistance in crops. Table 9.1 provides a list of genes that have been transferred to several cereal crops to enhance drought stress tolerance utilizing genetic engineering methods.
9.3.1.1 Rice (Oryza sativa)
As the impacts of drought stress become more pronounced, a greater number of rice genes alter expression, with about 5000 transcripts being upregulated and 6000 transcripts being downregulated (Bin Rahman and Zhang 2016). The vast majority of these genes belong to three categories: those that influence the membrane transport, those that affect signalling, and those that regulate transcription (Upadhyaya and Panda 2019; Kim et al. 2020). During drought conditions, their transcription regulates the majority of biochemical, physiological, and molecular processes in rice (Gupta et al. 2020). Multiple genes/transcription factors have been reported to be differentially expressed in rice and are being incorporated in transgenic plants to significantly improve drought stress responses (Kumar et al. 2017; Upadhyaya and Panda 2019). ABA-independent and also ABA-dependent regulatory mechanisms influence the majority of genes affected by drought (Gupta et al. 2020). OsJAZ1 reduces drought tolerance in rice by regulating ABA signalling, which concocts growth and development of plants under drought stress circumstances (Fu et al. 2017). Various genes associated with osmotic adjustment and late embryogenesis abundant (LEA) proteins have been described as being important in drought stress resilience in rice (Roychoudhury and Nayek 2014). Overexpression of Rab16A (group 2 LEA) gene in transgenic rice developed salinity and drought tolerance (Ganguly et al. 2012, 2020). In GM rice, the gene DRO1 stimulates lengthening of roots and deeper rooting (Uga et al. 2013). Certain genes, like EcNAC67 and OsPYL/RCAR5, slow down leaf rolling and increase root and shoot volume in water-stressed rice (Kim et al. 2014a, b; Rahman et al. 2016). Overexpression of OsDREB1F, CYP735A, and OsDREB2B improves root architectural plasticity in rice plants during drought stress (Kim et al. 2020). DREB2-like gene OsDRAP1 imparts drought resilience in rice, according to Huang et al. (2018). Enhanced grain production in rice during the episodes of water scarcity is critical, and it may be accomplished through transgenic strategies involving the incorporation of genes like OsWRKY47 (Raineri et al. 2015), OsbZIP71 (Liu et al. 2014), OsbZIP46 (Tang et al. 2012), OsNAC10 (Jeong et al. 2010), OsLEA3-1 (Xiao et al. 2007), and OsNAC5 (Hu et al. 2006). Introgression of genes like EDT1/HDG11 (Yu et al. 2013), AtDREB1A (Ravikumar et al. 2014), OsMIOX (Duan et al. 2012), and OsTPS1 (Duan et al. 2012) results in relatively high water use efficiency, cumulation of osmolytes, elevated antioxidant enzymatic activity, and augmented photosynthetic activity in transgenic rice (Li et al. 2011). In transgenic plants, OsCPK9 enhances drought stress tolerance via improving stomatal conductance and maintaining osmotic pressure (Wei et al. 2014). Transgenic plants overexpressing OsDREB2A have been shown to thrive better in conditions of severe drought and salinity (Cui et al. 2011). CDPK7 and CIPK03/CIPK12 modulate a number of regulatory enzymes, signalling pathways, and protein kinases in rice (Xiang et al. 2007). The WRKY proteins perform key functions in plant growth by responding to drought stress (Sahebi et al. 2018). Multiple genes are being studied in the laboratory or under glass house settings to understand if they may impart drought tolerance in rice. Nevertheless, prior to actually integrating such genetic traits in molecular breeding approaches, they should indeed be validated in the field.
9.3.1.2 Wheat (Triticum aestivum)
The diminution of resistance genes induced by genetic deterioration must be compensated for by using such efficient and consistent methods, which can transfer genes in a short span of time. It is worth noting that recombinant DNA technology has emerged as a potent tool for accomplishing this objective. It also has the advantage of stripping away genetic barriers, enabling genes from any wild relatives, land race, or other species to be introduced in the target species (Hussain 2015). TFs that control the primary enzymes of signal transduction pathways; genes that produce defensive chemicals including reactive oxygen species (ROS), proline, JA, and SA; and genes that manufacture defence protein are indeed strong candidates for dehydration stress resistance in wheat (Yang et al. 2010). It has been demonstrated that dehydration-responsive element-binding (DREB) transcription factors improve drought resilience in GM wheat (Saint Pierre et al. 2012). Using the RD29A and ubiquitin promoters, a DREB gene (GmDREB) from soybean (Glycine max) was particle bombarded into wheat, and transgenic plants with both promoters exhibited improved drought and salinity stress tolerance (Shiqing et al. 2005). Such heightened drought resilience has been ascribed to twice as much proline synthesis, the stay-green phenomena during drought, and survival and recovery on re-watering (SURV) following a dry period (Wang et al. 2006), implying a role for the signal transduction cascade downstream of proline biosynthesis. Owing to enhanced accumulation of soluble sugars and photosynthetic pigments (chlorophyll) in leaves, wheat engineered with a cotton-derived DREB (GhDREB) enhanced drought, salt, and cold tolerance (Gao et al. 2009). In greenhouse conditions, genetically engineered wheat plants containing the DREB1A gene showed substantially greater tolerance to dehydration stress than wild type, as indicated by slower wilting and leaf discolouration when water was discontinued (Pellegrineschi et al. 2004). HDG11, an HDZip IV transcription factor, was identified to improve drought tolerance by enhancing the expression of a multitude of drought-responsive proteins, notably genes implicated in calcium signalling pathway and the ABA biosynthesis (Cao et al. 2009). Following 30 days of exposure to drought stress environments, transgenic wheat overexpressing the AtHDG11 gene was analysed. Not only did transgenic plants overexpressing AtHDG11 gene improve physiological parameters, but they also significantly increased production, had a reduced water loss rate and stomatal density, and synthesized more proline.
Many crops have been genetically engineered with osmolytes including trehalose, mannitol, and glycine betaine to strengthen abiotic stress tolerance by stabilizing essential macromolecules. Proline, which is produced under stress, protects biomolecules from denaturation, helps reduce oxidative stress by scavenging ROS, and modulates cytosolic activity (Hayat et al. 2012). Drought tolerance has been documented in transgenic wheat plants overexpressing proline biosynthesis genes (Sawahel and Hassan 2002). GM wheat has been developed in yet another research by incorporating the betA gene, which encodes choline dehydrogenase, under the control of maize ubiquitin promoter (He et al. 2011). Although wheat crops do not generally cumulate mannitol, transgenic wheat developed via overexpressing mtlD gene transferred from the bacteria Escherichia coli cumulated it (Abebe et al. 2003). Drought, salt, and ABA stimulate late embryogenesis abundant (LEA) proteins, which are hydrophilic in nature. In barley, one of these proteins is called late embryogenesis abundant 3 (LEA3), which is transcribed by the Hordeum vulgare abundant protein 1 (HVA1) gene. GM crops that have the HVA1 gene and have been treated with ABA exhibited increased dehydration and salinity stress tolerance (Nguyen and Sticklen 2013). Genetic engineering approaches have been extensively utilized for introduction/incorporation of multiple genes including SNAC1, TaFER-5B, PEPC (phosphoenolpyruvate carboxykinase), and PPDK (pyruvate orthophosphate dikinase), AtOTS1 (OVERLY TOLERANT TO SALT-1) and TaPEPKR2 (phosphoenolpyruvate carboxylase kinase-related kinases) into transgenic wheat which showed increased tolerance to drought stress environments (Saad et al. 2013; Zang et al. 2017; Zhang et al. 2014a, b; Le Roux et al. 2019; Krugman et al. 2010).
9.3.1.3 Barley (Hordeum vulgare)
The prime end goal of genetic modification is to generate drought-tolerant plants with a single or many desirable traits that would be transmitted down through generations. Overexpression of TaDREB2 and TaDREB3 in barley transgenic lines, for example, improved drought tolerance by safeguarding cells from dehydration and damage (Morran et al. 2011). The HvP5CS gene, which encodes delta-1-pyrroline-5-carboxylate synthase (P5CS), has previously been cloned in barley as the principal drought-tolerant gene (Abu-Romman et al. 2011). The relative expression profile of HvP5CS gene was examined in the transgenic plants using semi-quantitative reverse transcription-PCR assay following drought, salinity stress, and abscisic acid (ABA) treatments. HvP5CS expression was shown to be elevated in leaf tissue following exposure to drought and salt stress, and this expression was greater in both scenarios than it was in response to just ABA treatment. Additionally, overexpression of HvSNAC1 in barley improved drought resistance and tolerance to other biotic stressors, like fungal infection of Ramularia collo-cygni, as per another study (Al Abdallat et al. 2014).
9.3.1.4 Maize (Zea mays)
Maize is one of the most essential crops; however, escalating drought stress across the globe is putting its production at risk. Regardless of the fact that there have been relatively few reports of drought-tolerant transgenic maize to date, research conducted in the private sector, in particular, seem to be very compelling. The very first drought-resilient GM crop is MON 87460, a maize (Zea mays L.) variety engineered by Monsanto Company in 2009 and reportedly planted in the United States in 2013. From then on, plantings have grown 5.5-fold, from 50,000 ha in 2013 to 275,000 ha in 2014 (http://www.isaaa.org/resources/publications/briefs/49/toptenfacts/default.asp). Drought tolerance is imparted by the inclusion of cold shock protein B (CSPB) from Bacillus subtilis in transgenic maize variety MON 87460. Furthermore, increased expression of a Nicotiana protein kinase (NPK1) gene with a potential function in oxidative stress management was shown to improve photosynthesis process and hence drought stress resilience in GM maize (Shou et al. 2004a, b). Overexpression of maize transcription factor, ZmNF-YB2, improved dehydration stress tolerance and productivity under drought conditions, as per a Monsanto Company study (Nelson et al. 2007). Virlouvet et al. (2011) showed that under both well-watered and drought-stressed circumstances in the field, overexpressing the ZmASR1 is manifested in higher dry leaf mass and total chlorophyll content and also enhanced maize kernel production. The study of Li et al. (2008) showed that integration of TsVP gene (vacuolar H+-pyrophosphatase (V-H+-PPase) from the dicotyledonous halophyte (Thellungiella halophila) into the maize (Z. mays L.) crop resulted in enhanced drought resistance of genetically engineered plants. In yet another genetic modification method, bacterial RNA chaperones imparted dehydration stress resilience in maize plants (Castiglioni et al. 2008). Wang et al. (2016) demonstrated that GM maize with ZmVPP1 gene overexpression is more drought resilient than untransformed counterparts.
9.3.1.5 Sorghum (Sorghum bicolor L.)
In response to environmental stress, sorghum exhibits physiological changes such as a deeper and more pervasive root system, leaf curling, and reduced stomatal conductance, as well as decreased metabolic activity, allowing it to withstand drought conditions (Schittenhelm and Schroetter 2014). As a result, sorghum is significantly tolerant to drought compared to other cereal crops. There have been barely a few reports of transgene introduction in sorghum to generate drought-tolerant cultivars. For instance, to initiate the mannitol biosynthetic cycle, the mtlD gene from E. coli, which encodes mannitol-1-phosphate dehydrogenase, was introduced into S. bicolor L. Moench cv. SPV462. Leaf samples from GM sorghum exhibited enhanced leaf water potential when challenged to polyethylene glycol 8000 (−2.0 MPa) and 1.7- to 2.8-fold greater shoot and root multiplication when subjected to NaCl stress (200 mmol/L) as compared to control plants (Maheswari et al. 2010). Nevertheless, a variety of drought stress tolerant genes originating from sorghum have been introduced to other model and agricultural crops. SbER2-1, a leucine-rich repeat-receptor-like kinase gene from sorghum (Sorghum bicolor L.), has been found to improve drought tolerance in maize (Li et al. 2019). Analogously, overexpression of SbSNAC1, a sorghum NAC transcription factor, conferred drought tolerance in transgenic Arabidopsis thaliana (Lu et al. 2013a, b). Furthermore, utilizing a population from BT × 642 and RT × 7000, four QTLs (stg1, stg 2, stg 3, and stg 4) for the stay-green phenotype were identified in sorghum. Under drought stress environments, RT × 7000 NILs with the stg2 gene from BT × 642 had greater green leaf area than other NILs, as per physiological analysis (Harris et al. 2007).
9.3.1.6 Pearl Millet (Pennisetum glaucum)
There have been no published studies on the establishment of a GM pearl millet variety that is resistant to abiotic stress. As a result, despite its economic importance, the development of GM pearl millet cultivars is still in its early stages, necessitating a concerted effort to produce and evaluate transgenic lines under a range of stress conditions (Shivhare and Lata 2017).
9.3.2 Salinity Stress
Cereals are cultivated in essentially every region of the world, and they are exposed to a multitude of environmental stressors that obstruct their growth and productivity. One of the most detrimental abiotic threats cereal crops face is salinity. Food security requires the deployment of countermeasures to this challenge. Plant scientists have leveraged a variety of approaches for improving crop yields on salt-affected soils. In the development of salt stress adaptable agricultural crops, traditional breeding, genetic engineering, marker-assisted selection, and, lately, genome editing have all proven effective. Improvements in salinity tolerance have been observed in rice and wheat, but there are few good instances for other cereals. Challenges to the generation of salt-tolerant cultivars/lines have been reported as a dearth of understanding regarding crop genetic structure, biochemical and physiological characteristics, vast diversity in environmental circumstances, and the intricate polygenic nature of the salinity tolerance trait. The application of transgenic methods to create salt-tolerant grain crops is discussed further below. A list of genes that have been incorporated to various cereal crops to enhance salt stress tolerance utilizing genetic engineering methods is shown in Table 9.1.
9.3.2.1 Rice (O. sativa)
In the large portion of studies, scientists introduced genes for vacuolar Na+/H+ transporters from various sources into rice and observed increased salt stress resilience (Zhao et al. 2006; Verma et al. 2007). The introduction of the vacuolar Na+/H+ antiporter AgNHX1 gene from Atriplex gmelinii, for instance, contributed in an eight-fold increase in the activities of the vacuolar-type Na+/H+ antiporter, improving rice seedling survivability from 50% to 100% under salinity environments (Ohta et al. 2002). PgNHX1 from Pennisetum glaucum (L.) R. Br., another vacuolar Na+/H+ antiporter gene, demonstrated expansive root architecture in GM rice seedlings and exhibited approximately 81% greater shoot and root lengths compared to untransformed control seedlings (Verma et al. 2007). Analogously, in salinity environments, GM rice seedlings with the yeast Na+/H+ antiporter SOD2 gene thrived effectively (Zhao et al. 2006). The second important approach that has been employed is genetic transformation to produce higher concentrations of compatible solutes. Proline over-accrual is also regarded as a considerable selection criterion for salinity tolerance. The P5CS, an essential proline biosynthesis intermediate gene isolated from moth bean (Vigna aconitifolia), was incorporated into rice, resulting in GM rice with higher proline contents than wild types in both control and salinity environments (Su and Wu 2004). Trehalose overproduction is another essential feature of salt-tolerant plants. Transgenic rice bearing the yeast trehalose gene (TPS1) exhibited improved salt tolerance as well as dehydration and cold stress tolerance (Garg et al. 2002). Furthermore, the chimeric gene Ubi1::TPSP in another GM rice variety led to significant concentrations of trehalose, thus providing salinity tolerance (Jang et al. 2003). The salt tolerance potential of GM rice lines was improved by overexpression of wheat TaSTRG in rice (Zhou et al. 2009). Recently, the A. thaliana AtDREB1A TF was introduced into rice under the control of two distinct promoters, CaMV35S and Lip9. The resulting transgenic plants were exposed to both normal and drought-stressed conditions. Plants with the Lip9-DREB1A and 35S-DREB1A genes were more fertile than wild-type plants; however, when salt stress was applied, Lip9-DREB1A plants outperformed 35S-DREB1A plants in terms of grain production (Hussain et al. 2018).
9.3.2.2 Wheat (T. aestivum)
On moderately saline soils, wheat productivity losses are significant (Shahbaz et al. 2012), and growth is considerably retarded (Perveen et al. 2012). GM crops, generated with the use of a range of genetic engineering techniques, have been described as a speedy way to produce crops that are resistant to unfavourable environmental conditions. Despite the fact that few experiments on the efficacy of stress-tolerant GM plants in natural settings have been undertaken, scientists are holding out hope to make headway in this area (Ashraf and Akram 2009). Salt tolerance is a multifaceted characteristic governed by a multitude of quantitative traits. Researchers have attempted to introduce specific genes to generate resistant GM varieties/lines of wheat to enhance salinity tolerance. For example, in wheat, higher expression of the vacuolar Na+/H+ antiporter gene AtNHX1 (from A. thaliana) boosted germination, biomass production, and productivity (Xue et al. 2004). Upon being exposed to salinity stress, the transgenic lines accumulated less Na+ in the leaves and more K+ in the leaves and had a 68% greater shoot dry mass and a 26% higher root dry weight than control plants (Xue et al. 2004). Bread wheat has a greater Na+ ion exemption capacity than durum wheat, which enhances its salinity tolerance capability. The TmNax2 locus from T. monococcum was transferred into durum wheat, which escalated salinity tolerance and production by up to 25% compared to control plants grown in high salinity soil (Munns et al. 2012). In wheat, high-affinity K+ transporters (HKTs) play a pivotal part in regulating Na+ accumulation (Munns and Gilliham 2015). Salinity tolerance was bolstered in GM wheat and Arabidopsis lines with high expression levels of TaAOC1, an allene oxide cyclase implicated in the α-linolenic acid metabolism pathway (Zhao et al. 2014). Indeed, genetically engineered wheat genotypes with higher TaCHP (a zinc finger protein) expression levels are more salt tolerant than the salinity-sensitive cultivar Jinan 177 (Li et al. 2010a, b). In genetically engineered wheat varieties, with higher expression level of TaOPR1, a 12-oxo-phytodienoic acid reductase, improves salinity stress tolerance (Dong et al. 2013).
9.3.2.3 Barley (H. vulgare)
Barley is an excellent model crop plant for understanding the physiological and molecular mechanisms underpinning salt stress tolerance in crop plants, because it is one of the most resistant crops when it comes to salt stress. Remarkably, there are few instances of transgenes being incorporated into barley to strengthen salt stress endurance. Several barley-derived genes, on the other hand, have been introduced into a variety of transgenic crops with the purpose of investigating salt stress resistance. Environmental stress tolerance in barley has stirred up interest in uncovering stress-responsive genes, which might be achieved using a number of omics methods, such as comparative genomics and genetic transformation to express stress-responsive genes (Gürel et al. 2016). Numerous barley transcription factors, including HvWRKY38 (Xiong et al. 2010), HvCBF4 (Oh et al. 2007), and HvDREB1 (Xu et al. 2009), are being constitutively overexpressed in transgenic plants, resulting in a significant increase in drought and salt stress tolerance, perhaps by modulating the transcription levels of stress tolerance genes, which have a greater affinity for DNA (Gürel et al. 2016). In addition, it has been shown that introducing the Arabidopsis vacuolar H+-pyrophosphatase gene (AVP1) into genetically engineered barley enhances shoot biomass and grain production under salinity stress conditions (Schilling et al. 2014). AVP1 gene is primarily engrossed in the production of compatible solutes (like glycine betaine and proline) and also enzymes implicated in the elimination of ROS, all of which contribute to improved crop salt stress tolerance (Roy et al. 2014). Similarly, it has been demonstrated that calcineurin-B-like interacting protein kinases (CIPK) are crucial in the modulation of Na+ buildup in Arabidopsis shoots. The A. thaliana CIPK gene has been overexpressed in barley, which resulted in decreased accumulation of Na+ ion in shoot and increased biomass yield under salt stress (Roy et al. 2013).
9.3.2.4 Maize (Z. mays)
Maize is grown under a variety of environmental situations. Genetic engineering has been used effectively in maize to incorporate salt stress-tolerant/stress-resistant genes from multiple sources. For instance, Yin et al. (2004) incorporated the vacuolar Na+/H+ antiporter AtNHX1 gene from A. thaliana into Z. mays, and the putative transgenic seedlings demonstrated significantly increased germination efficiency (80%) under salinity conditions (0.5% NaCl) than parental wild-type plants (13–57%). Li et al. (2010a, b) found that abolishing the als genes in AtNHX1 transgenic maize (marker-free transgenic maize) resulted in surprisingly high salinity tolerance, as evidenced by increased grain production, and boosted many important physiological features when compared to their respective control parental plants. Chen et al. (2007) had previously succeeded in developing salt-resilient maize by transferring the rice Na+/H+ antiporter gene (OsNHX1) into maize. When compared to non-engineered control plants, the GM line produced more biomass at a salt concentration of 200 mM. BADH gene originating from Suaedaliaotungensis Kitag and Atriplex micrantha has been incorporated into the maize genome through the pollen tube pathway and under the control of the maize ubiquitin promoter, separately, and conferred salinity stress tolerance (Wu et al. 2008; Di et al. 2015). Beltagi (2008) reported that GM maize containing a gene from Bacillus thuringiensis (Bt Corn) exhibited improved salt stress tolerance when subjected to varying concentrations of NaCl (50 mM, 100 mM, and 150 mM). The chlorophyll a concentration and chlorophyll stability index (CSI) of Bt transgenic maize were shown to be unaltered when compared to non-transgenic control plants.
9.3.2.5 Sorghum (S. bicolor L.)
Owing to the slow pace of advancement in developing transformation methodologies, the literature on genetically engineered sorghum to strengthen saline stress tolerance is limited (Shahbaz and Ashraf 2013). Primarily due to tissue culture constraints, a dearth of reference genomes, poor regeneration efficiency, and the difficulty to maintain regenerated plantlets via sub-cultures, progress in developing transgenic sorghum has lagged behind that of other cereals (Madhusudhana et al. 2015). Nevertheless, genetic modification has presented modest achievement in generating salt-tolerant sorghum. The mtlD gene, which encodes mannitol-1-phosphate dehydrogenase from E. coli, was introduced into the sorghum cv. SPV462 with the objectives of increasing tolerance to salt stress conditions (Maheswari et al. 2010). When exposed to NaCl stress (200 mmol L−1), the mtlD gene harbouring transgenic plants showed a 1.7–2.8-fold increase in root and shoot growth, respectively, compared to non-transgenic controls, exemplifying that integrating the mannitol biosynthetic process into sorghum can bestow elevated salt stress tolerance. SbDREB2, a dehydration response element-binding (DREB) transcription factor gene from sorghum, was identified by Bihani et al. (2011) and was found to be activated particularly during drought/salt stress.
9.3.2.6 Pearl Millet (P. glaucum)
Pearl millet (P. glaucum) is a staple grain and the world’s sixth most commonly cultivated cereal grain. It is acclaimed for its remarkable abiotic stress resistance and nutritional composition. Shinde and colleagues identified PgNAC21 TF as a potential salt stress-responsive candidate gene in P. glaucum. Plants with enhanced levels of expression of the PgNAC21 TF exhibited a greater germination rate, fresh weight, and root length under salt stress than their non-transgenic counterparts (Shinde et al. 2019). Higher levels of PgNAC21 TF expression in Arabidopsis plants enhanced transcription of stress-responsive genes like GSTF6 (GLUTATHIONE-S-TRANSFERASE 6), COR47 (COLD-REGULATED 47), and RD20 (RESPONSIVE TO DEHYDRATION 20). PgNAC21 functions as a stress-responsive NAC TF, according to Shinde et al. (2019), and may be utilized in GM techniques to improve salinity stress tolerance in agricultural plants.
9.3.3 Heat Stress
While traditional plant breeding methods and popular molecular biological strategies, such as molecular markers and genetic modification, have helped to functionally validate and/or revitalize plants with improved heat stress tolerance, documentation on the genetic core principle of heat stress tolerance is largely lacking. Transcriptional activation of specific proteins has been shown to enhance high temperature tolerance. Several GM plants with different degrees of heat stress tolerance are being developed, alongside scientific knowledge of the expression of heat shock proteins (HSPs)/chaperones and the manipulation of HSF expression patterns. Nonetheless, in comparison to experiments aimed at reconfiguring drought, salt, or cold stress tolerance, such investigations have been limited (Hemantaranjan et al. 2018). Table 9.1 shows a list of transgenes introduced into various cereal crops for heat stress tolerance.
9.3.3.1 Rice (O. sativa)
A substantial number of genes are involved in the synthesis of heat shock proteins (HSPs), which are activated by high temperature and play an essential role in heat stress recovery (Liu et al. 2007). Engineering of HSPs in GM plants has the ability to enhance temperature stress resilience and has a major effect on rice’s intrinsic genetic potential (Zou et al. 2011). Rice varietal reinforcement for thermotolerance via genetic engineering is a prospective methodology for rice production under undulating environmental circumstances (Zou et al. 2011). Nevertheless, high temperature tolerance in transgenic rice has been reported in just a few instances. Heightened thermotolerance has indeed been witnessed in engineered plants constitutively expressing HSPs. The significance of HSPs in conferring heat stress tolerance was substantiated by empirical observations from mutants and genetically engineered species (Queitsch et al. 2000). The cDNA of A. thaliana HSP101 (AtHSP101) was adeptly integrated into the indica rice variety Pusa basmati-1 (Katiyar-Agarwal et al. 2003). In terms of sustenance and development at high temperatures, T2 generation GM lines outperformed non-transformed plants. Analogously, the GM rice variety ‘Hoshinoyume’ that overexpresses HSP (sHSP17.7) exhibited enhanced thermal stress tolerance (Murakami et al. 2004). High temperature resilience has been seen in transgenic rice harbouring the FAD7 (dienoic fatty acid) gene, which enhanced growth rate and chlorophyll pigment concentration (Sohn and Back 2007). Thermal resistance was bolstered in GM rice plants with higher levels of expression of the mtHsp70 (mitochondrial gene), as evidenced by diminished programmed cell death and significantly lower ROS generation (Qi et al. 2011).
9.3.3.2 Wheat (T. aestivum)
Elevated temperature tolerance may be improved through genetic engineering, which can aid in mitigating the negative consequences of thermal stress (Chapman et al. 2012). It involves introducing beneficial traits into target plants in order to strengthen the plants’ adaptability to heat stress (Zheng et al. 2012). However, wheat genetic engineering efforts are difficult due to the complexity of the genomic architecture. Persistent heat stress increases elongation factor (EF-Tu) protein synthesis in chloroplasts, which is related to wheat’s resistance to severe temperatures. Overexpression of EF-Tu in GM wheat reduced leaf proteins from thermal agglomeration, reduced thylakoid membrane disintegration, enhanced photosynthetic efficiency, and safeguarded the plant from devastating microbial invasion (Fu et al. 2012). Wheat cultivars having higher EF-Tu levels tolerated temperature distress better than those with lower EF-Tu levels (Ristic et al. 2008). Overexpression of the maize phosphoenolpyruvate carboxylase gene (ZmPEPC) in wheat increased photocatalytic and antioxidant enzymatic activities, induced photosynthesis-related gene expression, slowed chlorophyll degeneration, altered proline and other metabolite concentrations, and improved heat tolerance (Qi et al. 2017). Plants that synthesize more glycine betaine after being transformed with the BADH gene have been suggested as a potential method for improving plant heat tolerance (Yang et al. 2005). Better osmotic adjustments and an improved antioxidative defence response have been shown to augment heat tolerance in GM wheat line T6 due to glycine-betaine overproduction (Wang et al. 2010).
9.3.3.3 Barley (H. vulgare)
One of the most significant abiotic stress factors influencing the production capacity of temperate cereal crops like barley is temperature. Certain heat shock factors (HSFs) and heat shock response regulators (HSRs) stimulate heat-responsive gene transcription in plants, enabling them to tolerate thermal stress. A recent study has shown that overexpression of a wheat heat shock factor (TaHSFA6b) in barley is linked with heat stress resilience (Poonia et al. 2020). Aside from incorporating genes from other origins into barley to strengthen heat stress tolerance through transgenic approach, a handful of H. vulgare genes have indeed been studied in various model plants, including tobacco. Higher levels of HvSHN1 [APETAL2/Ethylene Responsive Factor (AP2/ERF) family] expression in GM tobacco plants under the regulation of the 35S promoter bolstered resilience to salt, water, and heat stress (Djemal and Khoudi 2021). The resulting transgenic lines exhibited altered cuticle permeability and reduced stomatal density. HvSHN1 transgenic lines had enhanced catalase (CAT) and superoxide dismutase (SOD) activity and decreased MDA and H2O2 levels when challenged to thermal stress compared to non-transformed control plants (Djemal and Khoudi 2021). Increased expression levels of the HvAPX1 (ascorbate peroxidase 1) gene from barley elevated high temperature tolerance in A. thaliana (Shi et al. 2001).
9.3.3.4 Maize (Z. mays)
Numerous strategies have been undertaken to generate heat-tolerant transgenic maize by modulating (upregulation/downregulation) known pertinent genes obtained from various crop species, including maize. For instance, the overexpression of OsMYB55 in GM maize resulted in the upregulation of stress-responsive genes and improves heat and drought tolerance (Casaretto et al. 2016). Furthermore, a variety of maize-derived genes were tested using a transgenic approach by expressing them in various model plants to verify their potential role in heat stress endurance. When subjected to drought stress conditions, increased levels of ZmWRKY106 expression drastically enhanced drought and heat resilience in GM A. thaliana by inducing stress-related genes via the ABA signalling cascade and curbing ROS generation, which was accompanied by increases in SOD, peroxidase (POD), and CAT activities (Wang et al. 2018). Analogously, compared to untransformed plants, overexpression of ZmHSP16.9 in transgenic tobacco imparted heat and oxidative stress tolerance, as shown by higher seed germination rate, root length, and antioxidant enzyme activities (Sun et al. 2012).
9.3.3.5 Sorghum (S. bicolor L.)
Grain sorghum tolerates not only droughts but also severe temperatures better than other crops. Temperature stress has been shown to have a negative impact on sorghum reproductive development. However, at the time of drafting this book chapter, we have been unable to pinpoint any published literatures describing the use of genetic engineering techniques for the introgression of genes from other species into grain sorghum in order to enhance thermotolerance.
9.3.3.6 Finger Millet (Eleusine coracana L.)
Agricultural production is diminished as a consequence of the occurrence of numerous unfavourable circumstances as a result of global climate change. The identification and eventual transfer of genes from stress-tolerant plant species to cultivated cultivars may aid in agricultural production stabilization and improvement. Finger millet (E. coracana L.), a climate-resilient crop, could be a potential source of novel stress-tolerant cereal genes. Ramakrishna et al. (2018) reported EcbZIP17, a novel endoplasmic reticulum (ER) membrane-anchored bZIP transcription factor from finger millet. Transgenic tobacco plants overexpressing this gene exhibited greater vegetative growth and seed production than wild-type (WT) plants under optimal growth conditions, indicating activation of brassinosteroid signalling genes. The engineered plants outperformed WT plants in terms of germination rate, biomass production, primary and secondary root growth, and recovery rate when subjected to abiotic stressors such as water deprivation, heat stress, 400 mM mannitol, 250 mM NaCl, and 10% PEG6000 (Ramakrishna et al. 2018). In a similar line, EcDREB2A gene expression in transgenic tobacco increased resilience to heat stress 42 °C lasting up to 7 days by altering physiology and biochemical processes (Singh et al. 2021). EcDREB2A transgenics exposed to heat stress had higher stomatal conductance, chlorophyll and carotenoids levels, and other photosynthetic indices than WT plants (Singh et al. 2021).
9.3.4 Cold Stress
Plants’ gene expression patterns and protein product levels change significantly when they are exposed to cold temperatures. Non-freezing cold damages or kills a variety of tropical and subtropical plant species, including cereals, with symptoms such as discolouration, necrosis, and reduced development. Plants that are cold tolerant, on the other hand, may flourish in such conditions. Traditional breeding methods, such as inter-specific or inter-generic hybridization, have made little headway in improving the cold tolerance of important agricultural crops. Researchers have gained a better grasp of the complex mechanism that occurs under cold stress, thanks to recent study that combines whole genome profiling/sequencing, mutagenesis, and analyses of GM plants. When challenged to freezing conditions, changes in gene expression are accompanied by increases in the synthesis of hundreds of compounds (metabolites), the most of which have been shown to defend against the negative effects of cold stress. A number of low temperature inducible genes have been uncovered in plants. The bulk of the genes involved in cold stress endurance have been revealed, and many of them are controlled by transcription factors (TFs) called C-repeat binding factor/dehydration-responsive element-binding (CBF/DREB1). Significant modifications occur at the physiological and molecular levels during cold adaptation, implying that cold tolerance is a more complicated process entailing more than one mechanism. A list of transgenes incorporated into different cereal crops for cold stress tolerance is shown in Table 9.1.
9.3.4.1 Rice (O. sativa)
Genetic engineering, which involves the incorporation or disruption of certain gene sequences in rice plants, is a potential strategy to enhance cold stress resilience. The application of effective gene transfer methods and breakthroughs in recombinant DNA technology have culminated in speedy transformation and development of transgenic plants (Sanghera et al. 2011). The overexpression of a number of low-temperature stress-inducible genes resulted in GM rice plants with stress-tolerant characteristics. CBF/DREB1 TFs are among the most significant findings in the area of low-temperature acclimatization and signalling pathways (Sanghera et al. 2011). Overexpression of OsDREB1 or AtDREB1 in GM rice plants enhanced resilience to cold temperature, dehydration, and salt stress, as well as the accumulation of osmoprotectors like free proline and other soluble sugars (Ito et al. 2006). The rice DREB1 gene (OsDREB1D) was overexpressed in A. thaliana plants, producing GM plants with a link between cold stress and OsDREB1D transcription (Zhang et al. 2009). Increased levels of expression of a maize CBF gene (ZmCBF3) in rice plants resulted in GM plants that exhibited growth deceleration only at the nursery stage and had no production loss in outdoor environments. GM plants, as envisaged, were indeed cold stress resilient (Xu et al. 2011). TFs from a variety of families have indeed been introduced into rice plants in an effort to create transgenics that are more resistant to cold stress. In GM rice plants, expression of OsCOIN (O. sativa cold inducible), a TF from the bZIP zinc finger protein family, is manifested in improved tolerance to cold, salt, and drought stress treatment, as well as increased proline levels following cold treatment, than WT plants (Liu et al. 2007). Table 9.1 shows a list of genes that have been incorporated into rice through genetic engineering to improve cold stress resilience.
9.3.4.2 Wheat (T. aestivum)
Unexpected temperature fluctuation is linked to a high probability of devastating low-temperature episodes. Wheat is an important crop for addressing world food demands. Extreme temperatures caused by climate change significantly impact wheat’s vegetative and reproductive development, resulting in a reduction in productivity. Cold temperatures cause a variety of changes in the morphophysiological, biochemical, and molecular composition of wheat (Hassan et al. 2021). Crop plants power up their cold tolerance regimes in response to these changes which include the accumulation of soluble sugars, signalling pathways, and transcription of cold tolerance genes (Hassan et al. 2021). Among the various methods used for development of cold/freezing stress-tolerant wheat, transgenic approach is the most widely used. The transgenic strategy is the most routinely adopted of the many approaches used to produce cold/freezing stress-resilient wheat. Not only have genes from other sources been used to develop GM wheat, but a variety of wheat-derived genes have also been transferred to other crops. For instance, drought, high salt, and cold stress tolerance were substantially enhanced in GM wheat harbouring the cotton DRE-binding TF (GhDREB) maintained under the regulation of the ubi1 or rd29A promoters (Gao et al. 2009). Analogously, cold stress endurance was improved in GM wheat plants containing the barley lipid transfer protein, BLT101 (Choi and Hwang 2015). Huang et al. (2014) documented that the wheat aquaporin gene TaAQP7 imparts cold stress resilience in transgenic tobacco. Under cold exposure, TaAQP7 overexpression in tobacco plants culminated in enhanced root extension and growth in comparison to the wild-type (WT) plants. In GM strawberry leaves, overexpression of the wheat WCOR410, an acidic dehydrin gene, increases freezing stress resilience. In GM A. thaliana, overexpression of the wheat MYB TF gene TaMYB56-B improves resilience to chilling and salinity stress conditions (Zhang et al. 2012).
9.3.4.3 Barley (H. vulgare)
Strengthening the cold hardiness of winter grains is one of the most important challenges confronting plant breeders today. Therefore, analysing the genes that regulate cold acclimatization processes is critical. In a few instances, transgenic methodologies have been seamlessly used for functional studies of different cold stress-responsive regulatory genes. Many studies in a number of plant species have shown the involvement of CBF genes in cold adaptation. For example, GM barley with enhanced level of expression of TaCBF14, TaCBF15, and OsMYB4 boosts frost resilience and germination efficiency in cold environments (Soltész et al. 2012, 2013). In a related research, the cold-inducible expression of wheat TaDREB3 in barley and rice was optimized using two stress-inducible gene promoters, OsWRKY71 and TdCor39, with the intent of enhancing cold stress tolerance in barley. The promoters exhibited varying characteristics, such as activation period, strength, and ABA sensitivity (Kovalchuk et al. 2013). The stress-inducible promoters HDZI-3 and HDZI-4 were used to drive the transcription of two DREB/CBF genes, TaDREB3 and TaCBF5L, in GM wheat and barley. The promoters were obtained from durum wheat genes encoding HD-Zip class I subfamily c-clade TFs. Dehydration and cold resilience of GM barley seedlings increased with expression of the DREB/CBF genes under both promoters, as did frost endurance of GM wheat seedlings (Yang et al. 2019).
9.3.4.4 Maize (Z. mays)
Based on the existing literature, it can be inferred that relatively few researches have been conducted to date utilizing transgenic technology to improve cold stress tolerance in maize. According to oxidative stress and growth rate evaluations, evidence suggests that introgression and overproduction of A. thaliana iron superoxide dismutase (FeSOD) may result in higher methyl viologen concentrations as well as enhanced freezing tolerance in maize (Van Breusegem et al. 1999). When the E. coli-derived beta gene was introduced into maize via Agrobacterium-mediated plant transformation, the majority (four out of five) of the maize lines tested showed decreased cell membrane damage; increased photosynthetic activity; increased levels of amino acids such as alanine, glutamic acid, glycine, and serine; and improved survival rates under freezing stress as compared to their untransformed counterparts (Quan et al. 2004). In GM maize, low-level yet constitutive heterologous expression of a tobacco active mitogen-activated protein kinase (NPK1) gene is manifested in heightened freezing stress tolerance (Shou et al. 2004a, b). Drought and cold stress tolerance in A. thaliana transgenic plants were improved by enhanced expression of the maize ZmDBP3 gene, a candidate of the A-1 subgroup of the CBF/DREB subfamily (Wang and Dong 2009).
9.3.4.5 Sorghum (S. bicolor L.)
In temperate zones, soil temperatures <15 °C impede germination and establishment of sorghum seedlings during early-season planting. Producing short-duration sorghum cultivars is an essential breeding objective for temperate regions since low spring temperatures result in a protracted juvenile development (Anami et al. 2015). The dearth of relevant literature indicated that just a few efforts had been undertaken, with limited success in improving cold/freezing stress tolerance in grain sorghum crops thus far.
9.3.4.6 Pearl Millet (P. glaucum) and Finger Millet (E. coracana L.)
We did not find any noteworthy publications on the use of genetic engineering technology for transferring cold stress-responsive genes from other sources into pearl millet and finger millet at the time of preparing this book chapter.
9.3.5 Heavy Metal Tolerance
Among the various abiotic stresses to plants, heavy metal contamination is a serious environmental problem which affects the growth, productivity, and genome stability (Saini and Westgate 1999). The major cause of heavy metal contamination is the use of urban waste for crop production. In Asia, the urbanized or nearby urban areas are under major concern of increasing heavy metal toxicity because of soil and water contamination (Simmons et al. 2010). Exposure to high level of heavy metals results in the overproduction of ROS and ultimately leads to oxidative stress in crops.
Hyperaccumulation of heavy metal inside the plants and hypertolerance to it are the most practical means of combating heavy metal toxicity without affecting their viability (DalCroso et al. 2013). Though the research findings of heavy metal stress tolerance are majorly limited to the model plants such as tobacco, Arabidopsis, etc., the major outcomes of transgenic development in the case of cereals are limited in number. The transgenes or transcription factors used in these research findings are of great importance to understand the heavy metal tolerance and their downstream pathways. The major breakthrough achieved using transgenic approaches to develop heavy metal tolerance is summarized in Table 9.2.
9.3.6 Anoxia Stress/Oxygen Deficiency Stress Tolerance
Since plants are obligate aerobes, the deficiency of oxygen (hypoxia) or its absence (anoxia) causes various ecological stresses. The reason of stress condition may be due oxygen deprivation on hydromorphic and flooded soils leading to the poor solubility of oxygen and low diffusion rate in the water (Jackson et al. 2009; Vissere et al. 2003). Anoxia stress may cause the wilting and even can lead to the total damage of the crop. It appears that increased flooding tolerance is likely to result from ethanolic fermentation pathway upregulation in rice which is evident from several studies. The use of transgenic approach to oxygen deficiency has been employed in various cereals as well as in model plants. Important transgenic cereals targeting the ethanolic fermentation pathways and several other genes/TFs with improved hypoxia tolerance/resistance have been summarized in Table 9.3.
9.3.7 Ultraviolet-B (UV-B) Stress Tolerance
Plants respond to ultraviolet-B light (UV-B), which is one of the significant abiotic stresses and triggers their genetic programme and decrease in biomass production (Ulm and Jenkins 2015; Yin and Ulm 2017). The UV-B exposure induces an oxidative burst produced by higher level of ROS (Brosché and Strid 2003; Frohnmeyer and Staiger 2003). In the UV-B stress conditions plants accumulate ROS in the apoplast which is triggered by NADPH oxidase AtRBOHD and AtRBOHF subunit activation. The accumulation happens in the chloroplast as well because of the effect of UV-B on photosystem (PS) II function (Kulandaivelu and Noorudeen 1983; Larkum et al. 2001). The UV-B stress in crops has been mostly studied in combination with other abiotic stresses, and there is very limited information available on the development of transgenic cereals targeting UV-B stress tolerance. In some model plants and other plant species, transgene expression and their impact on the ROS and other oxidative accumulation have been elucidated. A few examples of transgenic approach used for UV-B stress tolerance development have been comprised in this section. In the report of Nagy et al. (2016), the transgenic barley lines expressing Medicago sativa aldose reductase (MsALR) gene showed accumulation of MsALR in the cytosol as well as chloroplast, which can increase the abiotic stress tolerance caused by ROS in the plants. The report of Wang et al. (2006) elucidates the enhanced tolerance to UV-B and heat stress overexpressing the cytosolic ascorbate peroxidase (cAPX) in transgenic tomato. While exposing the plants to UV-B, heat, and drought stresses, a several-fold higher level of APX activity was observed in the leaves of cAPX transgenic plants. The presence of higher APX activity in plants suggests the enhanced tolerance to the ROS as plant develops antioxidant enzymes such as SOD and APX to detoxify the ROS. Another interesting study on carrot by Jayaraj and Punja (2008) has been demonstrated to understand the expression of an algal β-carotene ketolase gene (bkt). The constitutive expression of pigments like astaxanthin, adonirubin, canthaxanthin, echinenone, adonixanthin, and β-cryptoxanthin in transgenic plants resulted in significant better growth than the wild type while exposed to the UV-B radiation. The transgenic tissue also accumulated lower amount of H2O2 following exposure to oxidative stresses, which suggests the quenching of ROS by the ketocarotenoids. Thus, it is well understood that the UV-B stress and oxidative stress caused by it have been attempted using transgenic plants, though it has not been studied in cereals.
9.4 Major Challenges of Transgenic Approach
The major challenge in the development of abiotic stress-tolerant transgenic cereal crops is the changing environmental condition. A particular genotype compatible in one particular environment may not be compatible in another. Moreover, all abiotic stresses can affect a plant depending upon its genetic makeup and adaptive response. The specific genotype × environment interaction leads to varied effects in response to different environments. Molecular genetic analysis of specific genes conferring tolerance against different abiotic stresses has produced many successful transgenic cereals. The challenge ahead lies in the identification of the natural variation in wild-type populations and using them to develop promising transgenic climate-resilient cereals. Another bottleneck in the development of future-ready abiotic stress-tolerant transgenic cereals lies in the crosstalk between many of the abiotic stresses. Combinations of these stresses like heat and drought can occur in the field causing unique effects that cannot be predicted individually (Suzuki et al. 2014). This may lead to varied physiological interactions which need novel interventions. Transgenic pyramiding can be a viable approach to solve this problem. For example, stacking of candidate genes for drought as well as salinity has been successfully done in rice (Gupta et al. 2018; James et al. 2018) and maize (Nguyen et al. 2013). Another challenge in the development of abiotic stress tolerant transgenic cereals is to understand the stress-responsive signalling pathways and the genes involved (Yoshida et al. 2014). Overexpression of transcription factors and other regulatory genes has been successfully used to develop transgenic cereals with improved stress tolerance and productivity (Mickelbart et al. 2015). Nevertheless, overexpression analysis might not fully represent the natural function of genes in the plant which may pose another difficulty.
9.5 Conclusion
Abiotic stresses will continue to be a challenge in agriculture. In the present scenario, with competing uses of land and the ever-increasing world population, the challenge is to produce the maximum in a limited area with depleting natural resources, confronted with climate change adversely affecting crop productivity. The transgenic approach has been successfully employed to engineer climate-resilient cereals. There are many instances where genetic engineering has come handy in producing environmentally stable crops that can yield more. Recent techniques like RNAi and CRISPR are now being used to develop future-ready cereals that can remain stable under changing climatic conditions. The challenge before us in crop improvement is to integrate information on abiotic stress response pathways, identify and fish out the different candidate genes, and use these to engineer environmentally stable cereals that can yield more under climatic constraints, to feed the growing population.
References
Abebe T, Guenzi AC, Martin B, Cushman JC (2003) Tolerance of mannitol-accumulating transgenic wheat to water stress and salinity. Plant Physiol 131(4):1748–1755
Abedin MJ, Cotter-Howells J, Meharg AA (2002) Arsenic uptake and accumulation in rice (Oryza sativa L.) irrigated with contaminated water. Plant Soil 240(2):311–319
Abu-Romman SM, Ammari TG, Irshaid LA, Salameh NM, Hasan MK, Hasan HS (2011) Cloning and expression patterns of the HvP5CS gene from barley (Hordeum vulgare). J Food Agric Environ 9(3/4 part 1):279–284
Acquaah G (2007) Principles of plant genetics and breeding. Blackwell, Oxford. ISBN: 978-0-470-66475-9
Ahmad R, Kim YH, Kim MD (2010) Simultaneous expression of choline oxidase, superoxide dismutase and ascorbate peroxidase in potato plant chloroplasts provides synergistically enhanced protection against various abiotic stresses. Physiol Plant 138:520–533
Ahmad I, Akhtar MJ, Zahir ZA, Jamil A (2012) Effect of cadmium on seed germination and seedling growth of four wheat (Triticum aestivum L.) cultivars. Pak J Bot 44(5):1569–1574
Al Abdallat AM, Ayad JY, Elenein JA, Al Ajlouni Z, Harwood WA (2014) Overexpression of the transcription factor HvSNAC1 improves drought tolerance in barley (Hordeum vulgare L.). Mol Breed 33(2):401–414
Amirjani MR (2011) Effect of salinity stress on growth, sugar content, pigments and enzyme activity of rice. Int J Bot 7:73–81
Anami SE, Zhang LM, Xia Y, Zhang YM, Liu ZQ, Jing HC (2015) Sweet sorghum ideotypes: genetic improvement of stress tolerance. Food Energy Secur 4(1):3–24
Anjum SA, Xie XY, Wang LC, Saleem MF, Man C, Lei W (2011) Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res 6(9):2026–2032
Ariel FD, Manavella PA, Dezar CA, Chan RL (2007) The true story of the HD-Zip family. Trends Plant Sci 12(9):419–426
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv 27:84–93
Ashraf M, Akram NA (2009) Improving salinity tolerance of plants through conventional breeding and genetic engineering: an analytical comparison. Biotechnol Adv 27(6):744–752
Banerjee A, Roychoudhury A (2017) Abscisic-acid-dependent basic leucine zipper (bZIP) transcription factors in plant abiotic stress. Protoplasma 254(1):3–16
Beltagi MS (2008) Molecular responses of Bt transgenic corn (Zea mays L.) plants to salt (NaCl) stress. Aust J Crop Sci 2(2):57–63
Bi H, Shi J, Kovalchuk N, Luang S, Bazanova N, Chirkova L, Zhang D, Shavrukov Y, Stepanenko A, Tricker P, Langridge P (2018) Overexpression of the TaSHN1 transcription factor in bread wheat leads to leaf surface modifications, improved drought tolerance, and no yield penalty under controlled growth conditions. Plant Cell Environ 41(11):2549–2566
Bihani P, Char B, Bhargava S (2011) Transgenic expression of sorghum DREB2 in rice improves tolerance and yield under water limitation. J Agric Sci 149(1):95–101
Bin Rahman AR, Zhang J (2016) Flood and drought tolerance in rice: opposite but may coexist. Food Energy Secur 5(2):76–88
Bonnet M, Camares O, Veisseire P (2000) Effects of zinc and influence of Acremonium lolii on growth parameters, chlorophyll a fluorescence and antioxidant enzyme activities of ryegrass (Lolium perenne L. cv Apollo). J Exp Bot. 51(346):945–953
Brosché M, Strid Å (2003) Molecular events following perception of ultraviolet-B radiation by plants. Physiol Plant 117:1–10
Bullmann T, Arendt T, Frey U, Hanashima C (2015) A transportable, inexpensive electroporator for in utero electroporation. Dev Growth Difer 57:369–377
Campo S, Baldrich P, Messeguer J, Lalanne E, Coca M, San SB (2014) Overexpression of a calcium-dependent protein kinase confers salt and drought tolerance in rice by preventing membrane lipid peroxidation. Plant Physiol 165(2):688–704
Canter LW (2018) Environmental impact of agricultural production activities. CRC Press, Broken Sound Parkway NW. ISBN: 9781351088695
Cao YJ, Wei Q, Liao Y, Song HL, Li X, Xiang CB, Kuai BK (2009) Ectopic overexpression of AtHDG11 in tall fescue resulted in enhanced tolerance to drought and salt stress. Plant Cell Rep 28(4):579–588
Casaretto JA, El-Kereamy A, Zeng B, Stiegelmeyer SM, Chen X, Bi YM, Rothstein SJ (2016) Expression of OsMYB55 in maize activates stress-responsive genes and enhances heat and drought tolerance. BMC Genomics 17(1):1–5
Castiglioni P, Warner D, Bensen RJ, Anstrom DC, Harrison J, Stoecker M et al (2008) Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physiol 147(2):446–455
Chapman SC, Chakraborty S, Dreccer MF, Howden SM (2012) Plant adaptation to climate change—opportunities and priorities in breeding. Crop Pasture Sci 63(3):251–268
Checker VG, Chhibbar AK, Khurana P (2012) Stress-inducible expression of barley Hva1 gene in transgenic mulberry displays enhanced tolerance against drought, salinity and cold stress. Transgenic Res 21(5):939–957
Chen M, Chen Q, Niu X, Zhang R, Lin H, Xu C, Wang X, Wang G, Chen J (2007) Expression of OsNHX1 gene in maize confers salt tolerance and promotes plant growth in the field. Plant Soil Environ 53(11):490
Chen NA, Xu Y, Wang XI, Du C, Du J, Yuan M, Xu Z, Chong K (2011) OsRAN2, essential for mitosis, enhances cold tolerance in rice by promoting export of intranuclear tubulin and maintaining cell division under cold stress. Plant Cell Environ 34(1):52–64
Chen Z, Pan Y, Wang S, Ding Y, Yang W, Zhu C (2012) Overexpression of a protein disulfide isomerase-like protein from Methanothermobacter thermoautotrophicum enhances mercury tolerance in transgenic rice. Plant Sci 197:10–20
Chen J, Xue B, Xia X, Yin W (2013a) A novel calcium-dependent protein kinase gene from Populus euphratica, confers both drought and cold stress tolerance. Biochem Biophys Res Commun 441(3):630–636
Chen LJ, Wuriyanghan H, Zhang YQ, Duan KX, Chen HW, Li QT, Lu X, He SJ, Ma B, Zhang WK, Lin Q (2013b) An S-domain receptor-like kinase, OsSIK2, confers abiotic stress tolerance and delays dark-induced leaf senescence in rice. Plant Physiol 163(4):1752–1765
Chen X, Wang Y, Lv B, Li J, Luo L, Lu S, Zhang X, Ma H, Ming F (2014) The NAC family transcription factor OsNAP confers abiotic stress response through the ABA pathway. Plant Cell Physiol 55(3):604–619
Chiang CM, Chen LFO, Shih SW, Lin KH (2015) Expression of eggplant ascorbate peroxidase increase the tolerance of transgenic rice plant to flooding stress. J Plant Biochem Biotechnol 24:257–267
Chinnusamy V, Zhu J, Zhu JK (2007) Cold stress regulation of gene expression in plants. Trends Plant Sci 12(10):444–451
Choi C, Hwang CH (2015) The barley lipid transfer protein, BLT101, enhances cold tolerance in wheat under cold stress. Plant Biotechnol Rep 9:197–207. https://doi.org/10.1007/s11816-015-0357-4
Christine Desfeux SJC, Bent AF (2000) Female reproductive tissues are the primary target of Agrobacterium-mediated transformation by the Arabidopsis floral-dip method1. Plant Physiol 123:895–900
Ciftci-Yilmaz S, Mittler R (2008) The zinc finger network of plants. Cell Mol Life Sci 65(7):1150–1160
Cramer GR, Schmidt CL, Bidart C (2001) Analysis of cell wall hardening and cell wall enzymes of salt-stressed maize (Zea mays) leaves. Aust J Plant Physiol 28:101–109
Cui M, Zhang W, Zhang Q, Xu Z, Zhu Z, Duan F, Wu R (2011) Induced over-expression of the transcription factor OsDREB2A improves drought tolerance in rice. Plant Physiol Biochem 49(12):1384–1391
Cui LG, Shan JX, Shi M, Gao JP, Lin HX (2014) The miR156-SPL9-DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J 80(6):1108–1117
DalCroso G, Manara A, Furini A (2013) An overview of heavy metal challenge in plants: from root to shoot. Metallomics 5(9):1117–1132
Delhaize E, Ryan PR, Hebb DM, Yamamoto Y, Sasaki T, Matsumoto H (2004) Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proc Natl Acad Sci U S A 101(42):15249–15254
Di H, Tian Y, Zu H, Meng X, Zeng X, Wang Z (2015) Enhanced salinity tolerance in transgenic maize plants expressing a BADH gene from Atriplex micrantha. Euphytica 206(3):775–783
Djemal R, Khoudi H (2021) The barley SHN1-type transcription factor HvSHN1 imparts heat, drought and salt tolerances in transgenic tobacco. Plant Physiol Biochem 164:44–53
Dong W, Wang M, Xu F, Quan T, Peng K, Xiao L, Xia G (2013) Wheat oxophytodienoate reductase gene TaOPR1 confers salinity tolerance via enhancement of abscisic acid signaling and reactive oxygen species scavenging. Plant Physiol 161(3):1217–1228
dos Reis SP, Lima AM, de Souza CRB (2012) Recent molecular advances on downstream plant responses to abiotic stress. Int J Mol Sci 13:8628–8647
Du X, He F, Zhu B, Ren M, Teng H (2020) NAC transcription factors from Aegilops markgrafii reduce cadmium concentration in transgenic wheat. Plant Soil 449:39–50
Duan J, Zhang M, Zhang H, Xiong H, Liu P, Ali J, Li J, Li Z (2012) OsMIOX, a myo-inositol oxygenase gene, improves drought tolerance through scavenging of reactive oxygen species in rice (Oryza sativa L.). Plant Sci 196:143–151
El-Esawi MA, Alayafi AA (2019) Overexpression of rice Rab7 gene improves drought and heat tolerance and increases grain yield in rice (Oryza sativa L.). Genes (Basel) 10
Ercoli L, Mariotti M, Massoni A, Arduini I (2004) Growth responses of sorghum plants to chilling temperatures and duration of exposure. Eur J Argon 21(2):93–103
Éva C, Solti Á, Oszvald M, Tomoskozi-Farkas R, Nagy B, Horvath GV, Tamas L (2016) Improved reactive aldehyde, salt and cadmium tolerance of transgenic barley due to the expression of aldo–ketoreductase genes. Acta Physiol Plant 38:99
Fang Y, Xiong L (2015) General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci 72(4):673–689
Fang Y, You J, Xie K, Xie W, Xiong L (2008) Systematic sequence analysis and identification of tissue-specific or stress-responsive genes of NAC transcription factor family in rice. Mol Genet Genom 280(6):547–563
Frohnmeyer H, Staiger D (2003) Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol 133:1420–1428
Fu J, Momcilovic I, Prasad PV, Josipovic S, Ludwig E (2012) Heat stress: causes, prevention and treatments. Nova Science Publishers Inc. ISBN: 978-1621001874
Fu J, Wu H, Ma S, Xiang D, Liu R, Xiong L (2017) OsJAZ1 attenuates drought resistance by regulating JA and ABA signaling in rice. Front Plant Sci 8:2108
Ganguly M, Datta K, Roychoudhury A, Gayen D, Sengupta DN, Datta SK (2012) Overexpression of Rab16A gene in indica rice variety for generating enhanced salt tolerance. Plant Signal Behav 7(4):502–509
Ganguly M, Roychoudhury A, Sengupta DN, Datta SK, Datta K (2020) Independent overexpression of OsRab16A and AtDREB1A exhibit enhanced drought tolerance in transgenic aromatic rice variety Pusa Sugandhi 2. J Plant Biochem Biotechnol 29:503–517
Gao SQ, Chen M, Xia LQ, Xiu HJ, Xu ZS, Li LC, Zhao CP, Cheng XG, Ma YZ (2009) A cotton (Gossypium hirsutum) DRE-binding transcription factor gene, GhDREB, confers enhanced tolerance to drought, high salt, and freezing stresses in transgenic wheat. Plant Cell Rep 28(2):301–311
Gao SQ, Chen M, Xu ZS, Zhao CP, Li L, Xu HJ, Tang YM, Zhao X, Ma YZ (2011) The soybean GmbZIP1 transcription factor enhances multiple abiotic stress tolerances in transgenic plants. Plant Mol Biol 75(6):537–553
Garg AK, Kim JK, Owens TG, Ranwala AP, Do Choi Y, Kochian LV, Wu RJ (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc Natl Acad Sci U S A 99(25):15898–15903
Ge LF, Chao DY, Shi M, Zhu MZ, Gao JP, Lin HX (2008) Overexpression of the trehalose-6-phosphate phosphatase gene OsTPP1 confers stress tolerance in rice and results in the activation of stress responsive genes. Planta 228(1):191–201
Gomez Mansur NM, Pena LB, Bossio AE, Lewi DM, Beznec AY, Blumwald E, Arbona V, Gómez-Cadenas A, Benavides MP, Gallego SM (2021) An isopentenyl transferase transgenic wheat isoline exhibits less seminal root growth impairment and a differential metabolite profile under Cd stress. Physiol Plant
Guo M, Ye J, Gao D, Xu N, Yang J (2019) Agrobacterium-mediated horizontal gene transfer: mechanism, biotechnological application, potential risk and forestalling strategy. Biotechnol Adv 37(1):259–270
Gupta BK, Sahoo KK, Ghosh A, Tripathi AK, Anwar K, Das P et al (2018) Manipulation of glyoxalase pathway confers tolerance to multiple stresses in rice. Plant Cell Environ 41(5):1186–1200
Gupta A, Rico-Medina A, Caño-Delgado AI (2020) The physiology of plant responses to drought. Science 368(6488):266–269
Gürel F, Öztürk ZN, Uçarlı C, Rosellini D (2016) Barley genes as tools to confer abiotic stress tolerance in crops. Front Plant Sci 7:1137
Gust AA, Brunner F, Nürnberger T (2010) Biotechnological concepts for improving plant innate immunity. Curr Opin Biotechnol 21(2):204–210
Guttieri MJ, Stein RJ, Waters BM (2013) Nutrient partitioning and grain yield of TaNAM-RNAi wheat under abiotic stress. Plant Soil 371:573–591
Hakala K, Jauhiainen L, Rajala AA, Jalli M, Kujala M, Laine A (2020) Different responses to weather events may change the cultivation balance of spring barley and oats in the future. Field Crops Res 259:107956
Harris K, Subudhi PK, Borrell A, Jordan D, Rosenow D, Nguyen H, Klein P, Klein R, Mullet J (2007) Sorghum stay-green QTL individually reduce post-flowering drought-induced leaf senescence. J Exp Bot 58(2):327–338
Hassan MA, Xiang C, Farooq M, Muhammad N, Yan Z, Hui X, Yuanyuan K, Bruno AK, Lele Z, Jincai L (2021) Cold stress in wheat: plant acclimation responses and management strategies. Front Plant Sci 12:1234
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7(11):1456–1466
He C, Yang A, Zhang W, Gao Q, Zhang J (2010) Improved salt tolerance of transgenic wheat by introducing betA gene for glycine betaine synthesis. Plant Cell Tissue Organ Cult (PCTOC) 101(1):65–78
He C, Zhang W, Gao Q, Yang A, Hu X, Zhang J (2011) Enhancement of drought resistance and biomass by increasing the amount of glycine betaine in wheat seedlings. Euphytica 177(2):151–167
Hede AR, Skovmand B, Reynolds MP, Crossa J, Vilhelmsen AL, Stolen O (1999) Evaluating genetic diversity for heat tolerance traits in Mexican wheat landraces. Genet Resour Crop Evol 46:37–45
Hemantaranjan A, Malik CP, Bhanu AN (2018) Physiology of heat stress and tolerance mechanisms—an overview. J Plant Sci Res 34(1)
Ho SL, Huang LF, Lu CA, He SL, Wang CC, Yu SP, Chen J, Yu SM (2013) Sugar starvation-and GA-inducible calcium-dependent protein kinase 1 feedback regulates GA biosynthesis and activates a 14-3-3 protein to confer drought tolerance in rice seedlings. Plant Mol Biol 81(4–5):347–361
Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, Xiong L (2006) Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc Natl Acad Sci U S A 103(35):12987–12992
Hu Y, Zhang L, Zhao L, Li J, He S, Zhou K, Yang F, Huang M, Jiang L, Li L (2011) Trichostatin A selectively suppresses the cold-induced transcription of the ZmDREB1 gene in maize. PLoS One 6(7):e22132
Hu T, Zhu S, Tan L, Qi W, He S, Wang G (2016) Overexpression of OsLEA4 enhances drought, high salt and heavy metal stress tolerance in transgenic rice (Oryza sativa L.). Environ Exp Bot 123:68–77
Hu XJ, Chen D, Lynne Mclntyre C, Fernanda Dreccer M, Zhang ZB, Drenth J, Kalaipandian S, Chang H, Xue GP (2018) Heat shock factor C2a serves as a proactive mechanism for heat protection in developing grains in wheat via an ABA-mediated regulatory pathway. Plant Cell Environ 41(1):79–98
Huang J, Sun S, Xu D, Lan H, Sun H, Wang Z, Bao Y, Wang J, Tang H, Zhang H (2012) A TFIIIA-type zinc finger protein confers multiple abiotic stress tolerances in transgenic rice (Oryza sativa L.). Plant Mol Biol 80(3):337–350
Huang C, Zhou S, Hu W, Deng X, Wei S, Yang G, He G (2014) The wheat aquaporin gene TaAQP7 confers tolerance to cold stress in transgenic tobacco. Zeitschrift Für Naturforschung C 69(3–4):142–148
Huang S, Weigel D, Beachy RN, Li J (2016) A proposed regulatory framework for genome-edited crops. Nat Genet 48:109–111
Huang X, Hou L, Meng J, You H, Li Z, Gong Z, Yang S, Shi Y (2018) The antagonistic action of abscisic acid and cytokinin signaling mediates drought stress response in Arabidopsis. Mol Plant 11(7):970–982
Hussain B (2015) Modernization in plant breeding approaches for improving biotic stress resistance in crop plants. Turk J Agric For 39(4):515–530
Hussain S, Zhang J, Zhong C, Zhu L, Cao X, Yu S, Bohr JA, Hu J, Jin Q (2017) Effects of salt stress on rice growth, development characteristics, and the regulating ways: a review. J Integr Agr 16(11):2357–2374
Hussain A, Shahzad A, Tabassum S, Hafeez H, Khattak JZ (2018) Salt stress tolerance of transgenic rice (Oryza sativa L.) expressing AtDREB1A gene under inducible or constitutive promoters. Biologia 73(1):31–41
Ibrahim IO, Kuaybe YK (2020) Particle bombardment technology and its applications in plants. Mol Biol Rep 47:9831–9847
Isayenkov SV, Maathuis FJM (2019) Plant salinity stress: many unanswered questions remain. Front Plant Sci 10:80
Ito Y, Katsura K, Maruyama K, Taji T, Kobayashi M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2006) Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol 47(1):141–153
Jackson MB, Ishizawa K, Ito O (2009) Evolution and mechanisms of plant tolerance to flooding stress. Ann Bot 200(103):137–142
James D, Borphukan B, Fartyal D, Ram B, Singh J, Manna M, Sheri V, Panditi V, Yadav R, Achary VMA, Reddy MK (2018) Concurrent overexpression of OsGS1;1 and OsGS2 genes in transgenic rice (Oryza sativa L.): impact on tolerance to abiotic stresses. Front Plant Sci 9:786
Jang IC, Oh SJ, Seo JS, Choi WB, Song SI, Kim CH, Kim YS, Seo HS, Choi YD, Nahm BH, Kim JK (2003) Expression of a bifunctional fusion of the Escherichia coli genes for trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase in transgenic rice plants increases trehalose accumulation and abiotic stress tolerance without stunting growth. Plant Physiol 131(2):516–524
Jayaraj J, Punja ZK (2008) Transgenic carrot plants accumulating ketocarotenoids show tolerance to UV and oxidative stresses. Plant Physiol Biochem 46(10):875–883
Jeong JS, Kim YS, Baek KH, Jung H, Ha SH, Do Choi Y, Kim M, Reuzeau C, Kim JK (2010) Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol 153(1):185–197
Jin XF, Xiong AS, Peng RH, Liu JG, Gao F, Chen JM, Yao QH (2010) OsAREB1, an ABRE-binding protein responding to ABA and glucose, has multiple functions in Arabidopsis. BMB Rep 43(1):34–39
Joo J, Choi HJ, Lee YH, Lee S, Lee CH, Kim CH, Cheong JJ, Do Choi Y, Song SI (2014) Over-expression of BvMTSH, a fusion gene for maltooligosyltrehalose synthase and maltooligosyltrehalose trehalohydrolase, enhances drought tolerance in transgenic rice. BMB Rep 47(1):27
Kapoor D, Bhardwaj S, Landi M, Sharma A, Ramakrishnan M, Sharma A (2020) The impact of drought in plant metabolism: how to exploit tolerance mechanisms to increase crop production. Appl Sci 10:5692
Karthikeyan A, Pandian SK, Ramesh M (2011) Transgenic indica rice cv. ADT 43 expressing a Δ 1-pyrroline-5-carboxylate synthetase (P5CS) gene from Vigna aconitifolia demonstrates salt tolerance. Plant Cell Tissue Organ Cult (PCTOC) 107(3):383–395
Katiyar-Agarwal S, Agarwal M, Grover A (2003) Heat-tolerant basmati rice engineered by over-expression of hsp101. Plant Mol Biol 51(5):677–686
Khan MA, Abdullah Z (2003) Salinity-sodicity induced changes in reproductive physiology of rice (Oryza sativa) under dense soil conditions. Environ Exp Bot 49:145–157
Khan GA, Bakhsh A, Husnain T, Riazuddin S (2011) Introduction of Cry1Ab gene in Gossypium hirsutum enhances resistance against lepidopteran pests. Span J Agri Res 9:296–330
Khan A, Sovero V, Gemenet D (2016) Genome-assisted breeding for drought resistance. Curr Genomics 17(4):330–342
Kikkert JR (1993) The biolistic PDS-1000/ He device. Plant Cell Tissue Organ Cult 33:221–226
Kim H, Lee K, Hwang H, Bhatnagar N, Kim DY, Yoon IS, Byun MO, Kim ST, Jung KH, Kim BG (2014a) Overexpression of PYL5 in rice enhances drought tolerance, inhibits growth, and modulates gene expression. J Exp Bot 65(2):453–464
Kim JS, Park HM, Chae S, Lee TH, Hwang DJ, Oh SD, Park JS, Song DG, Pan CH, Choi D, Kim YH (2014b) A pepper MSRB2 gene confers drought tolerance in rice through the protection of chloroplast-targeted genes. PLoS One 9(3):e90588
Kim Y, Chung YS, Lee E, Tripathi P, Heo S, Kim KH (2020) Root response to drought stress in rice (Oryza sativa L.). Int J Mol Sci 21(4):1513
Klein TM, Fromm M, Weissinger A, Tomes D, Schaaf S, Sletten M, Sanford JC (1988) Transfer of foreign genes into intact maize cells with high-velocity microprojectiles. Proc Natl Acad Sci U S A 85(12):4305–4309
Korres NE, Norsworthy JK, Burgos NR, Oosterhuis DM (2017) Temperature and drought impacts on rice production: an agronomic perspective regarding short- and long-term adaptation measures. Water Resour Rural Dev 9:12–27
Kovalchuk N, Jia W, Eini O, Morran S, Pyvovarenko T, Fletcher S, Bazanova N, Harris J, Beck-Oldach K, Shavrukov Y, Langridge P (2013) Optimization of TaDREB3 gene expression in transgenic barley using cold-inducible promoters. Plant Biotechnol J 11(6):659–670
Krugman T, Chagué V, Peleg Z, Balzergue S, Just J, Korol AB, Nevo E, Saranga Y, Chalhoub B, Fahima T (2010) Multilevel regulation and signalling processes associated with adaptation to terminal drought in wild emmer wheat. Funct Integr Genomic 10(2):167–186
Kulandaivelu G, Noorudeen AM (1983) Comparative study of the action of ultraviolet-C and ultraviolet- B radiation on photosynthetic electron transport. Physiol Plant 58:389–394
Kumar V, Shriram V, Kishor PK, Jawali N, Shitole MG (2010) Enhanced proline accumulation and salt stress tolerance of transgenic indica rice by over-expressing P5CSF129A gene. Plant Biotechnol Rep 4(1):37–48
Kumar P, Kamle M, Pandey A (2012) RNAi: new era of functional genomics for crop improvement. Front Recent Dev Plant Sci 1:24–38
Kumar A, Basu S, Ramegowda V, Pereira A (2017) Mechanisms of drought tolerance in rice. Burleigh Dodds Science Publishing Limited, pp 131–163
Kumar SVV, Verma RK, Yadav SK et al (2020) CRISPR-Cas9 mediated genome editing of drought and salt tolerance (OsDST) gene in indica mega rice cultivar MTU1010. Physiol Mol Biol Plants 26:1099–1110
Lacroix B, Citovsky V (2018) Beyond Agrobacterium-mediated transformation: horizontal gene transfer from bacteria to eukaryotes. Curr Top Microbiol Immunol 418:443–462
Larkum AW, Karge M, Reifarth F, Eckert HJ, Post A, Renger G (2001) Effect of monochromatic UV-B radiation on electron transfer reactions of Photosystem II. Photosynth Res 68:49–60
Le Roux ML, Kunert KJ, Van der Vyver C, Cullis CA, Botha AM (2019) Expression of a small ubiquitin-like modifier protease increases drought tolerance in wheat (Triticum aestivum L.). Front Plant Sci 10:266
Li B, Wei A, Song C, Li N, Zhang J (2008) Heterologous expression of the TsVP gene improves the drought resistance of maize. Plant Biotechnol J 6(2):146–159
Li B, Li N, Duan X, Wei A, Yang A, Zhang J (2010a) Generation of marker-free transgenic maize with improved salt tolerance using the FLP/FRT recombination system. J Biotechnol 145(2):206–213
Li C, Lv J, Zhao X, Ai X, Zhu X, Wang M, Zhao S, Xia G (2010b) TaCHP: a wheat zinc finger protein gene down-regulated by abscisic acid and salinity stress plays a positive role in stress tolerance. Plant Physiol 154(1):211–221
Li HW, Zang BS, Deng XW, Wang XP (2011) Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 234(5):1007–1018
Li H, Han X, Liu X, Zhou M, Ren W, Zhao B, Ju C, Liu Y, Zhao J (2019) A leucine-rich repeat-receptor-like kinase gene SbER2–1 from sorghum (Sorghum bicolor L.) confers drought tolerance in maize. BMC Genomics 20(1):1–5
Lisar SY, Motafakkerazad R, Hossain MM, Rahman IM (2012) Causes, effects and responses. Water stress 25(1)
Liu K, Wang L, Xu Y, Chen N, Ma Q, Li F, Chong K (2007) Overexpression of OsCOIN, a putative cold inducible zinc finger protein, increased tolerance to chilling, salt and drought, and enhanced proline level in rice. Planta 226(4):1007–1016
Liu C, Mao B, Ou S, Wang W, Liu L, Wu Y, Chu C, Wang X (2014) OsbZIP71, a bZIP transcription factor, confers salinity and drought tolerance in rice. Plant Mol Biol 84(1-2):19–36
Lu M, Zhang DF, Shi YS, Song YC, Wang TY, Li Y (2013a) Expression of SbSNAC1, a NAC transcription factor from sorghum, confers drought tolerance to transgenic Arabidopsis. PCTOC 115(3):443–455
Lu Y, Li Y, Zhang J, Xiao Y, Yue Y, Duan L, Zhang M, Li Z (2013b) Overexpression of Arabidopsis molybdenum cofactor sulfurase gene confers drought tolerance in maize (Zea mays L.). PLoS One 8(1):e52126
Luan M, Xu M, Lu Y, Zhang L, Fan Y, Yang L (2015) Expression of zma-miR169 miRNAs and their target ZmNF-YA genes in response to abiotic stress in maize leaves. Gene 555(2):178–185
Maas EV, Hoffman GJ (1977) Crop salt tolerance-current assessment. J Irrig Drain Div 103:115–134
Madhusudhana R, Rajendrakumar P, Patil JV (2015) Sorghum molecular breeding. Springer. ISBN 978-81-322-2422-8
Maheswari M, Varalaxmi Y, Vijayalakshmi A, Yadav SK, Sharmila P, Venkateswarlu B, Vanaja M, Saradhi PP (2010) Metabolic engineering using mtlD gene enhances tolerance to water deficit and salinity in sorghum. Biol Plant 54(4):647–652
Mantri N, Patade V, Penna S, Ford R, Pang E (2012) Abiotic stress responses in plants: present and future. In: Abiotic stress responses in plants. Springer, New York, pp 1–19
Marin AR, Pezeshki SR, Masscheleyn PH, Choi HS (1993) Effect of dimethylarsenic acid (DMAA) on growth, tissue arsenic and photosynthesis of rice plants. J Plant Nutr 16(5):865–880
Meyer T, Renoud S, Vigouroux A, Miomandre A, Gaillard V, Kerzaon I, Lavire C (2018) Regulation of hydroxycinnamic acid degradation drives Agrobacterium fabrum lifestyles. Mol Plant Microbe Interact 31(8):814–822. https://doi.org/10.1094/MPMI-10-17-0236-R
Mickelbart MV, Hasegawa PM, Bailey-Serres J (2015) Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat Rev Genet 16:237–251
Mishra GP, Singh B, Seth T, Singh AK, Halder J, Krishnan N, Tiwari SK, Singh PM (2017) Biotechnological advancements and begomovirus management in okra (Abelmoschus esculentus L.): status and perspectives. Front. Plant Sci 8:360
Moriwaki T, Yamamoto Y, Aida T, Funahashi T, Shishido T, Asada M, Prodhan SH, Komamine A, Motohashi T (2008) Overexpression of the Escherichia coli catalase gene, katE, enhances tolerance to salinity stress in the transgenic indica rice cultivar, BR5. Plant Biotechnol Rep 2(1):41–46
Morran S, Eini O, Pyvovarenko T, Parent B, Singh R, Ismagul A, Eliby S, Shirley N, Langridge P, Lopato S (2011) Improvement of stress tolerance of wheat and barley by modulation of expression of DREB/CBF factors. Plant Biotechnol J 9(2):230–249
Munns R, Gilliham M (2015) Salinity tolerance of crops—what is the cost? New Phytol 208(3):668–673
Munns R, James RA, Xu B, Athman A, Conn SJ, Jordans C, Byrt CS, Hare RA, Tyerman SD, Tester M, Plett D (2012) Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat Biotechnol 30(4):360–364
Murakami T, Matsuba S, Funatsuki H, Kawaguchi K, Saruyama H, Tanida M, Sato Y (2004) Over-expression of a small heat shock protein, sHSP17. 7, confers both heat tolerance and UV-B resistance to rice plants. Mol Breed 13(2):165–175
Nagy B, Majer P, Robert M, Pauk J, Horvath GV (2016) Stress tolerance of transgenic barley accumulating the alfalfa aldose reductase in the cytoplasm and the chloroplast. Phytochemistry 129:14–23
Nelson DE, Repetti PP, Adams TR, Creelman RA, Wu J, Warner DC, Anstrom DC, Bensen RJ, Castiglioni PP, Donnarummo MG, Hinchey BS (2007) Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc Natl Acad Sci U S A 104(42):16450–16455
Nguyen TX, Sticklen M (2013) Barley HVA1 gene confers drought and salt tolerance in transgenic maize (Zea mays L.). Adv Crop Sci Tech 1(105):2
Nguyen TX, Nguyen T, Alameldin H, Goheen B, Loescher W, Sticklen M (2013) Transgene pyramiding of the HVA1 and mtlD in T3 maize (Zea mays L.) plants confers drought and salt tolerance, along with an increase in crop biomass. Int J Agron:1–10
Niu X, Xiong F, Liu J, Sui Y, Zeng Z, Lu BR, Liu Y (2014) Co-expression of ApGSMT and ApDMT promotes biosynthesis of glycine betaine in rice (Oryza sativa L.) and enhances salt and cold tolerance. Environ Exp Bot 104:16–25
Noman A, Aqeel M, Deng J, Khalid N, Sanaullah T, Shuilin H (2017) Biotechnological advancements for improving floral attributes in ornamental plants. Front Plant Sci 8:530
OGTR (2008) The biology of Triticum aestivum L. em Thell. (bread wheat). Document prepared by the Office of the Gene Technology Regulator, Canberra, Australia. http://www.ogtr.gov.au/
Oh SJ, Kwon CW, Choi DW, Song SI, Kim JK (2007) Expression of barley HvCBF4 enhances tolerance to abiotic stress in transgenic rice. Plant Biotechnol J 5(5):646–656
Ohta M, Hayashi Y, Nakashima A, Hamada A, Tanaka A, Nakamura T, Hayakawa T (2002) Introduction of a Na+/H+ antiporter gene from Atriplex gmelini confers salt tolerance to rice. FEBS Lett 532(3):279–282
Ortiz D, Hu J, Salas Fernandez MG (2017) Genetic architecture of photosynthesis in sorghum bicolor under non-stress and cold stress conditions. J Exp Bot 68(16):4545–4557
Pan W, Shen J, Zheng Z, Yan X, Shou J, Wang W, Jiang L, Pan J (2018) Overexpression of the Tibetan Plateau annual wild barley (Hordeum spontaneum) HsCIPKs enhances rice tolerance to heavy metal toxicities and other abiotic stresses. Rice 11:51
Pavei D, Gonçalves-Vidigal MC, Schuelter AR, Schuster I, Vieira ES, Vendruscolo EC, Poletine JP (2016) Response to water stress in transgenic (‘p5cs’ gene) wheat plants (‘Triticum aestivum’ L.). Aust J Crop Sci 10(6):776–783
Pellegrineschi A, Reynolds M, Pacheco M, Brito RM, Almeraya R, Yamaguchi-Shinozaki K, Hoisington D (2004) Stress-induced expression in wheat of the Arabidopsis thaliana DREB1A gene delays water stress symptoms under greenhouse conditions. Genome 47(3):493–500
Perata P, Geshi N, Yamaguchi J, Akazawa T (1993) Effect of anoxia on the induction of α-amylase in cereal seeds. Planta 191:402–408
Perveen S, Shahbaz M, Ashraf M (2012) Is pre-sowing seed treatment with triacontanol effective in improving some physiological and biochemical attributes of wheat (Triticum aestivum L.) under salt stress? J Appl Bot Food Qual 85(1):41
Poonia A, Mishra S, Sirohi P, Chaudhary R, Germain H, Chauhan H (2020) Improved thermotolerance in transgenic barley by overexpressing a heat shock factor gene (TaHsfA6b) from wheat. Authorea Preprints
Prodhan SH, Hossain A, Nagamiya K, Komamine A, Morishima H (2008) Improved salt tolerance and morphological variation in indica rice (Oryza sativa L.) transformed with a catalase gene from E. coli. Plant Tissue Cult Biotechnol 18(1):57–63
Qi Y, Wang H, Zou Y, Liu C, Liu Y, Wang Y, Zhang W (2011) Over-expression of mitochondrial heat shock protein 70 suppresses programmed cell death in rice. FEBS Lett 585(1):231–239
Qi X, Xu W, Zhang J, Guo R, Zhao M, Hu L, Wang H, Dong H, Li Y (2017) Physiological characteristics and metabolomics of transgenic wheat containing the maize C 4 phosphoenolpyruvate carboxylase (PEPC) gene under high temperature stress. Protoplasma 254(2):1017–1030
Qiao K, Tian Y, Hu Z, Chai T (2019a) Wheat cell number regulator CNR10 enhances the tolerance, translocation, and accumulation of heavy metals in plants. Environ Sci Technol 53(2):860–867
Qiao K, Wang F, Liang S, Wang H, Hu Z, Chai T (2019b) Improved Cd, Zn and Mn tolerance and reduced Cd accumulation in grains with wheat-based cell number regulator TaCNR2. Sci Rep 9:870
Qin D, Wang F, Geng X, Zhang L, Yao Y, Ni Z, Peng H, Sun Q (2015) Overexpression of heat stress-responsive TaMBF1c, a wheat (Triticum aestivum L.) multiprotein bridging factor confers heat tolerance in both yeast and rice. Plant Mol Biol 87(1–2):31–45
Quan R, Shang M, Zhang H, Zhao Y, Zhang J (2004) Improved chilling tolerance by transformation with betA gene for the enhancement of glycinebetaine synthesis in maize. Plant Sci 166(1):141–149
Quan R, Hu S, Zhang Z, Zhang H, Zhang Z, Huang R (2010) Overexpression of an ERF transcription factor TSRF1 improves rice drought tolerance. Plant Biotechnol J 8(4):476–488
Queitsch C, Hong SW, Vierling E, Lindquist S (2000) Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 12(4):479–492
Rady MM (2011) Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci Hortic 129(2):232–237
Rahman M, Grover A, Peacock WJ, Dennis ES, Ellis MH (2001) Effects of manipulation of pyruvate decarboxylase and alcohol dehydrogenase levels on the submergence tolerance of rice. Funct Plant Biol 28:1231–1241
Rahman H, Ramanathan V, Nallathambi J, Duraialagaraja S, Muthurajan R (2016) Over-expression of a NAC 67 transcription factor from finger millet (Eleusine coracana L.) confers tolerance against salinity and drought stress in rice. BMC Biotechnol 16(1):7–20
Raineri J, Wang S, Peleg Z, Blumwald E, Chan RL (2015) The rice transcription factor OsWRKY47 is a positive regulator of the response to water deficit stress. Plant Mol Biol 88(4):401–413
Ramakrishna C, Singh S, Raghavendrarao S, Padaria JC, Mohanty S, Sharma TR, Solanke AU (2018) The membrane tethered transcription factor EcbZIP17 from finger millet promotes plant growth and enhances tolerance to abiotic stresses. Sci Rep 8(1):1–4
Ravikumar G, Manimaran P, Voleti SR, Subrahmanyam D, Sundaram RM, Bansal KC, Viraktamath BC, Balachandran SM (2014) Stress-inducible expression of AtDREB1A transcription factor greatly improves drought stress tolerance in transgenic indica rice. Transgenic Res 23(3):421–439
Reguera M, Peleg Z, Blumwald E (2012) Targeting metabolic pathways for genetic engineering abiotic stress-tolerance in crops. Biochim Biophys Acta Gene Regul Mech 1819(2):186–194
Ristic Z, Bukovnik U, Momčilović I, Fu J, Prasad PV (2008) Heat-induced accumulation of chloroplast protein synthesis elongation factor, EF-Tu, in winter wheat. J Plant Physiol 165(2):192–202
Rong W, Qi L, Wang A, Ye X, Du L, Liang H, Xin Z, Zhang Z (2014) The ERF transcription factor Ta ERF 3 promotes tolerance to salt and drought stresses in wheat. Plant Biotechnol J 12(4):468–479
Roy SJ, Huang W, Wang XJ, Evrard A, Schmöckel SM, Zafar ZU, Tester M (2013) A novel protein kinase involved in Na+ exclusion revealed from positional cloning. Plant Cell Environ 36(3):553–568
Roy SJ, Negrão S, Tester M (2014) Salt resistant crop plants. Curr Opin Biotechnol 26:115–124
Roychoudhury A, Nayek S (2014) Structural aspects and functional regulation of late embryogenesis abundant (LEA) genes and proteins conferring abiotic stress tolerance in plants. In: Ferro A (ed) Abiotic stress: role in sustainable agriculture, detrimental effects and management strategies. Nova Science Publishers, New York, pp 43–109
Roychoudhury A, Gupta B, Sengupta DN (2008) Trans-acting factor designated OSBZ8 interacts with both typical abscisic acid responsive elements as well as abscisic acid responsive element-like sequences in the vegetative tissues of indica rice cultivars. Plant Cell Rep 27(4):779–794
Roychoudhury A, Basu S, Sengupta DN (2012) Antioxidants and stress-related metabolites in the seedlings of two indica rice varieties exposed to cadmium chloride toxicity. Acta Physiol Plant 34(3):835–847
Saad ASI, Li X, Li HP, Huang T, Gao CS, Guo MW, Cheng W, Zhao GY, Liao YC (2013) A rice stress-responsive NAC gene enhances tolerance of transgenic wheat to drought and salt stresses. Plant Sci 203:33–40
Sahab S, Hayden MJ, Mason J, Spangenberg G (2019) Mesophyll protoplasts and PEG-mediated transfections: transient assays and generation of stable transgenic canola plants. Methods Mol Biol 1864:131–152
Sahebi M, Hanafi MM, Rafii MY, Mahmud TM, Azizi P, Osman M, Abiri R, Taheri S, Kalhori N, Shabanimofrad M, Miah G (2018) Improvement of drought tolerance in rice (Oryza sativa L.): genetics, genomic tools, and the WRKY gene family. BioMed Res Int 2018:3158474
Sahoo RK, Gill SS, Tuteja N (2012) Pea DNA helicase 45 promotes salinity stress tolerance in IR64 rice with improved yield. Plant Signal Behav 7:1042–1046
Saini HS, Westgate ME (1999) Reproductive development in grain crops during drought. Adv Agron 68:59–96
Saint Pierre C, Crossa JL, Bonnett D, Yamaguchi-Shinozaki K, Reynolds MP (2012) Phenotyping transgenic wheat for drought resistance. J Exp Bot 63(5):1799–1808
Sanford JC (1990) Biolistic plant transformation. Physiol Plant 79:206–209
Sanford JC, Klein TM, Wolf ED, Allen N (1987) Delivery of substances into cells and tissues using a particle bombardment process. J Part Sci Tech 5:27–37
Sanghera GS, Wani SH, Hussain W, Singh NB (2011) Engineering cold stress tolerance in crop plants. Curr Genomics 12(1):30
Sarangi S, Mandal C, Dutta S, Mukherjee P, Mondal R, Kumar SPJ, Choudhury PR, Singh VP, Tripathi DK, Mandal AB (2019) Microprojectile based particle bombardment in development of transgenic indica rice involving AmSOD gene to impart tolerance to salinity. Plant Gene 19:100183
Satish L, Rathinapriya P, Sagina A, Stanislaus R, Ceasar A, Prathibha M, Pandian S, Rameshkumar R, Ramesh M (2016) Effect of salinity stress on finger millet (Eleusine coracana (L.) Gaertn): histochemical and morphological analysis of coleoptile and coleorhizae. Flora: Morphol Distrib Funct Ecol Plants 222:111–120
Sawahel WA, Hassan AH (2002) Generation of transgenic wheat plants producing high levels of the osmoprotectant proline. Biotechnol Lett 24(9):721–725
Schilling RK, Marschner P, Shavrukov Y, Berger B, Tester M, Roy SJ, Plett DC (2014) Expression of the Arabidopsis vacuolar H+-pyrophosphatase gene (AVP 1) improves the shoot biomass of transgenic barley and increases grain yield in a saline field. Plant Biotechnol J 12(3):378–386
Schittenhelm S, Schroetter S (2014) Comparison of drought tolerance of maize, sweet sorghum and sorghum-sudangrass hybrids. J Agron Crop Sci 200(1):46–53
Shah N, Paulsen G (2003) Interaction of drought and high temperature on photosynthesis and grain-filling of wheat. Plant Soil 257:219–226
Shahbaz M, Ashraf M (2013) Improving salinity tolerance in cereals. Crit Rev Plant Sci 32(4):237–249
Shahbaz M, Ashraf M, Al-Qurainy F, Harris PJ (2012) Salt tolerance in selected vegetable crops. Crit Rev Plant Sci 31(4):303–320
Shan Q, Wang Y, Li J, Gao C (2014) Genome editing in rice and wheat using the CRISPR/Cas system. Nat Protoc 9:2395–2410
Shen CX, Que QZ, Xia YM, Tang N, Li D, He RH, Cao ML (2017) Knock out of the annexin gene OsAnn3 via CRISPR/Cas9-mediated genome editing decreased cold tolerance in rice. J Plant Biol 60(6):539–547
Shi WM, Muramoto Y, Ueda A, Takabe T (2001) Cloning of peroxisomal ascorbate peroxidase gene from barley and enhanced thermotolerance by overexpressing in Arabidopsis thaliana. Gene 273(1):23–27
Shi J, Gao H, Wang H, Lafitte HR, Archibald RL, Yang M, Hakimi SM, Mo H, Habben JE (2017) ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol 15(2):207–216
Shikov AE, Chirkova TV, Yemelyanov VV (2020) Post-anoxia in plants: reasons, consequences, and possible mechanisms. Russ J Plant Physiol 67:45–59
Shim JS, Oh N, Chung PJ, Kim YS, Choi Y, Do Kim J (2018) Overexpression of OsNAC14 improves drought tolerance in rice. Front Plant Sci 9:1–14
Shinde H, Dudhate A, Tsugama D, Gupta SK, Liu S, Takano T (2019) Pearl millet stress-responsive NAC transcription factor PgNAC21 enhances salinity stress tolerance in Arabidopsis. Plant Physiol Biochem 135:546–553
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58(2):221–227
Shiqing GAO, Huijun XU, Xianguo C, Ming C, Zhaoshi XU, Liancheng LI, Xingguo YE, Lipu DU, Xiaoyan HA, Youzhi MA (2005) Improvement of wheat drought and salt tolerance by expression of a stress-inducible transcription factor Gm DREB of soybean (Glycine max). Chin Sci Bull 50(23):2714–2723
Shivhare R, Lata C (2017) Exploration of genetic and genomic resources for abiotic and biotic stress tolerance in pearl millet. Front Plant Sci 7:2069
Shou H, Bordallo P, Fan JB, Yeakley JM, Bibikova M, Sheen J, Wang K (2004a) Expression of an active tobacco mitogen-activated protein kinase kinase kinase enhances freezing tolerance in transgenic maize. Proc Natl Acad Sci U S A 101(9):3298–3303
Shou H, Bordallo P, Wang K (2004b) Expression of the Nicotiana protein kinase (NPK1) enhanced drought tolerance in transgenic maize. J Exp Bot 55(399):1013–1019
Simmons RW, Ahmad W, Noble AD, Blummel M, Evans A, Weckenbrock P (2010) Effect of long-term un-treated domestic waste water reuse on soil quality, wheat grain and straw yields and attributes of fodder quality. Irrig Drain Syst 24(1–2):95–112
Singh S, Chopperla R, Shingote P, Chhapekar SS, Deshmukh R, Khan S, Padaria JC, Sharma TR, Solanke AU (2021) Overexpression of EcDREB2A transcription factor from finger millet in tobacco enhances tolerance to heat stress through ROS scavenging. J Biotechnol 336:10–24
Sohn SO, Back K (2007) Transgenic rice tolerant to high temperature with elevated contents of dienoic fatty acids. Biol Plant 51(2):340–342. https://doi.org/10.1007/s10535-007-0067-z
Soltész A, Vágújfalvi A, Rizza F, Kerepesi I, Galiba G, Cattivelli L, Coraggio I, Crosatti C (2012) The rice Osmyb4 gene enhances tolerance to frost and improves germination under unfavourable conditions in transgenic barley plants. J Appl Genet 53(2):133–143
Soltész A, Smedley M, Vashegyi I, Galiba G, Harwood W, Vágújfalvi A (2013) Transgenic barley lines prove the involvement of TaCBF14 and TaCBF15 in the cold acclimation process and in frost tolerance. J Exp Bot 64(7):1849–1862
Song SY, Chen Y, Chen J, Dai XY, Zhang WH (2011) Physiological mechanisms underlying OsNAC5-dependent tolerance of rice plants to abiotic stress. Planta 234(2):331–345
Su J, Wu R (2004) Stress-inducible synthesis of proline in transgenic rice confers faster growth under stress conditions than that with constitutive synthesis. Plant Sci 166(4):941–948
Su CF, Wang YC, Hsieh TH, Lu CA, Tseng TH, Yu SM (2010) A novel MYBS3-dependent pathway confers cold tolerance in rice. Plant Physiol 153(1):145–158
Sun L, Liu Y, Kong X, Zhang D, Pan J, Zhou Y, Wang L, Li D, Yang X (2012) ZmHSP16. 9, a cytosolic class I small heat shock protein in maize (Zea mays), confers heat tolerance in transgenic tobacco. Plant Cell Rep 31(8):1473–1484
Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R (2014) Abiotic and biotic stress combinations. New Phytol 203(1):32–43
Tang N, Zhang H, Li X, Xiao J, Xiong L (2012) Constitutive activation of transcription factor OsbZIP46 improves drought tolerance in rice. Plant Physiol 158(4):1755–1768
Tardieu F, Tuberosa R (2010) Dissection and modelling of abiotic stress tolerance in plants. Curr Opin Plant Biol 13(2):206–212
Tereshonok DV, Stepanova AY, Dolgikh YI, Osipova ES, Belyaev DV, Kudorarova GR, Vysotskaya LB, Vartapetian BB (2011) Effect of the ipt gene expression on wheat tolerance to root flooding. Russ J Plant Physiol 58:799
Tian F, Wang W, Liang C, Wang X, Wang G, Wang W (2017) Overaccumulation of glycine betaine makes the function of the thylakoid membrane better in wheat under salt stress. Crop J 5(1):73–82
Tigchelaar M, Battisti DS, Naylor RL, Ray DK (2018) Future warming increases probability of globally synchronized maize production shocks. Proc Natl Acad Sci U S A 115:6644–6649
Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, Inoue H (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 45(9):1097–1102
Ulm R, Jenkins GI (2015) Q&A: how do plants sense and respond to UV-B radiation? BMC Biol 3:45
Upadhyaya H, Panda SK (2019) Advances in rice research for abiotic stress tolerance. Woodhead Publishing. ISBN 9780128143322
Van Breusegem F, Slooten L, Stassart JM, Moens T, Botterman J, Van Montagu M, Inzé D (1999) Overproduction of Arabidopsis thaliana FeSOD confers oxidative stress tolerance to transgenic maize. Plant Cell Physiol 40(5):515–523
Varalaxmi Y, Reddy LA, Maheswari M (2015) In planta transformation of sorghum (Sorghum bicolor (L.) Moench) using TPS1 gene for enhancing tolerance to abiotic stresses. J Genet 94(3):425–434
Verma D, Singla-Pareek SL, Rajagopal D, Reddy MK, Sopory SK (2007) Functional validation of a novel isoform of Na+/H+ antiporter from Pennisetum glaucum for enhancing salinity tolerance in rice. J Biosci 32(3):621–628
Virlouvet L, Jacquemot MP, Gerentes D, Corti H, Bouton S, Gilard F, Valot B, Trouverie J, Tcherkez G, Falque M, Damerval C (2011) The ZmASR1 protein influences branched-chain amino acid biosynthesis and maintains kernel yield in maize under water-limited conditions. Plant Physiol 157(2):917–936
Vissere JW, Voesenec LAJ, Vartapetian BB, Jackson MB (2003) Flooding and plant growth. Ann Bot 91:107–109
Wang CT, Dong YM (2009) Overexpression of maize ZmDBP3 enhances tolerance to drought and cold stress in transgenic Arabidopsis plants. Biologia 64(6):1108–1114
Wang Y, Wisniewski M, Meilan R, Cui M, Fuchigami L (2006) Transgenic tomato (Lycopersicon esculentum) overexpressing cAPX exhibits enhanced tolerance to UV-B and heat stress. J Appl Hortic 8:87
Wang M, Zou J, Duan X, Jiang W, Liu D (2007) Cadmium accumulation and its effects on metal uptake in maize (Zea mays L.). Bioresour Technol 98(1):82–88
Wang GP, Hui Z, Li F, Zhao MR, Zhang J, Wang W (2010) Improvement of heat and drought photosynthetic tolerance in wheat by overaccumulation of glycinebetaine. Plant Biotechnol Rep 4(3):213–222
Wang F, Wang Z, Zhu C (2012) Heteroexpression of the wheat phytochelatin synthase gene (TaPCS1) in rice enhances cadmium sensitivity. Acta Biochim Biophys Sin 44:886–893
Wang X, Wang H, Liu S, Ferjani A, Li J, Yan J, Yang X, Qin F (2016) Genetic variation in ZmVPP1 contributes to drought tolerance in maize seedlings. Nat Genet 48(10):1233–1241
Wang CT, Ru JN, Liu YW, Li M, Zhao D, Yang JF, Fu JD, Xu ZS (2018) Maize WRKY transcription factor ZmWRKY106 confers drought and heat tolerance in transgenic plants. Int J Mol Sci 19(10):3046
Wei Q, Guo YJ, Cao HM, Kuai BK (2011) Cloning and characterization of an AtNHX2-like Na+/H+ antiporter gene from Ammopiptanthus mongolicus (Leguminosae) and its ectopic expression enhanced drought and salt tolerance in Arabidopsis thaliana. Plant Cell Tissue Organ Cul (PCTOC) 105(3):309–316
Wei S, Hu W, Deng X, Zhang Y, Liu X, Zhao X, Luo Q, Jin Z, Li Y, Zhou S, Sun T (2014) A rice calcium-dependent protein kinase OsCPK9 positively regulates drought stress tolerance and spikelet fertility. BMC Plant Biol 14(1):1–3
Wu W, Su Q, Xia XY, Wang Y, Luan YS, An LJ (2008) The Suaedaliaotungensiskitag betaine aldehyde dehydrogenase gene improves salt tolerance of transgenic maize mediated with minimum linear length of DNA fragment. Euphytica 159(1):17–25
Xiang Y, Huang Y, Xiong L (2007) Characterization of stress-responsive CIPK genes in rice for stress tolerance improvement. Plant Physiol 144(3):1416–1428
Xiao B, Huang Y, Tang N, Xiong L (2007) Over-expression of a LEA gene in rice improves drought resistance under the field conditions. Theor Appl Genet 115(1):35–46
Xiong X, James VA, Zhang H, Altpeter F (2010) Constitutive expression of the barley HvWRKY38 transcription factor enhances drought tolerance in turf and forage grass (Paspalumnotatum Flugge). Mol Breed 25(3):419–432
Xu ZS, Ni ZY, Li ZY, Li LC, Chen M, Gao DY, Yu XD, Liu P, Ma YZ (2009) Isolation and functional characterization of HvDREB1—a gene encoding a dehydration-responsive element binding protein in Hordeum vulgare. J Plant Res 122(1):121–130
Xu M, Li L, Fan Y, Wan J, Wang L (2011) ZmCBF3 overexpression improves tolerance to abiotic stress in transgenic rice (Oryza sativa) without yield penalty. Plant Cell Rep 30(10):1949–1957
Xu H, Xiao T, Chen CH, Li W, Meyer CA, Wu Q et al (2015) Sequence determinants of improved CRISPR sgRNA design. Genome Res 25:1147–1157
Xue ZY, Zhi DY, Xue GP, Zhang H, Zhao YX, Xia GM (2004) Enhanced salt tolerance of transgenic wheat (Tritivum aestivum L.) expressing a vacuolar Na+/H+ antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+. Plant Sci 167(4):849–859
Xue GP, Way HM, Richardson T, Drenth J, Joyce PA, McIntyre CL (2011) Overexpression of TaNAC69 leads to enhanced transcript levels of stress up-regulated genes and dehydration tolerance in bread wheat. Mol Plant 4(4):697–712
Xue GP, Drenth J, McIntyre CL (2015) TaHsfA6f is a transcriptional activator that regulates a suite of heat stress protection genes in wheat (Triticum aestivum L.) including previously unknown Hsf targets. J Exp Bot 66(3):1025–1039
Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57:781–803
Yang X, Liang Z, Lu C (2005) Genetic engineering of the biosynthesis of glycinebetaine enhances photosynthesis against high temperature stress in transgenic tobacco plants. Plant Physiol 138(4):2299–2309
Yang Z, Wu Y, Li Y, Ling HQ, Chu C (2009) OsMT1a, a type 1 metallothionein, plays the pivotal role in zinc homeostasis and drought tolerance in rice. Plant Mol Biol 70:219–229
Yang S, Vanderbeld B, Wan J, Huang Y (2010) Narrowing down the targets: towards successful genetic engineering of drought-tolerant crops. Mol Plant 3(3):469–490
Yang A, Dai X, Zhang WH (2012) A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. J Exp Bot 63(7):2541–2556
Yang X, Wang B, Chen L, Li P, Cao C (2019) The different influences of drought stress at the flowering stage on rice physiological traits, grain yield, and quality. Sci Rep 9:3742
Yin R, Ulm R (2017) How plants cope with UV-B: from perception to response. Curr Opin Plant Biol 37:42–48
Yin XY, Yang AF, Zhang KW, Zhang JR (2004) Production and analysis of transgenic maize with improved salt tolerance by the introduction of AtNHX1 gene. Acta Bot Sin 46(7):854–861
Yokotani N, Sato Y, Tanabe S, Chujo T, Shimizu T, Okada K, Yamane H, Shimono M, Sugano S, Takatsuji H, Kaku H (2013) WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. J Exp Bot 64(16):5085–5097
Yoshida T, Mogami J, Yamaguchi-Shinozaki K (2014) ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr Opin Plant Biol 12:133–139
You J, Hu H, Xiong L (2012) An ornithine δ-aminotransferase gene OsOAT confers drought and oxidative stress tolerance in rice. Plant Sci 197:59–69
Yourtchi MS, Bayat HR (2013) Effect of cadmium toxicity on growth, cadmium accumulation and macronutrient content of durum wheat (Dena CV.). Int J Agric Crop Sci 6(15):1099–1103
Yu L, Chen X, Wang Z, Wang S, Wang Y, Zhu Q, Li S, Xiang C (2013) Arabidopsis enhanced drought tolerance1/HOMEODOMAIN GLABROUS11 confers drought tolerance in transgenic rice without yield penalty. Plant Physiol 162(3):1378–1391
Yu TF, Xu ZS, Guo JK, Wang YX, Abernathy B, Fu JD, Chen X, Zhou YB, Chen M, Ye XG, Ma YZ (2017) Improved drought tolerance in wheat plants overexpressing a synthetic bacterial cold shock protein gene SeCspA. Sci Rep 7(1):1–4
Zang X, Geng X, Wang F, Liu Z, Zhang L, Zhao Y, Tian X, Ni Z, Yao Y, Xin M, Hu Z (2017) Overexpression of wheat ferritin gene TaFER-5B enhances tolerance to heat stress and other abiotic stresses associated with the ROS scavenging. BMC Plant Biol 17(1):1–3
Zang X, Geng X, He K, Wang F, Tian X, Xin M, Yao Y, Hu Z, Ni Z, Sun Q, Peng H (2018) Overexpression of the wheat (Triticum aestivum L.) taPEPKR2 gene enhances heat and dehydration tolerance in both wheat and Arabidopsis. Front Plant Sci 871:1–8
Zhang Y, Chen C, Jin XF, Xiong AS, Peng RH, Hong YH, Yao QH, Chen JM (2009) Expression of a rice DREB1 gene, OsDREB1D, enhances cold and high-salt tolerance in transgenic Arabidopsis. BMB Rep 42(8):486–492
Zhang S, Li N, Gao F, Yang A, Zhang J (2010) Over-expression of TsCBF1 gene confers improved drought tolerance in transgenic maize. Mol Breed 26(3):455–465
Zhang L, Zhao G, Xia C, Jia J, Liu X, Kong X (2012) Overexpression of a wheat MYB transcription factor gene, TaMYB56-B, enhances tolerances to freezing and salt stresses in transgenic Arabidopsis. Gene 505(1):100–107
Zhang H, Xu W, Wang H, Hu L, Li Y, Qi X, Zhang L, Li C, Hua X (2014a) Pyramiding expression of maize genes encoding phosphoenolpyruvate carboxylase (PEPC) and pyruvate orthophosphate dikinase (PPDK) synergistically improve the photosynthetic characteristics of transgenic wheat. Protoplasma 251(5):1163–1673
Zhang H, Zhang J, Wei P, Zhang B, Gou F, Feng Z et al (2014b) The CRISPR/Cas9 system produces specific and homozygous targeted gene editing in rice in one generation. Plant Biotechnol J 12:797–807
Zhang J, Zhang H, Srivastava AK, Pan Y, Bai J, Fang J, Shi H, Zhu JK (2018) Knockdown of rice microRNA166 confers drought resistance by causing leaf rolling and altering stem xylem development. Plant Physiol 176(3):2082–2094
Zhang A, Liu Y, Wang F et al (2019) Enhanced rice salinity tolerance via CRISPR/Cas9-targeted mutagenesis of the OsRR22 gene. Mol Breed 39:47
Zhao F, Wang Z, Zhang Q, Zhao Y, Zhang H (2006) RETRACTED ARTICLE: analysis of the physiological mechanism of salt-tolerant transgenic rice carrying a vacuolar Na+/H+ antiporter gene from Suaeda salsa. J Plant Res 119(2):95–104
Zhao FY, Liu W, Zhang SY (2009) Different responses of plant growth and antioxidant system to the combination of cadmium and heat stress in transgenic and non-transgenic rice. J Integr Plant Biol 51:942–950
Zhao Y, Dong W, Zhang N, Ai X, Wang M, Huang Z, Xiao L, Xia G (2014) A wheat allene oxide cyclase gene enhances salinity tolerance via jasmonate signaling. Plant Physiol 164(2):1068–1076
Zheng B, Chenu K, Fernanda Dreccer M, Chapman SC (2012) Breeding for the future: what are the potential impacts of future frost and heat events on sowing and flowering time requirements for Australian bread wheat (Triticum aestivum) varieties? Glob Change Biol 18(9):2899–2914
Zhou J, Deng K, Cheng Y, Zhong Z, Tian L, Tang X, Tang A, Zheng X, Zhang T, Qi Y, Zhang Y (2007) CRISPR-Cas9 based genome editing reveals new insights into microRNA function and regulation in rice. Front Plant Sci 8(1598)
Zhou W, Li Y, Zhao BC, Ge RC, Shen YZ, Wang G, Huang ZJ (2009) Overexpression of TaSTRG gene improves salt and drought tolerance in rice. J Plant Physiol 166(15):1660–1671
Zhou G, Delhaize E, Zhou M, Ryan PR (2013) The barley MATE gene, HvAACT1, increases citrate efflux and Al (3+) tolerance when expressed in wheat and barley. Ann Bot 112(3):603–612
Zhou G, Pereira JF, Delhaize E, Zhou M, Magalhaes JV, Ryan PR (2014) Enhancing the aluminium tolerance of barley by expressing the citrate transporter genes SbMATE and FRD3. J Exp Bot 65(9):2381–2390
Zorb C, Geilfus CM, Dietz KJ (2019) Salinity and crop yield. Plant Biol 21:31–38
Zou J, Liu C, Chen X (2011) Proteomics of rice in response to heat stress and advances in genetic engineering for heat tolerance in rice. Plant Cell Rep 30(12):2155–2165
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Das, D., Konwar, T., Sarma, S., Baldodiya, G.M., Chikkaputtaiah, C., Singha, D.L. (2022). Transgenic Strategies to Develop Abiotic Stress Tolerance in Cereals. In: Roychoudhury, A., Aftab, T., Acharya, K. (eds) Omics Approach to Manage Abiotic Stress in Cereals. Springer, Singapore. https://doi.org/10.1007/978-981-19-0140-9_9
Download citation
DOI: https://doi.org/10.1007/978-981-19-0140-9_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0139-3
Online ISBN: 978-981-19-0140-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)