Abstract
Both cadmium (Cd) contamination in agricultural soils and drought stress pose a serious problem for crop quality and human health. Owing to the specific physical and chemical characteristics, zinc oxide (ZnO) nanoparticles (NPs) can be used in agriculture as a nanofertilizer but their impact on Cd accumulation in wheat (Triticum aestivum) grains under normal and limited water conditions remains insufficient. In this study, the efficiency of ZnO NPs on Cd intake by wheat was investigated under normal and water-limited conditions grown in Cd-contaminated soil for 125 days after seed sowing. The lower biomass and higher oxidative stress were observed in the tissues of the control and drought stress further decreased the plant biomass and caused oxidative stress. Zinc oxide NP treatments increased the tissue dry weight and minimized the oxidative stress either Cd stress alone or combined with drought. Drought stress enhanced the Cd contents in wheat tissues and grains, while ZnO NPs significantly reduced the Cd accumulation in tissues and grains by reducing the soil bioavailable Cd and its accumulation by roots. These findings depicted that NP application to contaminated soils can promote wheat productivity and effectively alleviate soil Cd contamination either alone or under water-limited conditions. The baseline data demonstrated in this study provide insights that pave the way towards safer wheat production under combined drought and metal stress. However, the application of NPs at field levels with numerous crops and climatic conditions needs to be investigated before final recommendation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The contamination of soils with toxic trace elements has turned one of the serious environmental issues worldwide (Nagajyoti et al. 2010). Cadmium (Cd) is the toxic non-essential chemical element which mainly accumulates in the soils through anthropogenic sources and is a global agricultural contaminant (Gallego et al. 2012). The Cd is extremely toxic to humans, animals, and plants (Rehman et al. 2018; Wang et al. 2018). In humans, the major source of Cd is through food and Cd causes serious harmful effects including cancer (Baycu et al. 2017). In plants, Cd can readily enter through the roots and causes damages to photosynthetic system, impairing plant growth and nutrient accumulation by plants (Bashir et al. 2018). Cadmium negatively affects the redox homeostasis of the plant cells and enhance the release of reactive oxygen species (ROS) which affects the cell viability via lipid peroxidation, membrane damage, and inactivation of the enzymes. Although plants have a developed system of defense for quenching of ROS, this system fails at elevated Cd stress (Mittler 2002; Rizwan et al. 2019a). Therefore, it is necessary to minimize Cd accumulation by crops for the improvement in plant growth and reduction in human health risks.
Wheat (Triticum aestivum) is the main food crop in Pakistan and is one of major staple foods worldwide (Curtis and Halford 2014; Joy et al. 2017). Studies depicted that Cd not only accumulates in vegetative organs of wheat where it impairs the growth and yield but is also transferred to grains which is the major pathway of Cd accumulation to general population depending upon wheat products as a main food (Rizwan et al. 2016). The Cd exceeds the threshold level (0.2 mg/kg) specified for cereal grains even without causing negative impacts on vegetative growth, which may affect the health if the wheat-based products are being consumed (Rehman et al. 2015, 2019).
Drought stress is another one of the yields limiting abiotic stresses at world level. Drought negatively affects the wheat growth, especially latter growth stages of the plant which is called the terminal drought stress (Rizwan et al. 2015). The studies reported demonstrated that drought not only affects the plant growth but also the Cd uptake (Shi et al. 2015; Liu et al. 2017). The increase/decrease in Cd intake by drought-stressed plants depends upon the plant species, duration, and stages of the drought stress (Bauddh and Singh 2012; Xia et al. 2015). Recently, we have reported that drought stress during latter growth stages of the wheat increased the Cd in the vegetative tissues and grains (Abbas et al. 2018). The problem of drought stress and excess amount of Cd in grains of wheat is a prominent topic for research in recent time.
Zinc (Zn) is an essential micronutrient for crops, animals, and humans (Cakmak and Kutman 2018) and its deficiency in humans cause health complications as Zn is involved in approximately 10% of the human proteins (Krezel and Maret 2016). Zinc deficiency is mainly found in the people depending upon cereal-based foods worldwide (Rizwan et al. 2017a). The excessive amount of phosphorus (P) fertilizers is applied recently to enhance the growth of wheat and this excess amount of P reduced the bioavailability of Zn and its concentration in grains of wheat (Cakmak et al. 2010). Adequate amount of soil Zn is not only required for plant development but could also decrease the accumulation of non-essential elements such as Cd (Rizwan et al. 2019a, 2019b). Cadmium present in the soil is taken up by plants via the Zn transporters, due to the similarity between Cd and Zn, or by the transporters for the accumulation of other essential nutrients, i.e., iron (Fe) and calcium (Ca). It has been reviewed that Zn and Cd antagonistically or synergistically interact in several crop species (Rizwan et al. 2019a, 2019b). Various forms of Zn could be used to provide the Zn in the available forms in soil-plant system (Rizwan et al. 2017a; Hussain et al. 2018).
Recently, nanotechnology has received great attention due wide use of nanoparticles (NPs) in various sectors including agriculture (Liu and Lal 2015; Rizwan et al. 2017b). Nanoparticles can be employed as a controlled release of nutrients, considerably micronutrients because plants require only a small quantity of micronutrients which may limit their entry to other environmental compartments (Tripathi et al. 2015; Raliya et al. 2017; Hussain et al. 2019). It has been shown that Zn can be applied to the plants in the form of NPs and mainly zinc oxide (ZnO) NPs have been used for the studies (Sturikova et al. 2018). The positive impacts of ZnO NPs have been reported in several plant species and the efficiency of NPs varies with the methods and doses of the NPs (Sturikova et al. 2018; Ali et al. 2019). It has been reported that ZnO NPs reduced the Cd intake in wheat (Hussain et al. 2018). Zinc oxide NPs reduced the oxidative stress in plants (Mahajan et al. 2011; Venkatachalam et al. 2017). In addition, soil and foliar application of ZnO and other types of NP composite reduced the drought stress in soybean (Dimkpa et al. 2017b). In the colloidal solution of Cu/Zn NPs diminished the oxidative stress and enhanced the leaf chlorophyll contents in wheat (Taran et al. 2017). This demonstrates the positive effects of NPs on plants under metal and drought stress, but the efficiency of ZnO NPs under combined drought and Cd stress has not been investigated so far.
Hence, we assumed in the current study that ZnO NPs could be applied as a fertilization strategy for improving crop growth under Cd stress in different soil moisture conditions is logical. The main objective was to explore the impact of ZnO NPs on Cd uptake and oxidative burst in wheat under Cd and drought stress, which is likely to be a potential novel use of ZnO NPs in climate-smart agriculture.
Materials and methods
Soil selection and materials
A sandy loam soil (pH 7.71, 1:2.5 soil/water ratio) was sampled from surface layer (0–20-cm depth) of agricultural field located in the suburb of Multan (30° 12′ N, 71° 28′ E and 215 m from sea level) which was receiving raw city effluent as a source of water since long time. The samples were sieved at 2 mm to homogenize and remove any unwanted material and then air-dried prior to analyze and growth of the plants. The detailed information about the sampling site have been previously described (Abbas et al. 2018). The homogenized soil sample was used to analyze the soil for initial characteristics and the analytical results are given in Table 1. The texture of the soil was measured by using hydrometer (Bouyoucos 1962). The Walkley-Black method was employed to estimate the initial soil organic matter (Walkley and Black). The total and bioavailable contents of selected metals Cd, lead (Pb), Zn, and iron (Fe) were determined either by digesting the soil in HNO3 and HClO3 with specific ratio (Amacher 1996) and extracting the samples with ammonium bicarbonate diethylene triamine pentaacetic acid (AB-DTPA), respectively (Soltanpour 1985). In brief, to determine the AB-DTPA extractable metal concentrations in the soil, 10 g soil was extracted with 20 ml of AB-DTPA (pH 7.6) solution by horizontal shaking for 2 h at 180 rpm. Thereafter, the solution was filtered through a Whatman filter paper no. 42, and the filtrate was stored in plastic bottles and analyzed the metal concentration by an atomic absorption spectrophotometer (AAS) (Analytik Jena novAA 350).
Zinc oxide NPs (20–30 nm) were of Alfa Aesar containing a high purity (99%) and density of 5.606 g/cm3. Specified concentrations of ZnO NP solutions were made in d-H2O and the suspensions were ultrasonicated for 30 min to increase the dispersion of the particles. The final concentrations of NPs were 25, 50, and 100 mg/kg of soil and the soil was placed under ambient conditions after mixing the treatments for 2 weeks prior to the growth of wheat as detailed previously (Hussain et al. 2018).
Experimental setup
A pot experiment was performed under ambient conditions (68 ± 5% relative humidity and 29/20 °C day/night temperature, respectively, at the time of sowing) to better understand the effects of NPs under real environmental conditions at the Government College University, Faisalabad, Pakistan. Seeds of wheat (var. Lasani-2008) were surface disinfected with NaOCl solution of 0.5% by dipping the seeds in the solution for 5 min and subsequently washed many times by using d-H2O. Seed planting was done by using ten seeds in every pot containing 5 kg of soil amended with four (0, 25, 50, and 100 mg/kg) treatments of ZnO NPs. However, five uniform seedlings of wheat were maintained after 7 days of germination. All the pots were watered by using tap water to maintain about 70% of field water-holding capacity (WHC) by weighing every 3 days and water WHC was adjusted with tap water as per requirement. The soil in the pots was NPK-fertilized as a basal rate of 120/50/25 kg/ha at the time of seed sowing. The sources of NPK were urea, DAP (diammonium phosphate), and SOP (sulfate of potash). Only nitrogen (N) was given to the plants in two splits, i.e., half with PK and other half after 30 days of plant growth. Two water regimes were initiated after 50 days of seed placing in the pots. Half of the pots were irrigated at 70% of WHC termed as without drought stress and the remaining half pots were irrigated with 35% of WHC termed as drought stress. These water regimes were selected on the bases of our previous study (Abbas et al. 2018). Thus, all the treatments were duplicated as without and with drought stress and these treatments were randomly assigned. Each treatment was replicated four times, and finally, there were 32 pots with four treatments of NPs, two water regimes and four replicates in a complete randomize design with factorial arrangement. The selected soil moisture regimes were adjusted every 3 days with tap water by weighing the pots for the remaining duration of the study. The pots were rotated randomly and periodically, and finally, the weeds were checked and removed regularly.
Chlorophyll measurement
The fully expanded leaves were taken after 80 days of the seed sowing and the contents of chlorophyll a and b from the upper expanded leaf samples were determined by extracting the leaves (50 mg) in acetone (80%) in dark at 4 °C for about 24 h and then filtered the solution. The absorbance of the extracted solution was determined by a spectrophotometer (Halo DB-20/DB-20S, Dynamica Company, London, UK) at different wavelengths (470, 647, and 664.5 nm). The contents of the pigments were calculated by using the equations as described previously (Lichtenthaler 1987).
Estimation of EL, H2O2, MDA, and assays of enzymatic antioxidants
The above parameters were measured from the leaves and sampling was done at 80 days of seed sowing. All the samples were washed with d-H2O before further use of these leaf samples. Already developed procedure was employed to measure EL from the leaves (Dionisio-Sese and Tobita 1998). Carefully washed leaves were cut into pieces of appropriate size (about 200 mg and 1.0 cm) and transferred into the tubes and 10 ml of d-H2O was added in the tubes. All the tubes were placed for 2 h at 32 °C and EC of the solution in each tube was recorded which is termed as EC1. Subsequently, the same solution of each tubes was placed for 20 min at 121 °C, finally cooled at room temperature, and EC of the solution in each tube was recorded which is termed as EC2. A formula was employed to calculate the EL of each sample.
The Heath and Packer (1968) procedure was employed to calculate the MDA contents in the leaves. The level of MDA in leaves, which is correlated with lipid peroxidation (LPO), was determined as thiobarbituric acid–reactive substances (TBARS). Leaf samples (approx. 0.25 g) were ground in 5 ml of 0.1% trichloroacetic acid (TCA) and centrifuged at 13000g for 20 min at 4 °C. Of this solution, 1 ml was added in 4 ml of 0.5% thiobarbituric acid (TBA) in 20% TCA and the solution was heated for 30 min at 95 °C, cooled, and centrifuged (13,000g for 10 min). The concentration of TBA-MDA complex was measured by subtracting the absorption taken at 600 nm from that at 532 nm. Finally, MDA contents were estimated using an extinction coefficient (155 mM−1 cm−1).
Hydrogen peroxide concentration in the leaves was estimated with prescribed procedure; 3 ml of the extracted solution was added in 1.0 ml of titanium sulfate (0.1%) towards sulfuric acid (20% v/v). The final solution was centrifuged for 15 min at 6000g and the intensity of the solution was estimated at 410 nm, and subsequently, the concentration of H2O2 was calculated by using coefficient (0.28 μmol−1 cm−1).
For the measurement of superoxide dismutase (SOD) and peroxidase (POD) activities, the leaves (0.5 g approx.) were ground in ice in 10 ml of potassium phosphate buffer (50 mM) and homogenizing solution of 1.0% w/v polyvinylpyrrolidone (pH 7.8). Subsequently, the centrifugation was done for 30 min at 10,000g in 4 °C. The supernatant solution was employed for the estimation of SOD and POD activities, as described previously (Zhang 1992).
Plant harvesting and analysis
The harvesting of wheat was performed at physiological maturity (at 125 days of seed sowing). Before harvesting, the height of plants and spike lengths were recorded with the help of meter scale. The aboveground tissues were separated into shoots and grains and washed with d-H2O, oven-dried at about 70 °C until the constant weight. The roots were removed from the pots of each pot and washed with tap water followed by washing with dilute acid and finally with d-H2O. The roots were also oven-dried as described above.
Cd and Zn analysis of plants and post soil Cd
Dry biomass (approx. 0.5 g) of aboveground and belowground tissues taken in flasks and added the concentrated acids (HClO4-HNO3) at 1:3 ratio and placed the mixtures overnight. Subsequently, the mixtures were placed on a hot plate and digestion was processed until colorless solution and the final volume was made with d-H2O. The blank solutions (without plant samples) were digested in parallel to the sample digestions to ensure the quality of the results. The concentrations of Cd and Zn of the digested solutions were recorded with AAS.
The soil sampling was also performed after harvesting the wheat. This soil was extracted with AB-DTPA solution and Cd concentrations in the samples were recorded with AAS.
Translocation factors (TF) of shoots to roots and grains to shoots were recorded as below
Statistical analysis
All data reported in the study are the means of four replicates ± standard deviations (n = 4). Statistical analyses of the data were performed using the SPSS 21.0 software for windows. All parameters were examined using analysis of variance (ANOVA), followed by the least significant difference (LSD) test and significant differences were recorded at P < 0.05.
Results
Plant biomass and chlorophyll contents
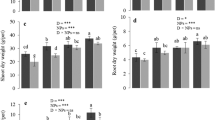
Nanoparticles and drought had a significant effect on dry biomass of shoots, roots, grains, and plant height and spike lengths of wheat (Fig. 1). The lowest values of these attributes were observed in the plants without any NPs (0 mg/kg NPs) under drought stress, whereas the highest values were reported in the 100 mg/kg NPs without drought stress. The NPs improved the growth and dry biomass yield of shoots, roots, and grains in a dose additive way either alone or combined with drought conditions. Overall, all of the NP treatments in Cd-contaminated soil increased the plant height which was increased by 6%, 18%, and 24% without drought and increased by 12%, 26%, and 34% with drought in 25, 50, and 100 mg/kg NPs, respectively, when compared with the respective controls. In NPs without and with drought, the spike length enhanced by 27% and 37% in 100 mg/kg NPs, respectively. The shoot, root, and grain dry biomasses enhanced by 37%, 40%, and 52% in the maximum NP treatment (100 mg/kg) without drought and increased by 54%, 46%, and 69% in the same NP treatment along with drought. Shoot, root, and grain dry weight of wheat had a strong positive correlation with plant height and spike length (Table 2).
Effect of soil amendment and drought stress on the height of plants (a), spike length (b), dry biomass yield of wheat shoot (c), root (d), and grains (e). Data presented in the figure is in an average of 4 replicates (n = 4) ± standard deviation. Different letters between NP and drought treatments showed significant differences at p ≤ 0.05
Adding NPs to the Cd-contaminated soil significantly affected the chlorophyll content of wheat leaves either alone or under drought (Fig. 2). Drought stress significantly decreased the photosynthetic pigment concentrations compared with the respective NP treatments without drought except at 100 mg/kg NPs where the non-significant decrease was observed. The highest increase in chlorophyll a and b concentrations was found in 100 NP treatments without drought, while the minimum values of the parameters were found in drought-stressed control. At 0 mg/kg NPs, the drought stress decreased the chlorophyll a content by 21%, and chlorophyll b concentration by 20% compared with the same NP treatment without drought. Without drought, the NPs increased the chlorophyll a concentration by 13%, 24%, and 39% and chlorophyll b concentration by 14%, 32%, and 44% in 25-, 50-, and 100-mg/kg NP levels as compared with the control. Under drought, the NPs increased the chlorophyll a concentration by 16%, 40%, 59%, and chlorophyll b concentration by 19%, 24%, and 49% in 25, 50, and 100-mg/kg NP levels as compared with the control. The chlorophyll contents had a strong positive correlation with dry weight and height of plants (Table 2).
Changes in chlorophyll a and b concentrations in wheat leaves under different rates of ZnO NPs with and without drought stress. Data presented in the figure is the average of 4 replicates (n = 4) ± standard deviation. Different letters between NP and drought treatments showed significant differences at p ≤ 0.05
Oxidative stress and antioxidant enzymes
The EL, H2O2, and MDA concentrations and enzyme activities (SOD, POD) in wheat shoots grown in the tested soils are reported in Figure 3. The drought stress increased the EL, H2O2, and MDA concentrations in the leaves compared with the respective treatments without drought stress (Fig. 3(a–c)). The highest values of these oxidative stress indicators were reported in the control leaves under drought whereas, the lowest values were reported in the 100-mg/kg NP treatment without drought. Soil application of different NP treatments decreased the values of EL, H2O2, and MDA in leaves in comparison with the control either alone or under drought stress and the results were significant, especially with higher levels of treatments (50 and 100 mg/kg). Additions of 100 mg/kg NPs without drought stress decreased the EL, H2O2, and MDA concentrations by 33%, 35%, and 49%, respectively when compared with the control. Under drought stress with 100 mg/kg NPs, the reduction in EL, H2O2, and MDA concentrations was 44%, 46%, and 48%, compared with drought-stressed control. The EL, H2O2, and MDA contents were negatively correlated with the plant height, chlorophyll contents, and dry biomasses of plant tissues and positively correlated with tissue Cd concentrations (Table 2).
Changes in electrolyte (EL), hydrogen peroxide (H2O2), lipid peroxidation (MDA), superoxide dismutase (SOD), and peroxidase (POD) activities in wheat leaves under different rates of ZnO NPs with and without drought stress. Data presented in the figure is the average of 4 replicates (n = 4) ± standard deviation. Different letters between NP and drought treatments showed significant differences at p ≤ 0.05. EL electrolyte leakage, MDA malondialdehyde, H2O2 hydrogen peroxide, SOD superoxide dismutase, POD peroxidase
All the NP treatments enhance the leaf SOD and POD activities in comparison with control irrespective of drought stress (Figure 3(d, e)). The drought stress reduced the activities of these enzymes in leaves in comparison with the respective NP treatments without drought. In control treatments, there was a significant reduction in these enzyme activities under drought stress than the normal control and this reduction was about 18% and 16% for SOD and POD activities, respectively. The leaf SOD and POD activities were positively correlated with the plant biomass and chlorophyll contents and negatively correlated with the Cd contents in the tissues of wheat (Table 2).
Cadmium and Zn concentrations in wheat
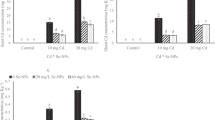
Figure 4 shows the concentration of Cd/Zn in wheat tissues grown in the tested soil and amended with different NPs under different soil moisture conditions. Cadmium accumulation in wheat mainly occurred in roots, followed by other tissues (shoots and grains). Cadmium ranged from 4.88 to 7.64 mg/kg in shoots and 8.6 to 16.73 mg/kg in roots and 0.19 to 1.38 mg/kg in grains (Fig. 4(a–c)). The highest Cd concentrations were observed in shoots, roots, and grains under drought stress without NPs, whereas the lowest Cd concentrations in these parts were found in the 100 mg/kg NPs without drought conditions. The drought stress enhanced the Cd in wheat than the same NP treatments without drought stress. In treatments without NPs, there was a significant enhancement in Cd concentrations in wheat shoots, roots and grains under drought stress in comparison without drought conditions. The NP soil amendments significantly decreased the Cd concentrations in wheat tissues over the control. There was a significant impact of drought, NPs, and drought × NPs except the Cd concentrations in roots where non-significant trend was observed for drought × NPs. The Cd concentrations in plants were negatively correlated to the plant biomass and chlorophyll contents and positively correlated with oxidative stress markers such as EL, H2O2, and MDA contents (Table 2).
Changes in Cd and Zn concentrations in wheat shoots, roots, and grains under different rates of ZnO NPs with and without drought stress. Data presented in the figure is the average of 4 replicates (n = 4) ± standard deviation. Different letters between NP and drought treatments showed significant differences at p ≤ 0.05
The NP amendment to Cd-contaminated soil potentially enhanced the Zn in wheat tissues (shoots, roots, grains) in comparison with that of control (Fig. 4(d–f)). The highest Zn accumulation by shoots, roots, and grains was achieved in the 100-mg/kg NP treatments under drought conditions. Limited water conditions were the most effective in enhancing Zn concentrations in wheat than the respective NP doses without drought stress. About 78%, 64%, and 103% greater Zn concentrations were observed in shoots, roots, and grains with the highest NPs (100 mg/kg) than with the control without drought. In comparison with the drought-stressed control, the Zn concentrations increased by 76%, in shoots, 93% in roots, and 96% in grains under the 100-mg/kg NP amendment under drought stress.
Soil bioavailable Cd and TF values
Cadmium mobility in the soil was considerably reduced (p < 0.05) with increasing levels of NPs compared with that of control (Fig. 5). The bioavailable soil Cd ranged from 0.61 to 1.19 mg/kg. The Cd concentration decreased by 45% and 37% in the 100-mg/kg NP treatment under alone or drought stress, respectively. Under the same NP amendments, there was a slight increase in Cd concentrations under water-limited conditions than normal water conditions.
Cadmium accumulated in wheat is absorbed by the roots from the soil and then transferred to the shoots and finally to grains. The Cd shoot to root TF increased as the NP levels increased in the soil which ranged from 0.46–0.57 to 0.47–0.54 in normal and drought stress conditions (Table 3). There was a significant increase in TF at the highest NPs compared with the control. The grain to shoot TF linearly decreased as the NP levels increased in the soil. There was a significant decrease in grain to shoot TF under the 50- and 100-mg/kg NP amendments compared with the control.
Discussion
Zinc has a beneficial role in growth and development of plants, whereas Cd is non-essential element for plants and caused oxidative stress through ROS production and damage at cellular levels (Rizwan et al. 2019a). Zinc improves the plant response under stressful environment through maintaining cell turgidity, homeostasis of nutrients, and improving cell structure and anatomical features in plants (Rizwan et al. 2019a). The purpose of the current study was to explore the efficiency of ZnO NPs on alleviation of Cd stress in wheat under different water conditions. The lower biomass was found in the control tissues and drought stress further minimized the plant biomass (Figure 1). Numerous studies highlighted that Cd-caused toxicities in crops and negatively affected the growth by stimulating the ROS generation (Nagajyoti et al. 2010). In current study, the minimum biomass observed in the control could be employed to the lower chlorophyll contents (Fig. 2), higher Cd concentrations in wheat (Fig. 4), and oxidative stress in leaves (Fig. 3). The drought stress further decreased the plant biomass (Fig. 1) and chlorophyll contents (Fig. 2). The studies reported that drought stress decreased the growth and plant biomass by affecting the nutrient intake by plants (Moosavi et al. 2015; Abbas et al. 2018) or altering the plant morphology (de Silva et al. 2012). It has been shown that drought combined with metal stress diminished the chlorophyll contents and gas exchange characteristics by altering the photosynthetic machinery (Xia et al. 2015; Ma et al. 2016).
The results clearly demonstrated that wheat biomass increased under NPs and the increasing trend was higher in higher levels of the treatments applied (Fig. 1). The colloidal solution of Cu and Zn NPs improved the drought-stressed wheat growth (Taran et al. 2017). The ZnO NPs improved the growth of wheat under Cd stress (Hussain et al. 2018). The positive impacts of ZnO NPs on plant biomass have been reported under metal (Venkatachalam et al. 2017; Ali et al. 2019) or drought stress (Taran et al. 2017). The composite supply of three micronutrient NPs (ZnO, B2O3, and CuO) increased the growth of drought-stressed soybean (Dimkpa et al. 2017a). The present study reported first time, to the best of our knowledge, that NPs have also beneficial role under combined Cd-drought stress. The major reason of variation in biomass and chlorophyll contents of the wheat was due to the Cd and Zn concentrations in the tissues.
Zinc plays an important role in cereals such as wheat and its high accumulation in required to alleviate abiotic and biotic stresses especially in Zn-deficient soils (Rizwan et al. 2019a). The results showed that ZnO NP soil amendment enhanced the Zn concentrations in wheat tissues and grains (Fig. 4(d–f)). The results are in line with the published reported demonstrating that NPs of Zn enhanced the Zn concentrations on the plants (Dimkpa et al. 2017b; Taran et al. 2017; Ali et al. 2019). These higher tissue Zn contents may minimize the oxidative stress as was depicted by the minimum generation of ROS in leaves (Fig. 3) and thus recovered wheat from Cd-induced oxidative stress. Thus, exogenous ZnO NPs might be the strategy to increase Cd tolerance in crops. The higher Zn concentration application in the soil in the form of NPs might be an efficient mean to minimize Cd intake by wheat due to the increased competition at the surface of roots which is due to the similar behavior of Zn and Cd (Rizwan et al. 2019b). The main reason for the low Cd content in tissues was due to the higher plant Zn contents. There is antagonism between Zn and Cd in plants but this effect depends upon the doses of Zn and Cd in the media (Rizwan et al. 2019a, 2019b). Cadmium accumulation by wheat is through roots and then is transferred to aboveground tissues. In the study, Cd concentrations were highest in roots then in shoots and were lowest in grains (Fig. 4). These results are consistent with the previous reports (Qayyum et al. 2017; Ali et al. 2019; Salam et al. 2019) indicating that roots are a main organ for Cd intake and accumulation.
The increase of Cd contents in wheat under limited water condition might be ascribed to the soil Cd availability (Abbas et al. 2018; Salam et al. 2019). Lower soil moisture conditions enhanced the Cu and Pb concentrations in corn tissues than higher moisture contents in the soil (Salam et al. 2019). These variations in metal uptake by plants could be due to the changes in metal mobile frictions in the soil as it was reported that higher moisture contents in the soil (80% of FC) decreased the mobile portion of Cu and Pb in sequential extraction compared with the lower moisture contents (Salam et al. 2019). The more concentrated solution under limited water conditions could be the reason of higher Cd accumulation by plants (Abbas et al. 2018). The reduction in mobility in moisture conditions might be ascribed to the precipitation of Cd with sulfides as Rinklebe et al. (2016) reported that Cu precipitated with sulfides under wet conditions. Furthermore, the difference in the biomass of the tissues (Fig. 1) may affect the Cd accumulation in wheat (Fig. 4).
The lower grain Cd concentrations could be explained by the reduction in Cd accumulation by roots and declined its upward roots to shoots movement and finally shoots to grains which is due to the decreasing Cd availability in the soil following the application of NPs (Figs. 4 and 5). In this study, shoot to root TF values increased with the increasing soil NP amendments (Table 3). It has been reported that translocation of metals from roots to shoots decreased when there are higher metal concentrations in the roots (Keller et al. 2015). This decreased translocation might be ascribed to the stimulation of metal-stressed plant defense system and plants tolerate metal stress by depositing the metals in the roots through different mechanisms such as chelation (Rizwan et al. 2018, 2019a, 2019b). However, under low metal concentrations, the plant defense system may not be active and the higher metal might be transported to the shoots. Our results depicted that Cd grain to shoot TF decreased with increasing NP levels (Table 3), suggesting that NPs can restrain transfer of Cd from shoots to grains. The higher Zn concentrations in grains may cause an antagonistic effect on Cd translocation to grains.
Conclusion
Zinc oxide NP application significantly increased the wheat yield and simultaneously reduced the wheat grain Cd accumulation, and the higher NP treatment (100 mg/kg) was the most effective in this regard. The reduction in grain Cd was related to the low bioavailability of soil Cd and Cd transfer from roots to shoots and then shoots to grains especially via the highest NP treatment. Overall, the application of NPs to Cd-contaminated soils under drought stress with the appropriate level could be an effective way to produce safe and quality food. The potential morpho-physiological and biogeochemical mechanisms occurring in the soil need to be explored, and field level tests are also helpful.
References
Abbas T, Rizwan M, Ali S, Adrees M, Mahmood A, Rehman MZ, Ibrahim M, Arshad M, Qayyum MF (2018) Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol Environ Saf 148:825–833
Ali S, Rizwan M, Noureen S, Anwar S, Ali B, Naveed M, Abd_Allah E, Alqarawi AA, Ahmad P (2019) Combined use of biochar and zinc oxide nanoparticle foliar spray improved the plant growth and decreased the cadmium accumulation in rice (Oryza sativa L.) plant. Environ Sci Pollut Res 26:11288–11299
Amacher MC (1996) Nickel, cadmium and lead. In: Sparks DL (ed) Methods of soil analysis. Part 3. Chemical methods, 3rd edn. SSSA/ASA, Madison, pp 739–768
Bashir A, Rizwan M, Ali S, Rehman MZ, Ishaque W, Riaz MA, Maqbool A (2018) Effect of foliar-applied iron complexed with lysine on growth and cadmium (Cd) uptake in rice under Cd stress. Environ Sci Pollut Res 25:20691–20699
Bauddh K, Singh RP (2012) Growth, tolerance efficiency and phytoremediation potential of Ricinus communis (L.) and Brassica juncea (L.) in salinity and drought affected cadmium contaminated soil. Ecotoxicol Environ Saf 85:13–22
Baycu G, Gevrek-Kürüm N, Moustaka J, Csatári I, Rognes SE, Moustakas M (2017) Cadmium-zinc accumulation and photosystem II responses of Noccaeacaerulescens to Cd and Zn exposure. Environ Sci Pollut Res 24:2840–2850
Bouyoucos GJ (1962) Hydrometer method improved for making particle- size analyses of soils. Agron J 54:464–465
Cakmak I, Kutman UB (2018) Agronomic biofortification of cereals with zinc: a review. Eur J Soil Sci 69:172–180
Cakmak I, Kalayci M, Kaya Y, Torun AA, Aydin N, Wang Y, Arisoy Z, Erdem H, Yazici A, Gokmen O, Ozturk L, Horst WJ (2010) Biofortification and localization of zinc in wheat grain. J Agric Food Chem 58:9092–9102
Curtis T, Halford NG (2014) Food security: the challenge of increasing wheat yield and the importance of not compromising food safety. Ann Appl Biol 164:354–372
de Silva NDG, Cholewa E, Ryser P (2012) Effects of combined drought and heavy metal stresses on xylem structure and hydraulic conductivity in red maple (Acer rubrum L.). J Exp Bot 63:5957–5966
Dimkpa CO, Bindraban PS, Fugice J, Agyin-Birikorang S, Singh U, Hellums D (2017a) Composite micronutrient nanoparticles and salts decrease drought stress in soybean. Agron Sustain Dev 37:1–13
Dimkpa CO, White JC, Elmer WH, Gardea-Torresdey J (2017b) Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J Agri Food Chem 65:8552–9
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hussain A, Ali S, Rizwan M, Rehman MZ, Javed MR, Imran M, Chatha SA, Nazir R (2018) Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ Pollut 242:1518–1526
Hussain A, Rizwan M, Ali Q, Ali S (2019) Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ Sci Pollut Res 26:7579-7588
Joy EJ, Ahmad W, Zia MH, Kumssa DB, Young SD, Ander EL, Watts MJ, Stein AJ, Broadley MR (2017) Valuing increased zinc (Zn) fertiliser-use in Pakistan. Plant Soil 411:139–150
Keller C, Rizwan M, Davidian JC, Pokrovsky OS, Bovet N, Chaurand P, Meunier JD (2015) Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 μM Cu. Planta 241:847–860
Krezel A, Maret W (2016) The biological inorganic chemistry of zinc ions. Arch Biochem Biophys 611:3–19
Lichtenthaler HK (1987) Chlorophylls and carotenoids-pigments of photosynthetic biomembranes. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 148. Academic Press, San Diego, pp 350–382
Liu R, Lal R (2015) Potentials of engineered nanoparticles as fertilizers for increasing agronomicproductions. Sci Total Environ 514:131–139
Liu C, Yu R, Shi G (2017) Effects of drought on the accumulation and redistribution of cadmium in peanuts at different developmental stages. Arch Agron Soil Sci 63:1049–1057
Ma Y, Rajkumar M, Zhang C, Freitas H (2016) Inoculation of Brassica oxyrrhina with plant growth promoting bacteria for the improvement of heavy metal phytoremediation under drought conditions. J Hazard Mater 320:36–44
Mahajan P, Dhoke SK, Khanna AS (2011) Effect of nano-ZnO particle suspension on growth of mung (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method. J Nano technol 2011:1–7. https://doi.org/10.1155/2011/696535
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Moosavi AA, Mansouri S, Zahedifar M (2015) Effect of soil water stress and nickel application on micronutrient status of canola grown on two calcareous soils. Plant Prod Sci 18:377–387
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Qayyum MF, Rehman MZ, Ali S, Rizwan M, Naeem A, Maqsood MA, Khalid H, Rinklebe J, Ok YS (2017) Residual effects of monoammonium phosphate, gypsum and elemental sulfur on cadmium phytoavailability and translocation from soil to wheat in an effluent irrigated field. Chemosphere 174:515–523
Raliya R, Saharan V, Dimkpa C, Biswas P (2017) Nanofertilizer for precision and sustainable agriculture: current state and future perspectives. J Agric Food Chem 66:6487–6503. https://doi.org/10.1021/acs.jafc.7b02178
Rehman MZ, Rizwan M, Ghafoor A, Naeem A, Ali S, Sabir M, Qayyum MF (2015) Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phyto-availability to wheat and rice under rotation. Environ Sci Pollut Res 22:16897–16906
Rehman MZ, Rizwan M, Ali S, Naeem A, Yousaf B, Lui G, Khalid H, Hafeez F, Azhar M (2018) A field study investigating the potential use of phosphorus combined with organic amendments on cadmium accumulation by wheat and subsequent rice. Arab J Geosci 11:1–9
Rehman MZ, Rizwan M, Rauf A, Ayub MA, Ali S, Qayyum MF, Waris AA, Naeem A, Sanaullah M (2019) Split application of silicon in cadmium (Cd) spiked alkaline soil plays a vital role in decreasing Cd accumulation in rice (Oryza sativa L.) grains. Chemosphere 226:454–462
Rinklebe J, Shaheen SM, Frohne T (2016) Amendment of biochar reduces the release of toxic elements under dynamic redox conditions in a contaminated floodplain soil. Chemosphere 142:41–47
Rizwan M, Ali S, Ibrahim M, Farid M, Adrees M, Bharwana SA, Rehman MZ, Qayyum MF, Abbas F (2015) Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: a review. Environ Sci Pollut Res 22:15416–15431
Rizwan M, Ali S, Qayyum MF, Ibrahim M, Rehman MZ, Abbas T, Ok YS (2016) Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: a critical review. Environ Sci Pollut Res 23:2230–2248
Rizwan M, Ali S, Hussain A, Ali Q, Shakoor MB, Rehman MZ, Farid M, Asma M (2017a) Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 187:35–42
Rizwan M, Ali S, Qayyum MF, Ok YS, Adrees M, Ibrahim M, Rehman MZ, Farid M, Abbas F (2017b) Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J Hazard Mater 322:2–16
Rizwan M, Ali S, Rehman MZ, Rinklebe J, Tsang DC, Bashir A, Maqbool A, Tack FM, Ok YS (2018) Cadmium phytoremediation potential of Brassica crop species: a review. Sci Total Environ 631:1175–1191
Rizwan M, Ali S, Rehman MZ, Maqbool A (2019a) A critical review on the effects of zinc at toxic levels of cadmium in plants. Environ Sci Pollut Res 26:6279–6289
Rizwan M, Ali S, Rehman MZ, Adrees M, Arshad M, Qayyum MF, Ali L, Hussain A, Chatha SA, Imran M (2019b) Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ Pollut 248:358–367
Salam A, Shaheen SM, Bashir S, Khan I, Wang J, Rinklebe J, Rehman FU, Hu H (2019) Rice straw-and rapeseed residue-derived biochars affect the geochemical fractions and phytoavailability of Cu and Pb to maize in a contaminated soil under different moisture content. J Environ Manag 237:5–14
Shi G, Xia S, Ye J, Huang Y, Liu C, Zhang Z (2015) PEG-simulated drought stress decreases cadmium accumulation in castor bean by altering root morphology. Environ Exp Bot 111:127–134
Soltanpour PN (1985) Use of AB-DTPA soil test to evaluate elemental availability and toxicity. Commun Soil Sci Plant Anal 16:323–338
Sturikova H, Krystofova O, Huska D, Adam V (2018) Zinc, zinc nanoparticles and plants. J Hazard Mater 349:101–110
Taran N, Storozhenko V, Svietlova N, Batsmanova L, Shvartau V, Kovalenko M (2017) Effect of zinc and copper nanoparticles on drought resistance of wheat seedlings. Nano Res Lett 12:1–6
Tripathi DK, Singh VP, Prasad SM, Chauhan DK, Dubey NK (2015) Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol Biochem 96:189–198
Venkatachalam P, Jayaraj M, Manikandan R, Geetha N, Rene ER, Sharma NC, Sahi SV (2017) Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: a physiochemical analysis. Plant Physiol Biochem 110:59–69
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining in soil organic matter, and a proposed modification of the chromic soil titration method. Soil Sci 37:29–38
Wang H, Xu C, Luo ZC, Zhu HH, Wang S, Zhu QH, Huang DY, Zhang YZ, Xiong J, He YB (2018) Foliar application of Zn can reduce Cd concentrations in rice (Oryza sativa L.) under field conditions. Environ Sci Pollut Res 25:29287–29294
Xia S, Wang X, Su G, Shi G (2015) Effects of drought on cadmium accumulation in peanuts grown in a contaminated calcareous soil. Environ Sci Pollut Res 22:18707–18717
Zhang XZ (1992) The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. Res Methodol Crop Physiol. Agriculture Press, Beijing, pp 208–211
Funding
This work is financially supported by the Government College University and the higher education commission of Pakistan (for the research funding via Project No. 5634/Punjab/NRPU/R&D/HEC/2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, Z.S., Rizwan, M., Hafeez, M. et al. The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ Sci Pollut Res 26, 19859–19870 (2019). https://doi.org/10.1007/s11356-019-05333-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05333-5