Abstract
Late embryogenesis abundant (LEA) proteins have been implicated in many stress responses of plants. In this report, a LEA protein gene OsLEA3-1 was identified and over-expressed in rice to test the drought resistance of transgenic lines under the field conditions. OsLEA3-1 is induced by drought, salt and abscisic acid (ABA), but not by cold stress. The promoter of OsLEA3-1 isolated from the upland rice IRAT109 exhibits strong activity under drought- and salt-stress conditions. Three expression constructs consisting of the full-length cDNA driven by the drought-inducible promoter of OsLEA3-1 (OsLEA3-H), the CaMV 35S promoter (OsLEA3-S), and the rice Actin1 promoter (OsLEA3-A) were transformed into the drought-sensitive japonica rice Zhonghua 11. Drought resistance pre-screening of T1 families at anthesis stage revealed that the over-expressing families with OsLEA3-S and OsLEA3-H constructs had significantly higher relative yield (yield under drought stress treatment/yield under normal growth conditions) than the wild type under drought stress conditions, although a yield penalty existed in T1 families under normal growth conditions. Nine homozygous families, exhibiting over-expression of a single-copy of the transgene and relatively low yield penalty in the T1 generation, were tested in the field for drought resistance in the T2 and T3 generations and in the PVC pipes for drought tolerance in the T2 generation. Except for two families (transformed with OsLEA3-A), all the other families (transformed with OsLEA3-S and OsLEA3-H constructs) had higher grain yield than the wild type under drought stress in both the field and the PVC pipes conditions. No significant yield penalty was detected for these T2 and T3 families. These results indicate that transgenic rice with significantly enhanced drought resistance and without yield penalty can be generated by over-expressing OsLEA3-1 gene with appropriate promoters and following a bipartite (stress and non-stress) in-field screening protocol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Continuous increase of world population, ever increasing deterioration of arable land, scarcity of fresh water, and global climate changes all underscore the importance of developing stress-resistant crops. Drought is one of the major abiotic stresses affecting plant growth and reducing crop productivity. It has been estimated that 70% of the crop yield loss can be attributed to abiotic stresses, especially drought (Bray et al. 2000). Due to the severe detrimental impact of drought on the crop yield, engineering drought resistant crops has become a challenging task for crop scientists. Conventional breeding of drought resistance has been a basic approach for a long time and some successes have been achieved in crops such as maize (Hoisington et al. 1996), rice (Zhang et al. 2006), and wheat (Zhao et al. 2000b). However, a big gap remains between the current resistance levels and what is needed for most of the major crops. This is especially true for rice because its yield stability is more sensitive to water scarcity than other upland crops.
In response to drought stress, plants have evolved mechanisms to perceive and transmit the stress signals to cellular machinery that activate adaptive responses (Thomashow 1999; Xiong et al. 2002). Drought resistance is a complex trait that is influenced by coordinated expression of a network of genes (Thomashow 1999; Xiong et al. 2002) and affected by a large number of environmental, anatomical, physiological, biophysical, biochemical and developmental factors (Soltis and Soltis 2003), making progress in genetic improvement of drought resistance quite slow. The rapid development of functional genomics and biotechnology in last decade has provided new opportunities in improving drought resistance. To date, quite a few reports suggest that increased expression of drought stress related genes could improve drought resistance to some extent in important crops (Xu et al. 1996; Zhang et al. 2001; Garg et al. 2002; Hu et al. 2006).
One efficient strategy for improving drought resistance of plants is to increase the content of soluble sugars and other compatible solutes through transgenic approaches. These compounds, such as proline, trehalose, betaine and mannitol, serve as osmoprotectants and, in some cases, stabilize functional molecules under stress conditions (Kishor et al. 1995; Hayashi et al. 1997; Shen et al. 1997; Garg et al. 2002). Late embryogenesis abundant (LEA) proteins have also been implicated in water deficit stress (Xu et al. 1996; Maqbool et al. 2002; Goyal et al. 2005). LEA proteins have been classified into five major groups based on amino acid sequences (Bake et al. 1988; Dure et al. 1989) and were re-examined recently using statistically based bioinformatics tools (Wise 2003). These proteins are part of evolutionarily conserved group of hydrophilic proteins termed “hydrophilins” involved in various adaptive responses to hyperosmotic conditions (Garay-Arroyo et al. 2000). The majority of LEA proteins display a preponderance of hydrophilic and charged amino acid residues. Expression of LEA genes, which often appears to be abscisic acid-dependent, was detected not only in seeds but also in vegetative tissues with water deficit associated with drought, salt, and cold stresses (Ingram and Bartels 1996; Thomashow 1998; Cuming 1999; Grelet et al. 2005). Both the pattern of expression and the structural features of LEA proteins suggest a general protective role in desiccation tolerance (Ingram and Bartels 1996). Despite massive data on the expression and protein structure (Raynal et al. 1999; NDong et al. 2002; Grelet et al. 2005), little work has been reported on the manipulation of LEA genes to improve drought resistance under field conditions. For example, the HVA1 gene encoding group 3 LEA protein from barley (Hordeum vulgare L.) was transformed into rice and the tolerance to water deficit and salt stress of the transgenic rice was improved under the greenhouse conditions (Xu et al. 1996).
In this study, a full-length cDNA clone of a drought and salt stress-responsive LEA gene, OsLEA3-1, was transformed into rice in order to assess the effect of its expression under the control of three different promoters on improving drought resistance under the field conditions. Our results indicate that drought resistance is significantly improved in the transgenic rice with expression of the OsLEA3-1 transgene controlled by a drought-inducible HVA1-like promoter and a constitutive promoter CaMV 35S.
Materials and methods
Isolation, construction and transformation of OsLEA3-1
In our previous studies on drought-stressed expression profiles in rice seedlings using a cDNA microarray containing about 9,000 unique expressed sequence tags (ESTs) (unpublished data), a cDNA gene showed strong induction by drought stress in the upland rice IRAT109 (Oryza sativa L. ssp japonica). The full-length cDNA of this gene, designated OsLEA3-1, was identified in the cDNA library of the indica rice Minghui 63 constructed by Chu et al (2003).
Three binary expression constructs (OsLEA3-S, OsLEA3-A, and OsLEA3-H) were generated by inserting the full-length cDNA (released from the cDNA vector pSPORT1 by BamHI and KpnI) into backbone vectors pCAMBIA1301S, pCAMBIA1301A, and pCAMBIA1301H, respectively. These backbone vectors were developed by inserting a double CaMV 35S, rice Actin1 and HVA1-like promoter, respectively, into the multiple cloning sites (HindIII and SacI) of pCAMBIA1301 (provided by the Center for the Application of Molecular Biology for International Agriculture, Australia). The HVA1-like promoter was isolated from the upland rice IRAT109 based on the genomic sequence of OsLEA3-1 promoter in rice cultivar Nipponbare using HindIII-adapted primer 5′-TCCAAGCTTAAGGGCCTCCATAACCTACG-3′ and SacI-adapted primer 5′-TCGGAGCTCACGCGCGAATGTTAGAACTC-3′. The HVA1-like promoter was also amplified by HindIII-adapted primer (5′-TCCAAGCTTGATCTGTGGTGATCGACTTG-3′) and BamHI-adapted primer (5′-TCGGGATCCACGCGCGAATGTTAGAACTC-3′) and fused to the GUS reporter gene in the vector pCAMBIA1391Z (named 1391-H) for assessing the stress-induced activity of the promoter.
All the expression vectors were introduced into japonica rice Zhonghua 11 (drought-sensitive) by Agrobacterium-mediated transformation (Hiei et al. 1994; Lin and Zhang 2005). The embryonic calli from Zhonghua 11 seeds were cultured for 3 days at 28°C with the Agrobacterium strain EHA105 that carried the cDNA or promoter constructs and then transferred to the selection medium containing 50 μg/ml hygromycin and 200 μg/ml carbenicillin. After 2–3 cycles (2 weeks per cycle) of selection, resistant calli were transferred to the pre-regeneration medium containing 40 μg/ml hygromycin. After 7 days, the resistant calli were transferred to the regeneration medium without hygromycin to regenerate plantlets.
PCR, southern and RNA gel blot analysis
Genomic DNA was extracted by using the CTAB method (Zhang et al. 1992). The hygromycin phosphotransferase (Hpt) gene-specific primers (5′-AGAAGAAGATGTTGGCGACCT-3′ and 5′-GTCCTGCGGGTAAATAGCTG-3′) were used to identify positive transgenic plants. PCR reaction was conducted in a volume of 20 μl containing 100 ng genomic DNA, 2 mM MgCl2, 0.2 mM of each dNTP, 1× PCR buffer, 0.2 μM of each primer, and 1 unit rTaq Polymerase (TaKaRa, Dalian, China). The PCR reaction was performed at 94°C for 5 min; then with 30 cycles of 94°C for 1 min, 55°C for 1 min, 72°C for 1 min; finally at 72°C for 5 min.
Copy number of the transgene was determined by Southern blot analysis using the Hpt gene as a probe (amplified by the two Hpt-specific primers). Three micrograms of genomic DNA from each sample was digested with EcoRI, fractionated on 0.7% agarose gel, and blotted onto nylon membranes, which were hybridized with a 32P-dCTP-labeled Hpt-specific probe using standard protocols (Sambrook et al. 1989).
Total RNA samples analyzed in this study were isolated from leaf tissues using TRIzol reagent (Life Technologies, Rockville, MD, USA). Mixed leaf tissues from normal growing (for OsLEA3-S and OsLEA3-A constructs) or drought-stressed (leaf rolled, for OsLEA3-H construct) transgenic T1 families were used for the expression identification of OsLEA3-1. Fifteen micrograms of total RNA from each sample were separated on 1.2% agarose gel containing formaldehyde and then transferred onto a nylon membrane, and hybridized with the 32P-labeled OsLEA3-1 gene-specific fragment obtained by digestion of the cDNA plasmid of this gene with BamHI and KpnI. Hybridization and washing conditions were based on standard protocols (Sambrook et al. 1989).
GUS activity assay of transgenic plants
GUS assay was performed using the standard protocol (Jefferson et al. 1987). Histochemical staining for GUS expression was performed essentially as described by Wu et al. (2003). Rice tissues were incubated in the GUS staining solution (50 mM sodium phosphate at pH 7.0, 10 mM EDTA, 0.1% Triton X-100, 1 mg/ml of X-Gluc, 100 μg/ml of chloramphenicol, 1 mM potassium ferricyanide, 1 mM potassium ferrocyanide, and 20% methanol), placed under a mild vacuum for 10 min, and then incubated at 37°C for about 36 h. After removing staining solution, tissues were fixed (50% ethanol, 5% acetic acid, and 3.7% formaldehyde) and examined.
Abiotic stress treatments at seedling stage
Abiotic stress and ABA treatments were conducted mainly according to Xiong and Yang (2003). The 3-week old seedlings of japonica rice IRAT109 were prepared by growing plants in plastic trays filled with sandy soil in the greenhouse with a 14-h light/10-h dark cycle at 28°C. Drought stress was conducted by withholding water from the trays, and seedling leaves were sampled at 1, 2, 4, 6, 7, 8 days after drought stress treatment. For salt stress, seedling roots were immersed in 200 mM NaCl solution, and seedling leaves were sampled at 1, 3, 6, 18, 36, 72 h after stress treatment. In ABA treatment, seedling leaves were sprayed with 100 μM ABA and sampled at 1, 3, 6, 24, 48 h after treatment. For cold stress, seedlings were transferred to a growth chamber at 4°C, and sampled at 1, 3, 6, 24, 48, 72 h after treatment.
Drought resistance testing of transgenic rice in the field
Thirty PCR-positive (checked at T0 generation) T1 families per construct were pre-screened for drought resistance in an isolated field equipped with a movable rain-off shelter (referred to shelter hereafter) located on the campus of Huazhong Agricultural University in 2004. Twenty plants from each family were planted in two rows (one plot). The wild type (WT) Zhonghua 11 was inserted after every five transgenic families for comparison. One month after transplanting, soil water was discharged through the outlets located 1.5 m below the top of concrete walls surrounding the field. Severe drought stress (soil water content was about 18%) occurred at flowering stage for the cultivar Zhonghua 11 grown in this field. Normal irrigation was resumed after pollination stage. A duplicate set of materials was planted in another isolated field with full irrigation to evaluate the difference of yield between the transgenic and WT rice.
Transgenic families for drought testing in the next generation were selected based on following criteria: no obvious morphological changes, lower yield penalty, increased drought resistance in terms of relative yield (yield under drought stress treatment/yield under normal growth conditions; Yue et al. 2006), over-expression (or drought-induced over-expression for OsLEA3-H) of a single copy transgene. For families satisfying such criteria, seeds from the plants with good drought resistance were harvested individually for testing in the T2 generation. To identify homozygous families for drought resistance testing, root segments of 7-day old T2 seedling were subjected to GUS assay. Only those families with more than 95% of GUS-positive seedlings (100 seedlings tested for each T2 family) were considered to be homozygous lines and used for following drought resistance testing. Homozygous T2 lines were tested under two drought stress treatments (sheltered field and PVC pipes), and control with normal-irrigation. Under the field conditions equipped with a movable shelter, selected homozygous lines and the wild type Zhonghua 11 were tested using a randomized complete block design with three replications. Each plot had 20 plants planted in two rows for each family with a space of 0.17 m between plants and 0.3 m between plots. Drought stress was applied as described above in the T1 generation. The same design was used for the normal irrigation treatment. The planting and drought treatment in the PVC pipes (1 m in length and 0.2 m in diameter) was essentially the same as described previously (Yue et al. 2006). For each homozygous T2 line, 20 plants were divided into two groups for drought stress and normal growth treatments, respectively, and planted individually in the PVC pipes placed under plastic tents (length × width × height; 26 × 6 × 3.6 m) with foldable roofs. The wild type plants were inserted after every ten transgenic plants for comparison. Drought stress was initiated at panicle development stage (ca. 2 weeks before flowering) by discharging water through a hole near the bottom of the pipes. Each plant was stressed to the same degree at which leaves of main tillers were completely rolled (observed at 6:00 p.m.), then irrigated thoroughly overnight and immediately subjected to another round of stress until complete leaf-rolling. After two rounds of drought stress, plants were irrigated to allow recovery at flowering and seed maturation stages.
Seeds were harvested from the homozygous T2 lines and drought resistance testing was conducted in the T3 generation in the field by following the same experimental design and stress treatment as in the T2 generation.
Data collection and statistical analysis
Grain yield per plant and spikelet fertility were used as the major criteria to evaluate the drought resistance performance of transgenic plants. For each T1 family or homozygous line (T2, T3) in the field, yield and spikelet fertility of 16 plants from each plot (excluding 4 plants, 2 on each side of the plot) were measured, and the mean value of the 16 plants in each plot was used for statistical analysis. Relative yield was the ratio of yield (the mean value of the 16 plants in each T1 family) under drought stress treatment to the yield under normal growth treatment (Yue et al. 2006). For the testing in the PVC pipes, yield and spikelet fertility of all the plants were individually measured, and the yield and spikelet fertility values of each plant under drought and normal growth conditions were used for statistical analysis.
The data on grain yield per plant, spikelet fertility, and relative yield were analyzed by one-way analysis of variance (ANOVA). The subsequent multiple comparisons among the means of transgenic families or lines and WT were examined based on the least significant difference (LSD) test. All statistical analysis was performed using SPSS package (version 12.0).
Results
Isolation and stress-induced expression of OsLEA3-1
We originally observed drought stress induction of OsLEA3-1 using a rice cDNA microarray (unpublished data). A full-length cDNA clone was identified for this gene, which encodes a predicted protein of 200 amino acids belonging to the group 3 LEA family in a cDNA library of the indica rice Minghui 63 (Chu et al. 2003). OsLEA3-1 protein sequence has 97% identity to OsLEA3 identified previously in rice (Moons et al. 1997) and OsLEA3-1 is considered to be an allele of OsLEA3 based on its location in the rice genome. The OsLEA3-1 protein has 56% identity to HVA1 from barley (Hong et al. 1988), 52% identity to group 3 LEA protein MGL3 from maize, and 53% identity to group 3 LEA protein pMA2005 from wheat (Curry et al. 1991; Curry and Walker-Simmons 1993) as revealed by ClustalW (Chenna et al. 2003) analysis (Fig. 1).
Alignment of deduced amino acid sequence of OsLEA3-1 with representative reported LEA proteins using ClustalW program (Chenna et al. 2003). Dashes were introduced to maximize sequence alignments. Identical (black) and conserved residues (grey) are highlighted. The accession numbers for the aligned sequences are as follows: OsLEA3-1, DQ789359; OsLEA3, AAV67829; HVA1, CAA31853; MGL3, CAA82632; pMA2005, CAA40204
RNA gel blot analysis was performed to investigate the expression of OsLEA3-1 in the seedling leaves of upland rice IRAT109 treated by drought, salt, ABA and cold (Fig. 2a). Transcript level of OsLEA3-1 began to increase at 6 days after water withholding and was strongly induced at 8 days after water withholding. After salt (200 mM NaCl) treatment, the OsLEA3-1 transcript was induced within 18 h and peaked at 72 h. The OsLEA3-1 was induced within 3 h after ABA treatment and its transcript level peaked at 48 h. However, the expression of OsLEA3-1 was not induced by low temperature (4°C). These results indicated that OsLEA3-1 was strongly induced by drought and salt stresses and ABA treatment but not by cold stress in the upland rice cultivar IRAT109.
Stress-inducible expression of OsLEA3-1 gene. a RNA gel blot analysis of OsLEA3-1 expression in 3-week old IRAT109 seedlings treated with drought, 200 mM NaCl, 100 μM ABA, and cold. Total RNA was isolated from leaf tissues at the time points specified on the top of the blots (d days, h hours). The relative amount of total RNA loaded in each lane is shown by ethidium bromide staining. The probe is the cDNA of OsLEA3-1 obtained by the digestion of the cDNA clone with BamHI and KpnI. b GUS activity assay of OsLEA3-1 promoter::GUS in 3-week old transgenic plants treated by drought stress (with water deprived from the hydroponic cultured plants), salt (200 mM NaCl), ABA (100 μM) and cold (4°C) stress. Values are mean of three replications
Identification of OsLEA3-1 promoter
Since OsLEA3-1 was strongly induced by drought in upland rice IRAT109, we isolated the promoter region (1,253 bp upstream of the transcribed sequence of the gene, designated HVA1-like promoter, accession no. DQ837728) from IRAT109 based on the genomic sequence of Nipponbare. Promoter sequence search against the PLACE database (http://www.dna.affrc.go.jp/PLACE/) suggested that more number of putative ABA responsive elements (ABRE) exist in the promoter sequence from IRAT109 than in Nipponbare (data not shown). The GUS reporter gene under the control of the HVA1-like promoter from IRAT109 was transformed into rice Zhonghua 11. GUS activity was strongly induced by drought and salt stresses and ABA treatment but not by cold in the transgenic plants (Fig. 2b), which agrees well with the results from RNA gel blot analysis of OsLEA3-1. This promoter was therefore used for making the stress-inducible cDNA construct used in this study.
Generation and identification of transgenic rice of OsLEA3-1
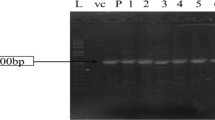
Three constructs, OsLEA3-S, OsLEA3-A and OsLEA3-H, were generated by fusing the cDNA of the OsLEA3-1 gene with CaMV 35S, Actin1 and HVA1-like promoter in the backbone vectors pCAMBIA1301S, pCAMBIA1301A, and pCAMBIA1301H, respectively (Fig. 3a). These constructs contain the GUS reporter gene under the control of CaMV 35S promoter, and the Hpt selection gene under the control of CaMV 35S promoter (Fig. 3a). Constructs were transformed into japonica rice Zhonghua 11, which is drought-sensitive compared to IRAT109 (unpublished data) and has a relatively high efficiency of transformation in comparison with indica rice cultivars (Lin et al. 2005). More than 600 transgenic plants, about 200 plants per construct, were generated. Transformed plants (T0 generation) were identified by PCR using primers specific to the hygromycin resistance gene (Hpt). Among 204 independent regenerants checked, an expected band (564 bp) was amplified in 187, suggesting that more than 90% of regenerants were transformed (Fig. 3b–d).
Molecular identification of transgenic plants. a Schematic diagram of the constructs for rice transformation. P represents CaMV 35S (S), Actin1 (A), or HVA1-like promoter (H). LB and RB represent T-DNA left and right border, respectively. The hygromycin phosphotransferase gene (Hpt) under the control of CaMV 35S promoter was used as a selective marker gene. E: EcoRI; B: BamHI; K: KpnI. b–d PCR (top in each panel), Southern (middle) and RNA blot (bottom) analysis of transgenic plants of OsLEA3-S (b), OsLEA3-A (c), and OsLEA3-H (d) constructs. A total of 120 plants were analyzed and only 15 plants for each construct were shown. PCR was conducted using Hpt gene-specific primers. The Hpt and OsLEA3-1 gene fragments were used as probes for Southern and RNA blot hybridization, respectively. M, 2 kb DNA marker; WT, wild type Zhonghua 11
Southern blot hybridization was performed using the Hpt-specific fragment as a probe. Among 90 independent transformants (30 plants per construct, Fig. 3b–d) checked, about 45% of the checked transformants harbored single copy of the transgene, and approximately 40% of the transformants contained 2–3 copies. These percentages are similar to previous reports (Garg et al. 2002; Wu et al. 2003). There were very few plants that appeared to be positive by PCR analysis but not positive by Southern hybridization. The hybridization pattern of each transgenic plant was unique, suggesting that these plants were derived from independent transformation events.
The expression level of OsLEA3-1 in the mixed leaf tissues of 20 plants from each T1 family derived from the T0 transgenic plants analyzed by Southern hybridization was checked by RNA gel blot analysis using the OsLEA3-1 gene as a probe (Fig. 3b–d). The percentage of over-expression (or drought-induced over-expression for OsLEA3-H) was 63, 56, and 46% for OsLEA3-S, OsLEA3-A, and OsLEA3-H, respectively. The transcript level of OsLEA3-1 in the wild type (WT) plants was almost undetectable under drought stress conditions (Fig. 3b).
Drought resistance pre-screening of T1 transgenic families
T1 families over-expressing the OsLEA3-1 gene from each construct (Fig. 3b–d) were pre-screened for drought resistance under the sheltered field (drought stress was applied at anthesis stage) in 2004. Morphology and yield comparison under normal irrigation conditions were also examined at that time. Under the normal growth conditions, the average values of grain yield and spikelet fertility of T1 families over-expressing the OsLEA3-1 gene for each construct (referred to over-expression group hereafter) were significantly (P < 0.01) lower than WT (Table 1). Only about 15% of transgenic families showed no significant difference in yield per plant compared to WT. The reduced yield of the transgenic plants under normal growth conditions (yield penalty) was mainly due to the reduced spikelet fertility (Table 1) as the number of spikelets per plant and the grain weight showed no significant difference between over-expression groups and WT (data not shown). Considering the yield penalty in T1 transgenic families, the relative yield (ratio of the yield in the stressed field to that in the normal irrigation field), a reliable parameter for evaluation of drought resistance according to Yue et al. (2006), was compared between each over-expression group and WT (Table 1). The results suggested that the relative yield of over-expression groups of the OsLEA3-S (52.34%) and OsLEA3-H (54.31%) constructs were significantly (P < 0.01) higher than that of WT (36.42%). However, there was no significant difference in the relative yield between the over-expression group of OsLEA3-A construct (35.73%) and WT. We also observed segregation of drought sensitivity (such as leaf rolling, Fig. 4a–b) for some T1 families over-expressing a single copy of the transgene. These results suggested that expression of OsLEA3-1 by a drought-inducible HVA1-like promoter and constitutive promoter CaMV 35S had significant effect on improving drought resistance in terms of relative yield, though a severe yield penalty existed in the T1 generation.
Drought resistance of transgenic families in the field. Photographs were taken just before re-watering for recovery after the drought stress at the anthesis stage in the field. a–b Segregation of drought resistance (degree of leaf-rolling) within T1 transgenic families over-expressing a single copy of the transgene with OsLEA3-S (a) and OsLEA3-H (b) constructs. c–d Field performance of homozygous T2 lines with OsLEA3-S (c) and OsLEA3-H (d) constructs under drought stress conditions. WT wild type Zhonghua 11
Drought resistance testing of homozygous T2 lines
To confirm the increased drought resistance of the T1 families, T2 seeds from the transgenic plants showing improved drought resistance within the T1 families were harvested to identify homozygous lines by GUS staining. The T1 families from which T2 seeds were harvested contained single copies of the transgenes which were over-expressed constitutively (OsLEA3-S) or during drought (OsLEA3-H) and exhibited relatively lower yield penalty. Three OsLEA3-S (S-6, S-18, S-21), two OsLEA3-A (A-2, A-29), and four OsLEA3-H (H-8, H-14, H-23, H-24) homozygous T2 lines were selected for drought resistance testing in the sheltered field and PVC pipes in 2005.
In the field with normal irrigation, grain yield per plant of all the transgenic lines (28.5–30.8 g) and WT (31.1 g) was not significantly different (Table 2), suggesting very little yield penalty, if any, in these lines. Based on this result, grain yield was used for comparing drought resistance between these transgenic lines and WT. Under severe drought-stressed field conditions, the homozygous transgenic lines with OsLEA3-S and OsLEA3-H constructs showed less rolled or dead leaves than WT at the flowering stage (Fig. 4c–d). The grain yield per plant of all seven homozygous lines with the OsLEA3-S and OsLEA3-H constructs (15.2–17.8 g) was higher or significantly (P < 0.05 for S-18, H-8, H-24) higher than that of WT (12.1 g), whereas the two lines (A-2, A-29) with OsLEA3-A construct (12.0–12.4 g) showed no difference compared to WT. These transgenic lines were also tested for drought tolerance, a major mechanism for drought resistance, by growing the plants in PVC pipes according to Yue et al. (2006). Except for A-2 and A-29 lines, grain yield of all the other seven lines (20.4–23.6 g per plant) was higher or significantly (P < 0.05 for S-18, H-8, H-24) higher than that of WT (17.8 g per plant) under drought stress conditions (Table 2). Under normal irrigation conditions, all the lines and WT showed no significant difference in yield (Table 2).
In addition to grain yield, spikelet fertility (%) of the seven homozygous T2 lines for OsLEA3-S and OsLEA3-H constructs was higher than WT under drought stress conditions in both the field and the PVC pipes. Significantly (P < 0.05) higher spikelet fertility than WT was observed in five lines (S-6, S-18, H-8, H-14, H-24) in the sheltered field experiment and four lines (S-18, H-8, H-14, H-24) in the PVC pipes experiment. No significant difference was detected between any transgenic lines and WT under the normal irrigation conditions (Table 2). Moreover, no significant difference was detected for the other two yield component traits (number of spikelets per plant and grain weight) between transgenic lines and WT in all treatments.
Drought resistance testing of homozygous T3 lines
To verify the improved drought resistance of the T2 lines described above, nine homozygous T3 lines derived from the nine T2 lines tested in 2005 were tested under both drought stress and normal growth conditions in the field in 2006. The results showed that six T3 lines (S-6, S-18, H-8, H-14, H-23, H-24) with the OsLEA3-S and OsLEA3-H constructs had significantly (P < 0.05) higher grain yield per plant than WT under the drought stress conditions, and the spikelet fertility of all the homozygous T3 lines with OsLEA3-S and OsLEA3-H constructs was significantly (P < 0.05) higher than that of WT (Table 3). No significant difference in grain yield and spikelet fertility was detected between these T3 lines and WT under normal growth conditions (Table 3). For the A-2 and A-29 lines containing the OsLEA3-A construct, the yield and spikelet fertility were not significantly (P < 0.05) different from those of WT under drought stress conditions.
Two other yield components (number of spikelets per plant and grain weight) were also investigated under both drought stress and normal field conditions. In both cases, no significant difference was detected between the T3 lines and WT, suggesting that the better yield performance of transgenics under drought stress conditions was mainly due to the relatively higher spikelet fertility.
Discussion
Improved drought resistance of OsLEA3-1-overexpressed transgenic rice in the field
To date, there have been many reports of the development of transgenic plants with improved drought resistance by manipulation of the expression of stress related genes in laboratory or greenhouse conditions (Holmström et al. 1996; Xu et al. 1996; Shen et al. 1997; Garg et al. 2002; Dubouzet et al. 2003; Xiong and Yang 2003; Park et al. 2005). However, there are very few studies in which drought resistance of transgenic plants has been tested in the field (Wang et al. 2005; Hu et al. 2006). The results obtained under the laboratory or greenhouse conditions may be partially consistent with those obtained in the field, but must be further confirmed in drought-stressed field environments (Shao et al. 2005).
In this study, transgenic T1 families over-expressing OsLEA3-1 gene were pre-screened for drought resistance in terms of relative yield in the field, then nine homozygous T2 and T3 lines were tested for drought resistance in the consecutive 2 years (T2 in 2005 and T3 in 2006) in the field. Among the nine lines, three lines (S-18, H-8, H-24) showing significantly improved drought resistance in terms of yield in the T2 generation continued to display drought resistance in the T3 generation. Three lines (S-6, H-14, H-23) showing higher (but not significant) yield than WT under drought stress conditions in T2 generation displayed significant drought resistance in the T3 generation. The remaining three lines (S-21, A-2, A-29) did not show improved drought resistance in both years (Tables 2, 3). Some lines such as H-8 exhibited even better drought resistance in the T3 generation (significance level P < 0.01) than in the T2 generation (significance level P < 0.05). These results suggested that drought resistance of transgenic lines over-expressing the OsLEA3-1 gene can be improved through selections in terms of the relative yield in the T1 generation or yield in the T2 or later generations under the field conditions.
Data from a number of previous studies suggested accumulation of LEA proteins was correlated with stress tolerance in oat (Maqbool et al. 2002), rice (Moons et al. 1995, 1997; Xu et al. 1996), wheat (Ried and Walker-Simmons 1993), and tobacco (Kim et al. 2005). Delayed leaf symptoms (such as wilting and dying of old leaves) caused by water-deficit stress were observed in the juvenile R1 transgenic rice over-expressing a barley group 3 LEA gene (HVA1) (Xu et al. 1996). Here we also observed delayed leaf wilting in the transgenic rice lines over-expressing OsLEA3-1 gene at the flowering stage (Fig. 4c–d). The delay of leaf wilting may allow more spikelets of the transgenic plants to develop and flower normally. Our data indeed indicated that transgenic lines had significantly higher spikelet fertility than WT.
It is interesting to note that the OsLEA3-1 gene is located within the interval (between RM421 and RM274 on chromosome 5) of a drought tolerance-related QTL qRSF5 for relative spikelet fertility in the Zhenshan 97/IRAT109 population (Yue et al. 2006). We are generating a near-isogenic line (IRAT109 allele in Zhenshan 97’s background) to verify the function of this gene and its relationship with the QTL.
Effect of OsLEA3-1 controlled by different promoters in improving drought resistance at the reproductive stage of rice
In this study, three different promoters (CaMV 35S, Actin1, HVA1-like) were used to drive the expression of OsLEA3-1 gene in transgenic rice for enhancing drought resistance. Grain yield and spikelet fertility were used to evaluate drought resistance under field conditions. T2 and T3 homozygoues lines over-expressing OsLEA3-1 under the control of the CaMV 35S or the drought-inducible HVA1-like promoter produced significantly higher yield than WT under drought stress conditions. Generally, promoters from drought-inducible genes such as the OsLEA3-1 in this study, rd29A (Yamaguchi-Shinozaki and Shinozaki 1994), cor15a (Baker et al. 1994), kin1 and cor6.6 (Wang and Cutler 1995; Wang et al. 1995) have advantages in maximizing the effects of transgenes on stress resistance improvement compared to constitutive promoters such as rice Actin1 and maize Ubiquitin (Yamaguchi-Shinozaki and Shinozaki 1994; Su et al. 1998; Zhao et al. 2000a; Garg et al. 2002). Transgenes controlled by drought-inducible promoters may have strong expression only under drought stress conditions and low level of expression under normal irrigation conditions, thus minimizing possible side effects resulting from the over-expression of the target gene (Zhao et al. 2000a). We indeed observed a much higher frequency of abnormal T0 plants (such as dwarfism or sterility) with the constitutive OsLEA3-A and OsLEA3-S constructs than that with the inducible OsLEA3-H construct (data not shown). In this study, the yield of some selected drought-resistance-improved lines with the OsLEA3-S construct was significant higher than that of WT under drought stress conditions (Tables 2, 3), suggesting that the OsLEA3-S construct with constitutive promoter CaMV 35S can also be used to generate transgenic lines with improved drought resistance.
Previously, Xu et al. (1996) reported that the over-expression of the barley LEA gene HVA1 driven by the Actin1 promoter conferred drought resistance to rice seedlings. However, in our study, the two homozygous lines over-expressing OsLEA3-1 gene by Actin1 promoter had no significant effect on improving drought resistance, though the yield of transgenic lines under drought stress were slightly higher than that of WT under drought stress conditions (Table 3). There are three possible explanations for this discrepancy. First, different traits measured in different environments were used to evaluate the drought resistance for the two genes. We used yield or relative yield under drought-stressed field conditions as criteria in order to evaluate the potential usefulness of the OsLEA3-1 gene for drought resistance breeding, while the drought resistance effect measured by Xu et al. (1996) was based on growth rate and leaf damage symptoms under the water-deficit conditions in greenhouse. We also observed that some of OsLEA3-A over-expressors exhibited delayed leaf rolling during the process of drought stress development in the field (data not shown). Second, the number of T1 families (16) over-expressing OsLEA3-1 gene by Actin1 promoter may be limited for pre-screening, considering the fact that not all the lines exhibiting over-expression of the transgene showed significantly improved drought resistance in terms of yield (e.g., OsLEA3-S line S-21). Therefore, it may be possible to identify drought-resistant lines by screening more T1 families. Third, the strong activity of Actin1 promoter (McElroy et al. 1990) may interfere with the endogenous gene expression or translation of the gene (Zhao et al. 2000a) in the two lines.
Yield penalty associated with Agrobacterium-mediated transformation in rice
Although the T1 families selected for drought resistance testing exhibited the same phenotypes as WT for the majority of morphological traits (e.g., plant height, plant structure, number of tillers, and flowering time), most of these transgenic families had significantly lower grain yield than WT under normal irrigation conditions (Table 1). This yield penalty may be due to several effects associated with the Agrobacterium-mediated transformation of rice. First, the transgenic plants were derived from tissue culture, which may have potential detrimental effects, particularly in the T0 generation, on the growth and productivity (Stam et al. 1997; Liu et al. 1998; Kasuga et al. 1999; Chen et al. 2005). Second, since Agrobacterium-mediated transformation generates random insertions of T-DAN into the recipient genome (Hiei et al. 1994; Wu et al. 2003) and yield is associated with many genes (Yoon et al. 2006), it is possible that genes related to yield were disrupted. Third, introduction of the transgene may lead to genetic or physiological incompatibility (Holmström et al. 1996; Romero et al. 1997; Meyer 2000; Stempak et al. 2005). While some morphological changes (e.g., plant height, leaf color, erectness of stem) can be easily observed during vegetative development, changes in yield and yield-related traits may be difficult to observe.
In this study, T1 families used for pre-screening exhibited normal development during vegetative growth, but the yield of most families was significantly reduced compared to WT under the non-stress conditions. Thus, selection of transgenic families for drought resistance testing should be based on not only morphological characters but also yield or yield-related traits, especially for major crops (e.g., rice, wheat, maize) in which the yield is the ultimate goal for crop production, and yield performance in drought stressed field is the most important criterion for assessing drought resistance (Turner 1979, 1997). Promising transgenic lines for drought resistance breeding should meet the following criteria: significantly improved drought resistance, no phenotypic changes, no yield penalty, and over-expression of a single copy transgene. Having a single copy of the transgene is very important for transgenic breeding because multiple gene copies can lead to instability of expression and inheritance of transgene or even gene silencing (Stam et al. 1997; Chen et al. 2005).
Although only 30 independent T1 families for each construct (about half of them have single copy of the transgene) were pre-screened in the both stressed and non-stressed fields, a few promising families meeting the above criteria were identified. If more T1 families over-expressing a single copy of the transgene are included in the pre-screening, it may be possible to obtain transgenic lines with better drought resistance than the lines described here. Using this protocol, a limited number of homozygous T2 and T3 lines with improved drought resistance (in terms of yield and spikelet fertility) and no yield penalty were identified for formal field trail. We believe that such a bipartite (stress and non-stress) in-field screening protocol can be successfully applied to the field-testing of other transgenic crops with minor modification.
References
Bake J, Steele C, Dure LI (1988) Sequence and characterization of 6 LEA proteins and their genes from cotton. Plant Mol Biol 11:277–291
Baker SS, Wilhelm KS, Thomashow MF (1994) The 5′-region of Arabidopsis thaliana cor15a has cis-acting elements that confer cold-, drought- and ABA-regulated gene expression. Plant Mol Biol 24:701–713
Bray EA, Bailey-Serres J, Weretilnyk E (2000) Responses to abiotic stresses. In: Gruissem W, Buchannan B, Jones R (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, pp 1158–1249
Chen H, Tang W, Xu C, Li X, Lin Y, Zhang Q (2005) Transgenic indica rice plants harboring a synthetic cry2A* gene of Bacillus thuringiensis exhibit enhanced resistance against lepidopteran rice pests. Theor Appl Genet 111:1330–1337
Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31:3497–3500
Chu ZH, Peng KM, Zhang LD, Zhou B, Wei J, Wang SP (2003) Construction and characterization of a normalized whole-life-cycle cDNA library of rice. Chin Sci Bull 48:229–235
Cuming AC (1999) LEA proteins. In: Shewry PR, Casey R (eds) Seed proteins. Kluwer, Dordrecht, pp 753–780
Curry J, Walker-Simmons MK (1993) Unusual sequence of group 3 LEA (II) mRNA inducible by dehydration stress in wheat. Plant Mol Biol 21:907–912
Curry J, Morris CF, Walker-Simmons MK (1991) Sequence analysis of a cDNA encoding a group 3 LEA mRNA inducible by ABA or dehydration stress in wheat. Plant Mol Biol 16:1073–1076
Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J 33:751–763
Dure LIII, Crouch M, Harada J, Ho T-H, Mundy J, Quatrano RS, Thomas T, Sung ZR (1989) Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol Biol 12:475–486
Garay-Arroyo A, Colmenero-Flores JM, Garciarrubio A, Covarrubias AA (2000) Highly hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water deficit. J Biol Chem 275:5668–5674
Garg AK, Kim JK, Owens TG, Ranwala AP, Choi YD, Kochian LV, Wu RJ (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc Natl Acad Sci USA 99:15898–15903
Goyal K, Walton LJ, Tunnacliffe A (2005) LEA proteins prevent protein aggregation due to water stress. Biochem J 388:151–157
Grelet J, Benamar A, Teyssier E, Avelange-Macherel MH, Grunwald D, Macherel D (2005) Identification in pea seed mitochondria of a late-embryogenesis abundant protein able to protect enzymes from drying. Plant Physiol 137:157–167
Hayashi H, Alia, Mustardy L, Deshnium P, Ida M, Murata N (1997) Transformation of Arabidopsis thaliana with the codA gene for choline oxidase; accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. Plant J 12:133–142
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Hoisington D, Jiang C, Khairallah M, Ribaut JM, Bohn M, Melchinger A, Willcox M, Gonzalez-de-Leon D (1996) QTL for insect resistance and drought tolerance in tropical maize: prospects for marker assisted selection. Symp Soc Exp Biol 50:39–44
Holmström K-O, Mäntylä E, Welin B, Mandal A, Palva ET, Tunnela OE, Londesborough J (1996) Drought tolerance in tobacco. Nature 379:683–684
Hong B, Uknes SJ, Ho T-HD (1988) Cloning and characterization of a cDNA encoding a mRNA rapidly induced by ABA in barley aleurone layers. Plant Mol Biol 11:495–506
Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, Xiong L (2006) Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc Natl Acad Sci USA 103:12987–12992
Ingram J, Bartels D (1996) The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol 47:377–403
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1999) Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 17:287–291
Kim HS, Lee JH, Kim JJ, Kim CH, Jun SS, Hong YN (2005) Molecular and functional characterization of CaLEA6, the gene for a hydrophobic LEA protein from Capsicum annuum. Gene 344:115–123
Kishor P, Hong Z, Miao GH, Hu C, Verma D (1995) Over-expression of Δ-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108:1387–1394
Lin YJ, Zhang Q (2005) Optimising the tissue culture conditions for high efficiency transformation of indica rice. Plant Cell Rep 23:540–547
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10:1391–1406
Maqbool B, Zhong H, El-Maghraby Y, Ahmad A, Chai B, Wang W, Sabzikar R, Sticklen B (2002) Competence of oat (Avena sativa L.) shoot apical meristems for integrative transformation, inherited expression, and osmotic tolerance of transgenic lines containing hva1. Theor Appl Genet 105:201–208
McElroy D, Zhang W, Cao J, Wu R (1990) Isolation of an efficient actin promoter for use in rice transformation. Plant Cell 2:163–171
Meyer P (2000) Transcriptional transgene silencing and chromatin components. Plant Mol Biol 43:221–234
Moons A, Bauw G, Prinsen E, Van Montagu M, Van der Straeten D (1995) Molecular and physiological responses to abscisic acid and salts in roots of salt-sensitive and salt-tolerant indica rice varieties. Plant Physiol 107:177–186
Moons A, De Keyser A, Van Montagu M (1997) A group 3 LEA cDNA of rice, responsive to abscisic acid, but not to jasmonic acid, shows variety-specific differences in salt stress response. Gene 191:197–204
NDong C, Danyluk J, Wilson KE, Pocock T, Huner NP, Sarhan F (2002) Cold-regulated cereal chloroplast late embryogenesis abundant-like proteins: molecular characterization and functional analyses. Plant Physiol 129:1368–1381
Park S, Li J, Pittman JK, Berkowitz GA, Yang H, Undurraga S, Morris J, Hirschi KD, Gaxiola RA (2005) Up-regulation of a H+-pyrophosphatase (H+-P Pase) as a strategy to engineer drought-resistant crop plants. Proc Natl Acad Sci USA 102:18830–18835
Raynal M, Guilleminot J, Gueguen C, Cooke R, Delseny M, Gruber V (1999) Structure, organization and expression of two closely related novel Lea (late-embryogenesis-abundant) genes in Arabidopsis thaliana. Plant Mol Biol 40:153–165
Ried JL, Walker-Simmons MK (1993) Group 3 late embryogenesis abundant proteins in desication-tolerant seedlings of wheat (Triticum aestivum L.). Plant Physiol 102:125–131
Romero C, Belles J, Vaya J, Serrano R, Culianez-Macia F (1997) Expression of the yeast trehalose-6-phosphate synthase gene in transgenic tobacco plants: pleiotropic phenotypes include drought tolerance. Planta 201:293–297
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor, New York
Shao HB, Liang ZS, Shao MA (2005) LEA proteins in higher plants: structure, function, gene expression and regulation. Colloids Surf B Biointerfaces 45:131–135
Shen B, Jensen RG, Bohnert HJ (1997) Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiol 113:1177–1183
Soltis DE, Soltis PS (2003) The role of phylogenetics in comparative genetics. Plant Physiol 132:1790–1800
Stam M, Mol JN, Kooter JM (1997) The silence of genes in transgenic plants. Ann Bot 79:3–12
Stempak JM, Sohn KJ, Chiang EP, Shane B, Kim YI (2005) Cell and stage of transformation-specific effects of folate deficiency on methionine cycle intermediates and DNA methylation in an in vitro model. Carcinogenesis 26:981–990
Su J, Shen Q, David Ho TH, Wu R (1998) Dehydration-stress-regulated transgene expression in stably transformed rice plants. Plant Physiol 117:913–922
Thomashow MF (1998) Role of cold-responsive genes in plant freezing tolerance. Plant Physiol 118:1–8
Thomashow MF (1999) Plant Cold Acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50:571–599
Turner NC (1979) Drought resistance and adaptation to water deficits in crop plants. In: Mussall H (ed) Stress physiology in crop plants, Wiley, New York, pp 343–372
Turner NC (1997) Further progress in crop water relations. Adv Agron 58:293–339
Wang H, Cutler AJ (1995) Promoters from kin1 and cor6.6, two Arabidopsis thaliana low-temperature- and ABA-inducible genes, direct strong β-glucuronidase expression in guard cells, pollen and young developing seeds. Plant Mol Biol 28:619–634
Wang H, Datla R, Georges F, Loewen M, Cutler AJ (1995) Promoters from kin1 and cor6.6, two homologous Arabidopsis thaliana genes: transcriptional regulation and gene expression induced by low temperature, ABA, osmoticum and dehydration. Plant Mol Biol 28:605–617
Wang Y, Ying J, Kuzma M, Chalifoux M, Sample A, McArthur C, Uchacz T, Sarvas C, Wan J, Dennis DT, McCourt P, Huang Y (2005) Molecular tailoring of farnesylation for plant drought tolerance and yield protection. Plant J 43:413–424
Wise (2003) Leaping to conclusions: a computational reanalysis of late embryogenesis abundant proteins and their possible roles. BMC Bioinformatics 4:52
Wu C, Li X, Yuan W, Chen G, Kilian A, Li J, Xu C, Zhou DX, Wang S, Zhang Q (2003) Development of enhancer trap lines for functional analysis of the rice genome. Plant J 35:418–427
Xiong L, Yang Y (2003) Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid-inducible mitogen-activated protein kinase. Plant Cell 15:745–759
Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14(Suppl):S165–S183
Xu D, Duan X, Wang B, Hong B, Ho T, Wu R (1996) Expression of a late embryogenesis abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol 110:249–257
Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6:251–264
Yoon DB, Kang KH, Kim HJ, Ju HG, Kwon SJ, Suh JP, Jeong OY, Ahn SN (2006) Mapping quantitative trait loci for yield components and morphological traits in an advanced backcross population between Oryza grandiglumis and the O. sativa japonica cultivar Hwaseongbyeo. Theor Appl Genet 112:1052–1062
Yue B, Xue W, Xiong L, Yu X, Luo L, Cui K, Jin D, Xing Y, Zhang Q (2006) Genetic basis of drought resistance at reproductive stage in rice: separation of drought tolerance from drought avoidance. Genetics 172:1213–1228
Zhang HX, Hodson JN, Williams JP, Blumwald E (2001) Engineering salt-tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc Natl Acad Sci USA 98:12832–12836
Zhang Q, Saghai Maroof MA, Lu TY, Shen BZ (1992) Genetic diversity and differentiation of indica and japonica rice detected by RFLP analysis. Theor Appl Genet 83:495–499
Zhang X, Zhou S, Fu Y, Su Z, Wang X, Sun C (2006) Identification of a drought tolerant introgression line derived from Dongxiang common wild rice (O. rufipogon Griff.). Plant Mol Biol 62:247–259
Zhao HW, Chen YJ, Hu YL, Gao Y, Lin ZP (2000a) Construction of a trehalose-6-phosphate synthase gene driven by drought-responsive promoter and expression of drought-resistance in transgenic tobacco. Acta Bot Sin 42:616–619
Zhao SH, Wang FZ, Lu L, Zhang HY, Zhang XY (2000b) Breeding and selection of drought resistant and salt tolerant wheat variety Cang 6001. Acta Agric Boreall Sin 15:113–117
Acknowledgments
We thank Drs. Abraham Blum and Qifa Zhang for suggestions on drought resistance testing protocol of rice in the field. This research was supported by the National Program on Basic Research and Development, the National Special Key Project on Functional Genomics of Rice, the National Natural Science Foundation of China, and the Rockefeller Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Paterson.
Rights and permissions
About this article
Cite this article
Xiao, B., Huang, Y., Tang, N. et al. Over-expression of a LEA gene in rice improves drought resistance under the field conditions. Theor Appl Genet 115, 35–46 (2007). https://doi.org/10.1007/s00122-007-0538-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-007-0538-9