Abstract
Zinc oxide (ZnO) nanoparticles are among the most promising nanoparticles used in precision agriculture. Since they are made of an essential element Zn, their potential applications are most vital in agricultural regions where the natural concentrations of bioavailable Zn are low, and crop plants suffer from the deficiency of this essential micronutrient. Also, a large number of genes requires Zn to protect cells from the detrimental effects of environmental stress to regulate and maintain their expression. ZnO nanoparticles are more tuneable in their properties, such as size, shape, dissolution rate, and surface properties, compared to conventional ionic formulas. Thus, they pose an effective way to supplement plants with Zn. Their nanoparticulate characteristics, such as photocatalysis, may provide additional beneficial effects for crop plants. Precise application of nanoparticles may reduce chemical inputs to agricultural fields, helping with long-term agricultural sustainability, environmental protection, and higher nutritional value of crops. In this chapter, we describe the influence of ZnO nanoparticles on the stress tolerance of crop plants. Since their effect is dependent on their properties, we elaborated on the importance of size, shape, crystal structure, and nanoparticle surface coating. Different modes of application show varying effects on crops, and foliar application may have direct positive effects connected to the photocatalytic properties of ZnO nanoparticles. We provide a summary of the positive impacts of ZnO NP on crop plants associated with the alleviation of biotic stresses (herbivores, pathogens) and abiotic stresses (heavy metals, heat, cold, drought, and salt). In mainly laboratory or pot studies, several beneficial effects of ZnO nanoparticles were identified. Future field experiments are needed to further our knowledge and adapt agricultural techniques to changing local and global climatic conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Engineered nanoparticles are increasingly used in many diverse human activities. The ability to adjust their properties through a change in their size, shape, crystallinity, and surface properties is highly beneficial for new technological applications. The small size of nanoparticles means that large fractions of atoms that build up the nanoparticles are on the surface. Nanoparticle ’s surface to volume ratio dramatically changes with their size, which affects their properties and smaller nanoparticles exhibit forces typical for the atomic and molecular world, e.g. various quantum effects (Nel et al. 2006; González-Melendi et al. 2008; Nair et al. 2010; Gogos et al. 2012; Strambeanu et al. 2015; Mallakpour and Madani 2015; Rasmussen et al. 2018; Faraz et al. 2020). Nanomaterials are usually defined as materials with at least one dimension between 1 and 100 nm. They are subdivided into three types (1) nanosheets with one dimension below 100 nm, (2) nanofibres and nanotubes with two dimensions lower than 100 nm, and (3) nanoparticles with three dimensions between 1 and 100 nm (Wang et al. 2015). Due to their highly tunable properties, engineered nanoparticles are of high interest in various technological applications, including precision agriculture, where they may improve the health and yields of crops, even under suboptimal conditions of environmental stress.
Biotic and abiotic stress has a considerable influence on the growth of plants and agricultural production. Through millions of years of evolution, plants have developed various physiological responses that improve their ability to tolerate biotic and abiotic stresses (Almutairi 2019). It is, thus, essential to understand plants’ stress tolerance mechanisms. The response mechanisms start with fast recognition of the nature of the stress. Afterwards, a complex cascade of signals triggers the plant’s defences. Accumulation of reactive oxygen species (ROS), activation of gene networks, specific ion channels, and kinase cascades are involved in early stress response (Rejeb et al. 2014; Czarnocka and Karpiński 2018; Almutairi 2019). Also, the response leads to an elevated release of hormones such as abscisic acid (ABA), ethylene (Et), jasmonic acid (JA), and salicylic acid (SA) (Bari and Jones 2009; Davies 2010).
During the normal metabolism of oxygen, ROS are produced as a by-product of aerobic metabolism and are kept at low levels by cells’ antioxidant chemicals. Furthermore, ROS are used as signalling molecules. Although ROS are necessary for basic biological processes such as cellular proliferation and differentiation, their elevated levels may have undesired effects. When under stress, ROS levels increase and impose oxidative stress in the cells. To cope with the increased levels of ROS, plants elevate the production of antioxidants through various defence systems (Allen 1995; Apel and Hirt 2004; Mittler 2017).
Plants activate multiple complex signalling pathways during different types of abiotic and biotic stresses. Signalling cascades induced by the sensing of stress trigger and change expressions of specific genes used in stress defence. Proteins and enzymes participating in these pathways function as agents for ROS detoxification; they induce signalling cascades such as nitrogen-activated kinase and salt overly sensitive kinase; they play a role in transcriptional control, and alter water and ion uptake and transport (Blumwald 2000; Scandalios 2005; Saibo et al. 2008; Choudhury et al. 2013; Flowers and Colmer 2015; Jain et al. 2018; Kosová et al. 2018).
In recent days, enhancing plant production through nanotechnology has shown promise as a new emerging strategy. Nanotechnology can be supplemental in alleviation of nutrition deficiencies, improvement of resistance to diseases and tolerance to hostile environments. Nanoparticle interaction with plants has been studied on various levels, including genetics, physiology, plant development, and changes in the morphology of plant organs, etc. Literature reports effects ranging from negative through neutral to positive, with some higher concentrations toxic to plants and lower concentrations having positives effects. Recently, nanoparticles have also been applied to mitigate the adverse effects of both biotic and abiotic stresses. Unlike conventional agricultural chemicals, the effectiveness of nanoparticles depends not only on their chemistry, dosage, repetition and time of application but also on nanoparticle size, shape, crystallinity, and surface properties (Misra et al. 2016; Wang et al. 2016a; Faraz et al. 2020; Landa 2021). There is a trend in agriculture to reduce bulk fertilizers in particle size to nanometer sizes to increase their efficiency. Liu and Lal (2015) proposed to categorise these nanofertilizers according to plant nutrition into (1) macronutrient-nanofertilizers that incorporate elements such as P, K, N, Mg, and Ca; (2) micronutrient fertilizers incorporating Zn, Mn, Fe, Cu, Mo, etc.; (3) nanomaterials-enhanced fertilizers, such as nutrient-loaded zeolites with silica, carbon-coated Fe, polymers etc.; and (4) plant-growth enhancers with unclear mechanisms of action , e.g. TiO2 or carbon nanotubes.
Zinc is an element, a micronutrient, essential for most organisms living on the planet, including plants, fungi, animals, and humans. There is a wide range of studies concerned with the effects of zinc on the growth and proliferation of plants since it is an essential part of more than 300 enzymes in organisms. It is the only metal that is included in all six groups of enzymes, and as such, it is present in oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases (Alloway 2008, 2009). Most of the processes in plants, such as photosynthesis and production of DNA and RNA, depend on zinc being present in some form. Therefore, amendment with zinc is essential, and it is used to support cereal, vegetable, and forage production (Alloway 2008; Faraz et al. 2020). In plants, zinc is required to metabolise carbohydrates, synthesise enzymes, maintain the integrity of cell membranes, regulate auxin synthesis, and create pollen (Alloway 2008). It also regulates the expression of genes important in tolerance toward environmental stresses, such as high intensity of light or high temperature. Zinc deficiency in plants is expressed by abnormal growth of plant structures. During acute deficiency, visible signs include slowed growth, chlorosis of leaves, reduction of leaf area, and sterility. Quality of crops is threatened, including protein content, appearance, and size of the fruit or seeds. Tolerance towards heightened intensity of light, and some fungal infections is diminished. Under slight deficiency of zinc in soils, quality and quantity of produced crops is diminished without visible hinderance in growth of the plants (Alloway 2008, 2009). For humans and higher organisms, zinc is referred to as a “type 2” nutrient , meaning that its concentration in the blood does not decrease in proportion to its deficiency in the organism. Zinc deficiency results in slowed physical growth, and its secretion is lowered since the organism strives to retain it. Many children with this deficiency have stunted growth. The recommended daily intake of Zn is between 3 and 16 mg∙day−1, and this value depends on age, sex, diet and several other factors. Roughly, one-third of the human population suffers from zinc deficiency in their diet. The relative size of the population afflicted is highly specific for each country and ranges from 4% to 73% (Alloway 2009).
Zinc oxide (ZnO) is an amphoteric oxide with low solubility in pure water. However, it is easily soluble in many acids. It crystallizes almost exclusively in the hexagonal wurtzite type structure known in the mineral classification system as zincite (Fig. 12.1). On rare occasions, it crystalises in cubic structure (Borysiewicz 2019). In lattice, Zn2+ cation is coordinated four O2− anions in tetrahedron arrangement (Fig. 12.1), and reciprocally, each anion of O2− can be thought of as a tetrahedron coordinated with four cations of Zn2+. Zincite has sp3 hybridized bonds with almost equivalent covalent and ionic nature (Borysiewicz 2019). As such, ZnO is a wide gap semiconductor (Eg = 3.37 eV) (Klingshirn et al. 2010). ZnO NPs have many useful properties, such as high binding energy, high refractive index, high thermal conductivity, piezoelectric properties, high absorbance of UV light, and antibacterial properties and it is applied in a wide variety of uses and products (Moezzi et al. 2012). As a nanomaterial, ZnO exhibits a broad range of shapes, from flowerlike structures to nanorods and nanoparticles . And since it is easy to manipulate its shape and to dope it, ZnO nanomaterials have been used in, for example, rubber production, concrete composites, electronics, solar panels, cosmetics, medicine, biosensors, food packaging and food products, and agriculture (Moezzi et al. 2012; Sabir et al. 2014). ZnO nanomaterials have been produced in a volume of approximately 30 kilotons in 2014, with a predicted rise in production (Future Markets Inc 2016). ZnO nanoparticle (ZnO NP ) input in the environment was predicted to be in the range of 0.01–0.03 μg∙kg−1∙y−1 for soils (unintentional release), and levels of ZnO NPs of 0.05–0.29 μg∙l−1 were predicted for fresh waters (Sun et al. 2014). Properties of ZnO NPs related to their UV protection, easily adjustable size, shape, and surface properties, and their antimicrobial and antifungal properties were shown to positively impact the growth and health of plants (Tarafdar et al. 2012; Raliya and Tarafdar 2013; Sabir et al. 2014; Raliya et al. 2015, 2016, 2018), and to help plants with coping with environmental stresses (Saxena et al. 2016; Hussain et al. 2018; Rizwan et al. 2019a, c). Under appropriate concentrations, ZnO NPs were found to increase seed germination (García-López et al. 2018), growth (Singh et al. 2019), photosynthesis (Faizan et al. 2018), activity of antioxidant enzymes (Venkatachalam et al. 2017), production of chlorophyll (Reddy Pullagurala et al. 2018a), proteins (Venkatachalam et al. 2017; Salama et al. 2019), oil, and seeds (Kolenčík et al. 2019, 2020), and they increased uptake of micronutrients (Peralta-Videa et al. 2014). They were also found to alleviate abiotic stresses, e.g. drought (Kolenčík et al. 2019; Dimkpa et al. 2020a), heavy metals (Rizwan et al. 2019c), salt (Torabian et al. 2016; Wan et al. 2020), and temperature (Fig. 12.2) (Hassan et al. 2018).
Geometrical arrangement of atoms with tetrahedron coordination in unit cell of ZnO (Zn in grey, O in red) constructed and visualized using VESTA program in space-filling regime (Momma and Izumi 2011)
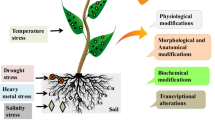
ZnO NPs play an important role in plant development, photosynthesis, and other critical physiological systems of plants. Also, ZnO NPs were applied under stress conditions and found to increase the tolerance to both biotic (herbivores and pathogens) and abiotic (heavy metals, heat, cold, drought, flooding, and salts). Up-to-date knowledge on the positive effects of ZnO NPs on (crop) plants is shortly summarized. We discuss current knowledge gaps connected to the research on environmental stress alleviation and propose research areas that may further our understanding and help with best practices in applying ZnO NPs.
12.2 Effect of ZnO Nanoparticles ’ Properties on Biological Interaction in Soils and Colloids
One of the most important properties of ZnO NPs is their ability to be transported in soils and their interaction with soil constituents. In an acidic environment, ZnO NPs can create large aggregates and dissolve easily (partially or fully), and the behaviour of released Zn ions is the most important in plant bioavailability (Bian et al. 2011; Mohd Omar et al. 2014; Sirelkhatim et al. 2015; Šebesta et al. 2020a). In more alkaline environments, the dissolution decreases dramatically, and under some circumstances, ZnO NPs can be more easily transported compared to ionic Zn (Šebesta et al. 2020a), even though the binding to various soil constituents may be similar to both forms (Šebesta et al. 2020b). The most important soil constituents that interact with ZnO NPs are living and dead organic matter, oxyhydroxides of Al, Fe and Mn, CaCO3 and clay fraction (Fig. 12.3) (Bian et al. 2011; Zhao et al. 2012; Mohd Omar et al. 2014; Han et al. 2016; Polák et al. 2019; Šebesta et al. 2019, 2020a). The soils pore water characteristics, such as ionic strength, concentration of Ca2+, SO42−, and different phosphates also play an important role, mainly in ZnO NPs’ transformation and aggregation (Sivry et al. 2014; Xu et al. 2016; Peng et al. 2017). The behaviour of ZnO NPs was also dependent on the properties such as concentration (Yung et al. 2015; Šebesta et al. 2020a), particle size and surface area (Bian et al. 2011), and surface coating (Gelabert et al. 2013).
The size of ZnO NPs is important to several processes that happen in soils and during interaction with plants. The dissolution behaviour of ZnO NPs depends on their size, and smaller nanoparticles dissolve more readily (Meulenkamp 1998; Bian et al. 2011; Chang et al. 2012; Mudunkotuwa et al. 2012). The size of the nanoparticles and their aggregates is also important when absorption by plants is considered, and smaller nanoparticles can be more readily absorbed since they do not need to dissolve (Dietz and Herth 2011; Molnárová et al. 2015). Transfer of nanoparticles is hindered by the pores of plant cell walls, and they most probably only allow a passage of particles smaller than 20 nm (Wang et al. 2016a) or 5 nm (Gogarten 1988) to the apoplast. Because of the changes in dissolution and ability to penetrate deeper into plant tissues, nanoparticle size is also important in toxicity, including toxicity towards plants (Nair et al. 2009; Chang et al. 2012; Nemček et al. 2020). Size of ZnO NPs was important in the test with fava bean (Vica fabia) where it was found that the toxicity is linked to a greater dissolution of NPs where 25 nm ZnO NPs released ca 30% more Zn2+ than the 70 nm ZnO NPs. The ROS generation was very similar between the two NPs under the conditions used in the experiment (Pedruzzi et al. 2020).
The ZnO nanomaterials have highly tunable shape and form one-dimensional, two-dimensional, and three-dimensional structures (Thorny Chanu and Upadhyaya 2019). One dimensional ZnO structures are the most diverse and include belts, combs, helixes, nanorods, needles, ribbons, rings, springs, tubes, and wires (Pan et al. 2001; Kong et al. 2004; Liu et al. 2005; Huang et al. 2006; Chen et al. 2007; Wahab et al. 2007; Frade et al. 2012; Xu et al. 2012; Nikoobakht et al. 2013). Nanoplates/nanosheets and nanopellets belong to ZnO two-dimensional structures (José-Yacamán et al. 2005; Chiu et al. 2010). The three-dimensional structures are variously shaped with several different shapes such as coniferous urchin-like forms, dandelions, flowers, snowflakes, etc. (Liu et al. 2006; Bitenc and Crnjak Orel 2009; Polshettiwar et al. 2009). In a study comparing the effect of shape, Zhou and Keller (2010) found that spherical ZnO NPs had higher critical coagulation concentration than the mixture of ZnO NPs in the shape of rods and platelets. ZnO nanorods also dissolved more readily than the spherical ZnO NPs of similar volume, which is related to their uncompensated surface energies (Joo and Zhao 2017). Hexagonal ZnO NPs were shown to have a slightly higher positive effect on the growth of tomato plants compared to spherical particles (Pérez Velasco et al. 2020). In theory, the interaction of NPs with plant surfaces is related to (1) the chemistry and crystallinity of the nanoparticles ; (2) nanoparticle surface in contact with the surrounding environment, and its shape, roughness, charge, and surface energy; and (3) the physicochemical properties of the environment the nanoparticles interact with, such as chemistry, input energy, e.g. sunlight or other types radiation, and changes in temperature (Konvičková et al. 2018; Holišová et al. 2019, 2021; Kolenčík et al. 2021).
Therefore, ZnO NPs are often surface modified to adjust their properties like aggregation and interaction with other constituents in the system. Nanoparticles with negative surface charge often behave more similarly to conservative tracers (such as Br−) in model porous media, whereas positively charged nanoparticles are retained to a much higher degree (Yecheskel et al. 2016). Also, surface capping changed the toxicity of ZnO NPs toward E. coli bacteria and the cancer cell line, where starch-capped ZnO NPs had the lowest toxicity (Nair et al. 2009). The surface coating was found to be an important factor affecting the toxicity of ZnO NPs, and different coatings either increased or decrease cell damage or stress (Le et al. 2016). Beans (Phaseolus vulgaris) were exposed to bare and Z-COTE HP1® coated ZnO NPs. Coated NPs promoted more root growth and increased the concentration of nutritional elements (B, Mg, Mo, and S) compared to bare ZnO NPs (Medina-Velo et al. 2017; López-Moreno et al. 2018). Surface defects also increase the toxicity of ZnO NPs (Persaud et al. 2020).
In an experiment measuring oxidative stress response, ZnO NPs doped with Mn and Co showed increased response compared to pristine uncoated ZnO NPs. The Fe doped ZnO NPs showed a similar response when compared with pristine uncoated ZnO NPs (Le et al. 2016). Se doped ZnO NPs exhibited decreased toxicity to Esheria coli even though they showed higher production of reactive oxygen species due to Se leaching from NPs in culture media (Dutta et al. 2014).
To create more benign ZnO NPs for agricultural application , a biologically induced synthesis with plant, fungi or microbial extracts was used in studies. There is some evidence that there may be a synergy of nanoparticle-sized effect together with residual effects of organic extracts that are bound to nanoparticles surfaces (Gebre and Sendeku 2019). For example, Chaudhuri and Malodia (2017) biosynthesised ZnO NPs with leaf extract of Calotropis gigantea. They applied the created ZnO NPs on three plant species (Azadirachta indica, Alstonia pinnata, a Pongamia scholaris). All three species showed improvement in height after 6 months when compared to control. Biosynthesis is a new trend in the application of ZnO NPs in agriculture that may lead to better crop production with lower side effects.
12.3 Multiple Effects of Exposure Pathways
Several pathways of exposure are typical for the agricultural application of ZnO NPs. ZnO NPs were applied (1) on seeds to evaluate germination and early growth of seedling (Umavathi et al. 2020; Khan et al. 2021; Rai-Kalal and Jajoo 2021), (2) into the soil (or growth medium) to evaluate the root uptake and its toxicological and beneficial effects (Nemček et al. 2020), and lastly, (3) foliar application , that applies ZnO NPs directly on plants, mainly leaves, and may be good in reducing the number of nanoparticles needed to induce beneficial effects in plants (Kolenčík et al. 2020; Adrees et al. 2021).
12.3.1 Seed Application
Application of ZnO NPs on seeds was tested on various plants, and low concentrations had a generally positive effect on germination, seed vigour index, and the photosynthesis of seedlings (Dileep Kumar et al. 2020; Itroutwar et al. 2020b; Maslobrod et al. 2020; Rafique et al. 2020; Rani et al. 2020; Rawashdeh et al. 2020; Younes et al. 2020; Awan et al. 2021; Khan et al. 2021). Submerging seeds in ZnO NPs suspensions for 1, 2, 3, or 18 hours had a positive effect at a range of concentrations from 0.05 mg∙l−1 to 2000 mg∙l−1, and the concentrations that improved the germination the most were very plant species-specific (Itroutwar et al. 2020b; Kasivelu et al. 2020; Awan et al. 2021; Rai-Kalal and Jajoo 2021). In Tymoszuk and Wojnarowicz (2020), Allium cepa seeds were grown on the modified Murashige and Skoog (MS) medium spiked with ZnO NP at concentrations of 50, 100, 200, 400, 800, 1600, and 3200 mg∙l−1 and the highest germination was recorded at 800 mg∙l−1. ZnO NP concentration of 3200 mg∙l−1 decreased the germination. Youssef and Elamawi (2020) found that concentrations of 50 mg∙l−1 of ZnO NPs were positively affecting germination of Vicia faba, while higher concentrations, higher than 100 mg∙l−1, had a negative effect. Corn (Zea mays) was primed with 2, 4, 8, and 16 mg∙l−1 of 16 to 20 nm ZnO NPs biosynthesized with Bacillus sp. for 24 h. Root length, shoot length, and protein concentration was increased after growth in pots, and the maximum increase was observed at 8 mg∙l−1 (Sabir et al. 2020). Generally, priming with higher concentrations of ZnO NPs has an inhibitory effect on germination and the early growth of plants, and lower concentrations have a positive impact. The positive effect of ZnO NPs was found to be higher than in their ionic counterparts, at least for lentil (Lens esculentum) and chick pea (Cicer arietinum) (Choudhary and Khandelwal 2020) and higher than bulk ZnO when applied on corn plants (Zea mays) (Esper Neto et al. 2020).
12.3.2 Soil Application
ZnO NPs can be applied to soils to ameliorate the Zn deficiency that is one of the most widespread deficiencies in plants, and it affects up to one-third of agricultural soils, mainly in tropical and subtropical regions (Alloway 2008, 2009). The germination of black mustard (Brassica nigra) in soils contaminated with high concentrations of ZnO NPs (200, 400, and 600 mg∙kg−1) caused inhibition in seed germination and had a negative effect on the root length and height of plants. Also, phenolics and flavonoids, which play a vital role in the detoxification of ROS, were increased compared to control without applied ZnO NPs. However, an increase in leaf area was observed for 200 and 600 mg∙kg−1 (ur Rehman et al. 2020). Similarly, an increase of leaf area and also the stem height, number of leaves, number of branches, and number of nodes per black mustard plant was observed in a study by Zafar et al. (2020) when 200, 400, and 600 mg∙kg−1 ZnO NPs were applied to soil, but a decrease in seed diameter and number of pods per plant was also observed. Application of ZnO NPs also resulted in a higher accumulation of Ca, Co, and Zn in seeds along with protein, glucosinolates and erucic acid (Zafar et al. 2020). When black mustard (Brassica nigra) was grown for 30 days in ZnO NPs enriched soil, concentrations below 400 mg∙kg−1 ZnO NPs had a positive effect on root length, and higher concentrations caused increased oxidative stress (Zafar et al. 2019). In an early growth experiment with barley (Hordeum vulgare), only high concentrations of ZnO NPs (2000 mg Zn∙kg−1 as ZnO NP ) had a negative effect on average fresh and dry weight and root and sprout length (Nemček et al. 2020). In a 35-day growth experiment with cilantro plants (Coriandrum sativum), 100, 200, and 400 mg∙kg−1 of ZnO NPs were used, and concentration of 100 and 200 mg∙kg−1 increased the concentration of chlorophyll by at least 50% and changed the carbinolic-based compounds. The concentration of 400 mg∙kg−1 of ZnO NPs had a negative effect and decreased lipid peroxidation by 70%. Application of ZnO NPs to soils alone or in combination with organic matter did not influence soil chemical properties, however, they may influence microbial properties and may support bacterial growth over fungal growth (Aziz et al. 2019). Nanoparticles may enhance the nutrient mobilization in soils via influence on the soil microbial population and extracellular enzymes secretion such as urease or phosphatase activity in soils which play an important role in the regulation of plant available nitrogen and phosphorus (Olander and Vitousek 2000; Raliya and Tarafdar 2013; Raliya et al. 2016, 2018). Nonetheless, there are indications that ZnO NPs may enhance microbial activity and increase their biomass that could lead to N immobilization in soils, and thus, decreasing its plant uptake (Aziz et al. 2019). Higher levels of chlorophyll, nitrogen and micronutrients such as zinc, magnesium, and potassium were observed when lettuce (Lactuca sativa) and carrot (Daucus carota subsp. sativus) plants were grown in commercial soil substrate spiked with 1, 5, 20, 100, and 1000 mg∙kg−1 ZnO NPs, where the highest concentration had negative effects and the other concentrations showed mostly positive or neutral effects on both plants (Song and Kim 2020). After 120 days of growing in soil, soybean (Glycine max cv. Kowsar) seed yield was evaluated in experiments with different concentrations of ZnO NPs with different sizes and morphologies and Zn2+ to compare ionic vs nanoparticle influence. All Zn compounds (ZnO NPs, and Zn2+) increased seed yield when applied at a concentration up to 160 mg Zn∙kg−1. At higher concentrations, ZnO NPs were toxic, with the highest toxicity elucidated by spherical 38 nm NPs and evidence suggested some nano-specific toxicological effect when compared with Zn2+ (Yusefi-Tanha et al. 2020). In experiments with either foliar or soil application of ZnO NPs, tomato plants (Solanum lycopersicum) were positively influenced by both application , i.e. their height, stem diameter and leaves, stem and root dry weight was increased, with little actual difference between the two applications (Pérez Velasco et al. 2020). Similarly, soil and foliar application led to similar Zn distribution of zinc in wheat grain (Doolette et al. 2020). Umar et al. (2020) found that foliar application of ZnO NPs on corn had the highest positive effect on the concentration of Zn in grains when compared to both soil application of ZnO NPs and more conventional Zn fertilizers and also ZnO NPs application promoted plant growth and seed yield.
12.3.3 Foliar Application
In general, uptake of nutrients via leaves, including uptake from nanoparticles and nutrient distribution in leaves, is still less well-known in comparison to nutrient translocation in root systems in the soil environment (Li et al. 2019). It depends on several factors such as concentration, particle size, the chemical composition of NPs, the timing and number of applications, plant species, etc. (Nair et al. 2010; Servin and White 2016; Wang et al. 2016a).
Currently, foliar application of ZnO NPs to plants has two distinct benefits: (1) decrease in amounts of agrochemicals used, and (2) gradual release of Zn from the NPs (Prasad et al. 2014; Wang et al. 2016a; Li et al. 2019). In contrast, corresponding conventional ionic Zn fertilizers are absorbed faster through leaves and are more readily metabolized in plants. Additionally, our research indicates (Kolenčík et al. 2019, 2020) that in the case of ZnO NPs, similarly to TiO2 NPs, their nano-domain effect may contribute to elevated photosynthetic activity in plants via the protection of chloroplasts or their photocatalytic properties (Siddiqui et al. 2019; Rizwan et al. 2019b). Sunlight exposition may also support photo-corrosion and dissolution of ZnO NPs (Ma et al. 2014), and the released ionic zinc is easily absorbed and utilized by the plant. However, there are still knowledge gaps when it comes to their application in field conditions. In this context, their foliar application may be an effective measure in precision agriculture that aids in adaptation to climate change (Kolenčík et al. 2019, 2020).
There are three potential pathways for foliar absorption of ZnO NPs or Zn ions released from them: cuticular, stomatal, or through trichomes (Li et al. 2019). After the application of ZnO NPs on the sunflower, there was a qualitative change in leaves in the flower bud stage of the life cycle of the plants. Trichomes diversity, ratio, width, and length were change, and a new type of trichomes was observed – capitate glandular trichomes (Kolenčík et al. 2019). Leaf part with different structures and the semi-quantitative analysis of leaf surface chemistry is shown in Fig. 12.4.
(a) Sunflower leaf surface visualized by scanning electron microscopy with two types of trichomes – non-glandular trichomes (NGTs) and linear glandular trichomes (LGTs); (b) details of NGTs; semiquantitative analysis of element by energy-dispersive X-ray spectroscopy on surface of control (a) and ZnO NPs foliated leaves (b)
Zinc released from ZnO NPs or conventionally applied zinc, such as ZnSO4, is transformed after the absorption to leaves via complexation with carboxylic groups (oxalate, pectine, citrate), phytate and cysteine, or it stays in Zn2+ form, with forms and their relative concentrations varying in different plants and plant stages (Li et al. 2019; Kolenčík et al. 2019).
Foliar application of 10 mg∙l−1 ZnO NPs, 15–52 nm in size, applied twice, before and after flowering, improved grain weight, seed length, seed thickness, and seed in rice (Oryza sativa) (Itroutwar et al. 2020a). Foliar application of ZnO NPs also leads to transfer of Zn to grains, as was observed in the work of Doolette et al. (2020), where 40–50 nm ZnO NPs were applied on wheat (Triticum aestivum). ZnO NP application at 750 mg∙l−1 led to a higher grain yield than in ZnCl2 application . However, the authors did not have an explanation for this phenomenon, and all other measured parameters did not show a better efficacy compared to the conventional application of Zn (ZnCl2 and Zn EDTA). Read et al. (2019) found that ZnEDTA had higher foliar uptake than ZnO NP in wheat (Triticum estivum), and already taken up ZnO NP and ZnEDTA were transported in a similar way to newly formed leaves. When applied on corn, ZnO NPs showed a higher promotion of growth and seed yield compared to more conventional fertilizers (Bala et al. 2019). Two application of low concentration of ZnO NPs at crucial points of foxtail millet (Setaria italica) growth positively affected several parameters necessary for crop production (Kolenčík et al. 2019). Foxtail millet plant grains had significantly higher oil and total nitrogen contents and a significantly lower crop water stress index (Kolenčík et al. 2019). Two applications of ZnO NPs at low concentrations had a positive effect on head diameter, dry-seed head weight, yield and thousand seed weight of sunflower, and also on sunflower physiological responses (Helianthus annuus) (Kolenčík et al. 2020). When applied on sunflowers at 60 mg∙l−1 on day 25 and 45 after sawing, ZnO NP decreased the uptake of Cr and Pb and increased the uptake of Fe and had an even better effect when applied at 30 mg∙l−1 in combination with rice straw biochar and cow manure biochar and additionally reduced Cu, Ni, and Cd concentrations in plants (Seleiman et al. 2020). Generally, the foliar application is preferred as less of the ZnO NP is needed overall, and the ZnO NP do not contaminate the soil to such a degree that it could be detrimental to the growth of other, more susceptible plant species grown later at the same field.
12.3.4 Effect of Applied Nanoparticle Concentration and Soil Properties
Despite multiple studies showing that ZnO NPs can have an influential role in the growth and development of plants, they also may have a detrimental effect on plants and the environment when applied at too high concentrations and by an inappropriate method. Release of high concentrations of Zn ions and production of ROS due to ZnO NPs higher reactivity can inflict damage to organisms in contaminated environment (Manke et al. 2013; Rajput et al. 2018). The toxic effects of ZnO NPs were studied on a large variety of organisms, including bacteria, fungi, plants, invertebrates and vertebrates, and their effect on soil health was also studied (Rajput et al. 2018). Under natural sunlight, ZnO NPs became much more toxic than during laboratory light and dark conditions to free-living nematode Caenorhabditis elegans and the increased toxicity was related to ROS generation (Ma et al. 2011). Seed soaking and exposure of roots to higher concentrations of ZnO NPs may lead to diminished root growth (Yang et al. 2015), reduction of plant growth, and photosynthesis (Wang et al. 2016b), reduction in catalase and ascorbate peroxidase in roots (Mukherjee et al. 2014). Tripathi et al. (2017a) found a decrease in ascorbate peroxidase, glutathione reductase, dehydroascorbate reductase, and monodehydroascorbate reductase, whereas ascorbic acid þ dehydroascorbic acid, and ascorbic acid increased when ZnO NPs were applied on wheat seedlings. The toxicity may come from the dissolution of Zn inside and/or outside roots and from damage caused by direct contact of roots with ZnO NPs (Lin and Xing 2008).
When applied at lower, appropriate concentrations, ZnO NPs may have a positive effect on plant growth. At higher dosages (>500 mg∙kg−1), ZnO NPs may often have a toxic effect on plants via the release of Zn ions and ROS production. While at lower concentrations (50 mg∙kg−1) or when applied on leaves or through seed coating or priming applications, they often have beneficial effects and are promising plant growth promoters, nanofertilizers, or nanopesticides. When studying impacts on plant growth, it is also advisable to ascertain that the ZnO NPs do not have an inhibitory effect on beneficial soil bacteria (Reddy Pullagurala et al. 2018b). The positive effects of ZnO NPs include an increase in shoot length, root length, fresh and dry biomass, protein content, an increase in other phytochemicals of agricultural use and an increase in photosynthetic activity. Upon application of ZnO NPs, expression of genes is altered, and various effects on biochemistry, physiology, and plant morphology have been observed (Tripathi et al. 2017b; Thorny Chanu and Upadhyaya 2019; Kolenčík et al. 2019, 2020; Faraz et al. 2020).
A different number of applications of ZnO NPs, two and four, were applied on pinto bean (Phaseolus vulgaris) cultivars, and the four applications (0.05%, 0.1%, or 0.15% w/v) showed an increase in plant height and internode length compared to two applications of ZnO NPs and also compared to two and four applications of ZnSO4 and chelated Zn at similar concentrations. Four applications of 0.05% ZnO NPs were more effective than two applications of 0.1% or 0.15% for several measured parameters (Mahdieh et al. 2018), hinting to possibility that a higher number of applications may be more beneficial for plants, even if the total concentration of the applied ZnO NPs is similar.
Soil factors influence the response of plants to metallic nanoparticles . Both chemical factors, such as pH, organic matter, and ionic strength, and biological factors such as plant root exudates, microbes, and microbial activities, heavily influence what effects nanoparticles have on the growth and health of plants (Dimkpa 2018). One of the most important factors, soil pH, has a considerable influence on the effect of ZnO NPs on plants. More acidic pH leads to their dissolution, and their association with soil chemicals lead to similar behaviour compared to the application of ionic Zn (Wang et al. 2013). Wheat was grown in both alkaline and acidic soil, and the soil-applied ZnO NPs had a positive effect in alkaline soil but a negative effect in acidic soil (Watson et al. 2015; Anderson et al. 2017). Similar effect was observed by García-Gómez et al. (2018b) in nine plant crop species. This behaviour can be reverted by creating ZnO NP- alginate complexes that release Zn more slowly in acidic soils and thus can lower their toxicity and increase their usefulness (Martins et al. 2020). Similarly, soil enzymes produced by soil microbes were adversely affected by ZnO NPs in acidic soil more than in calcareous soil (García-Gómez et al. 2018a), which can also negatively affect the growth and health of plants.
When ZnO NPs were applied on sewage sludge-amended soil, they enhanced root growth at a concentration of 50, 250, and 500 mg∙kg−1 compared to the soil with just sludge-amendment. The root growth enhancement at even a relatively high concentration may be due to a high organic matter content (Oleszczuk et al. 2019). Similar results were observed in collected manure-amended soil where higher concentrations of ZnO NPs (1000 mg∙kg−1) affected shoot weight negatively in unamended soil and positively in manure-amended soil (Moghaddasi et al. 2017).
12.4 Amelioration of Stress by ZnO NP
Plant stress is described as any unfavourable condition that affects the metabolism, growth or/and development of a plant. Plant stress can be caused by multiple factors, which are generally divided into two categories, biotic and abiotic stresses (Kranner et al. 2010). Biotic stresses are induced by living organisms like microorganisms, insects, viruses or other plant species, and abiotic stresses are initiated by environmental factors, for example, drought, salinity, and temperature (Hakim et al. 2018; Thakur et al. 2019). As a response to various types of stresses, plants have evolved immune systems and defence responses that increase their tolerance to environmental stress. Therefore, a broader study and understanding of plant tolerance mechanisms can benefit agriculture (Almutairi 2019).
The alleviation of environmental stress on crops by applying NPs has been a significant trend in the agricultural research of the last decade, as nanotechnology has been shown to be a promising tool for enhancing plant production by improving disease resistance and plant tolerance to a harsh environment. Various sources of stress, which are discussed in the following subchapters, can be mitigated by NPs (Almutairi 2019). This mitigation role of NPs depends on NPs’ size, shape and dosage, as some concentrations have been toxic for plants while lower concentrations have positive effects (Jha and Pudake 2016; Siddiqi and Husen 2017).
12.4.1 Biotic Stress
Biotic stresses, like herbivore grazing and pathogen infection, are essential factors affecting crop production. The attacker has to defeat many defence strategies that plants deploy against the intruder (Thordal-Christnsen 2003; Zhao et al. 2020). In the case of pathogen infection, it requires the interactions of a susceptible host, virulent pathogen and conducive environment. Even though conventional pesticides can significantly increase agricultural production, they can also cause health and environmental risks. Therefore, the application of various engineered metal NPs, including ZnO NPs, was considered a more gentle way to protect plants from pathogen invasion or pest and insect attacks (Poschenrieder et al. 2006; Zhao et al. 2020).
12.4.1.1 Herbivores
Higher metal ion activity in the soil or on the plant surface may deter, kill or inhibit the development of herbivores. Especially for chewing herbivores, consuming polluted leaves can lead to suffering from the detrimental effects of metals. Also, herbivores eating plant tissues with high metal concentrations can be affected by the toxicity or the evocation of an aversion response. Therefore insects can learn to avoid feeding on plants with higher concentrations of metals through a post-ingestive feedback mechanism (Eeva et al. 1998; Behmer et al. 2005; Poschenrieder et al. 2006). There are many studies (Noret et al. 2005; Stolpe et al. 2017) dealing with zinc applications against stress caused by herbivores. For example, the performance of caterpillars, either chewing or sucking species, on Arabidopsis halleri (Brassicaceae) was reduced on plants grown on zinc-amended soil compared to plants grown on unamended soil (Stolpe et al. 2017). Therefore, we find it essential that ZnO-NPs, a form of particulate Zn, should also be studied. Its more gradual release in soils may be advantageous, and also foliar application may be more effective because, unlike ionic Zn that is readily absorbed by leaves, they may stay for a longer period of time on leaf surfaces and thus have a longer-lasting protective effect on the plants.
12.4.1.2 Pathogens
At least 25% of crop losses worldwide is due to plant parasites. Conventional synthetic fungicides are considered to be the most effective for plant diseases (Pandey et al. 2018; Malandrakis et al. 2019). However, pathogens can become resistant to fungicides because of long-term exposure, and residues of fungicides are also dangerous for human health and the environment (Zhang et al. 2015). NPs are promising in resolving this challenge in the future by providing a novel eco-friendly alternative to synthetic fungicides. ZnO NPs have been shown to be very effective antibacterial and antifungal agents against numerous species due to their unique physicochemical properties (Pandey et al. 2018; Sun et al. 2018; Malandrakis et al. 2019).
According to Malandrakis et al. (2019), ZnO NPs were able to inhibit in vitro mycelial growth of fungal strains in a dose-response manner. ZnO NPs were also more toxic at the spore germination level than at mycelial growth and more effective than the commercial fungicide containing Cu(OH)2. Hafez et al. (2020) tested the application of bio-agent Bacillus subtilis with ZnO NPs to control powdery mildew in cucumber plants caused by Podosphaera xanthii. The application reduced electrolyte leakage, and the disease severity was correlated with the production of defence-related enzymes and early elevation of ROS levels. Total chlorophyll content and yield production were increased, along with most morphological and physiological characteristics and improved fruit yield. Savi et al. (2015) studied ZnO NPs treatment onto spikelets at the anthesis stage on wheat, inoculated with Fusarium graminearum. Results showed a reduction in the number of colonies of Fusarium graminearum in samples treated with ZnO NPs when compared to control. Deoxynivalenol (mycotoxin) formation in the grains was also reduced. The concentration of Zn remained within the internationally recommended levels for consumption, and the ZnO NPs treatment did not cause any damage to wheat grains. Biologically synthesized ZnO NPs using Parthenium hysterophorus reported maximum inhibition for Aspergillus niger and A. flavus. It was confirmed that smaller ZnO NPs have greater antifungal activity against fungal pathogens (Rajiv et al. 2013; Ingle et al. 2020). Still, more studies are needed to find the best ways of ZnO NPs application under field conditions and also to investigate their effects on a diverse range of pathogens.
12.4.2 Abiotic Stress
Abiotic stresses are estimated to be the primary factor of crop-production drops worldwide (Bajguz and Hayat 2009; Zhu 2016). ZnO NPs may enhance the defence mechanisms of plants against abiotic stresses by stimulating the activities of antioxidant enzymes and bettering the accumulation of osmolytes, free amino acids, and nutrients (Torabian et al. 2016; Hassan et al. 2018; Rizwan et al. 2019a). In Table 12.1., we show some of the known effects ZnO NPs have on the amelioration of abiotic stresses.
12.4.2.1 Heavy Metals
Heavy metal stress has become a global phenomenon causing various toxic effects at high concentrations and, thus, growth inhibition of crop plants. Although some heavy metals act as nutrients at lower concentrations, their excess in plants can lead to oxidative stress. High concentrations of some heavy metals in soil/growth medium can also increase ROS generation, denaturation of cell structures, cell membranes, and biomolecules (Sharma et al. 2012; Chibuike and Obiora 2014; Khan et al. 2017). For example, Cd can enter through roots and cause damage to the photosynthetic system, impairing plants growth and nutrient uptake and accumulation. Further, Cd affects the redox homeostasis of the plant cells and enhances ROS production. Even though plants have developed a defence system, it fails at elevated Cd stress (Bashir et al. 2018; Rizwan et al. 2019c, a). To combat the heavy metal stress, NPs have been applied to soils, and they were found to be effective in alleviating heavy metals stress in plants. They can easily penetrate into a contaminated zone due to their small size and large surface area and have a strong affinity to metals, where the metals make bonds with NPs, they are either adsorbed on their surfaces or chemically bound in NPs and are, thus, immobilized and are less bioavailable to plants. ZnO NPs also release Zn ions that compete with Cd, Cu or other ions in soil solutions and limit their uptake (Khan et al. 2017; Tripathi et al. 2015; Worms et al. 2012).
Hussain et al. (2018), Khan et al. (2019) and Rizwan et al. (2019a, c) tested the effect of ZnO NPs on wheat under Cd stress. Both results showed increased dry weights of shoot, roots, spikes, and grains. The concentration of Cd in roots, shoots and grains were significantly reduced with ZnO NPs treatment. Rizwan et al. (2019a) further showed that ZnO NPs positively affected the photosynthesis of wheat and reduced the electrolyte leakage and superoxide dismutase and peroxidase activities in leaves of Cd-stressed wheat. Shah et al. (2021) showed that the combined application of ZnO NPs and Bacillus fortis IAGS 223 modulated the activity of antioxidant enzymes besides upregulation of the biochemicals and growth parameters of Cd stressed plants. They also found a decreased amount of stress markers (H2O2 and MDA) and a reduction of Cd content in shoots. In rice, foliar application of ZnO NPs decreased Cd uptake, and lower Cd content was found in rice roots and shoots (Ali et al. 2019). However, contrary to the studies mentioned above, Zhang et al. (2019, 2020) discovered that Cd bioavailability increased in high ZnO NPs (500 mg∙kg−1) treatments.
Priyanka et al. (2021) tested the application of ZnO NPs (0–200 mg∙l−1) on the development of Cd and Pb tolerance mechanism in cotton seedlings. ZnO NPs applications significantly promoted shoot and root growth as well as biomass under Cd and Pb stress. It also up-regulated chlorophyll a,b and carotenoids contents in leaves under Cd and Pb stress, along with the accumulation of antioxidant defence enzymes (CAT, POX, APX, SOD) and MDA contents. This indicates that the addition of ZnO NPs protects cotton seedlings by alleviating Cd and Pb stress. Sharifan et al. (2020) showed similar results after the application of ZnO NPs (100 mg∙l−1) on different leafy greens (spinach, parsley and cilantro) under Cd and Pb stress. Results by Seleiman et al. (2020) showed positive effects after foliar applications of ZnO NPs (60 mg∙l−1), rice straw biochar and cow-manure biochar on sunflowers under Pb, Cr, Cu and Cd stress. The application of the combination treatment reduced the availability of Pb, Cr, Cu and Cd in the soil by 78.6, 115.3, 153.3, and 178.5% in comparison to untreated plots, and it also reduced the Pb, Cr, Cu and Cd in plant biomass by 1.13, 5.19, 3.88, and 0.26 mg∙kg−1, respectively.
Wu et al. (2020) described the role of ZnO NPs (10–200 mg∙l−1) in alleviating As stress in rice germination and early seedling growth. ZnO NPs increased the germination rate (2.3–8.9%), shoot weight (18.2–42.4%), root weight (5.2–23.9%), and chlorophyll content (3.5–40.1%), while elevated the SOD (2.2–22.8%) and CAT (7.2–60.7%) activities and reduced the MDA content (17.5–30.8%). The concentration of As was decreased by 8.4–72.3% in rice roots and 10.2–56.6% in rice shoots. ZnO NPs amendment increased As adsorption and promoted biomass of rice. Similarly, in the study by Wang et al. (2018), ZnO NPs reduced the accumulation of As(III) in rice roots and shoots when the As was applied as As(III) and As(V), and As(V) in rice roots. However, the concentration of As(V) in rice shoots was unaffected.
Interaction of heavy metals with ZnO NPs in plants still has gaps in knowledge, and, therefore, ZnO NPs application was studied in plants affected by Pb. Raghib et al. (2020) applied ZnO NPs, and ZnO NPs in combination with arbuscular mycorrhizal fungi. Both applications increased the growth and biochemical attributes of wheat and decreased the Pb uptake from contaminated soil. The combined formula of ZnO NPs and fungi has shown the best results, increasing growth parameters like plant height, fresh weight, dry weight, and total chlorophyll content. Also, the application of ZnO NPs with fungi had a positive effect on plant metabolism and increased proline content, H2O2 content, the SOD and CAT enzymes’ activity, and increased lipid peroxidation content. The Pb concentration was reduced in both roots and shoots of wheat after applying ZnO NPs with fungi.
ZnO NPs have the potential to alleviate heavy metal stress in plants. However, more field studies are needed where the best mode of application is found. ZnO NPs also show promise in combined formulas with other treatments where they positively enhance the treatment effects.
12.4.2.2 Heat
Heat stress is defined as “the rise in temperature of both soil and air above the level of the threshold for a limited time such that permanent harm occurs to plants” (Lipiec et al. 2013). Generally, an impermanent phase when the temperature exceeds temperature tolerance by 10–15 °C is referred to as heat stress/shock, which reduces plant growth and crop productivity (Wahid 2007). Higher temperature also increases ROS production, and it causes oxidative stress and limits plant growth and yields. The chlorophyll content is also affected by heat stress, and low chlorophyll content is mainly present in leaves (Møller et al. 2007; Mathur et al. 2014; Faizan et al. 2020b). This could be caused by inhibited chlorophyll biosynthesis or enhanced degradation of chlorophyll pigments. The damaged chlorophyll biosynthesis under heat stress is a consequence of the presence of many heat-sensitive enzymes in the chlorophyll biosynthesis pathway (Mathur et al. 2014). An increase in leaf temperature can also lead to a deactivation of the heat-sensitive enzyme Rubisco (the enzyme responsible for CO2 fixation during photosynthesis), initiating the photorespiratory pathway and generating H2O2 (a by-product of the pathway) (Sharkey 2005; Allakhverdiev et al. 2008).
According to Hassan et al. (2018), the application of ZnO NPs enhanced heat tolerance in wheat by maintaining ROS production and the stability of biomembranes and proteins. The treatment of ZnO NPs on wheat also reduced the permeability of the leaf cells’ plasma wall, resulting in a decrease in lipid peroxidation and protecting the cellular wall against heat stress. The mechanism of heat stress amelioration by ZnO NPs is still poorly understood since we were able to find only one study that examined it. The heat tolerance may come from sufficient nutrition with Zn that increased levels of antioxidants, as was shown in chickpeas and winter wheat supplemented with ionic Zn (Peck and McDonald 2010; Ullah et al. 2019). However, Ag NPs also helped to increase heat tolerance in wheat (Iqbal et al. 2019) with a not well-understood process that may be linked to their nano-size. Therefore, ZnO NPs may be superior in protecting plants from heat stress compared to more conventional ionic zinc formulations.
12.4.2.3 Cold
Cold stress is abiotic stress, which can cause difficulties in plant growth and production. It is caused by temperatures cool enough (0–15 °C) to damage plants without forming ice crystals in plant tissues, whereas freezing stress (<0 °C) results in the formation of ice crystal in plant tissues (Hasanuzzaman et al. 2013). Plants exposed to cold stress suffer from loss of fluidity of membranes, leakage of solutes, poor growth and germination, and reduced crop yield. It also causes inhibition in chlorophyll levels, CO2 assimilation, transpiration rate and degradation of Rubisco (Welti et al. 2002; Suzuki et al. 2008; Liu et al. 2012). Enhancement of carboxylation of Rubisco, the light absorption capacity of chloroplasts, electron transport rate, and inhibition of ROS generation have been described as alleviating effects of NPs on cold stress in plants (Gao et al. 2006; Giraldo et al. 2014; Khan et al. 2017; Ze et al. 2011). Foliar application of ZnO NPs may mitigate ROS generation by increasing enzymatic activities of superoxide dismutase, catalase, and peroxidase, and, more generally, they may prevent photoinhibition (Elsheery et al. 2020).
Elsheery et al. (2020) tested a foliar application of ZnO NPs (50 mg∙l−1) on sugarcane in an open field experiment. Results showed that during a cold front, chlorophyll a and b contents were significantly reduced, but the rate of reduction was lower in seedlings treated with ZnO NPs than that of the control group. In contrast, carotenoids were increased during the cold front. These effects demonstrate that ZnO NPs can mitigate the negative impact of cold stress in sugarcane. Maslobrod et al. (2020) treated winter wheat seeds with water dispersions of ZnO NPs and a mixture of bismuth, copper, zinc oxide NPs. Both treatments increased seed thermal stability, seed germination energy and length of coleoptiles while being exposed to low temperature (+4 °C).
12.4.2.4 Drought
Drought events are becoming more common as a result of anthropogenic influence on climate change that severely limits crop production. During these events, plants experience insufficient water uptake and, consequently, nutrient uptake that is related to a changed condition in a soil environment with limited amounts of capillary water present. During drought, plant growth and development are affected (Faizan et al. 2020a, 2021). Drought seriously disturbs plant growth, reducing the rate of cell division, leaf expansion, stem elongation, and water use efficiency. Drought stress also impairs enzyme activities, results in loss of turgor, root proliferation, plant water and nutrients. Likewise, diminished agricultural productivity and prolonged maturation of plants are caused by droughts (Poormohammad Kiani et al. 2007; Farooq et al. 2009; Faizan et al. 2020b). Plant macronutrients (N, P, K) have low uptake efficiencies (<50%) under normal soil moisture. These efficiencies are even lower during drought event, which further reduces fertilizer efficacy. Therefore, a reduction in grain yield and nutritional quality caused by drought stress can lead to food and nutrition insecurity (Baligar et al. 2001; Fischer et al. 2019). Among numerous techniques used to alleviate drought stress in crops, the application of ZnO NPs is considered to be an effective treatment. ZnO NPs help protect chlorophyll and other pigments, reduce ROS generation, and provide plants with Zn, which is important for many enzymes related to drought stress response mechanisms.
Dimkpa et al. (2020a, b) studied wheat performance after application of ZnO NPs under drought condition. Results showed that drought significantly reduced chlorophyll levels (6%), but ZnO NPs alleviated some stress by increasing chlorophyll levels (16%) compared to control. Drought delayed (3 days) panicle emergence, and ZnO NPs accelerated (5 days) panicle emergence under drought condition. Grain yield was unaffected by ZnO NPs under drought stress but increased (88%) under non-drought condition. Adrees et al. (2021) studied the foliar application of ZnO NPs (20, 50, and 100 mg∙l−1) on wheat under drought stress (35% of water holding capacity). The foliar exposure of ZnO NPs elevated leaf chlorophyll contents and also decreased oxidative stress, and enhanced the leaf superoxide dismutase and peroxidase activities. They further showed that ZnO NPs decreased Cd concentrations in grains under water deficit conditions by 35, 66, and 81%, respectively. Furthermore, Linh et al. (2020) demonstrated that ZnO NPs treatment effectively helped soybean plants at an early vegetative stage to adapt to drought stress, and (Dimkpa et al. 2019) showed similar findings with sorghum plants.
12.4.2.5 Flooding
Similarly to drought, flooding is also a source of major abiotic stress and can have adverse effects on plant growth and development, albeit they are very different. It affects soils by altering soils structure, depleting O2, accumulating CO2, and inducing anaerobic decomposition of organic matter. Lack of O2 around plant roots can cause severe damage and affect physiological processes in plants (Kozlowski 1997). Anaerobic respiration via ethanolic fermentation is considered to be an essential mechanism for plants to deal with the lack of O2. Switching from aerobic respiration to anaerobic fermentation under flooding stress seems to be an important mechanism that helps plants survive O2 deficiency (Drew 1997). Foliar application of Zn, especially after flooding, was shown to increase the growth of plants since Zn becomes more immobile during flood-related soil conditions (Hafeez et al. 2013). Therefore, ZnO NPs may pose an efficient way of supplying Zn to affected plants.
For example, in soybean, flooding damages plant growth mainly by damaging root length due to the loss of root tips in waterlogged soil and also reducing hypocotyl pigmentation, which leads to low intracellular O2 levels and the synthesis of proteins related to anaerobic metabolic pathways (Russell et al. 1990; Huang et al. 2005; Hashiguchi et al. 2009). To alleviate some aspects of flood stress, Mustafa et al. (2015) exposed soybeans to various NPs, including ZnO NPs (5, 50, and 500 mg∙l−1). After ZnO NP (50 mg∙l−1) treatment, soybean showed a lower fresh weight of plants under flood stress compared to control. Soybeans treated with 5 and 500 mg∙l−1 ZnO NPs experienced a further decrease in the fresh weight of plants. Also, under treatment with 50 mg∙l−1 ZnO NPs, the length of root, including hypocotyl, was increased after 2 days of stress but then decreased during the remaining days of the treatment period. Soybean treated with 5 and 500 mg∙l−1 of ZnO NPs showed a decreased length of root and hypocotyl compared to the flooding-stressed plants during the treatment period. The results of the experiments have shown that treating soybean with ZnO NPs before flooding did not alleviate the stress caused by flooding. However, this was a laboratory experiment, where 2 days-old soybeans were submerged in reverse osmosis water. For rice, there are known times of application in field, when Zn treatment can have the highest positive effect even before flooding of the plants, with preplant incorporated, and delayed preemergence stages having the highest positive effect (Slaton et al. 2005). Therefore, we believe that studying the application of ZnO NPs may positively affect the growth of rice and other plants that may face flooding stress (Elshayb et al. 2021).
12.4.2.6 Salts
Crop production worldwide faces an increase in land area with heightened salinity as a result of the industrialization of agriculture, incorrect agricultural practices, and changing climate. Salt affected lands increased by >100 Mha between 1986 and 2016 (Ivushkin et al. 2019). Stress from salinity affects plant growth and causes severe problems, mainly in arid and semi-arid lands (Hussain et al. 2019). The increased amount of soil salts hinders germination, morpho-physiological traits and crop yield. It can lead to extensive accumulation of ions (Na+, Cl−) and inhibit K+ and Ca2+ uptake and result in ionic imbalance. Furthermore, salt causes the accumulation of ROS in plant cells, creating oxidative and osmotic stress (Astaneh et al. 2018; Isayenkov and Maathuis 2019). Osmotic stress causes the lower availability to take up water, which leads to dwarfed growth, while oxidative stress inhibits plant transpiration and damages cells in the transpiring leaves (Munns 2005; Amirjani 2011).
The application of ZnO NPs could be beneficial for reducing the adverse effects of salt stress. ZnO NPs positively affected the growth ratings in salt-stressed plants because ZnO NPs treatment synthesizes the indole acetic acid (IAA) and thus activates cell division and enlargement (Ali and Mahmound 2013; Latef et al. 2016; Faizan et al. 2020b). It can also be beneficial for maintaining the structural integrity of biomembranes, improving protein synthesis, increasing shoot length, chlorophyll, nutrient content, antioxidant enzyme activity, photosynthetic rate, etc. (He et al. 2015; Landa et al. 2015; Torabian et al. 2016; Hussein and Abou-Baker 2018). Moreover, Soliman et al. (2015) showed that ZnO NPs reduce Na+ and Cl− contents and increase N, P, K+, Ca2+, Mg2+, Fe and Zn in Moringa peregrina.
Faizan et al. (2021) studied the foliar application of ZnO NPs (10, 50, and 100 mg∙l−1) in the presence/absence of NaCl (150 mM) on tomato plants. Results showed that foliar spray of ZnONPs significantly increased shoot length, root length, biomass, leaf area, chlorophyll content and photosynthetic attributes of tomato plants in the presence/absence of salt stress. The application of ZnONPs also ameliorate the negative effects of salt stress and enhanced protein content and antioxidative enzyme activity under salt stress. Alabdallah and Alzahrani (2020) showed that ZnO NPs (10 mg∙l−1) treatment increased the salinity tolerance in okra plants. The folial application of ZnO NPs increased the contents of photosynthetic pigments and the activity of antioxidant enzymes. ZnO NPs seed priming (5 and 10 mg∙l−1) of wheat increased growth parameters such as photosynthetic pigments, indole-3-acetic acid, phenol contents, and organic antioxidant enzyme activities. The treatments also significantly decreased lipid peroxidation (El-Bassiouny et al. 2020). Other studies (Gaafar et al. 2020; Singh et al. 2021) showed positive effects of ZnO NPs (50 mg∙l−1) under salt stress at soybean and Linum usitassimum, respectively. Overall, these studies suggest that the application of ZnO NPs at the appropriate dosage can be beneficial for enhancing the plant’s toleration and antioxidant activity to decrease the damage caused by salt stress.
12.5 Conclusion and Future Outlook
Exposure to engineered NPs, including ZnO NPs, may have both negative or positive effects on plants and the environment. From an agricultural perspective, nanotechnology may mainly help with nutrient management that leads to more environmentally friendly agriculture, where applied nanomaterials have more targeted effects, reduce the number of chemicals used thanks to a more controlled release of nutrients and potential benefits related to the nanostructure of applied chemicals. It may help create more effective formulas with better plant responses against biotic and abiotic stresses during their life cycles. Such a limited, precise application of nanomaterials can have positive environmental impacts together with potential cost-saving measures for agriculture. However, there is also inherent risk connected to their unknown long-term application spanning several generations, detailed effects on the food chain, unclear impact on soil edaphon, and the quality of agricultural products as viewed by biogeochemical transport and transformations of NPs and their residues. Equally important is the absence of a more precise international legislative framework that determines levels of concentrations of NPs that are toxic and that determines which individual properties of NPs play the major role in their toxicity with standardised tests for not only toxicity in agricultural and model plants, but also for soil microorganisms and animals, including the impact on human health.
ZnO NPs have been shown to improve the growth of crops functioning as micronutrient nano fertilizer, nano growth promoters or nano pesticides that also protect plants from abiotic and biotic stress. A wide variety of effects, including alteration of several biochemical, metabolic, and physiological processes, e.g. production of reactive oxygen species, photosynthesis, water status, root hydraulic conductance, stress signalling and hormonal pathways, and transport and distribution of solutes in plants were found for ZnO NPs. The direct knowledge of the processes governing ZnO NPs interaction with various species of crops is growing but is not exhaustive, although much can be inferred from the action of ionic Zn in plants. ZnO NPs have the potential to alleviate different types of abiotic and biotic stresses in plants. However, more research is needed to fully understand the interaction between different species of plants and ZnO NPs with corresponding macro-sized or ionic forms, where the molecular and transcriptional alterations at the level of the plant are still not to be fully understood. Also, metabolic and proteomic changes in different plant organs need to be fully described. Mechanisms underlying the ability of ZnO NPs to alleviate abiotic stress in plants need to be characterised at the molecular and genetic levels and their actions compared to ionic forms of Zn to elucidate nano specific actions of ZnO NPs. Finally, more attention should be put on the development of strategic tools for ZnO NPs application in fields conditions in the context of climate change to further the knowledge gained from greenhouse and laboratory studies.
References
Adrees M, Khan ZS, Hafeez M et al (2021) Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous Cd and water deficient stress. Ecotoxicol Environ Saf 208:111627. https://doi.org/10.1016/j.ecoenv.2020.111627
Alabdallah NM, Alzahrani HS (2020) The potential mitigation effect of ZnO nanoparticles on [Abelmoschus esculentus L. Moench] metabolism under salt stress conditions. Saudi J Biol Sci 27:3132–3137. https://doi.org/10.1016/j.sjbs.2020.08.005
Ali EA, Mahmound AM (2013) Effect of foliar spray by different salicylic acid and zinc concentrations on seed yield and yield components of mungbean in Sandy soil. Asian J Crop Sci 5:33–40
Ali S, Rizwan M, Noureen S et al (2019) Combined use of biochar and zinc oxide nanoparticle foliar spray improved the plant growth and decreased the cadmium accumulation in rice (Oryza sativa L.) plant. Environ Sci Pollut Res 26:11288–11299. https://doi.org/10.1007/s11356-019-04554-y
Allakhverdiev SI, Kreslavski VD, Klimov VV et al (2008) Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res 98:541–550. https://doi.org/10.1007/s11120-008-9331-0
Allen RD (1995) Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol 107:1049–1054. https://doi.org/10.1104/pp.107.4.1049
Alloway BJ (2008) Zinc in soils and crop nutrition. International Zinc Association, Brussels
Alloway BJ (2009) Soil factors associated with zinc deficiency in crops and humans. Environ Geochem Health 31:537–548. https://doi.org/10.1007/s10653-009-9255-4
Almutairi ZM (2019) Plant molecular defense mechanisms promoted by nanoparticles against environmental stresses. Int J Agric Biol 21:259–270
Amirjani MR (2011) Effect of salinity stress on growth, sugar content, pigments and enzyme activity of rice. Int J Bot 7:73–81
Anderson A, McLean J, McManus P, Britt D (2017) Soil chemistry influences the phytotoxicity of metal oxide nanoparticles. Int J Nanotechnol 14:15–21. https://doi.org/10.1504/IJNT.2017.082438
Apel K, Hirt H (2004) REACTIVE OXYGEN SPECIES: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. https://doi.org/10.1146/annurev.arplant.55.031903.141701
Astaneh RK, Bolandnazar S, Nahandi FZ, Oustan S (2018) The effects of selenium on some physiological traits and K, Na concentration of garlic (Allium sativum L.) under NaCl stress. Inf Process Agric 5:156–161. https://doi.org/10.1016/j.inpa.2017.09.003
Awan S, Shahzadi K, Javad S et al (2021) A preliminary study of influence of zinc oxide nanoparticles on growth parameters of Brassica oleracea var italic. J Saudi Soc Agric Sci 20:18–24. https://doi.org/10.1016/j.jssas.2020.10.003
Aziz Y, Shah GA, Rashid MI (2019) ZnO nanoparticles and zeolite influence soil nutrient availability but do not affect herbage nitrogen uptake from biogas slurry. Chemosphere 216:564–575. https://doi.org/10.1016/j.chemosphere.2018.10.119
Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47:1–8. https://doi.org/10.1016/j.plaphy.2008.10.002
Bala R, Kalia A, Dhaliwal SS (2019) Evaluation of efficacy of ZnO nanoparticles as remedial zinc Nanofertilizer for Rice. J Soil Sci Plant Nutr 19:379–389. https://doi.org/10.1007/s42729-019-00040-z
Baligar VC, Fageria NK, He ZL (2001) Nutrient use efficiency in plants. Commun Soil Sci Plant Anal 32:921–950. https://doi.org/10.1081/CSS-200047048
Bari R, Jones JDG (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69:473–488. https://doi.org/10.1007/s11103-008-9435-0
Bashir A, Rizwan M, Ali S et al (2018) Effect of foliar-applied iron complexed with lysine on growth and cadmium (cd) uptake in rice under cd stress. Environ Sci Pollut Res 25:20691–20699. https://doi.org/10.1007/s11356-018-2042-y
Behmer ST, Lloyd CM, Raubenheimer D et al (2005) Metal hyperaccumulation in plants: mechanisms of defence against insect herbivores. Funct Ecol 19:55–66. https://doi.org/10.1146/annurev-arplant-042809-112156
Bian S-WW, Mudunkotuwa IA, Rupasinghe T, Grassian VH (2011) Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 27:6059–6068. https://doi.org/10.1021/la200570n
Bitenc M, Crnjak Orel Z (2009) Synthesis and characterization of crystalline hexagonal bipods of zinc oxide. Mater Res Bull 44:381–387. https://doi.org/10.1016/j.materresbull.2008.05.005
Blumwald E (2000) Sodium transport and salt tolerance in plants. Curr Opin Cell Biol 12:431–434. https://doi.org/10.1016/S0955-0674(00)00112-5
Borysiewicz MA (2019) ZnO as a functional material, a review. Crystals 9. https://doi.org/10.3390/cryst9100505
Chang Y-N, Zhang M, Xia L et al (2012) The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials (Basel) 5:2850–2871. https://doi.org/10.3390/ma5122850
Chaudhuri SK, Malodia L (2017) Biosynthesis of zinc oxide nanoparticles using leaf extract of Calotropis gigantea: characterization and its evaluation on tree seedling growth in nursery stage. Appl Nanosci 7:501–512. https://doi.org/10.1007/s13204-017-0586-7
Chen WJ, Liu WL, Hsieh SH, Tsai TK (2007) Preparation of nanosized ZnO using α brass. Appl Surf Sci 253:6749–6753. https://doi.org/10.1016/j.apsusc.2007.01.091
Chibuike GU, Obiora SC (2014, 2014) Heavy metal polluted soils : effect on plants and bioremediation methods. Appl Environ Soil Sci. https://doi.org/10.1155/2014/752708
Chiu WS, Khiew PS, Cloke M et al (2010) Photocatalytic study of two-dimensional ZnO nanopellets in the decomposition of methylene blue. Chem Eng J 158:345–352. https://doi.org/10.1016/j.cej.2010.01.052
Choudhary PK, Khandelwal V (2020) Comparative efficacy of Zn supplement and zinc oxide nanoparticles over the seed germination of lentil and Chick pea. J Pure Appl Microbiol 14:673–678. https://doi.org/10.22207/JPAM.14.1.69
Choudhury S, Panda P, Sahoo L, Panda SK (2013) Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav 8:e23681. https://doi.org/10.4161/psb.23681
Czarnocka W, Karpiński S (2018) Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic Biol Med 122:4–20. https://doi.org/10.1016/j.freeradbiomed.2018.01.011
Davies PJ (2010) The plant hormones: their nature, occurrence, and functions. In: Davies PJ (ed) Plant hormones: biosynthesis, signal transduction, action! Springer, Dordrecht, pp 1–15
Dietz K-J, Herth S (2011) Plant nanotoxicology. Trends Plant Sci 16:582–589. https://doi.org/10.1016/j.tplants.2011.08.003
Dileep Kumar G, Raja K, Natarajan N et al (2020) Invigouration treatment of metal and metal oxide nanoparticles for improving the seed quality of aged chilli seeds (Capsicum annum L.). Mater Chem Phys 242:122492. https://doi.org/10.1016/j.matchemphys.2019.122492
Dimkpa CO (2018) Soil properties influence the response of terrestrial plants to metallic nanoparticles exposure. Curr Opin Environ Sci Heal 6:1–8. https://doi.org/10.1016/J.COESH.2018.06.007
Dimkpa CO, Singh U, Bindraban PS et al (2019) Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci Total Environ 688:926–934. https://doi.org/10.1016/j.scitotenv.2019.06.392
Dimkpa CO, Andrews J, Fugice J et al (2020a) Facile coating of urea with low-dose ZnO nanoparticles promotes wheat performance and enhances Zn uptake under drought stress. Front Plant Sci 11:168. https://doi.org/10.3389/fpls.2020.00168
Dimkpa CO, Andrews J, Sanabria J et al (2020b) Interactive effects of drought, organic fertilizer, and zinc oxide nanoscale and bulk particles on wheat performance and grain nutrient accumulation. Sci Total Environ 722:137808. https://doi.org/10.1016/j.scitotenv.2020.137808
Doolette CL, Read TL, Howell NR et al (2020) Zinc from foliar-applied nanoparticle fertiliser is translocated to wheat grain: a 65Zn radiolabelled translocation study comparing conventional and novel foliar fertilisers. Sci Total Environ 749:142369. https://doi.org/10.1016/j.scitotenv.2020.142369
Drew MC (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Biol 48:223–250. https://doi.org/10.1146/annurev.arplant.48.1.223
Dutta RK, Nenavathu BP, Talukdar S (2014) Anomalous antibacterial activity and dye degradation by selenium doped ZnO nanoparticles. Colloids Surf B Biointerf 114:218–224. https://doi.org/10.1016/j.colsurfb.2013.10.007
Eeva T, Lehikoinen E, Ronka M (1998) Air pollution fades the plumage of the Great Tit. Funct Ecol 12:607–612
El-Bassiouny HMS, Abdallah MM-S, El-Enany MAM, Sadak MS (2020) Nano-zinc oxide and arbuscular mycorrhiza effects on physiological and biochemical aspects of wheat cultivars under saline conditions. Pakistan J Biol Sci 23:478–490. https://doi.org/10.3923/pjbs.2020.478.490
Elshayb OM, Farroh KY, Amin HE, Atta AM (2021) Green synthesis of zinc oxide nanoparticles: fortification for Rice grain yield and nutrients uptake enhancement. Molecules 26. https://doi.org/10.3390/molecules26030584
Elsheery NI, Sunoj VSJ, Wen Y et al (2020) Foliar application of nanoparticles mitigates the chilling effect on photosynthesis and photoprotection in sugarcane. Plant Physiol Biochem 149:50–60. https://doi.org/10.1016/j.plaphy.2020.01.035
Esper Neto M, Britt DW, Lara LM et al (2020) Initial development of corn seedlings after seed priming with nanoscale synthetic zinc oxide. Agronomy:10. https://doi.org/10.3390/agronomy10020307
Faizan M, Faraz A, Yusuf M et al (2018) Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 56:678–686. https://doi.org/10.1007/s11099-017-0717-0
Faizan M, Hayat S, Pichtel J (2020a) Effects of zinc oxide nanoparticles on crop plants: a perspective analysis. In: sustainable agriculture reviews 41 nanotechnology for plant growth and development. Springer, Cham, pp 83–100
Faizan M, Yu F, Chen C et al (2020b) Zinc oxide nanoparticles help to enhance plant growth and alleviate abiotic stress: a review. Curr Protein Pept Sci 21:1–15. https://doi.org/10.2174/1389203721666201016144848
Faizan M, Bhat JA, Chen C et al (2021) Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol Biochem 161:122–130. https://doi.org/10.1016/j.plaphy.2021.02.002
Faraz A, Faizan M, Fariduddin Q, Hayat S (2020) Nanotechnology for plant growth and development. Springer, Cham
Farooq M, Wahid A, Basra SMA (2009) Improving water relations and gas exchange with brassinosteroids in rice under drought stress. J Agron Crop Sci 195:262–269. https://doi.org/10.1111/j.1439-037X.2009.00368.x
Fischer S, Hilger T, Piepho HP et al (2019) Do we need more drought for better nutrition? The effect of precipitation on nutrient concentration in East African food crops. Sci Total Environ 658:405–415. https://doi.org/10.1016/j.scitotenv.2018.12.181
Flowers TJ, Colmer TD (2015) Plant salt tolerance: adaptations in halophytes. Ann Bot 115:327–331. https://doi.org/10.1093/aob/mcu267
Frade T, Melo Jorge ME, Gomes A (2012) One-dimensional ZnO nanostructured films: effect of oxide nanoparticles. Mater Lett 82:13–15. https://doi.org/10.1016/j.matlet.2012.05.028
Future Markets Inc (2016) The global market for zinc oxide nanoparticles, Edinburgh
Gaafar R, Diab R, Halawa M et al (2020) Role of zinc oxide nanoparticles in ameliorating salt tolerance in soybean. Egypt J Bot 60:733–747. https://doi.org/10.21608/ejbo.2020.26415.1475
Gao F, Hong F, Liu C et al (2006) Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach: inducing complex of rubisco-rubisco activase. Biol Trace Elem Res 111:239–253. https://doi.org/10.1385/BTER:111:1:239
García-Gómez C, Fernández MD, García S et al (2018a) Soil pH effects on the toxicity of zinc oxide nanoparticles to soil microbial community. Environ Sci Pollut Res 25:28140–28152. https://doi.org/10.1007/s11356-018-2833-1
García-Gómez C, Obrador A, González D et al (2018b) Comparative study of the phytotoxicity of ZnO nanoparticles and Zn accumulation in nine crops grown in a calcareous soil and an acidic soil. Sci Total Environ 644:770–780. https://doi.org/10.1016/j.scitotenv.2018.06.356
García-López JI, Zavala-García F, Olivares-Sáenz E et al (2018) Zinc oxide nanoparticles boosts phenolic compounds and antioxidant activity of capsicum annuum L during germination. Agronomy:8. https://doi.org/10.3390/agronomy8100215
Gebre SH, Sendeku MG (2019) New frontiers in the biosynthesis of metal oxide nanoparticles and their environmental applications: an overview. SN Appl Sci 1:928. https://doi.org/10.1007/s42452-019-0931-4
Gelabert A, Sivry Y, Ferrari R et al (2013) Uncoated and coated ZnO nanoparticle life cycle in synthetic seawater. Environ Toxicol Chem 33:341–349. https://doi.org/10.1002/etc.2447
Giraldo JP, Landry MP, Faltermeier SM et al (2014) Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat Mater 13:400–408. https://doi.org/10.1038/nmat3890
Gogarten JP (1988) Physical properties of the cell wall of photoautotrophic suspension cells fromChenopodium rubrum L. Planta 174:333–339. https://doi.org/10.1007/bf00959518
Gogos A, Knauer K, Bucheli TD (2012) Nanomaterials in plant protection and fertilization: current state, foreseen applications, and research priorities. J Agric Food Chem 60:9781–9792. https://doi.org/10.1021/jf302154y
González-Melendi P, Fernández-Pacheco R, Coronado MJ et al (2008) Nanoparticles as smart treatment-delivery systems in plants: assessment of different techniques of microscopy for their visualization in plant tissues. Ann Bot 101:187–195. https://doi.org/10.1093/aob/mcm283
Hafeez B, Khanif YM, Saleem M (2013) Role of zinc in plant nutrition- a review. Am J Exp Agric 3:374–391. https://doi.org/10.9734/AJEA/2013/2746
Hafez YM, Attia KA, Kamel S et al (2020) Bacillus subtilis as a bio-agent combined with nano molecules can control powdery mildew disease through histochemical and physiobiochemical changes in cucumber plants. Physiol Mol Plant Pathol 111:101489. https://doi.org/10.1016/j.pmpp.2020.101489
Hakim UA, Hussain A et al (2018) Osmotin: a plant defense tool against biotic and abiotic stresses. Plant Physiol Biochem 123:149–159. https://doi.org/10.1016/j.plaphy.2017.12.012
Han Y, Hwang G, Kim D et al (2016) Transport, retention, and long-term release behavior of ZnO nanoparticle aggregates in saturated quartz sand: role of solution pH and biofilm coating. Water Res 90:247–257. https://doi.org/10.1016/j.watres.2015.12.009
Hasanuzzaman M, Nahar K, Fujita M (2013) Extreme temperature responses, oxidative stress and antioxidant defense in plants. In: Vahdati K, Leslie C (eds) Abiotic stress – plant responses and applications in agriculture. InTech Open Access Publisher
Hashiguchi A, Sakata K, Komatsu S (2009) Proteome analysis of early-stage soybean seedlings under flooding stress. J Proteome Res 8:2058–2069. https://doi.org/10.1021/pr801051m
Hassan NS, El Din TAS, Hendawey MH, Borai IH, Mahdi AA (2018) Magnetite and zinc oxide nanoparticles alleviated heat stress in wheat plants. Curr Nanomater 3:32–43
He XC, Lin M, Li F et al (2015) Advances in studies of nanoparticle-biomembrane interactions. Nanomedicine 10:121–141. https://doi.org/10.2217/nnm.14.167
Holišová V, Konvičková Z, Kratošová G et al (2019) Phytosynthesis of Au and Au/ZrO 2 bi-phasic system nanoparticles with evaluation of their colloidal stability. J Nanosci Nanotechnol 19:2807–2813. https://doi.org/10.1166/jnn.2019.15851
Holišová V, Urban M, Konvičková Z et al (2021) Colloidal stability of phytosynthesised gold nanoparticles and their catalytic effects for nerve agent degradation. Sci Rep 11:4071. https://doi.org/10.1038/s41598-021-83460-1
Huang S, Greenway H, Colmer T, Millar AH (2005) Protein synthesis by Rice coleoptiles during prolonged anoxia: implications for glycolysis, growth and energy utilization. Ann Bot 96:703–715. https://doi.org/10.1093/aob/mci222
Huang Y, He J, Zhang Y et al (2006) Morphology, structures and properties of ZnO nanobelts fabricated by Zn-powder evaporation without catalyst at lower temperature. J Mater Sci 41:3057–3062. https://doi.org/10.1007/s10853-006-6978-9
Hussain A, Ali S, Rizwan M et al (2018) Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ Pollut 242:1518–1526. https://doi.org/10.1016/j.envpol.2018.08.036
Hussain S, Shaukat M, Ashraf M, et al (2019) Salinity stress in arid and semi-arid climates: effects and management in field crops. In: Climate Change and Agriculture
Hussein MM, Abou-Baker NH (2018) The contribution of nano-zinc to alleviate salinity stress on cotton plants. R Soc Open Sci 5. https://doi.org/10.1098/rsos.171809
Ingle AP, Biswas A, Vanlalveni C et al (2020) Biogenic synthesis of nanoparticles and their role in the management of plant pathogenic fungi. In: Pai M, Golinsa P (eds) Microbial nanotechnology, 1st edn. CRC Press, Boca Raton, pp 135–161
Iqbal M, Raja NI, Mashwani Z-U-R et al (2019) Effect of silver nanoparticles on growth of wheat under heat stress. Iran J Sci Technol Trans A Sci 43:387–395. https://doi.org/10.1007/s40995-017-0417-4
Isayenkov SV, Maathuis FJM (2019) Plant salinity stress: many unanswered questions remain. Front Plant Sci 10:80. https://doi.org/10.3389/fpls.2019.00080
Itroutwar PD, Govindaraju K, Tamilselvan S et al (2020a) Seaweed-based biogenic ZnO nanoparticles for improving agro-morphological characteristics of rice (Oryza sativa L.). J Plant Growth Regul 39:717–728. https://doi.org/10.1007/s00344-019-10012-3
Itroutwar PD, Kasivelu G, Raguraman V et al (2020b) Effects of biogenic zinc oxide nanoparticles on seed germination and seedling vigor of maize (Zea mays). Biocatal Agric Biotechnol 29:101778. https://doi.org/10.1016/j.bcab.2020.101778
Ivushkin K, Bartholomeus H, Bregt AK et al (2019) Global mapping of soil salinity change. Remote Sens Environ 231:111260. https://doi.org/10.1016/j.rse.2019.111260
Jain S, Muneer S, Guerriero G et al (2018) Tracing the role of plant proteins in the response to metal toxicity: a comprehensive review. Plant Signal Behav 13:e1507401. https://doi.org/10.1080/15592324.2018.1507401
Jha S, Pudake R (2016) Molecular mechanism of plant–nanoparticle interactions. In: Kole C, Kumar DS, Khodakovskaya MV (eds) Plant nanotechnology: principles and practices. Springer, pp 155–181
Joo SH, Zhao D (2017) Environmental dynamics of metal oxide nanoparticles in heterogeneous systems: A review. J Hazard Mater 322(Part):29–47. https://doi.org/10.1016/j.jhazmat.2016.02.068
José-Yacamán M, Gutierrez-Wing C, Miki M et al (2005) Surface diffusion and coalescence of mobile metal nanoparticles. J Phys Chem B 109:9703–9711. https://doi.org/10.1021/jp0509459
Kasivelu G, Selvaraj T, Malaichamy K et al (2020) Nano-micronutrients [γ-Fe2O3 (iron) and ZnO (zinc)]: green preparation{,} characterization{,} agro-morphological characteristics and crop productivity studies in two crops (rice and maize). New J Chem 44:11373–11383. https://doi.org/10.1039/D0NJ02634D
Khan MN, Mobin M, Abbas ZK et al (2017) Role of nanomaterials in plants under challenging environments. Plant Physiol Biochem 110:194–209. https://doi.org/10.1016/j.plaphy.2016.05.038
Khan ZS, Rizwan M, Hafeez M et al (2019) The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ Sci Pollut Res 26:19859–19870. https://doi.org/10.1007/s11356-019-05333-5
Khan MI, Fatima N, Shakil M et al (2021) Investigation of in-vitro antibacterial and seed germination properties of green synthesized pure and nickel doped ZnO nanoparticles. Phys B Condens Matter 601:412563. https://doi.org/10.1016/j.physb.2020.412563
Klingshirn C, Fallert J, Zhou H et al (2010) 65 years of ZnO research – old and very recent results. Phys Status Solidi 247:1424–1447. https://doi.org/10.1002/pssb.200983195
Kolenčík M, Ernst D, Komár M et al (2019) Effect of foliar spray application of zinc oxide nanoparticles on quantitative, nutritional, and physiological parameters of foxtail millet (Setaria italica l.) under field conditions. Nanomaterials 9:1559. https://doi.org/10.3390/nano9111559
Kolenčík M, Ernst D, Urík M et al (2020) Foliar application of low concentrations of titanium dioxide and zinc oxide nanoparticles to the common sunflower under field conditions. Nano 10. https://doi.org/10.3390/nano10081619
Kolenčík M, Nemček L, Šebesta M et al (2021) Effect of TiO2 as plant growth-stimulating nanomaterial on crop production. In: Singh VP, Singh S, Prasad SM et al (eds) Plant responses to nanomaterials. Springer, Cham, pp 129–144
Kong XY, Ding Y, Yang R, Wang ZL (2004) Single-crystal nanorings formed by epitaxial self-coiling of polar nanobelts. Science 80(303):1348–1351. https://doi.org/10.1126/science.1092356
Konvičková Z, Holišová V, Kolenčík M et al (2018) Phytosynthesis of colloidal Ag-AgCl nanoparticles mediated by Tilia sp. leachate, evaluation of their behaviour in liquid phase and catalytic properties. Colloid Polym Sci 296:677–687. https://doi.org/10.1007/s00396-018-4290-2
Kosová K, Vítámvás P, Urban MO et al (2018) Plant abiotic stress proteomics: the major factors determining alterations in cellular proteome. Front Plant Sci 9:122. https://doi.org/10.3389/fpls.2018.00122
Kozlowski TT (1997) Responses of woody plants to flooding and salinity. Tree Physiol 17:490. https://doi.org/10.1093/treephys/17.7.490
Kranner I, Minibayeva FV, Beckett RP, Seal CE (2010) What is stress? Concepts, definitions and applications in seed science. New Phytol 188:655–673. https://doi.org/10.1111/j.1469-8137.2010.03461.x
Landa P (2021) Positive effects of metallic nanoparticles on plants: overview of involved mechanisms. Plant Physiol Biochem 161:12–24. https://doi.org/10.1016/j.plaphy.2021.01.039
Landa P, Prerostova S, Petrova S et al (2015) The transcriptomic response of Arabidopsis thaliana to zinc oxide: a comparison of the impact of nanoparticle, bulk, and ionic zinc. Environ Sci Technol 49:14537–14545. https://doi.org/10.1021/acs.est.5b03330
Latef AAHA, Jan S, Abd-Allah EF et al (2016) Soybean under abiotic stress. Plant Environ Interact 2:28–42. https://doi.org/10.1002/9781119081005.ch2
Le TC, Yin H, Chen R et al (2016) An experimental and computational approach to the development of ZnO nanoparticles that are safe by design. Small 12:3568–3577. https://doi.org/10.1002/smll.201600597
Li C, Wang P, van der Ent A et al (2019) Absorption of foliar-applied Zn in sunflower (Helianthus annuus): importance of the cuticle, stomata and trichomes. Ann Bot 123:57–68. https://doi.org/10.1093/aob/mcy135
Lin D, Xing B (2008) Root uptake and Phytotoxicity of ZnO nanoparticles. Environ Sci Technol 42:5580–5585. https://doi.org/10.1021/es800422x
Linh TM, Mai NC, Hoe PT et al (2020) Metal-based nanoparticles enhance drought tolerance in soybean. J Nanomater 2020:13. https://doi.org/10.1155/2020/4056563
Lipiec J, Doussan C, Nosalewicz A, Kondracka K (2013) Effect of drought and heat stresses on plant growth and yield: a review. Int Agrophys 27:463–477. https://doi.org/10.2478/intag-2013-0017
Liu R, Lal R (2015) Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci Total Environ 514:131–139. https://doi.org/10.1016/j.scitotenv.2015.01.104
Liu J, Huang X, Duan J et al (2005) A low-temperature synthesis of multiwhisker-based zinc oxide micron crystals. Mater Lett 59:3710–3714. https://doi.org/10.1016/j.matlet.2005.06.043
Liu J, Huang X, Li Y et al (2006) Selective growth and properties of zinc oxide nanostructures. Scr Mater 55:795–798. https://doi.org/10.1016/j.scriptamat.2006.07.010
Liu YF, Qi MF, Li TL (2012) Photosynthesis, photoinhibition, and antioxidant system in tomato leaves stressed by low night temperature and their subsequent recovery. Plant Sci 196:8–17. https://doi.org/10.1016/j.plantsci.2012.07.005
López-Moreno ML, Cedeño-Mattei Y, Bailón-Ruiz SJ et al (2018) Environmental behavior of coated NMs: physicochemical aspects and plant interactions. J Hazard Mater 347:196–217. https://doi.org/10.1016/j.jhazmat.2017.12.058
Ma H, Kabengi NJ, Bertsch PM et al (2011) Comparative phototoxicity of nanoparticulate and bulk ZnO to a free-living nematode Caenorhabditis elegans: the importance of illumination mode and primary particle size. Environ Pollut 159:1473–1480. https://doi.org/10.1016/j.envpol.2011.03.013
Ma H, Wallis LK, Diamond S et al (2014) Impact of solar UV radiation on toxicity of ZnO nanoparticles through photocatalytic reactive oxygen species (ROS) generation and photo-induced dissolution. Environ Pollut 193:165–172. https://doi.org/10.1016/j.envpol.2014.06.027
Mahdieh M, Sangi MR, Bamdad F, Ghanem A (2018) Effect of seed and foliar application of nano-zinc oxide, zinc chelate, and zinc sulphate rates on yield and growth of pinto bean (Phaseolus vulgaris) cultivars. J Plant Nutr 41:2401–2412. https://doi.org/10.1080/01904167.2018.1510517
Malandrakis AA, Kavroulakis N, Chrysikopoulos CV (2019) Use of copper, silver and zinc nanoparticles against foliar and soil-borne plant pathogens. 670:292–299
Mallakpour S, Madani M (2015) A review of current coupling agents for modification of metal oxide nanoparticles. Prog Org Coat 86:194–207. https://doi.org/10.1016/j.porgcoat.2015.05.023
Manke A, Wang L, Rojanasakul Y (2013) Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int 2013:942916. https://doi.org/10.1155/2013/942916
Martins NCT, Avellan A, Rodrigues S et al (2020) Composites of biopolymers and ZnO NPs for controlled release of zinc in agricultural soils and timed delivery for maize. ACS Appl Nano Mater 3:2134–2148. https://doi.org/10.1021/acsanm.9b01492
Maslobrod SN, Lupashku GA, Gavzer SI et al (2020) Evaluation of stimulatory, antifungal and Thermo-resistant action of aqueous dispersions of nanoparticles on seeds of parental forms and reciprocal hybrids of winter wheat. In: Tiginyanu I, Sontea V, Railean S (eds) 4th international conference on nanotechnologies and biomedical engineering. Springer, Cham, pp 137–141
Mathur S, Agrawal D, Jajoo A (2014) Photosynthesis: response to high temperature stress. J Photochem Photobiol B Biol 137:116–126. https://doi.org/10.1016/j.jphotobiol.2014.01.010
Medina-Velo IA, Barrios AC, Zuverza-Mena N et al (2017) Comparison of the effects of commercial coated and uncoated ZnO nanomaterials and Zn compounds in kidney bean (Phaseolus vulgaris) plants. J Hazard Mater 332:214–222. https://doi.org/10.1016/j.jhazmat.2017.03.008
Meulenkamp EA (1998) Size dependence of the dissolution of ZnO nanoparticles. J Phys Chem B 102:7764–7769. https://doi.org/10.1021/jp982305u
Misra P, Shukla PK, Pramanik K et al (2016) Nanotechnology for crop improvement. In: Kole C, Kumar DS, Khodakovskaya MV (eds) Plant nanotechnology: principles and practices. Springer, Cham, pp 219–256
Mittler R (2017) ROS are good. Trends Plant Sci 22:11–19. https://doi.org/10.1016/j.tplants.2016.08.002
Moezzi A, McDonagh AM, Cortie MB (2012) Zinc oxide particles: synthesis, properties and applications. Chem Eng J 185–186:1–22. https://doi.org/10.1016/j.cej.2012.01.076
Moghaddasi S, Fotovat A, Khoshgoftarmanesh AH et al (2017) Bioavailability of coated and uncoated ZnO nanoparticles to cucumber in soil with or without organic matter. Ecotoxicol Environ Saf 144:543–551. https://doi.org/10.1016/j.ecoenv.2017.06.074
Mohd Omar F, Abdul Aziz H, Stoll S (2014) Aggregation and disaggregation of ZnO nanoparticles: influence of pH and adsorption of Suwannee River humic acid. Sci Total Environ 468–469:195–201. https://doi.org/10.1016/j.scitotenv.2013.08.044
Møller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481. https://doi.org/10.1146/annurev.arplant.58.032806.103946
Molnárová M, Filová A, Peško M (2015) Iónové a nanočasticové formy ťažkých kovov v prostredí a ich interakcia s fotosyntetizujúcimi organizmami
Momma K, Izumi F (2011) VESTA3 for three-dimensional visualization of crystal, volumetric and morphology data. J Appl Crystallogr 44:1272–1276. https://doi.org/10.1107/S0021889811038970
Mudunkotuwa IA, Rupasinghe T, Wu C-M, Grassian VH (2012) Dissolution of ZnO nanoparticles at Circumneutral pH: a study of size effects in the presence and absence of citric acid. Langmuir 28:396–403. https://doi.org/10.1021/la203542x
Mukherjee A, Peralta-Videa JR, Bandyopadhyay S et al (2014) Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics 6:132–138. https://doi.org/10.1039/C3MT00064H
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663. https://doi.org/10.1111/j.1469-8137.2005.01487.x
Mustafa G, Sakata K, Komatsu S (2015) Proteomic analysis of flooded soybean root exposed to aluminum oxide nanoparticles. J Proteome 128:280–297. https://doi.org/10.1016/j.jprot.2015.08.010
Nair S, Sasidharan A, Divya Rani VV et al (2009) Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J Mater Sci Mater Med 20:235–241. https://doi.org/10.1007/s10856-008-3548-5
Nair R, Varghese SH, Nair BG et al (2010) Nanoparticulate material delivery to plants. Plant Sci 179:154–163. https://doi.org/10.1016/j.plantsci.2010.04.012
Nel A, Xia T, Mädler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311(80):622–627. https://doi.org/10.1126/science.1114397
Nemček L, Šebesta M, Urík M et al (2020) Impact of bulk ZnO, ZnO nanoparticles and dissolved Zn on early growth stages of barley—a pot experiment. Plants 9. https://doi.org/10.3390/plants9101365
Nikoobakht B, Wang X, Herzing A, Shi J (2013) Scalable synthesis and device integration of self-registered one-dimensional zinc oxide nanostructures and related materials. Chem Soc Rev 42:342–365. https://doi.org/10.1039/C2CS35164A
Noret N, Meerts P, Tolrà R et al (2005) Palatability of Thlaspi caerulescens for snails: influence of zinc and glucosinolates. New Phytol 165:763–772. https://doi.org/10.1111/j.1469-8137.2004.01286.x
Olander LP, Vitousek PM (2000) Regulation of soil phosphatase and chitinase activityby N and P availability. Biogeochemistry 49:175–191. https://doi.org/10.1023/A:1006316117817
Oleszczuk P, Czech B, Kończak M et al (2019) Impact of ZnO and ZnS nanoparticles in sewage sludge-amended soil on bacteria, plant and invertebrates. Chemosphere 237:124359. https://doi.org/10.1016/j.chemosphere.2019.124359
Pan ZW, Dai ZR, Wang ZL (2001) Nanobelts of semiconducting oxides. Science 291(80):1947–1949. https://doi.org/10.1126/science.1058120
Pandey S, Giri K, Kumar R et al (2018) Nanopesticides: opportunities in crop protection and associated environmental risks. Proc Natl Acad Sci India Sect B Biol Sci 88:1287–1308. https://doi.org/10.1007/s40011-016-0791-2
Peck AW, McDonald GK (2010) Adequate zinc nutrition alleviates the adverse effects of heat stress in bread wheat. Plant Soil 337:355–374. https://doi.org/10.1007/s11104-010-0532-x
Pedruzzi DP, Araujo LO, Falco WF et al (2020) ZnO nanoparticles impact on the photosynthetic activity of Vicia faba: effect of particle size and concentration. NanoImpact 19:100246. https://doi.org/10.1016/j.impact.2020.100246
Peng Y-H, Tsai Y-C, Hsiung C-E et al (2017) Influence of water chemistry on the environmental behaviors of commercial ZnO nanoparticles in various water and wastewater samples. J Hazard Mater 322:348–356. https://doi.org/10.1016/J.JHAZMAT.2016.10.003
Peralta-Videa JR, Hernandez-Viezcas JA, Zhao L et al (2014) Cerium dioxide and zinc oxide nanoparticles alter the nutritional value of soil cultivated soybean plants. Plant Physiol Biochem 80:128–135. https://doi.org/10.1016/j.plaphy.2014.03.028
Pérez Velasco EA, Betancourt Galindo R, Valdez Aguilar LA et al (2020) Effects of the morphology, surface modification and application methods of ZnO-NPs on the growth and biomass of tomato plants. Molecules 25. https://doi.org/10.3390/molecules25061282
Persaud I, Raghavendra AJ, Paruthi A et al (2020) Defect-induced electronic states amplify the cellular toxicity of ZnO nanoparticles. Nanotoxicology 14:145–161. https://doi.org/10.1080/17435390.2019.1668067
Polák F, Urík M, Matúš P (2019) Low molecular weight organic acids in soil environment. Chem List 113:307–314
Polshettiwar V, Baruwati B, Varma RS (2009) Self-assembly of metal oxides into three-dimensional nanostructures: synthesis and application in catalysis. ACS Nano 3:728–736. https://doi.org/10.1021/nn800903p
Poormohammad Kiani S, Grieu P, Maury P et al (2007) Genetic variability for physiological traits under drought conditions and differential expression of water stress-associated genes in sunflower (Helianthus annuus L.). Theor Appl Genet 114:193–207. https://doi.org/10.1007/s00122-006-0419-7
Poschenrieder C, Tolrà R, Barceló J (2006) Can metals defend plants against biotic stress? Trends Plant Sci 11:288–295. https://doi.org/10.1016/j.tplants.2006.04.007
Prasad R, Kumar V, Prasad KS (2014) Nanotechnology in sustainable agriculture: present concerns and future aspects. Afr J Biotechnol 13:705–713
Priyanka N, Geetha N, Manish T et al (2021) Zinc oxide nanocatalyst mediates cadmium and lead toxicity tolerance mechanism by differential regulation of photosynthetic machinery and antioxidant enzymes level in cotton seedlings. Toxicol Rep 8:295–302. https://doi.org/10.1016/j.toxrep.2021.01.016
Rafique M, Tahir R, Gillani SSA et al (2020) Plant-mediated green synthesis of zinc oxide nanoparticles from Syzygium Cumini for seed germination and wastewater purification. Int J Environ Anal Chem 0:1–16. https://doi.org/10.1080/03067319.2020.1715379
Raghib F, Naikoo MI, Khan FA et al (2020) Interaction of ZnO nanoparticle and AM fungi mitigates Pb toxicity in wheat by upregulating antioxidants and restricted uptake of Pb. J Biotechnol 323:254–263. https://doi.org/10.1016/j.jbiotec.2020.09.003
Rai-Kalal P, Jajoo A (2021) Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol Biochem 160:341–351. https://doi.org/10.1016/j.plaphy.2021.01.032
Rajiv P, Rajeshwari S, Venckatesh R (2013) Bio-fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens. Spectrochim Acta Part A Mol Biomol Spectrosc 112:384–387. https://doi.org/10.1016/j.saa.2013.04.072
Rajput VD, Minkina TM, Behal A et al (2018) Effects of zinc-oxide nanoparticles on soil, plants, animals and soil organisms: a review. Environ. Nanotechnology. Monit Manag 9:76–84
Raliya R, Tarafdar JC (2013) ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in clusterbean (Cyamopsis tetragonoloba L.). Agric Res 2:48–57. https://doi.org/10.1007/s40003-012-0049-z
Raliya R, Nair R, Chavalmane S et al (2015) Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 7:1584–1594. https://doi.org/10.1039/C5MT00168D
Raliya R, Tarafdar JC, Biswas P (2016) Enhancing the mobilization of native phosphorus in the mung bean rhizosphere using ZnO nanoparticles synthesized by soil fungi. J Agric Food Chem 64:3111–3118. https://doi.org/10.1021/acs.jafc.5b05224
Raliya R, Saharan V, Dimkpa C, Biswas P (2018) Nanofertilizer for precision and sustainable agriculture: current state and future perspectives. J Agric Food Chem 66:6487–6503. https://doi.org/10.1021/acs.jafc.7b02178
Rani P, Kaur G, Rao KV et al (2020) Impact of green synthesized metal oxide nanoparticles on seed germination and seedling growth of Vigna radiata (mung bean) and Cajanus cajan (red gram). J Inorg Organomet Polym Mater 30:4053–4062. https://doi.org/10.1007/s10904-020-01551-4
Rasmussen K, Rauscher H, Mech A et al (2018) Physico-chemical properties of manufactured nanomaterials – characterisation and relevant methods. An outlook based on the OECD testing Programme. Regul Toxicol Pharmacol 92:8–28. https://doi.org/10.1016/J.YRTPH.2017.10.019
Rawashdeh RY, Harb AM, AlHasan AM (2020) Biological interaction levels of zinc oxide nanoparticles; lettuce seeds as case study. Heliyon 6:e03983. https://doi.org/10.1016/j.heliyon.2020.e03983
Read TL, Doolette CL, Cresswell T et al (2019) Investigating the foliar uptake of zinc from conventional and nano-formulations: a methodological study. Environ Chem 16:459. https://doi.org/10.1071/EN19019
Reddy Pullagurala VL, Adisa IO, Rawat S et al (2018a) ZnO nanoparticles increase photosynthetic pigments and decrease lipid peroxidation in soil grown cilantro (Coriandrum sativum). Plant Physiol Biochem 132:120–127. https://doi.org/10.1016/j.plaphy.2018.08.037
Reddy Pullagurala VL, Adisa IO, Rawat S et al (2018b) Finding the conditions for the beneficial use of ZnO nanoparticles towards plants-a review. Environ Pollut 241:1175–1181. https://doi.org/10.1016/j.envpol.2018.06.036
Rejeb IB, Pastor V, Mauch-Mani B (2014) Plant responses to simultaneous biotic and abiotic stress: molecular mechanisms. Plan Theory 3:458–475. https://doi.org/10.3390/plants3040458
Rizwan M, Ali S, Ali B et al (2019a) Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214:269–277. https://doi.org/10.1016/J.CHEMOSPHERE.2018.09.120
Rizwan M, Ali S, ur Rehman MZ, Maqbool A (2019b) A critical review on the effects of zinc at toxic levels of cadmium in plants. Environ Sci Pollut Res 26:6279–6289. https://doi.org/10.1007/s11356-019-04174-6
Rizwan M, Ali S, ur Zia Rehman M et al (2019c) Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ Pollut 248:358–367. https://doi.org/10.1016/j.envpol.2019.02.031
Russell DA, Wong DML, Sachs MM (1990) The anaerobic response of soybean. Plant Physiol 92:401–407. https://doi.org/10.1104/pp.92.2.401
Sabir S, Arshad M, Chaudhari SK (2014) Zinc oxide nanoparticles for revolutionizing agriculture: synthesis and applications. Sci World J 2014:8. https://doi.org/10.1155/2014/925494
Sabir S, Zahoor MA, Waseem M et al (2020) Biosynthesis of ZnO nanoparticles using bacillus subtilis: characterization and nutritive significance for promoting plant growth in Zea mays L. Dose-Response 18:1559325820958911. https://doi.org/10.1177/1559325820958911
Saibo NJM, Lourenço T, Oliveira MM (2008) Transcription factors and regulation of photosynthetic and related metabolism under environmental stresses. Ann Bot 103:609–623. https://doi.org/10.1093/aob/mcn227
Salama DM, Osman SA, Abd El-Aziz ME et al (2019) Effect of zinc oxide nanoparticles on the growth, genomic DNA, production and the quality of common dry bean (Phaseolus vulgaris). Biocatal Agric Biotechnol 18:101083. https://doi.org/10.1016/j.bcab.2019.101083
Savi GD, Piacentini KC, de Souza SR et al (2015) Efficacy of zinc compounds in controlling fusarium head blight and deoxynivalenol formation in wheat (Triticum aestivum L.). Int J Food Microbiol 205:98–104. https://doi.org/10.1016/j.ijfoodmicro.2015.04.001
Saxena R, Tomar RS, Kumar M (2016) Exploring Nanobiotechnology to mitigate abiotic stress in crop plants. J Pharm Sci Res 8:974–980
Scandalios JG (2005) Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res 38:995–1014. https://doi.org/10.1590/S0100-879X2005000700003
Šebesta M, Kolenčík M, Urík M et al (2019) Increased colloidal stability and decreased solubility – sol-gel synthesis of zinc oxide nanoparticles with humic acids. J Nanosci Nanotechnol 19:3024–3030. https://doi.org/10.1166/jnn.2019.15868
Šebesta M, Nemček L, Urík M et al (2020a) Partitioning and stability of ionic, nano- and microsized zinc in natural soil suspensions. Sci Total Environ 700:134445. https://doi.org/10.1016/j.scitotenv.2019.134445
Šebesta M, Urík M, Kolenčík M et al (2020b) Sequential extraction resulted in similar fractionation of ionic Zn, nano- and microparticles of ZnO in acidic and alkaline soil. Forests 11. https://doi.org/10.3390/f11101077
Seleiman MF, Alotaibi MA, Alhammad BA et al (2020) Effects of ZnO nanoparticles and biochar of Rice straw and cow manure on characteristics of contaminated soil and sunflower productivity, oil quality, and heavy metals uptake. Agronomy 10. https://doi.org/10.3390/agronomy10060790
Servin AD, White JC (2016) Nanotechnology in agriculture: next steps for understanding engineered nanoparticle exposure and risk. NanoImpact 1:9–12. https://doi.org/10.1016/j.impact.2015.12.002
Shah AA, Aslam S, Akbar M et al (2021) Combined effect of Bacillus fortis IAGS 223 and zinc oxide nanoparticles to alleviate cadmium phytotoxicity in Cucumis melo. Plant Physiol Biochem 158:1–12. https://doi.org/10.1016/j.plaphy.2020.11.011
Sharifan H, Moore J, Ma X (2020) Zinc oxide (ZnO) nanoparticles elevated iron and copper contents and mitigated the bioavailability of lead and cadmium in different leafy greens. Ecotoxicol Environ Saf 191:110177. https://doi.org/10.1016/j.ecoenv.2020.110177
Sharkey TD (2005) Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ 28:269–277. https://doi.org/10.1111/j.1365-3040.2005.01324.x
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and Antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1–26. https://doi.org/10.1155/2012/217037
Siddiqi KS, Husen A (2017) Plant response to engineered metal oxide nanoparticles nanoscale. Res Lett:12. https://doi.org/10.1186/s11671-017-1861-y
Siddiqui ZA, Parveen A, Ahmad L, Hashem A (2019) Effects of graphene oxide and zinc oxide nanoparticles on growth, chlorophyll, carotenoids, proline contents and diseases of carrot. Sci Hortic (Amsterdam) 249:374–382. https://doi.org/10.1016/j.scienta.2019.01.054
Singh J, Kumar S, Alok A et al (2019) The potential of green synthesized zinc oxide nanoparticles as nutrient source for plant growth. J Clean Prod 214:1061–1070. https://doi.org/10.1016/j.jclepro.2019.01.018
Singh P, Arif Y, Siddiqui H et al (2021) Nanoparticles enhances the salinity toxicity tolerance in Linum usitatissimum L. by modulating the antioxidative enzymes, photosynthetic efficiency, redox status and cellular damage. Ecotoxicol Environ Saf 213:112020
Sirelkhatim A, Mahmud S, Seeni A et al (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett 7:219–242. https://doi.org/10.1007/s40820-015-0040-x
Sivry Y, Gelabert A, Cordier L et al (2014) Behavior and fate of industrial zinc oxide nanoparticles in a carbonate-rich river water. Chemosphere 95:519–526. https://doi.org/10.1016/j.chemosphere.2013.09.110
Slaton NA, Norman RJ, Wilson CE Jr (2005) Effect of zinc source and application time on zinc uptake and grain yield of flood-irrigated rice. Agron J 97:272–278. https://doi.org/10.2134/agronj2005.0272
Soliman AS, El-feky SA, Darwish E (2015) Alleviation of salt stress on Moringa peregrina using foliar application of nanofertilizers. J Hortic For 7:36–47. https://doi.org/10.5897/JHF2014.0379
Song U, Kim J (2020) Zinc oxide nanoparticles: a potential micronutrient fertilizer for horticultural crops with little toxicity. Hortic Environ Biotechnol 61:625–631. https://doi.org/10.1007/s13580-020-00244-8
Stolpe C, Krämer U, Müller C (2017) Heavy metal (hyper)accumulation in leaves of Arabidopsis halleri is accompanied by a reduced performance of herbivores and shifts in leaf glucosinolate and element concentrations. Environ Exp Bot 133:78–86. https://doi.org/10.1016/j.envexpbot.2016.10.003
Strambeanu N, Demetrovici L, Dragos D, Lungu M (2015) Nanoparticles: definition, classification and general physical properties. In: Lungu M, Neculae A, Bunoiu M, Biris C (eds) Nanoparticles’ promises and risks: characterization, manipulation, and potential hazards to humanity and the environment. Springer, Cham, pp 3–8
Sun TY, Gottschalk F, Hungerbühler K, Nowack B (2014) Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ Pollut 185:69–76. https://doi.org/10.1016/j.envpol.2013.10.004
Sun Q, Li J, Le T (2018) Zinc oxide nanoparticle as a novel class of antifungal agents: current advances and future perspectives. J Agric Food Chem 66:11209–11220. https://doi.org/10.1021/acs.jafc.8b03210
Suzuki K, Nagasuga K, Okada M (2008) The chilling injury induced by high root temperature in the leaves of rice seedlings. Plant Cell Physiol 49:433–442. https://doi.org/10.1093/pcp/pcn020
Tarafdar JC, Agrawal A, Raliya R et al (2012) ZnO nanoparticles induced synthesis of polysaccharides and phosphatases by aspergillus fungi. Adv Sci Eng Med 4:324–328. https://doi.org/10.1166/asem.2012.1160
Thakur M, Bhattacharya S, Khosla PK, Puri S (2019) Improving production of plant secondary metabolites through biotic and abiotic elicitation. J Appl Res Med Aromat Plants 12:1–12. https://doi.org/10.1016/j.jarmap.2018.11.004
Thordal-Christnsen H (2003) Fresh insight into processes of nonhost resistance. Curr Opin Plant Biol 6:351–357
Thorny Chanu T, Upadhyaya H (2019) Chapter 3: Zinc oxide nanoparticle-induced responses on plants: a physiological perspective. In: Tripathi DK, Ahmad P, Sharma S et al (eds) Nanomaterials in plants, algae and microorganisms. Academic, pp 43–64
Torabian S, Zahedi M, Khoshgoftarmanesh A (2016) Effect of foliar spray of zinc oxide on some antioxidant enzymes activity of sunflower under salt stress. J Agric Sci Technol 18
Tripathi DK, Singh VP, Prasad SM et al (2015) Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol Biochem 96:189–198. https://doi.org/10.1016/j.plaphy.2015.07.026
Tripathi D, Mishra R, Singh S et al (2017a) Nitric oxide ameliorates zinc oxide nanoparticles phytotoxicity in wheat seedlings: implication of the ascorbate-glutathione cycle. Front Plant Sci 8:1. https://doi.org/10.3389/fpls.2017.00001
Tripathi DK, Shweta SS et al (2017b) An overview on manufactured nanoparticles in plants: uptake, translocation, accumulation and phytotoxicity. Plant Physiol Biochem 110:2–12. https://doi.org/10.1016/j.plaphy.2016.07.030
Tymoszuk A, Wojnarowicz J (2020) Zinc oxide and zinc oxide nanoparticles impact on in vitro germination and seedling growth in Allium cepa L. Materials (Basel) 13. https://doi.org/10.3390/ma13122784
Ullah A, Romdhane L, Rehman A, Farooq M (2019) Adequate zinc nutrition improves the tolerance against drought and heat stresses in chickpea. Plant Physiol Biochem 143:11–18. https://doi.org/10.1016/j.plaphy.2019.08.020
Umar W, Hameed MK, Aziz T et al (2020) Synthesis, characterization and application of ZnO nanoparticles for improved growth and Zn biofortification in maize. Arch Agron Soil Sci 0:1–13. https://doi.org/10.1080/03650340.2020.1782893
Umavathi S, Mahboob S, Govindarajan M et al (2020) Green synthesis of ZnO nanoparticles for antimicrobial and vegetative growth applications: a novel approach for advancing efficient high quality health care to human wellbeing. Saudi J Biol Sci. https://doi.org/10.1016/j.sjbs.2020.12.025
ur Rehman R, Khan B, Aziz T et al (2020) Postponement growth and antioxidative response of Brassica nigra on CuO and ZnO nanoparticles exposure under soil conditions. IET Nanobiotechnol 14:423–427. https://doi.org/10.1049/iet-nbt.2019.0357
Venkatachalam P, Priyanka N, Manikandan K et al (2017) Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.). Plant Physiol Biochem 110:118–127. https://doi.org/10.1016/j.plaphy.2016.09.004
Wahab R, Ansari SG, Kim Y-S et al (2007) Room temperature synthesis of needle-shaped ZnO nanorods via sonochemical method. Appl Surf Sci 253:7622–7626. https://doi.org/10.1016/j.apsusc.2007.03.060
Wahid A (2007) Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J Plant Res 120:219–228. https://doi.org/10.1007/s10265-006-0040-5
Wan J, Wang R, Bai H et al (2020) Comparative physiological and metabolomics analysis reveals that single-walled carbon nanohorns and ZnO nanoparticles affect salt tolerance in Sophora alopecuroides. Environ Sci Nano 7:2968–2981. https://doi.org/10.1039/D0EN00582G
Wang P, Menzies NW, Lombi E et al (2013) Fate of ZnO nanoparticles in soils and cowpea (Vigna unguiculata). Environ Sci Technol 47:13822–13830. https://doi.org/10.1021/es403466p
Wang H, Adeleye AS, Huang Y et al (2015) Heteroaggregation of nanoparticles with biocolloids and geocolloids. Adv Colloid Interf Sci 226:24–36. https://doi.org/10.1016/j.cis.2015.07.002
Wang P, Lombi E, Zhao F-J, Kopittke PM (2016a) Nanotechnology: a new opportunity in plant sciences. Trends Plant Sci 21:699–712. https://doi.org/10.1016/j.tplants.2016.04.005
Wang X, Yang X, Chen S et al (2016b) Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in arabidopsis. Front Plant Sci 6:1243. https://doi.org/10.3389/fpls.2015.01243
Wang X, Sun W, Zhang S et al (2018) Elucidating the effects of cerium oxide nanoparticles and zinc oxide nanoparticles on arsenic uptake and speciation in Rice (Oryza sativa) in a hydroponic system. Environ Sci Technol 52:10040–10047. https://doi.org/10.1021/acs.est.8b01664
Watson J-L, Fang T, Dimkpa CO et al (2015) The phytotoxicity of ZnO nanoparticles on wheat varies with soil properties. Biometals 28:101–112. https://doi.org/10.1007/s10534-014-9806-8
Welti R, Li W, Li M et al (2002) Profiling membrane lipids in plant stress responses: role of phospholipase Dα in freezing-induced lipid changes in arabidopsis. J Biol Chem 277:31994–32002. https://doi.org/10.1074/jbc.M205375200
Worms IAM, Boltzman J, Garcia M, Slaveykova VI (2012) Cell-wall-dependent effect of carboxyl-CdSe/ZnS quantum dots on lead and copper availability to green microalgae. Environ Pollut 167:27–33. https://doi.org/10.1016/j.envpol.2012.03.030
Wu F, Fang Q, Yan S et al (2020) Effects of zinc oxide nanoparticles on arsenic stress in rice (Oryza sativa L.): germination, early growth, and arsenic uptake. Environ Sci Pollut Res 27:26974–26981. https://doi.org/10.1007/s11356-020-08965-0
Xu T, Ji P, He M, Li J (2012) Growth and structure of pure ZnO micro/nanocombs. J Nanomater 2012:797935. https://doi.org/10.1155/2012/797935
Xu H, Li L, Lv H et al (2016) pH-dependent phosphatization of ZnO nanoparticles and its influence on subsequent lead sorption. Environ Pollut 208:723–731. https://doi.org/10.1016/J.ENVPOL.2015.10.052
Yang Z, Chen J, Dou R et al (2015) Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants, maize (Zea mays L.) and rice (Oryza sativa L.). Int J Environ Res Public Health 12:15100–15109. https://doi.org/10.3390/ijerph121214963
Yecheskel Y, Dror I, Berkowitz B (2016) Transport of engineered nanoparticles in partially saturated sand columns. J Hazard Mater 311:254–262. https://doi.org/10.1016/j.jhazmat.2016.03.027
Younes NA, Hassan HS, Elkady MF et al (2020) Impact of synthesized metal oxide nanomaterials on seedlings production of three Solanaceae crops. Heliyon 6:e03188. https://doi.org/10.1016/j.heliyon.2020.e03188
Youssef MS, Elamawi RM (2020) Evaluation of phytotoxicity, cytotoxicity, and genotoxicity of ZnO nanoparticles in Vicia faba. Environ Sci Pollut Res 27:18972–18984. https://doi.org/10.1007/s11356-018-3250-1
Yung MMN, Wong SWY, Kwok KWH et al (2015) Salinity-dependent toxicities of zinc oxide nanoparticles to the marine diatom Thalassiosira pseudonana. Aquat Toxicol 165:31–40. https://doi.org/10.1016/J.AQUATOX.2015.05.015
Yusefi-Tanha E, Fallah S, Rostamnejadi A, Pokhrel LR (2020) Zinc oxide nanoparticles (ZnONPs) as a novel nanofertilizer: influence on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Sci Total Environ 738:140240. https://doi.org/10.1016/j.scitotenv.2020.140240
Zafar H, Abbasi BH, Zia M (2019) Physiological and antioxidative response of Brassica nigra (L.) to ZnO nanoparticles grown in culture media and soil. Toxicol Environ Chem 101:281–299. https://doi.org/10.1080/02772248.2019.1691555
Zafar H, Aziz T, Khan B et al (2020) CuO and ZnO nanoparticle application in synthetic soil modulates morphology, nutritional contents, and metal analysis of Brassica nigra. ACS Omega 5:13566–13577. https://doi.org/10.1021/acsomega.0c00030
Ze Y, Liu C, Wang L et al (2011) The regulation of TiO2 nanoparticles on the expression of light-harvesting complex II and photosynthesis of chloroplasts of arabidopsis thaliana. Biol Trace Elem Res 143:1131–1141. https://doi.org/10.1007/s12011-010-8901-0
Zhang Z, Jiang W, Jian Q et al (2015) Residues and dissipation kinetics of triazole fungicides difenoconazole and propiconazole in wheat and soil in Chinese fields. Food Chem 168:396–403. https://doi.org/10.1016/j.foodchem.2014.07.087
Zhang W, Long J, Li J et al (2019) Impact of ZnO nanoparticles on Cd toxicity and bioaccumulation in rice (Oryza sativa L.). Environ Sci Pollut Res 26:23119–23128. https://doi.org/10.1007/s11356-019-05551-x
Zhang W, Long J, Li J et al (2020) Effect of metal oxide nanoparticles on the chemical speciation of heavy metals and micronutrient bioavailability in paddy soil. Int J Environ Res Public Health 17. https://doi.org/10.3390/ijerph17072482
Zhao L, Peralta-Videa JR, Ren M et al (2012) Transport of Zn in a sandy loam soil treated with ZnO NPs and uptake by corn plants: electron microprobe and confocal microscopy studies. Chem Eng J 184:1–8. https://doi.org/10.1016/j.cej.2012.01.041
Zhao L, Lu L, Wang A et al (2020) Nano-biotechnology in agriculture: use of nanomaterials to promote plant growth and stress tolerance. J Agric Food Chem 68:1935–1947. https://doi.org/10.1021/acs.jafc.9b06615
Zhou D, Keller AA (2010) Role of morphology in the aggregation kinetics of ZnO nanoparticles. Water Res 44:2948–2956. https://doi.org/10.1016/j.watres.2010.02.025
Zhu J (2016) Review abiotic stress signaling and responses in plants. Cell 167:313–324. https://doi.org/10.1016/j.cell.2016.08.029
Acknowledgements
Presented work was financially supported by Culture and Education Grant Agency of the Ministry of Education, Science, Research and Sports of the Slovak Republic and the Slovak Academy of Sciences via grant KEGA 013SPU-4/2019, by Science Grant Agency of the Ministry of Education, Science, Research and Sports of the Slovak Republic and the Slovak Academy of Sciences via grants VEGA 1/0146/18 and 1/0292/21, and by Comenius University’s grant for young scientists Grant UK UK/48/2021.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Šebesta, M., Kurtinová, S., Kolenčík, M., Illa, R. (2021). Enhancement of Stress Tolerance of Crop Plants by ZnO Nanoparticles. In: Faizan, M., Hayat, S., Yu, F. (eds) Sustainable Agriculture Reviews 53. Sustainable Agriculture Reviews, vol 53. Springer, Cham. https://doi.org/10.1007/978-3-030-86876-5_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-86876-5_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-86875-8
Online ISBN: 978-3-030-86876-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)