Abstract
OsWRKY47 is a divergent rice transcription factor belonging to the group II of the WRKY family. A transcriptomic analysis of the drought response of transgenic rice plants expressing P SARK ::IPT, validated by qPCR, indicated that OsWRKY47 expression was induced under drought stress in P SARK ::IPT plants. A PCR-assisted site selection assay (SELEX) of recombinant OsWRKY47 protein showed that the preferred sequence bound in vitro is (G/T)TTGACT. Bioinformatics analyses identified a number of gene targets of OsWRKY47; among these two genes encode a Calmodulin binding protein and a Cys-rich secretory protein. Using Oswrk47 knockout mutants and transgenic rice overexpressing OsWRKY47 we show that the transcription of these putative targets were regulated by OsWRKY47. Phenotypic analysis carried out with transgenic rice plants showed that Oswrky47 mutants displayed higher sensitivity to drought and reduced yield, while plants overexpressing OsWRKY47 were more tolerant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
WRKY transcription factors (TF) are defined by the presence of a 60 amino acid conserved region containing a WRKY and a zinc-finger-like motif. WRKY TFs have been classified in three groups according to the number of WRKY domains that they encode and to the zinc finger motif pattern. Members of group I exhibit two WRKY motifs and those of group II have only one motif (Eulgem et al. 2000); both groups I and II have a zinc finger type C2–H2 (C–X4–5–C–X22–23–H–X1–H). A third group, group III, is formed by proteins with one WRKY motif and a zinc finger type C2–HC(C–X7–C–X23–H–X1–C). Sequence alignment tools and phylogenetic analyses have been used for the identification and classification of WRKY TFs in a few species such as Arabidopsis thaliana (Eulgem et al. 2000), Oryza Sativa (Wu et al. 2005; Xie et al. 2005; Ramamoorthy et al. 2008), Hordeum vulgare (Mangelsen et al. 2008) and Heilanthus annuus (Giacomelli et al. 2010), among others. In rice, the WRKY family comprises 102 proteins (Wu et al. 2005). All the known WRKY TFs tested so far, bind in vitro a sequence named W-box containing an invariant TGAC core, and variations outside this core (Eulgem et al. 2000).
WRKY TFs play pivotal roles in the complex signaling mediating plant responses to biotic and abiotic stresses (Ulker and Somssich 2004; Eulgem and Somssich 2007; Rushton et al. 2010; Chen et al. 2012; Tripathi et al. 2014; Schluttenhofer and Yuan 2015). Overexpression of soybean GmWRKY13 in Arabidopsis conferred higher sensitivity to salinity and osmotic stress in the transgenic plants (Zhou et al. 2008). On the other hand, the expression of the barley HvWRKY38 in Paspalum notatum Flugge increased the drought tolerance of the transgenic plants (Xiong et al. 2010). Similar results were obtained when the Glycine Soja GsWRKY20 was expressed in Arabidopsis (Luo et al. 2013) and the maize ZmWRKY58 was overexpressed in transgenic rice (Cai et al. 2014). In rice, the overexpression of OsWRKY7 (Ramamoorthy et al. 2008), OsWRKY05, OsWRKY43, OsWRKY1, OsWRKT2 (Berri et al. 2009) and OsWRKY11 (Wu et al. 2009) was shown to improved drought tolerance.
A number of mechanisms associated with the ability of plants to tolerate water deficit stress have been described (Chen et al. 2012; Reguera et al. 2012; Suzuki et al. 2014). They involve regulatory molecules, TFs and hormones such as ABA, cytokinin (CK), ethylene and their crosstalk mechanisms (Peleg and Blumwald 2011). Among these, the CK-induced delayed senescence has been shown to be effective in increasing the tolerance of plants to water deficit (Rivero et al. 2007). Transgenic plants expressing the IPT gene, encoding a key enzyme in CK synthesis, under the control of SARK (a stress- and maturity-induced promoter) displayed enhanced photosynthesis and high yields under water stress (Rivero et al. 2007; Peleg et al. 2011b; Reguera et al. 2013). A Gene expression profile of flag leaves from wild-type plants and transgenic rice plants expressing P SARK ::IPT, highlighted the differential expression of OsWRKY47 in the P SARK ::IPT plants under water stress (Peleg et al. 2011b). The correlation between the enhanced stress tolerance of the transgenic P SARK ::IPT plants and the enhanced OsWRKY47 expression suggested a possible role of OsWRKY47 in water stress tolerance.
OsWRKY47 is a TF divergent from its more related proteins in rice and other species. It presents 50 and 42 % similarity with WRKY38 and WRKY70 of Setaria italica, respectively; 42 % similarity with BdWRKY70 of Brachypodium distachyon and 46 % similarity with the barley HvWRKY4. The Arabidopsis TFs most related to OsWRKY47 are AtWRKY46, AtWRKY54 and AtWRKY70; however, the sequence similarity with these proteins is relatively low. AtWRKY70 and AtWRKY54 have been associated with leaf senescence in Arabidopsis (Besseau et al. 2012). Atwrky70 mutant plants displayed an early senescence phenotype, and a role as a negative regulator of senescence was suggested for this TF (Ülker et al. 2007). In spite of the high sequence and structure similarity between AtWRKY70 and AtWRKY54, Atwrky54 mutant plants did not show changes in senescence (Besseau et al. 2012). Nevertheless, double Atwrky54-Atwrky70 knockout mutant plants showed an increased early senescence as compared to that displayed by Atwrky70, thus suggesting a possible cooperative interaction between AtWRKY70 and AtWRKY54 negatively regulating leaves senescence (Besseau et al. 2012).
Here, we describe experiments aimed at elucidating OsWRKY47 function(s) in the enhanced drought tolerance of transgenic P SARK ::IPT rice plants.
Results
OsWRKY47 expression is induced in water stressed P SARK ::IPT plants
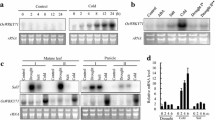
Transcriptome analysis of wild-type (WT) and transgenic P SARK ::IPT rice plants (Peleg et al. 2011b) showed induced expression of OsWRKY47 under WS in P SARK -IPT plants. qPCR validation showed that the OsWRKY47 transcript levels increased sixfold after 3 days of WS and decreased during the 4th day of stress in P SARK- IPT plants (Fig. 1). These levels were significant higher than those shown by the WT plants under WS. The OsWRKY47 levels of expression returned to WW levels after re-watering (Fig. 1).
Relative expression of OsWRKY47 in WT and transgenic P SARK ::IPT plants. BS before stress (BS), 3DS and 4DS, days of water stress and ReW, 3 days after re-watering. WW well-watered, WS water-stress, WT wild type rice; 5T, P SARK ::IPT plants. Samples were harvested at the indicated periods. Values were calculated and normalized using the rice transcription elongation factor as internal control. Values are the mean ± SD (n = 6)
OsWRKY47 preferentially binds the sequence GTTGACC in vitro
In order to identify signal pathway(s) components associated with OsWRKY47 in P SARK -IPT plants, we identified DNA sequences that could be specifically bound by OsWRKY47. A PCR-assisted binding site selection (SELEX) assay was applied as an experimental strategy. The binding domain of OsWRKY47 was expressed in E. coli cells as a fusion with S. Japonicum glutathione S-transferase and purified by affinity chromatography. Seven SELEX rounds were performed, and since no additional improvement was detected after the 7th round, the PCR products were cloned and 14 individual clones were randomly selected for sequencing. The sequences were aligned (Fig. 2a) and the alignments resulted in a 7 bp consensus (G/A)TTGAC(T/C) or its reverse complement (A/G)GTCAA(T/C), containing the canonical W box (T)TGAC(C/T). Notably, in addition to this conserved core, positions −4, −5, +9 and +10 (taking the first G/A as +1) showed a highly conserved A, extending the target to a 14 bp sequence AANNN(G/A)TTGAC(T/C)NAA (Fig. 2a).
OsWRKY47 binds in vitro the sequence (G/A)TTGAC (T/C) containing a canonical W-box. a Compilation of the sequences of random clones obtained after cloning the selected oligonucleotide population. The sequences of 14 different clones, obtained from the last SELEX step performed with the recombinant OsWRKY47, were aligned to obtain the best consensus (G/A)TTGAC(T/C). Clone numbers (arbitrary) are indicated on the left. Below, a table indicating nucleotide frequencies at each position and the derived consensus sequence. b EMSA assay performed with OsWRKY47 with four variants of the consensus obtained after SELEX. Lane 1 GTTGACT, Lane 2 GTTGACC, Lane 3 ATTGACT, Lane 4 ATTGACC. c Competition EMSA assay performed with OsWRKY47 with labeled oligonucleotides containing GTTGACT or GTTGACC alone or in the presence of the same unlabeled oligonucleotides. Each double stranded oligonucleotide (10,000 cpm) was incubated during 20 min as described in Methods with equal aliquots of W47 before loading. In the competition assays 100-fold unlabeled double-stranded oligonucleotides containing the indicated sequences were incubated with the protein during 10 min before the labelled oligonucleotide was added
In order to solve the slight incertitude in the central core flanking nucleotides (Fig. 2a), synthetic double strand oligonucleotides bearing variants 1 (GTTGACC), 2 (GTTGACT), 3 (ATTGACT) or 4 (ATTGACC) of the consensus sequence were labeled and confronted to the recombinant OsWRKY47. EMSA assays performed with and without unlabeled competitors (Fig. 2b, c) indicated that G was clearly preferred than A in the +1 position (Fig. 2b) while C was slightly preferred as compared to T in position 7 (Fig. 2c). These results were consistent with the abundance of each nucleotide observed when the analysis of the SELEX was carried out (Fig. 2a). Altogether these results indicated that GTTGACC was the OsWRKY47 preferred sequence, at least in vitro.
Identification of OsWRKY47 targets
Transcriptome analysis of the transgenic P SARK ::IPT rice plants, combined with the SELEX results, allowed the identification of few putative target genes. For the identification of putative targets of OsWRKY, the transcriptome of transgenic P SARK ::IPT was analyzed aiming at the identification of genes with expression patterns similar to OsWRKY47. We considered genes exhibiting up- or down-regulation under WS in P SARK ::IPT vs. WT or in P SARK ::IPT plants under WS vs. WW. This first selection resulted in 356 genes (Fig. S1). These 356 genes were investigated for the presence of OsWRKY47-bound sequences in their promoters, specifically for the presence of GTTGACC or GTTGACT domains. This second selection further reduced the number of candidate genes to 82 (Fig. S1; Table S1). An in silico analysis using Blas2GO (www.blast2go.com) and Mapman (www.mapman.gabipd.org) did not contribute to the identification of specific process/metabolism enriched with these 82 genes. In addition, we examined genes that were co-expressed with OsWRKY47 in other tissues/organs using in silico analysis of publicly available rice microarray databases. This analysis reduced the putative targets to 26 genes (Table S2), of which eight targets were selected for validation by qRT-PCR (Fig. S2). These genes included: Cys Rich Repeat Secretory Protein 55 Precursor (CRRSP; LOC_Os03g16950); Calmodulin-Binding Protein (CaMBP; LOC_Os12g36110); Receptor-like Kinase (LOC_Os06g36270); Metal (Zn) Cation Transporter (LOC_Os03g29850); Cys Rich domain containing protein (LOC_Os03g01210); Rhodanese-Like (LOC_Os02g06290); Receptor-like protein kinase (LOC_Os05g25350) and Brassinosteroid insensitive 1-associated receptor kinase 1 (BAK1; LOC_Os08g07760). Two of these genes, CaMBP and CRRSP were selected for further analysis.
OsWRKY47 binds in vitro and in vivo the promoters of CaBP and CRRSP
Segments of the promoters of CaMBP and CRRSP containing the DNA sequences identified as bound by OsWRKY47 were labeled and used in EMSA assays with the recombinant WRKY47 protein. The protein was able to specifically bind to the segment GTTGACT in the CaMBP promoter and the sequence TTTGACT in the CRRSP promoter (Fig. 3). Increasing concentrations of the unlabeled DNA fragment, used as a competitor, significantly diminished the binding while the competition with other unlabeled sequences did not, supporting the specificity of the binding (Fig. 3).
The CRRSP-rich protein and the CaBP promoters are bound by OsWRKY47 in vitro. Segments of the CRRSP-rich protein (137 bp) and calmodulin-binding protein (142 bp) promoters, containing the W-boxes were labeled and used as probes in EMSA assays. OsWRKY47 was confronted during 20 min with the DNA labeled segments (10,000 cpm each) as described in Methods. For competition assays 100-fold unlabeled DNA containing the sequences indicated below was incubated with the protein during 10 min before adding the labelled promoter
To confirm these interactions in vivo, Nicotiana benthaniana leaves were transiently co-transformed with P 35S ::WRKY47 and different constructs bearing the CaMBP or the CRRSP promoters directing the reporter GUS expression or the same promoters where the putative active W-boxes were mutated or deleted (Fig. 4). GUS activity was evaluated fluorometrically (Fig. 4a) and histochemically (Fig. 4b). GUS activity was detected when tobacco leaves were transformed with the constructs bearing either the CaMBP or the CRRSP promoter directing it even in the absence of OsWRKY47, suggesting that other WRKY TFs could be also involved in the activation of CaMBP and CRRSP. However, GUS activity was clearly increased (threefold to fourfold change) when P 35S ::WRKY47 was used to co-transform the leaves. The GUS expression strongly decreased when the core of the W-boxes (4 bp) was deleted or when the C in position +6 was mutated to T, indicating the specific binding of OsWRKY47 to this sequence activating GUS expression (Fig. 4). Positive and negative transient transformation controls were performed using 35S::GUS and 35S::null constructs, respectively, yielded the expected results (Fig. 4).
OsWRKY47 regulates the expression of CaBP and CRRSP in transient co-expression assays a GUS activity was evaluated by fluorometry after transient co-expression assays of tobacco-leaf disks. An empty vector 35S::null (negative control) or 35S::OsWRKY47 (W47) were co-transformed with the complete promoters of the cys-rich protein (Prom Cys, 1448 bp upstream the +1) or of the calmodulin-binding-protein (Prom Cal, 667 bp upstream the +1) or mutated versions of the same promoters (Prom CaBP del, Prom CRRSP del, Prom CaBP C1464→T) all fused to the GUS reporter gene. b Illustrative photographs of the leaf-disks assayed by GUS histochemistry
CaMBP and CRSSP transcript levels were evaluated by qRT-PCR in a WRKY47 knockdown mutant (Oswrky47, termed Osw47-1) and in plants overexpressing WRKY47. We obtained Osw47-1, a WRKY mutant showing a transposon-DNA insertion in the first intron of the OsWRKY47 gene (Fig. 5a), resulting in a significant decrease in OsWRKY47 transcripts (Fig. 5b, c). Both CaMBP and CRSSP genes were down-regulated in the Osw47-1 mutant (Fig. 5d, e) and up-regulated in the WRKY47-overexpressing plants (Fig. 5g, h), as compared to the rice WT plants, supporting the notion of the regulation of CaMBP and CRSSP expression by WRKY47. Both CaMBP and CRSSP displayed higher transcript levels in OE-1 and OE-2 plants even before the drought treatment, indicating their responsiveness to WRKY47 (Fig. 5g, h). Consistent with this notion, CaMBP and CRSSP expression was repressed in Osw47-1 grown under well-watered conditions and remained low during the stress episode and after re-watering (Fig. 5d, e).
CaBP and CRRSP are repressed in OsWRKY47 mutants and induced in overexpressors OsWRKY47. a OsWRKY47 gene structure and position of the transposon insertion. Exons are represented by boxes and introns by lines. Insertion positions of the transposon insertion in mutant Osw47-1 and start codon (ATG) are shown. b PCR detection of transposon insertion in the mutant Osw47-1 using the primers F1, R1, and T shown in (a). c OsWRKY47 transcript levels in WT and in mutant Osw47-1 plants. d Calmodulin binding protein (CaMBP) transcript levels in WT and in mutant Osw47-1 plants. e Cysteine-rich repeat secretory protein (CRRSP) transcript levels in WT and in mutant Osw47-1 plants. f OsWRKY47 transcript levels in WT and in transgenic plants overexpressing OsWRKY47 (OE-1 and OE-2) plants. g CaMBP transcript levels in WT and in transgenic plants overexpressing OsWRKY47 (OE-1 and OE-2) plants; h CRRSP transcript levels in WT and in transgenic plants overexpressing OsWRKY47 (OE-1 and OE-2) plants. The plants were subjected to 5-day water stress and then re-watering. Samples were harvested before treatment (BS), 3 days post stress (3DS) and after re-watering (REW). Values were calculated and normalized by using the transcription elongation factor as internal control. Values are the mean ± SD (n = 6)
Effects of silencing and overexpressing OsWRKY47 on drought tolerance
Two independent transgenic rice plants (OE-1 and OE-2) expressing the P Ubi ::OsWRKY47 were tested for drought tolerance (Fig. 6). Under WW conditions, the transgenic rice plants did not showed significant morphological or developmental differences as compared with the WT plants (Fig. 6a). However, a yield reduction of ~20 % was observed at the end of the experiment (Fig. 6c). Under WS conditions WT plants displayed a significant yield reduction of ~70 %, whereas the transgenic plants displayed only a 15–20 % yield reduction (Fig. 6c).
OsWRKY47 overexpressors display higher yield than controls under drought stress. a–c The phenotype of WT and OsWRKY47 overexpressors (OE-1 and OE-2) subjected to 5-day water stress (left) and then re-watered (right). Photographs were taken before treatment (a), 3 days post stress (b) and after re-watering (c). d Relative grain yield of wild type (WT) and mutant Osw47-1 plants subjected to water stress (WS) or well watered (WW). The average grain yield of WT plants under water well condition was set as 100 %. The values (mean ± SD) were calculated relatively to the average grain yield of WT plants. Twenty plants were tested for each line. Asterisks indicate significance levels as compared to WT (P ≤ 0.001)
Under WW conditions, no phenotypical differences were observed between WT and Osw47-1 mutant plants (Fig. 7a). The WS episode induced a typical leaf rolling phenotype (Fig. 7b) with a small reduction in the total chlorophyll content of the flag leaves of the Osw47-1 plants (Fig. 7d), and a slight reduction (albeit not significant) in grain yield (Fig. 7e). After re-watering, the WT chlorophyll content remained constant after 6 d of re-watering (Fig. 7c), while it decreased further in Osw47-1 flag leaves (Fig. 7d). Under WS conditions, grain yield of the transgenic Osw47-1 plants showed a significant reduction (~60 %) as compared with only 20 % reduction in the WT plants (Fig. 7e). It should be noted that when the response to WS of the Osw47-1 and WT plants was compared, watering was halted for only 3 days because the Osw47-1 plants displayed early stress symptoms (i.e. leaf rolling). Hence, the apparent differences in yield penalty of the WT plants in Fig. 6c (5 days of WS) versus Fig. 7e (3 days of WS).
Mutant Osw47-1 plants exhibit lower yield than controls after drought stress. a–c The phenotype of WT and mutant Osw47-1 plants subjected to 3-day water stress (left) and then re-watered (right). Photographs were taken before treatment (a), 3 days post stress (b) and after re-watering (c). d Total chlorophyll content of flag leaves. The values are the Mean ± SD (n = 20). Asterisks denote significance levels as compared to WT (P ≤ 0.05)
Discussion
WRKY TFs were first identified by their ability to bind the cis-element W-box, (T)(T)TGAC(C/T), a core with varying flanking sequences (Rushton et al. 2010). Only, a few exceptions in the binding sequence specificity have been reported (Sun et al. 2003; Verk et al. 2008). Flanking sequences have been shown to be specific determinants for binding. Here, we demonstrated that OsWRKY47 specifically binds GTTGACT/C, at least in vitro, while it is also able to bind, albeit with lesser affinity, ATTGACT/C. Although SELEX assays could not absolutely distinguish between these sequences, EMSA assays clearly indicated that an A in the 5′ flanking position was almost forbidden while competition experiments showed that T or C were similar in the 3′ flanking position, although C seemed to be preferred. Binding preferences of WRKY TFs have not been deeply explored so far. However, some interesting examples are available in the literature; AtWRKY18 which is involved in ABA signaling and salt tolerance (Chen et al. 2010; Shang et al. 2010) binds the sequence (C/A)TTGAC(T/G). AtWRKY6, a negative regulator of the response to low Pi levels and a positive regulator to low brassinosteroid levels (Chen et al. 2009; Kasajima et al. 2010), binds specifically the sequence GTTGACC, a sequence similar to that bound by OsWRKY47 (Ciolkowski et al. 2008). The same cis-element bound by OsWRKY47 is also bound by AtWRKY11 (Ciolkowski et al. 2008) which is a positive regulator of the drought response (Wu et al. 2009). Although other WRKY TFs have been associated with enhanced plant tolerance to drought, no binding assays for these WRKY TFs have been reported.
Among the genes containing a W-box that were co-expressed with OsWRKY47 (Table S1), eight were chosen for confirmation by qPCR (Table S2 and Fig. S2), BAK1 (LOC_Os08g07760), the receptor-like protein kinase (LOC_Os06g36270), with an Arabidopsis homolog (At3g46290) (Guo et al. 2009), and the Cys-rich domain containing protein (LOC_Os05g25350) are associated with BR-mediated signaling (Li et al. 2007). The co-expression of genes involved in BR-regulation and signaling and OsWRKY47 is noteworthy. A comparison between the P SARK ::IPT and WT plants revealed the enhanced expression of BR regulation- and BR signaling-associated genes (Peleg et al. 2011a, b). These results indicated an interaction between CK and BR (Peleg et al. 2011a, b). The CK-dependent induction of OsWRKY47 and its association with BR-regulated genes support this notion.
The binding of OsWRKY47 to W-box containing genes was demonstrated in a gene encoding a CaM-binding protein (CaMBP, Os12g36110) and a Cysteine-Rich Repeat Secretory Protein 55 Precursor (CRRSP, Os03g16950). CaM can bind to a highly conserved Ca2+-dependent CaM-binding domain (CaMBD) in the WRKY TFs belonging to the Group IId (Park et al. 2005). Although OsWRKY47 does not contain typical CaM binding domains, its interaction with a CaMBP would suggest a possible role in the propagation of signals triggered by changes in cellular Ca2+ homeostasis. Three Arabidopsis genes (At5g57580, At4g31000 and At5g26920) displayed high similarity to Os12g36110. At5g57580 belongs to the Group II of CaM-binding proteins playing a role during the heat shock-mediated elevation of cytosolic Ca2+ (Reddy et al. 2002). At4g31000 was shown to be BR-regulated. At5g26920 encodes a CaMBP (CBP60g) that conferred drought tolerance when overexpressed (Wan et al. 2012). Moreover, similarly to OsWRKY47, the transgenic plants overexpressing CBP60g displayed a higher tolerance to bacterial infection. CRRSP expression was induced by water stress in P SARK ::IPT rice plants (Peleg et al. 2011b). The expression of At5g48540, an Arabidopsis CRRSP homolog, was induced in the mutants abi4 and abi5 (Nakabayashi et al. 2005) and in plants subjected to hypoxia treatments (Klok et al. 2002). Similar phenotypes, i.e. increased biomass (Kerchev et al. 2011), were seen in both abi4 and water stressed P SARK ::IPT rice plants.
For crop-plants the reproductive stage is the most drought sensitive developmental stage (Blum 2009; Peleg et al. 2011a). In the current study we applied a short water-stress treatment at the pre-anthesis stage (booting stage, panicle elongation), by withholding water until the stress symptoms appeared in the transgenic plants (leaf rolling, leaf senescence and reduction of photosynthetic activity), follow re-watering (Peleg et al. 2011b). The constitutive expression of OsWRKY47 did not affect any morphological changes of flowering delay in the transgenic plants. Nevertheless the two transgenic lines expressing OsWRKY47 displayed a yield reduction of about 20 % when the plants were well-watered. Negative effects are not uncommon when constitutive promoters (and not inducible promoters) drive the expression of key regulatory genes such as transcription factors, and pleiotropic effects on growth and development under control conditions have been reported (Reguera et al. 2012, and references therein). The expression of OsWRKY47 resulted in a smaller stress-induced yield loss in the transgenic plants in comparison to the wild-type plants that lost 70 % of the grain yield. The role of OsWRKY47 in water-deficit stress tolerance was further supported by the marked reduction in chlorophyll content in Osw47-1 knockdown mutants and the reduction in grain yield after watering was held for 3 days during pre-anthesis.
In conclusion, the enhanced expression of OsWRKY47 correlated well with the expression of proteins associated with CK- and BR-mediated signaling. Our data supports the involvement of OsWRKY47 in DNA binding of these genes during the water-deficit stress episode and suggest role(s) of OsWRKY47 activating genes associated with the inhibition of stress-induced senescence. Among the genes putatively regulated by OsWRKY47, a few encode proteins with regulatory roles including protein kinases, cation- and phosphate- transporters and TFs (Table S2). It has been shown that receptor-like-protein kinases (RLK) are targeted by WRKY TFs (Du and Chen 2000). AtWRKY6 is a TF mediating the induction of the senescence- and pathogen defense-associated PR1 and SIRK promoter activities. The latter encodes a receptor-like protein kinase whose expression is specifically induced during leaf senescence. Atwrky6 knockout mutants showed a drastic growth reduction, and AtWRKY6 overexpression led to increased SIRK expression. Notably, the SIRK promoter comprises a W-box indicating a direct activation by WRKY6 in vivo (Robatzek and Somssich 2002). WRKY53 has been related to senescence and several target genes, among these, other WRKY TFs, senescence-associated genes and others similar to the putatve OsWRKY47 targets, indicating similar functions of these Arabidopsis and rice TFs. (Miao et al. 2004).
Accession numbers
Sequence data from this article can be found in the EMBL/GenBank data libraries under accession number: OsWRKY47: GenBank AK110900.1 and in MSU Rice Genome Annotation Project Database and Resource under accession numbers: OsCRRSPrich promoter LOC_Os03g16950 and OsCaM promoter LOC_Os12g36110.
Materials and Methods
Plant material and growth conditions
Seeds of the transposon insertion line RdSpm6084I_3.1 (termed as Osw47-1) were obtained from the Rice Sequence Indexed Transposon Insertion Library (http://www.plb.ucdavis.edu/Labs/sundar/projects/riceGenomics.html). The insertions are assumed to be stable, because the transposase was segregated away in the selection for insertion lines. Seeds of wild-type (WT) rice (O. sativa japonica cv. Kitaake), transgenic plants expressing P SARK ::IPT (Peleg et al. 2011a), transgenic plants overexpressing OsWRKY47 (OE-1 and OE-2), and transposon insertion mutant Osw47-1 were sown on moist germination papers for 4 d at room temperature in the dark. Seedlings were transplanted into 2 l pots filled with a mix of 80 % sands and 20 % peat. Greenhouse conditions were controlled at 12 h/12 h day/night under an illumination of 1200 μmol photons m−2 s−1 at 30 °C/20 °C. Water-stress (WS) treatments were carried out at the pre-anthesis stage (end of booting stage toward panicle emerging) by withholding water for 5 days. Plants were re-watered when visual stress symptoms (i.e. leaf rolling) appeared in the transgenic plants.
Nicotiana benthamiana seeds were grown on soil in a culture room at 28 °C under long-day photoperiods (16 h of illumination) with a mixture of cool-white and GroLux fluorescent lamps) at 300 µmol photons m−2 s−1.
Constructs
ProCRRSP::GUS and ProCaMBP::GUS
The CaMBP and CRRSP promoter regions, identified in the OSIRIS database, were amplified by PCR using rice genomic DNA extracted from 45-d-old leaves as template and the oligonucleotides CysF/CysR and CalF/CalR, respectively. The amplified fragments of 1450 and 668 nucleotides, upstream the transcription initiation site, were cloned into the pGEM®-T Easy vector (Promega). Once verified, the promoter fragments were released by XhoI and XbaI restriction and cloned into SalI and XbaI sites of the pKGWFS7 vector. The resulting constructs direct the expression of the reporter GUS by the specific promoters CRRSP and CaMBP.
ProCRRSPΔwbox::GUS
The entire W-box of the CRRSP promoter was deleted following the technique described by Ho et al. (1989). Two flanking DNA segments surrounding the element to be deleted were amplified by PCR in separate reactions using the wild type CRRSP promoter as template and the oligonucleotides CysF and CysdelR and CysdelF and CysR respectively. CysdelR and CysdelF exhibited a 18 bp overlapped region. The resulting PCR products were mixed in a Taq polymerase buffer with 0.5 mM of each dNTP, 2.5 mM MgCl2 and 5 units of Taq DNA polymerase. Hybridization and extension of the segments were carried out during 10 cycles of 30 s at 94 °C, 90 s at 62 °C and 2 min at 72 °C. A normal PCR amplification was performed using CysF and CysR as primers (Table S3). Once verified, the promoter fragments were digested with XhoI and XbaI restriction and cloned into SalI and XbaI sites of the pKGWFS7 vector. The resulting constructs direct the expression of the reporter GUS by the specific promoter CRRSP Δwbox .
ProCaBPΔwbox::GUS
The mutated CaMBP promoter was obtained by the procedure described for ProCRRSP Δwbox ::GUS using the WT CaMBP promoter as template and the oligonucleotides CalF and CaldelR and CaldelF and CalR (Table S3). Once checked, the promoter fragments were digested with XhoI and XbaI restriction and cloned into SalI and XbaI sites of the pKGWFS7 vector. The resulting construct direct the expression of the reporter GUS by the specific promoter CaMBP Δwbox .
ProCaBPC→T::GUS
Substitution of C in position-1460 (inside the W-box) was obtained by the procedure described for ProCRRSP Δwbox ::GUS using the WT CaMBP promoter as template and the oligonucleotides CalF and CalsustR and CalsustF and CalR (Table S3). Once verified, the promoter fragments were digested with XhoI and XbaI restriction and cloned into SalI and XbaI sites of the pKGWFS7 vector. The resulting construct direct the expression of the reporter GUS by the specific promoter ProCaMBP C→T .
ProCRRSPshort
A short version of the CRRSP promoter (147 bp) containing the Wbox was amplified by PCR with the CysFshort and CysFshort primers using the WT CRRSP promoter as template and cloned into the pGEM®-T Easy vector (Promega).
ProCaMBPshort
A short version of the CaMBP promoter (132 bp) containing the Wbox was amplified by PCR with the CalFshort and CalFshort primers using the WT CaMBP promoter and as template cloned into the pGEM®-T Easy vector (Promega).
pOsW47
The cDNA of OsWRKY47 was cloned in pENTR/D-TOPO and the WRKY domain (W47, amino acids 23–219) was obtained by restricting with XhoI and SalI, and inserted in frame into pGEX-4T-3 (Smith and Johnson 1988). To express the polypeptide, E. coli cells [strain BL21 (DE3)] bearing the corresponding plasmid were grown and induced with 1 mM IPTG as described previously (Palena et al. 1998). The recombinant protein was purified through GST-Sepharose (Research AG) as previously described (Smith and Johnson 1988), with a few modifications (Palena et al. 1998).
35S::OsW47
The complete OsWRKY47 cDNA sequence was amplified by PCR with the W47R and W47F primers using the Oswrky47cd clone and as template cloned into the pBI122 vector (Capella et al. 2014) in the BamHI and KpnI restriction sites (Table S3). This construct was used to transform tobacco leaves.
Ub::OsWRKY47
For overexpression in rice of OsWRKY47 gene (LOC_Os07g48260), we constructed the binary vector pH7m24GW-Ub::OsWRKY47 through Gateway Cloning (Life Technologies). We cloned the Maize ubiquitin promoter from pCAMBIA-pMUb-CtHSR to entry vector pDONRP4P1r. OsWRKY47 were cloned by RT-PCR into entry vector pDONR207. These entry vectors were recombined with pH7m24GW by LR reaction. The constructed vector pH7m24GW-Ub:OsWRKY47 was transformed in agrobacterium EHA105. The constructs 35S::OsW47, ProCRRSP::GUS, ProCaMBP::GUS, ProCaMBP Δwbox ::GUS, ProCRRSP Δwbox ::GUS and ProCaMBP C→T ::GUS were initially introduced in the BL21 (DE3) E. coli strain and then transferred to Agrobacterium tumefaciens (strain LBA4404) by electroporation using a gene Gene PulserTM (Bio-Rad).
Sequence analysis
The correct insertion and sequence of all the obtained clones was verified by sequencing (Macrogen Korea).
PCR-assisted binding site selection
To select DNA molecules specifically bound by the purified recombinant GST::OsWRKY47, the random oligonucleotide selection technique (SELEX; Oliphant et al. 1989) was applied, using procedures described by Blackwell and Weintraub (1990). A 32P-labeled (30,000 cpm) 51-mer double stranded oligonucleotide containing a 12-bp random central core [5′-GATGAAGCTTCCTGGACAAT(12N)GCAGTCACTGAAGAATTCT-3′] was incubated with purified GST::OsWRKY47 binding domain (BD). Binding reactions (20 µl) containing 20 mM of HEPES (pH 7.5), 50 mM of KCl, 2 mM of MgCl2, 0.5 mM of EDTA, 1.0 mM of DTT, 0.5 % Triton X-100, 22 ng/ml of BSA, 1 mg of poly(dI-dC), and 10 % glycerol were incubated for 15 min at room temperature, supplemented with 2.5 % Ficoll, and immediately loaded onto a running gel (5 % acrylamide, 0.08 % bis-acrylamide in 0.53 TBE plus 2.5 % glycerol; 13 TBE is 90 mM of Tris–borate, pH 8.3, and 2 mM of EDTA). The gel was run in 0.53 TBE at 20 mA for 2 h and dried before autoradiography. Bound DNA molecules were separated by Electrophoretic mobility shift assay (EMSA) and eluted from gel slices with 0.5 ml of 0.5 M NH4Ac, 10 mM MgCl2, 1 mM EDTA, and 0.1 % (w/v) SDS. The selected DNA molecules were amplified using oligonucleotides SelexF and SelexR (Table S3). Amplification reactions were performed as follows: 18 cycles of 1 min at 94 °C, 1 min at 53 °C, and 1 min at 72 °C. The number of cycles was decreased to 12 after the fourth round. After purification through polyacrylamide gels, the amplified molecules were subjected to new cycles of labelling, binding, elution, and amplification. Enrichment in sequences bound specifically by OsWRKY47 was monitored by binding and competition analysis in EMSA. After seven rounds of selection, the population of oligonucleotides was cloned into the pCR 2.1-TOPO vector (Invitrogen). Eighteen randomly picked clones were sequenced. DNA Binding Assays For EMSA performed with synthetic probes, aliquots of the purified proteins were incubated with double-stranded DNA (0.3–0.6 ng, 30,000 cpm, labelled with [α-32P] dATP by filling in the 3′ends using the Klenow fragment of DNA polymerase) generated by hybridization of the complementary synthetic oligonucleotides named Oligo1, Oligo2, Oligo3 and Oligo4 (Table S3) with modifications within the binding sequence as described in the Results. When competition assays were performed, 100-fold unlabelled double-stranded oligonucleotides were included in the binding reaction mix previously described and incubated for 10 min before the addition of the selected labelled oligonucleotide. For competition EMSAs, fresh DTT was added in the binding reactions to avoid the formation of the double band typically caused by GST oligomerization.
For assays with promoter fragments containing W-boxes, DNA was labelled by annealing oligonucleotides CalShort-F or CysShort-F and filling in the 3′ end using Klenow fragment.
Quantitative PCR analysis
RNA were extracted from flag leaves of wild-type and transgenic plants under well-watered and water-stress conditions. Total RNA was extracted from plant tissue using RNeasy Mini Kit (Qiagen, Valencia, CA). The quality of RNA was measured by using a Nanodrop ND-1000. First strand cDNA was synthesized with the QuantiTect Reverse Transcription Kit (Qiagen). Quantitative PCR were performed as described previously (Peleg et al. 2011b). The \(2^{{ - \varDelta \varDelta C_{\text{T}} }}\) method (Livak and Schmittgen 2001) was used to normalize and calibrate expression values relative to the endogenous rice transcription elongation factor (TEF) gene. The different sets of primers used in qPCR are listed in Supplemental Table S3.
Identification of promoter fragments
The promoters of the selected genes were identified using the OSIRIS database (http://www.bioinformatics2.wsu.edu/cgi-bin/Osiris/cgi/home.pl). OSIRIS allows identification of promoter regions in genes maps as well as transcription initiation sites and translated regions. The selected promoters exhibit between 700 and 2000 bp upstream the +1 and correspond to the japonica variety.
In silico expression analysis of OsWRKY47
In silico co-expression analysis was carried out by using the following databases: HanaDB-Os (http://www.evolver.psc.riken.jp/seiken/OS/co-express.html); Rice Oligonucleotide Array Database (http://www.ricearray.org); Rice Genome Annotation Project (http://www.rice.plantbiology.msu.edu) and co-expressed biological processes (http://www.webs2.kazusa.or.jp/kagiana/cop0911).
Transient transformation of tobacco leaves
For agroinfiltration, A. tumefaciens strain LBA4404 containing individual constructs were grown overnight at 28 °C in LB supplemented with 100 μg/ml streptinomicin, 50 μg/ml rifampicin and 100 μg/ml kanamycin or 100 μg/ml spectinomycin, depending of the vector. Cells were collected by centrifugation and incubated in 10 ml Agrobacterium induction media (100 mM MgCl2 and 100 μM acetosyringone). After further incubation, when cells reached a OD600 = 0.5, the Agrobacterium suspensions were mixed [half bearing the promoter construct and half bearing 35S::OsWRKY47 or empty vector (pBI101) as control]. The mixes were infiltrated into the abaxial side of fully expanded of 5-week-old young tobacco leaves using a needleless disposable syringe. After agro-infiltration, the leaves were kept in a growth chamber at 22 °C under a 16 h light regime for 48 h (Jung et al. 2006).
Stable transformation of rice plants
Rice (Oryza sativa L. ssp. Japonica cv. Kitaake) were transformed with pH7m24GW-Ub:OsWRKY47 in the UC Davis Plant Transformation Facility (http://www.ucdptf.ucdavis.edu/). Transposon-DNA insertion was identified with PCR. The expression of OsWRKY47 was examined by quantitative RT-PCR.
GUS activity assays
Specific GUS activity in protein extracts was measured using the fluorogenic substrate 4-Methylumbelliferyl-β-d-glucuronide (4-MUG) essentially as described by Welchen and Gonzalez (2005). Total protein extracts were prepared by grinding tissues in extraction buffer [50 mM NaPO4 buffer, pH 7.0, 10 mM β-mercaptoethanol, 10 mM EDTA, 0.1 % (w/v) sodium lauryl sarcosine, and 0.1 % (w/v) Triton X-100], followed by centrifugation at 13,000 g for 10 min. GUS activity was measured with 1 mM 4-methylumbelliferyl b-d-glucuronide and 20 % methanol. 20 µl of tobacco extracts were added to 180 µl of fluorometric solution and the reactions were carried out for 20 min at 37 °C, and finally stopped with 800 µl of 0.2 M Na2CO3. A fluorescence spectrophotometer (Hitachi, model F-2000, Hitachi, Tokyo, Japan) was used to quantify the amount of 4-methylumbelliferone (4-MU) cleaved from 4-MUG.
GUS histochemical staining
In situ assays of GUS activity were performed as described by Jefferson (1987). Leaf disks were immersed in a 1 mM 5-bromo-4-chloro-3-indolyl-b-glucuronic acid solution in 100 mM sodium phosphate pH 7.0 and 0.1 % Triton X-100 and, after applying vacuum for 5 min; and incubated at 37 °C overnight. Chlorophyll was cleared from green tissues by immersing them in 70 % ethanol.
Chlorophyll measurements
The flag leaves were weighed and ground in liquid N2 and chlorophyll was extracted in 80 % acetone. The absorbance at 663 and 645 nm were measured and total chlorophyll contents were calculated as described elsewhere (Porra 2002).
References
Berri S, Abbruscato P, Faivre-Rampant O, Brasileiro A, Fumasoni I, Satoh K, Kikuchi S, Mizzi L, Morandini P, Pe M, Piffanelli P (2009) Characterization of WRKY co-regulatory networks in rice and Arabidopsis. BMC Plant Biol 9:120
Besseau S, Li J, Palva ET (2012) WRKY54 and WRKY70 co-operate as negative regulators of leaf senescence in Arabidopsis thaliana. J Exp Bot 63:2667–2679
Blackwell TK, Weintraub H (1990) Differences and similarities in DNA binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science 250:1104–1110
Blum A (2009) Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res 112:119–123
Cai R, Zhao Y, Wang Y, Lin Y, Peng X, Li Q, Chang Y, Jiang H, Xiang Y, Cheng B (2014). Overexpression of a maize WRKY58 gene enhances drought and salt tolerance in transgenic rice. Plant Cell Tiss Organ Cult 119:565–577
Capella M, Re DA, Arce AL, Chan RL (2014) Plant homeodomain-leucine zipper I transcription factors exhibit different functional AHA motifs that selectively interact with TBP or/and TFIIB. Plant Cell Rep 33:955–967
Chen Y-F, Li L-Q, Xu Q, Kong Y-H, Wang H, Wu W-H (2009) The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. Plant Cell 21:3554–3566
Chen H, Lai Z, Shi J, Xiao Y, Chen Z, Xu X (2010) Roles of Arabidopsis WRKY18, WRKY40 and WRKY60 transcription factors in plant responses to abscisic acid and abiotic stress. BMC Plant Biol 10:281
Chen L, Song Y, Li S, Zhang L, Zou C, Yu D (2012) The role of WRKY transcription factors in plant abiotic stresses. Biochim Biophys Acta Gene Regul Mech 1819:120–128
Ciolkowski I, Wanke D, Birkenbihl RP, Somssich IE (2008) Studies on DNA-binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function. Plant Mol Biol 68:81–92
Du L, Chen Z (2000) Identification of genes encoding receptor-like protein kinases as possible targets of pathogen- and salicylic acid-induced WRKY DNA-binding proteins in Arabidopsis. Plant J 24:837–847
Eulgem T, Somssich IE (2007) Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol 10:366–371
Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5:199–206
Giacomelli I, Ribichich KF, Dezar CA, Chan RL (2010) Expression analyses indicate the involvement of sunflower WRKY transcription factors in stress responses, and phylogenetic reconstructions reveal the existence of a novel clade in the Asteraceae. Plant Sci 178:398–410
Guo H, Li L, Ye H, Yu X, Algreen A, Yin Y (2009) Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc Natl Acad Sci USA 106:7648–7653
Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Jung HW, Lim CW, Hwang BK (2006) Isolation and functional analysis of a pepper lipid transfer protein III (CALTPIII) gene promoter during signaling to pathogen, abiotic and environmental stresses. Plant Sci 170:258–266
Kasajima I, Ide Y, Yokota Hirai M, Fujiwara T (2010) WRKY6 is involved in the response to boron deficiency in Arabidopsis thaliana. Physiol Plant 139:80–92
Kerchev PI, Pellny TK, Vivancos PD, Kiddle G, Hedden P, Driscoll S, Vanacker H, Verrier P, Hancock RD, Foyer CH (2011) The transcription factor ABI4 Is required for the ascorbic acid-dependent regulation of growth and regulation of jasmonate-dependent defense signaling pathways in Arabidopsis. Plant Cell 23:3319–3334
Klok EJ, Wilson IW, Wilson D, Chapman SC, Ewing RM, Somerville SC, Peacock WJ, Dolferus R, Dennis ES (2002) Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. Plant Cell 14:2481–2494
Li F, Asami T, Wu X, Tsang EW, Cutler AJ (2007) A putative hydroxysteroid dehydrogenase involved in regulating plant growth and development. Plant Physiol 145:87–97
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−DDCT method. Methods 25:402–408
Luo X, Bai X, Sun XL, Zhu D, Liu BH, Ji W, Cai H, Cao L, Wu J, Hu MR, Liu X, Tang LL, Zhu YM (2013) Expression of wild soybean WRKY20 in Arabidopsis enhances drought tolerance and regulates ABA signaling. J Exp Bot 64:2155–2169
Mangelsen E, Kilian J, Berendzen KW, Kolukisaoglu ÜH, Harter K, Jansson C, Wanke D (2008) Phylogenetic and comparative gene expression analysis of barley (Hordeum vulgare) WRKY transcription factor family reveals putatively retained functions between monocots and dicots. BMC Genom 9:194
Miao Y, Laun T, Zimmermann P, Zentgraf U (2004) Targets of the WRKY53 transcription factor and its role during leaf senescence in Arabidopsis. Plant Mol Biol 55:853–867
Nakabayashi K, Okamoto M, Koshiba T, Kamiya Y, Nambara E (2005) Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: epigenetic and genetic regulation of transcription in seed. Plant J 41:697–709
Oliphant AR, Brandl CJ, Struhl K (1989) Defining the sequence specificity of DNA-binding proteins by selecting binding sites from random sequence oligonucleotides: analysis of yeast GCN4 protein. Mol Cell Biol 9:2944–2949
Palena CM, Gonzalez DH, Guelman SA, Chan RL (1998) Expression of sunflower homeodomain containing proteins in Escherichia coli: purification and functional studies. Protein Expr Purif 13:97–103
Park CY, Lee JH, Yoo JH, Moon BC, Choi MS, Kang YH, Lee SM, Kim HS, Kang KY, ChungWS Lim CO, Moo JC (2005) WRKY group IId transcription factors interact with calmodulin. FEBS Lett 579:1545–1550
Peleg Z, Blumwald E (2011) Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol 14:290–295
Peleg Z, Apse MP, Blumwald E (2011a) Engineering salinity and water-stress tolerance in crop plants: getting closer to the field. Adv Bot Res 57:405–443
Peleg Z, Reguera M, Tumimbang E, Walia H, Blumwald E (2011b) Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotechnol J 9:747–758
Porra RJ (2002) The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res 73:149–156
Ramamoorthy R, Jiang S-Y, Kumar N, Venkatesh PN, Ramachandran S (2008) A comprehensive transcriptional profiling of the WRKY Gene family in rice under various abiotic and phytohormone treatments. Plant Cell Physiol 49:865–879
Reddy VS, Ali GS, Reddy ASN (2002) Genes encoding Calmodulin-binding proteins in the Arabidopsis genome. J Biol Chem 277:9840–9852
Reguera M, Peleg Z, Blumwald E (2012) Targeting metabolic pathways for genetic engineering abiotic stress-tolerance in crops. Biochim Biophys Acta Gene Regul Mech 1819:186–194
Reguera M, Peleg Z, Abdel-Tawab YM, Tumimbang EB, Delatorre CA, Blumwald E (2013) Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiol 163:1609–1622
Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E (2007) Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc Natl Acad Sci USA 104:19631–19636
Robatzek S, Somssich IE (2002) Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev 16:1139–1149
Rushton PJ, Somssich IE, Ringler P, Shen QJ (2010) WRKY transcription factors. Trends Plant Sci 15:247–258
Schluttenhofer C, Yuan L (2015) Regulation of specialized metabolism by WRKY transcription factors. Plant Physiol 167:295–306
Shang Y, Lu Y, Liu Z.-Q, Cao Z, Mei C, Xin Q, Wu F.-Q, Wang X.-F, Du S.-Y, Jiang T, Zhang X.-F, Zhao R, Sun H.-L, Liu R, Yu Y.-T, Zhang D.-P (2010) The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription represors to relieve ABA-responsive genes of inhibition. Plant Cell 22:1909–1935
Smith DB, Johnson KS (1988) Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67:31–40
Sun C, Palmqvist S, Olsson H, Bore´n M, Ahlandsberg S, Jansson C (2003) A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar responsive elements of the iso1 promoter. Plant Cell 15:2076–2090
Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R (2014) Abiotic and biotic stress combinations. New Phytol 203:32–43
Tripathi P, Rabara R, Rushton P (2014) A systems biology perspective on the role of WRKY transcription factors in drought responses in plants. Planta 239:255–266
Ülker B, Mukhtar S, Somssich IE (2007) The WRKY70 transcription factor of Arabidopsis influences both the plant senescence and defense signaling pathways. Planta 226:125–137
Ulker B, Somssich IE (2004) WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol 7:491–498
Verk MC, Pappaioannou D, Neeleman L, Bol JF, Linthorst HJM (2008) A novel WRKY transcription factor is required for induction of PR-1A gene expression by salicylic acid and bacterial elicitors. Plant Physiol 146:1983–1995
Wan D, Li R, Zou B, Zhang Z, Cong J, Wang R, Xia Y, Li G (2012) Calmodulin-binding protein CBP60 g is a positive regulator of both disease resistance and drought tolerance in Arabidopsis. Plant Cell Rep 31:1269–1281
Welchen E, Gonzalez DH (2005) Differential expression of the Arabidopsis cytochrome c genes Cytc-1 and Cytc-2. Evidence for the involvement of TCP-domain protein-binding elements in anther- and meristem-specific expression of the Cytc-1 gene. Plant Physiol 139:88–100
Wu K-L, Guo Z-J, Wang H-H, Li J (2005) The WRKY family of transcription factors in rice and Arabidopsis and their origins. DNA Res 12:9–26
Wu X, Shiroto Y, Kishitani S, Ito Y, Toriyama K (2009) Enhanced heat and drought tolerance in transgenic rice seedlings overexpressing OsWRKY11 under the control of HSP101 promoter. Plant Cell Rep 28:21–30
Xie Z, Zhang ZL, Zou X, Huang J, Ruas P, Thompson D, Shen QJ (2005) Annotations and functional analyses of the rice WRKY gene superfamily reveal positive and negative regulators of abscisic acid signaling in aleurone cells. Plant Physiol 137:176–189
Xiong X, James VA, Zhang HN, Alpeter F (2010). Constitutive expression of the barley HvWRKY38 transcription factor enhances drought in turf and forage grass (Paspalum notatum Flugge). Mol Breed 25:419–432
Zhou QY, Tiam AG, Zou HF, Xie ZM, Lei G, Huang J, Wang CM, Wang HW, Zhang JS, Chen SY (2008) Soybean WRKY-type transcription genes, GmWRKY13, GmWRKY21 and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol J 6:486–503
Acknowledgments
This research was supported by funds from the UC Office of the President, UC Discovery Grant # 212976 (EB). The research of the Argentinean group was supported by Agencia Nacional de Promoción Científica y Tecnológica (PICT 2011 0850 and PICT 2012 0955) and Universidad Nacional del Litoral (UNL). JR is a CONICET Ph. D. Fellow and RLC is a career member of the same institution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jesica Raineri and Songhu Wang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1.
Schematic representation of the in silico analysis performed to detect putative targets of OsWRKY47. Supplementary material 1 (JPEG 38 kb)
Fig. S2.
Validation of the expression of candidate genes targeted by OsWRKY47 by qRT-PCR. Supplementary material 2 (PDF 115 kb)
Table S1.
List of genes containing OsWRKY47-bound sequences in their promoters. Supplementary material 3 (XLSX 14 kb)
Table S2.
List of genes co-expressed with OsWRKY47 according to in silico analysis. Supplementary material 4 (XLSX 12 kb)
Table S3.
List of oligonucleotides used in this work for cloning or for RT-qPCR. Supplementary material 5 (DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Raineri, J., Wang, S., Peleg, Z. et al. The rice transcription factor OsWRKY47 is a positive regulator of the response to water deficit stress. Plant Mol Biol 88, 401–413 (2015). https://doi.org/10.1007/s11103-015-0329-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-015-0329-7