Abstract
Zinc (Zn) deficiency and heat stress affect the productivity of cereal crops in many semi arid regions of the world. Zinc nutrition affects membrane integrity and activity of superoxide dismutase (SOD) enzymes which are also important to a plant’s ability to cope with high temperatures. Two experiments were conducted to examine the interaction between Zn nutrition and heat stress at two growth stages. Wheat was grown at deficient and adequate levels of Zn at 22/16°C before being exposed to a short period of high temperature (40/20°C). One experiment examined the response to a 3 day heat stress treatment at 10 days after anthesis and the second experiment examined the response to 6 days of heat stress in 30-day old seedlings. Varieties differing in thermotolerance and sensitivity to Zn deficiency were compared. Zinc deficiency and heat stress reduced kernel growth rates, kernel weight and grain yield and the greatest reductions were measured in Zn-deficient, heat stressed plants. The greater reduction in kernel growth rates from heat stress in Zn deficient plants occurred in both thermotolerant and sensitive varieties and was negatively associated with grain Zn concentration. Genetic differences in tolerance to Zn deficiency did not have consistent effects on the response to heat stress. Chlorophyll fluorescence and chlorophyll content were reduced and chloroplast ultrastructure was disrupted by heat stress and the effect was exacerbated by low supplies of Zn. While the responses to heat stress and low Zn were largely additive effects, the experiments demonstrated that low levels of Zn nutrition can exacerbate the adverse effects of short periods of heat stress on kernel growth and chloroplast function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the field environment plants are exposed to multiple and often coincident stresses. Consequently, the ability to respond to one stress may be influenced by the severity of other stresses. While interactions between different environmental stresses are frequently acknowledged when yield limitations of crop plants are discussed, they are seldom studied. Even the interactive effects of the common abiotic stresses, drought and heat stress, are not well understood (Barnabas et al. 2008). The experiments described in this paper examined the interactions between heat stress and zinc (Zn) deficiency, two abiotic stresses that are important limitations to wheat yields in many cereal growing regions of the world where alkaline soils occur.
Heat stress is a major cause of reductions in yield and quality in many of the world’s major wheat-growing regions (Paulsen 1994). The risk of yield losses from heat stress is also likely to increase in a number of regions with the predicted increases in mean temperatures and in the frequency of hot days associated with global warming (Easterling et al. 2007; Ainsworth et al. 2008). The optimum temperature for vegetative growth and grain filling of wheat is between 15°C and 20°C (Paulsen 1994) and temperatures significantly above this range are regularly experienced in semi arid regions of Australia, West Asia and North America. As well, crops are often exposed to short periods (2–5 days) of very high temperatures (≥35°C) during grain filling and such periods of heat shock further damage crops and reduce grain quality (Stone and Nicolas 1994).
Heat stress induces many responses in the plants, but one of the important causes of injury is by changes in membrane structure, which results in loss of function and increases membrane leakiness (Sayed 2003; Wahid et al. 2007). While the integrity of all membranes is reduced by heat stress, the tonoplast is relatively more heat stable compared with the photosynthetic membranes (Weigel 1983). High temperatures can damage the photosynthetic apparatus of plants and inhibit the thylakoid membrane activity of chloroplasts before the visual symptoms of high temperature occur and this is reflected in changes in chlorophyll fluorescence (Berry and Björkman 1980; Al-Khatib and Paulsen 1990). Lesions in the chloroplast envelope caused by short periods of very high temperature also result in the loss of chloroplast contents (McCain et al. 1989). This damage to plant membranes by heat stress is associated with increased generation of reactive oxygen species (ROS) (Liu and Huang 2000; Barnabas et al. 2008). To reduce the accumulation of ROS, plants employ a system of physiological antioxidants, one of which is the enzyme, superoxide dismutase (SOD), which scavenges the superoxide radical molecule (O2·−) in chloroplasts (Foyer and Harbinson 1994). Of the three isoenzymes of SOD (CuZnSOD, MnSOD and FeSOD) CuZnSOD and FeSOD are found in chloroplasts and play an important role in protecting the membranes from peroxidation of membrane lipids and maintaining membrane integrity (Cakmak and Marschner 1988; Yu et al. 1998; Cakmak 2000). Therefore, adequate Zn may help maintain photosynthetic activity and preserve membrane integrity under heat stress (Cakmak and Marschner 1988; Cakmak 2000).
Zinc deficiency is prevalent in West Asia, southern Australia and South Asia (Cakmak 2000; Rashid and Ryan 2004; Alloway 2008), regions that regularly experience high temperature stress. Given the role of Zn in maintaining membrane integrity it is feasible that adequate Zn nutrition helps to protect plants from the damaging effects of high temperature stress. For example, in a field study on the effects of sowing date and Zn nutrition on grain filling, in which the number of days with temperatures greater than 35°C during grain filling increased from 4 to 9 with late sowing, kernel weight in the central spikelets was lower at the later sowing date (36.0 mg seed−1 to 32.4 mg seed−1) under a low supply of Zn whereas plants grown with supplementary Zn showed no significant difference in kernel weight (35.3 mg seed−1 and 37.1 mg seed−1; LSD = 3.2 mg seed−1) (Graham 2004). However, there is little information on the interaction between Zn nutrition and heat stress. The present study was designed to investigate the role of Zn nutrition in thermotolerance. Genotypes that differ in thermotolerance and sensitivity to Zn were used to examine whether these is a genetic link between tolerance to the two abiotic stresses.
Materials and methods
Two experiments were conducted during the reproductive and vegetative phases of plant development. The first experiment examined the effects of a single period of heat shock 10 days after anthesis and the second examined the effects of high temperature during vegetative growth. In both experiments, plants were grown at different levels of Zn, which were 0.2 and 2 mg Zn kg−1 (Zn0.2 and Zn2; Experiment 1) and 0, 0.2, 2 and 20 mg Zn kg−1 (Zn0, Zn0.2, Zn2 and Zn20, Experiment 2). In the first experiment the interest was in examining responses during grain filling in plants that were marginally Zn deficient rather than having growth greatly impaired by severe Zn deficiency. The aim was to generate differences in the Zn concentrations in the leaf tissue without causing large differences in growth which, because of factors such as differences in stem number or in water use, may have affected the response to high temperatures. The second experiment extended the range of Zn treatments to generate more severe deficiency and very high Zn levels in the plants.
Soil preparation
The plants were grown in a Zn-deficient siliceous sandy soil (Laffer sand, DTPA-extractable Zn = 0.07 mg kg−1) collected from a naturally vegetated area near Tintinara, in the southeast of South Australia. The soil was passed through a 2 mm stainless steel sieve, washed three times with deionised water and air dried in a glasshouse. Soil was weighed into polythene-lined pots, and calcium carbonate powder (0.3% w/w) was added to raise the pH to 8.0. The following basal nutrients were applied in solution separately to each pot (in mg kg−1 dry soil): NH4NO3, 350; KH2PO4, 150; K2SO4, 120; MgSO4.7H2O, 90; CuSO4.5H2O, 5; MnSO4.4H2O, 7; H3BO3, 1; CoSO4.7H2O, 1; FeSO4.7H2O, 0.7; H2MoO4.H2O, 0.5 and NiSO4.6H20, 0.15. Zinc was applied as a solution of ZnSO4.7H2O to correspond to the Zn treatments selected for each experiment. These nutrients were thoroughly mixed through the soil before nanopure water (18.2 Mohms cm−1 resistivity) was added to bring the soil moisture content to 12% (w/w), which corresponded to field capacity. The soil was allowed to equilibrate in the pots for 24 h before seeds were sown. In Experiment 1, solutions of NH4NO3 (120 mg N kg−1 dry soil) and K2SO4 (54 mg K kg−1 dry soil) were applied to each pot at four and six weeks after sowing, respectively, and in Experiment 2 a solution of NH4NO3 (120 mg N kg−1 dry soil) was applied to each pot at 22 days after sowing (DAS).
Genotypes
Four wheat genotypes differing in their tolerance to Zn deficiency (defined as Zn efficiency; (Graham 1984)) and to high temperature were used: Frame (moderately thermotolerant, moderately Zn efficient); Goldmark (moderately thermosensitive, Zn inefficient); Halberd (heat tolerant, Zn efficient) and Meering (heat sensitive, Zn efficiency). The Zn efficiency of the genotypes was based on previous screening of germplasm (McDonald et al. 2001) and the classification of the heat tolerance of Halberd and Meering was based on their responses to heat stress during grain filling (Wrigley et al. 1994; Stone and Nicolas 1995b) while those of Frame and Goldmark were based on previous studies with these varieties (Graham 2004). With the exception of Halberd all are semi-dwarf varieties of bread wheat released by breeding programs in southern Australia. Each of the genotypes had a seed Zn content of between 500 and 550 ng seed−1.
Seed preparation and sowing
Uniformly-sized seeds were pre-germinated on filter paper at 20°C for 24 h in sterile Petri dishes after being surface sterilised by soaking in 70% (v/v) ethanol for 1 min, followed by sodium hypochlorite (3% active chlorine, v/v) for 6 min and rinsed three times in nanopure water. In Experiment 1, the plants were grown in 3 kg of washed sand in 2 L pots and in Experiment 2 plants were grown in 1.4 kg of washed sand in 1.2 L pots. Uniformly-germinated seeds were planted 15 mm deep. A 1 cm layer of acid washed black polypropylene beads was placed on the soil surface of each pot. Pots were watered to weight daily with nanopure water to maintain the soil water content at field capacity (12% (w/w)).
Heat treatment
Plants were initially grown in a growth room at 22/16°C day/night temperature and a 14 h photoperiod. The intensity of photosynthetically active radiation (PAR) varied from 600 to 725 μmol s−1 m−2 at the plant canopy level. A second growth room (light intensity 715–825 μmol s−1 m−2 at the plant canopy level) was used to impose the heat stress treatments (40/20°C). Pots were transferred to the high temperature growth room at the appropriate growth stage at the beginning of the night cycle. Rather than have a rapid transition between the night and day temperatures, the day temperature was gradually increased from 20°C to 40°C at a rate of 7°C h−1 after which it was maintained at 40°C for 8 h. The temperature was then reduced at a rate of 7°C h−1. This resulted in a mean temperature during the 14 h. photoperiod of 34°C. During the heat stress treatment the plants were watered to 12% moisture content four times per day to ensure that water stress did not confound the effect of heat stress.
Experiment 1: Heat stress during early grain filling
Ten pre-germinated seeds were sown in each pot and thinned to eight uniform plants per pot after emergence. The youngest emerged leaf blade (YEB) was sampled from one plant of each replicate at 38 DAS, and analysed for Zn and other nutrient concentrations by ICP spectrometry (Zarcinas et al. 1987).
Each main stem ear was labelled with the date of the first anther exertion (growth stage 60 (Zadoks et al. 1974)) and the date of all subsequent operations was related to the average anthesis date for each genotype. Only plants that flowered within 3 days of one another were used in the experiment. Heat treatments were applied for 3 days starting from 10 days after anthesis (DAA).
Chlorophyll fluorescence measurements were made 4–5 h into the light period on the flag leaves of two plants per pot at regular intervals from 5 days prior to anthesis until the leaves began to senesce. Measurements were made using a Hansatech Plant Efficiency Analyser (PEA) (Hansatech Instruments Ltd., King’s Lynn, UK) after dark-adapting the leaves for 30 min. Measurements were made approximately 2 cm from the leaf tip. Each leaf was illuminated with saturating light of 3,000 μmol m−2 s−1 at 650 nm for a period of 5 s.
One plant from each pot was harvested at 10, 13, 20 and 35 DAA, and separated into main stem and tillers. The grains from the basal florets of the four central spikelets of the main stem (two on each side), the remainder of the gains from the main stem and the tillers were removed. The grains and remainder of the plant parts were oven dried at 80°C for 48 h and weighed. Flag leaf area was determined at ten DAA using a Paton Electronic Planimeter (Paton Industries, Pty Ltd., South Australia).
Leaf chlorophyll content was measured on the flag leaves of two plants per pot at two-day intervals from nine DAA until leaf senescence. Leaf chlorophyll content was estimated non-destructively by measuring leaf greenness using a portable SPAD (Soil Plant Analysis Development)-502® chlorophyll meter (Minolta Camera Co. Ltd., Japan). Three measurements were made along the leaf blade, midway between the mid-rib and leaf margin whenever possible.
At maturity (50 DAA), two plants per pot were harvested from each replicate, and separated into main stem and tillers. Grain weight from three parts of the plant was recorded: four grains from the two basal florets in the two central spikelets, the main stem and the whole plant. The grain, straw and chaff were oven dried at 80°C for 48 h and weighed. Grain nutrient concentration was determined by ICP spectrometry after digestion in nitric acid-perchloric acid (Zarcinas et al. 1987).
The experiment was conducted in adjoining growth rooms and prior to the transfer to the heated growth room the treatments were arranged as a 4 × 2 × 2 factorial, randomised block design with four replicates. Although the heated plants were only removed from the growth room for 3 days, the two temperature treatments were considered as separate ‘environments’ and the data was analysed as a combined analysis over the two heat treatments with Heat treatment, Variety and Zinc level considered as fixed effects (McIntosh 1983). Data were analysed by ANOVA using the Genstat statistical program. Treatment means were compared using LSD at the 5% probability level.
Experiment 2: heat stress during vegetative growth
Fourteen uniformly germinated seeds were sown into each pot, and later thinned to 12 uniform plants per pot. The temperature regime in Experiment 2 was altered from that used in Experiment 1. The recovery from the short period of heat stress in Experiment 1 was rapid so it was decided to expose the plants to a longer period of heat stress. Therefore, plants were exposed to high temperatures for 6 days rather than 3 days. Heat stress commenced 30 days after sowing (DAS) using the method described in Experiment 1.
Leaf chlorophyll content was measured on the youngest emerged leaf blades (YEBs) of two plants per pot at 39 DAS. Chlorophyll fluorescence measurements were made on YEBs of two plants per pot at regular intervals from 25 DAS until the end of the experiment at 39 DAS.
Two plants from each pot were harvested prior to the heat stress treatment (28 DAS), and again at the end of the experiment (39 DAS) and dried at 80°C for 48 h and weighed. YEBs from two plants of each replicate were sub-sampled at 39 DAS, and analysed for nutrient concentrations by ICP spectrometry (Zarcinas et al. 1987).
The YEBs from two plants per pot of Frame and Goldmark were harvested at 28, 31 and 39 DAS, 7 h into the light period, and placed immediately in liquid N and stored at −80°C. The activity of SOD (EC 1.15.1.1) was assayed using the method of Giannapolis and Reis (1977) as modified by Dhindsa et al. (1981). Seven hundred milligrams of leaf tissue (without necrotic areas) was homogenised on ice for 2 min in a mortar and pestle containing 7 mL of 50 mM Hepes buffer and 0.1 mM Na2EDTA at pH 7.6. The homogenate was centrifuged twice at 13,200 g for 15 min at 4°C and the supernatant was used for the assay. The reaction mixture (5 mL) contained 50 mM Hepes (pH 7.6), 0.1 mM EDTA (pH 8.0), 50 mM Na2CO3 (pH 10.4), 13 mM methionine, 0.025% (w/v) Triton X-100, 75 μM NBT, 2 μM riboflavin and the enzyme aliquot (0 to 500 μL). Riboflavin was added last and the tubes were shaken before being placed in front of a light bank consisting of two 15 W fluorescent lamps. The reaction mixtures were illuminated for 10 min. at a light intensity of 270 μmole m−2 s−1 after which the tubes were covered with a black cloth. One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of the rate of NBT reduction measured at 560 nm. Identical reaction mixtures that had not been illuminated were used to correct for background absorbance. Activity of CuZnSOD is inhibited by cyanide, while Fe-SOD is not sensitive to cyanide but is inhibited by H2O2. MnSOD is unaffected by both H2O2 and cyanide. Activity of MnSOD was measured after addition of potassium cyanide (KCN) and H2O2 to the assay solution at a final concentration of 3 mM and 5 mM, respectively. Preliminary studies of FeSOD activity, obtained by subtracting MnSOD activity from the activity yielded in the presence of 3 mM KCN only, found the activity of FeSOD was not measurable. Activity of CuZnSOD was therefore determined by subtracting MnSOD activity from the total SOD activity in assay solutions containing no KCN or H2O2.
Leaf pieces of approximately 1 cm2, excluding necrotic areas, were harvested 2 cm from the tip of the YEBs of Frame and Goldmark at 33 DAS, 7 h into the light period and were placed immediately in 2.9 mL of EM fixative (1.25% glutaraldehyde, 4% sucrose, 4% paraformaldehyde in phosphate buffered saline, pH 7.2) and stored at 4°C before examination by transmission electron microscopy. Leaves from two treatments, Zn0 and Z2, were used. Leaf segments were recut to 1 mm2 under glutaraldehyde and washed twice in phosphate buffered saline plus 4% sucrose, for 10 min each time. Samples were post-fixed in 1% osmium tetroxide for 2 h and dehydrated in a graded series of acetone (70%, 90%, 95%, 100%) and then infiltrated overnight with a mixture of epoxy resin and 100% acetone (1:1), followed by three changes of 100% epoxy resin of 8 h each, and embedded in fresh resin. Polymerisation was completed in 24 h at 70°C. Ultra thin sections of 70 nm were cut on a diamond knife (Diatome, Fort Washington, PA, USA) using a Reichert Ultracut E Ultramicrotome (Leica-Reichert, Vienna, Austria), and collected from the knife’s water bath on 200 mesh Cu/Pd grids. The sections were stained for 20 min with 5% uranyl acetate in 70% ethanol, followed by 20 min with 0.4% lead citrate (Reynolds 1963). Sections were examined with a Philips CM100 Transmission Electron Microscope (Philips Electron Optics, Eindhoven, The Netherlands) at an accelerating voltage of 80 kV, and photographed with an analysis® Mega-View II digital image capture system. Qualitative observations were made of approximately 120–150 randomly chosen mesophyll cells in each plant, photographs were taken of at least three random sites in three sections, and representative pictures are presented.
Prior to and after imposition of the heat stress treatment the pots were arranged as a 4 × 4 × 2 factorial, completely randomised block design with four replicates. The sowing of each replicate was staggered, 7 days apart, in order to allow sufficient time for all measurements to be taken. The statistical model and analysis was the same as that described for Experiment 1.
Results
Experiment 1
Growth prior to heat stress
At 38 DAS the mean Zn concentration of the YEBs in plants grown at 0.2 mg Zn kg−1 was 17 mg kg−1, which confirmed the plants were marginally deficient in Zn (Reuter and Robinson 1997). There was no significant difference among the four varieties. By comparison, at 2 mg Zn kg−1 the Zn concentration in the YEB was 50 mg kg−1. The lower Zn supply only had a relatively small effect on shoot growth. At 10 DAA reducing the supply of Zn significantly reduced the mean flag leaf area from 15.4 cm2 to 13.7 cm2 (P = 0.05). There was no Zn x Variety interaction. The dry weight and tiller number were unaffected by Zn treatment or Variety and the mean values were 3.1 g plant−1 and 2.4 tillers plant−1, respectively.
Chlorophyll content
Both heat stress and Zn treatment affected green leaf retention, but with few significant interactions between them. The varieties differed significantly in their response to high temperature (Fig. 1). Frame showed no response to high temperature and Halberd showed a small response with a significant difference between the two heat treatments only occurring at 35 days. In contrast Goldmark showed a large and immediate decline in leaf chlorophyll content during the period of heat stress and Meering showed a significant decline 10 days after the heat treatment. The area under the curves was used to assess the duration of chlorophyll retention: it was not significantly reduced by heat stress in Frame and Halberd but was reduced significantly in Goldmark (33% reduction) and in Meering (17%).
The effect of a period of high temperature stress imposed 10 days after anthesis on changes in the chlorophyll content of the flag leaf of four varieties of bread wheat. Plant were grown at 22°/16° C (black circle) or exposed to 40°/20°C (black square) for 3 days. The period of heat stress is indicated by the horizontal bar. Significant differences are shown by an asterisk
Initially, leaf chlorophyll content was significantly lower in plants grown under low Zn supply, but later, senescence was significantly delayed (Fig. 2). Consequently there was no significant effect of Zn treatment on the duration of chlorophyll retention. The only time when there was a significant Zinc × Heat treatment interaction was immediately after the end of the heat treatment, 13 days after anthesis, when the reduction in leaf chlorophyll content due to high temperature was significantly greater (17% reduction) under low zinc supply than under adequate zinc supply (10%).
The changes in leaf chlorophyll content for plants grown under low (white circle) or high Zn supply (black circle). Values are the means of the Zn main effects averaged over variety and heat treatments. Significant differences between Zn treatments are indicated as asterisks (P < 0.05) or + (P < 0.10)
Chlorophyll fluorescence
Zinc deficiency did not affect the chlorophyll fluorescence ratio (Fv/Fm) prior to exposure to heat stress and all varieties showed a similar value. The Fv/Fm values decreased sharply within 24 h. of exposure to heat stress followed by a slower reduction or, in the case of Frame, no further decline until the end of the heat stress treatment (Fig. 3). Meering showed the greatest reduction and Halberd the least. Zinc deficiency exacerbated the decline in all varieties except Meering. Once the heat stress was removed, there was a rapid recovery in all varieties to values similar to or greater than that of the control. Zinc nutrition did not affect the recovery.
The effect of a period of high temperature stress imposed 10 days after anthesis on changes in Fv/Fm of the flag leaf of four varieties of bread wheat grown at two levels of soil Zn. Plants were grown with 0.2 mg Zn kg−1 (white circle, white square) or 2 mg Zn kg−1 (black circle, black square) and maintained at 22°/16°C (black circle, white circle) or exposed to 40°/20°C (black square, white square) for 3 days. The period of heat stress is indicated by the horizontal bar. Error bars are the LSD (P = 0.05)
Heat stress increased initial fluorescence (F0) and reduced the maximum fluorescence (Fm) and the values recovered to the control levels once exposure to high temperatures ceased (data not presented). The effects of Zn deficiency on F0 and Fm were small and non-significant.
Grain yield and kernel growth
While the trends in the effects of heat stress and Zn treatment were similar in the main stem and tillers, the magnitude of the effects were smaller in the tillers. The timing and duration of the heat treatment was based on the commencement of anthesis on the main stem and therefore exposure to stress in the tillers would have been different. Therefore, the results will concentrate on the changes in the main stem rather than the whole plant.
Zinc deficiency reduced grain yield of the main stem by an average of 11% (1,067 mg c.f. 963 mg, P = 0.013) with no significant difference among genotypes in the effect of Zn. Heat stress at 10 DAA significantly reduced the grain yield in Goldmark and Meering by approximately 20% but did not affect the yield of Frame and Halberd (Table 1). Heat stress reduced grain yield by the same amount at both levels of Zn: at Zn0.2, yield was reduced by 10% from 1,005 mg plant−1 to 913 mg plant−1, while at Zn2, the reduction from heat stress was also 10%, from 1,129 mg plant−1 to 1,013 mg plant−1. Consequently, when the two stresses occurred together (heat stress at low Zn), the combined loss in yield was 20%, from 1,129 mg plant−1 to 913 mg plant−1.
Reducing the supply of Zn decreased the number of grains set in the main stem ear slightly, from 41.8 grains spike−1 to 38.9 grains spike−1 (P < 0.01). Exposure to 3 days of heat stress did not significantly affect the number of kernels set (41.1 c.f. 39.5 grains spike−1 at 22/15°C and 40/20°C respectively) so variation in yield due to heat stress was due to the effects of high temperature on kernel weight. Kernel weight was not reduced by heat stress in Frame and Halberd, but was reduced by 15%–18% in Goldmark and Meering. Zinc deficiency reduced average kernel weight in the main stem at maturity by 4% (26.2 mg at 20/15°C c.f 24.7 mg at 40/20°C) and the response was the same in both the control and heat stress treatments. As with grain yield, the responses to heat and Zn were additive and lowest kernel weight occurred in Zn-deficient plants exposed to heat stress.
Heat stress for 3 days slowed grain growth, but the response differed among the varieties and depended on the level of Zn nutrition (Fig. 4). The thermotolerance of Frame, Halberd and Goldmark was evident at the higher level of Zn but when the varieties were marginally Zn-deficient the sensitivity of kernel growth to temperature stress increased. Meering showed the greatest sensitivity to heat stress among the four varieties irrespective of the Zn supply.
The effect of heat stress imposed at 10 days after anthesis on the growth of the kernels on the main stem of four varieties of wheat grown at 0.2 mg Zn kg−1 (-Zinc) or 2 mg Zn kg−1 (+Zinc) . Plants were grown at 22/16°C (white circle) or exposed to 3 days of heat stress (40/20°C) for 3 days (black circle). The period of heat stress is indicated by the horizontal line
The initial effects of heat stress on kernel growth were examined by calculating the kernel growth rates at the end of the heat treatment, 13 DAA, from the slope of the fitted quadratic regression. Under Zn deficiency, heat stress reduced kernel growth rate by between 28% and 42%, but when the Zn supply was adequate the reductions in growth rate were between 3% and 14% (Table 2). The variation in kernel growth rate among the four genotypes during the period of heat stress were positively correlated with Fv/Fm, but the reduction in kernel growth rate was greater among the plants growing under low Zn (Fig. 5). At 20/16°C all Fv/Fm values were high and there was no association between chlorophyll fluorescence and kernel growth rates.
The relationships between the mean Fv/Fm and kernel growth rate during the 3-day period of heat stress among four varieties of wheat. The plants were grown at 0.2 mg kg−1 Zn (white circle, white square) or 2 mg kg−1 Zn (black circle, black square) at either 20/16°C (white circle, black circle) or 40/20°C (white square, black square)
Grain Zn concentration
Adding Zn increased grain Zn from 13.1 mg kg−1 to 45.4 mg kg−1 on average, and the heat stress treatment did not affect this response (Zinc × Heat stress interaction non-significant). There were significant differences in grain Zn among the genotypes, but the differences depended on the Zn treatment (Genotype × Zn interaction P < 0.001). Without additional Zn Halberd had the highest grain Zn concentration (15.6 mg kg−1) and this was significantly greater than the remaining three varieties, which had similar grain Zn concentrations—Goldmark (13.0 mg kg−1), Frame (12.5 mg kg−1) and Meering (11.1 mg kg−1; LSD = 2.85). When Zn was applied, greater differences were evident among the genotypes: Halberd again had the highest concentration (55.4 mg kg−1) but Goldmark (46.2 mg kg−1) had a significantly higher Zn concentration than Frame (40.2 mg kg−1) and Meering (39.8 mg kg−1; LSD = 2.85). The heat stress treatment did not affect this response (Heat stress × Genotype × Zn interaction non-significant). There was an inconsistent response in grain Zn concentration to heat stress among the four genotypes with Goldmark showing a significant increase, Halberd a significant decrease and Frame and Meering showing no significant change in grain Zn concentration with high temperature (Table 1).
Experiment 2
Growth prior to exposure to heat stress
At 28 DAS all genotypes showed a similar response to the application of Zn (Genotype × Zinc interaction non-significant) with shoot biomass increasing up to 2 mg Zn kg−1 before declining at 20 mg Zn kg−1 (Table 3). Subsequent tissue analysis indicated that the magnesium (Mg) and sulphur (S) concentrations of plants grown at 20 mg Zn kg−1 were close to their critical levels (see below) which may have contributed to the reduction in growth at the highest Zn concentration. Halberd produced the largest seedlings (mean shoot dry matter = 476 mg plant−1) while the other genotypes produced similar biomass (365–375 mg plant−1).
Nutrient concentrations of the YEBs
Heat stress increased the Zn concentration in the YEBs at Zn2 and Zn20 in all varieties except Goldmark, but had no effect on Zn concentration at Zn0 or Zn0.2 (Fig. 6). The greatest increase in Zn concentration was measured in Frame. Adding Zn significantly reduced the concentrations of a number of other nutrients, the most notable of which were Mg and S where the concentrations at Zn20 were reduced to values close to the critical values for deficiency. Magnesium was reduced from 2,070 mg kg−1 (Zn0) to 1,490 mg kg−1 (Zn0.2, and Zn2) and 1,270 mg kg−1 (Zn20). Sulphur concentrations were 3,050 mg kg−1 (Zn0), 2,500 mg kg−1 (Zn0.2, and Zn2) and 2,400 mg kg−1 (Zn20). The concentrations of all other nutrients were within the adequate range for growth.
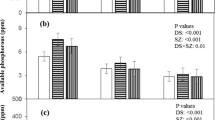
The effect of soil zinc concentration on the Zn concentration of the youngest emerged leaf blades of four genotypes of wheat grown at 20/15°C (black circle) or exposed to 40/20°C (black square) for 6 days. Leaves were sampled at the completion of the period of heat stress. The bars are the LSD (P = 0.05) for (a) comparisons within temperature treatments and (b) other comparisons
Chlorophyll content
High temperature significantly reduced the chlorophyll content in the YEBs of Goldmark (20%) and Meering (10%) but not in Frame and Halberd (Table 4). Chlorophyll content was significantly lower at Zn0.2 and Zn20 (Table 3). There was no significant interaction between heat stress and Zn treatment on chlorophyll content.
Chlorophyll fluorescence
There was an immediate decline in Fv/Fm when plants were exposed to high temperature, followed by a slow recovery until the heat stress was removed (Fig. 7). There was no significant difference between varieties in the response to heat stress. No evidence of permanent injury due to heat stress was evident because the plants recovered to the control level within 24 h after the heat stress ended.
Changes in fluorescence ratio (Fv/Fm), initial fluorescence (F0) and maximum fluorescence (Fm) in wheat grown at different levels of soil Zn and exposed to high temperature stress (40/20°C) for 6 days (open symbols) or maintained at 22/16°C (closed symbols). The period of heat stress is indicated by the horizontal bar. Error bars are the LSD (P = 0.05). The Zn treatments are 0 mg Zn kg−1 (black circle, white circle) 0.2 mg Zn kg−1 (black square, white square), 2 mg Zn kg−1 (black diamond, white diamond), and 20 mg Zn kg−1 (black triangle, white triangle)
Zn fertilisation affected the Fv/Fm ratio of the heat-treated plants during the first 4 days of the heat stress period (Temperature × Zinc interaction significant, P < 0.05), with no difference in the response among the varieties. The reduction in Fv/Fm was least at Zn2 and Zn0 and greatest at Zn0.2 and Zn20, and this difference was maintained during recovery from heat stress. The effects of Zn treatment on Fv/Fm mirrored those observed in chlorophyll content (Table 3). Measurements of Fv/Fm of plants maintained at 20/16°C were not influenced by Zn treatment.
Initial fluorescence (F0) increased immediately with heat stress and the magnitude of the response depended on the Zn treatment (Fig. 4). The greatest increases occurred at Zn0.2 and Zn20, while the smallest change occurred in Zn2. Leaves recovered quickly and were similar to the control values after 3–4 days. The decline in Fm with heat stress was also affected by the level of Zn nutrition, but there was little recovery in Fm until the heat stress was removed.
Shoot growth
Shoot growth was significantly affected by heat stress and Zn supply, with no interaction between the two. The effect of heat stress differed among the varieties: it significantly reduced shoot dry matter in Halberd by 15% but had no significant effect on the other genotypes (Table 4). Growth rate was reduced by heat stress, with Halberd (25% reduction) and Meering (21%) showing significant reductions and Frame and Goldmark unaffected (Table 3).
Applying Zn up to 2 mg Zn kg−1 increased shoot dry matter production and growth rates increased but it then declined at 20 mg Zn kg−1 (Table 3). There was no significant difference between varieties or a Heat stress × Zinc interaction.
CuZnSOD activity
There was no effect of Zn treatment on Mn SOD (data not presented) and the variation in total SOD activity was caused by the changes in CuZnSOD activity. At 28 DAS activity of CuZnSOD increased with the level of Zn nutrition (Table 5) and the mean activity of Frame was significantly greater than Goldmark (21.1 vs. 12.8 units g−1 FW, P < 0.01). The higher SOD activity with increased Zn supply was maintained at 31 DAS. Heat stress significantly increased SOD activity in plants that were grown at the two lowest levels of Zn, but not in plants grown at 2 mg Zn kg−1 or 20 mg Zn kg−1. There was no significant difference in SOD activity between Frame (16.0 units g−1 FW) and Goldmark (14.4 units g−1 FW).
At 39 DAS, 3 days after the heat stress treatment, CuZnSOD activity in the control plants increased as Zn supply increased from 0.2 to 20 mg Zn kg−1, but surprisingly, the CuZnSOD activity at 0 mg Zn kg−1 was high, and not significantly different to that measured at 2 and 20 mg Zn kg −1. The CuZnSOD activity in the heat stressed also increased with Zn supply and they were also significantly lower than the control at 0 mg Zn kg−1 and 0.2 mg Zn kg−1. Activity declined between 31 DAS and 39 DAS in the plants exposed to heat stress and grown at 0 mg Zn kg−1 and 0.2 mg Zn kg−1, whereas in the control plants CuZnSOD activity increased.
Chloroplast ultra-structure
Both heat stress and Zn nutrition altered the ultrastructure of chloroplasts (Fig 8). The effects, summarised in Table 6, indicate that heat stress and Zn deficiency had slightly different effects on the type of damage observed. However, when both stresses occurred together, the severity of damage increased markedly.
Transmission electron micrographs of wheat mesophyll chloroplasts at 33 DAS, from two varieties grown at 22/16°C (A-D) or 40/20°C (E-H) (×19,000). a, e Frame, supplied with adequate Zn (2 mg kg−1 soil); b, f Goldmark, supplied with adequate Zn (2 mg kg−1 soil); (c, g) Frame grown with no supplementary Zn (0 mg kg−1 soil); d, h Goldmark grown with no supplementary Zn (0 mg kg−1 soil). Abbreviations: cw = cell wall; st = starch grains; g = grana stacks; t = tonoplast; p = plastoglobuli; arrow indicates ruptured chloroplast envelope
Mesophyll cell chloroplasts from plants grown with adequate Zn fertilisation at 22/16°C were well developed, with distinct chloroplast envelope membranes (Fig 8a, b). Numerous grana and stromal thylakoid membranes were distributed evenly throughout the granular stromal matrix, and starch grains were also obvious within the stroma. Thylakoid granum stacks lay parallel to the longitudinal axis of these chloroplasts and small, electron-dense, plastoglobuli were apparent within the stroma
Chloroplasts from Zn deficient plants at 22/16°C were swollen (Fig. 8c, d) and in many cases the envelope had ruptured completely, allowing leakage of the stroma contents into the cytoplasm. There was an increase in the number of plastoglobuli within the stroma of these chloroplasts, as well as evidence of a reduced number of thylakoids per granum stack. Furthermore many grana had undergone a change in spatial orientation, becoming perpendicular or at an angle to the longitudinal axis of the chloroplast. Goldmark, had a greater number of ruptured chloroplasts than Frame. There was also evidence of swelling of the thylakoid membranes within the chloroplasts of Goldmark, resulting in an increase in the volume of the intrathylakoid space.
When subject to heat stress but grown at adequate level of Zn, the thylakoid membranes of both varieties were swollen and the intrathylakoid spaces increased (Fig. 8e, f). Large plastoglobuli were present within the stroma, and the starch grains within the chloroplasts were smaller and fewer compared with those maintained at 22/16°C. The number of thylakoids per granum stack had increased, and many grana were irregular in shape. Both Frame and Goldmark exhibited similar abnormalities in response to heat stress.
Chloroplasts from wheat plants grown under both Zn deficiency and high temperature stress suffered a much greater level of damage than those subjected to either stress independently (Fig. 8g, h). These chloroplasts were both swollen and ruptured and were virtually devoid of starch grains. The combined stresses resulted in thylakoids that were extremely swollen. The number of thylakoids per granum stack was increased, and these grana were highly irregular in shape and orientation. Very large, densely stained, plastoglobuli were evident throughout the stroma. The simultaneous stresses also caused some damage to other parts of the cell that were not obvious when each stress occurred alone. This damage included distortion of the cell wall (observed predominantly in the Zn-inefficient variety, Goldmark) and rupture of the tonoplast, which resulted in the cytoplasmic contents leaking into the vacuole. Goldmark exhibiting a greater degree of structural injury than Frame when subjected to both Zn deficiency and high temperature stress.
Discussion
Individually both Zn deficiency and high temperature stress can reduce growth and grain yield in cereals but the interaction between the two stresses has not been much studied. This work has provided evidence that some responses to heat stress can be modified by the severity of Zn stress and that adequate levels of Zn nutrition can alleviate the detrimental effects of high temperatures.
Grain yield of the main stem was reduced by a 3-day exposure to high temperature from 10 DAA in two of the four wheat varieties (Goldmark and Meering) due to a reduction in kernel weight. However, growing plants under a low supply of Zn increased the sensitivity of the developing kernel to heat stress, even in thermotolerant varieties (Fig. 3, Table 2). The two genotypes that showed the greatest reduction in kernel growth rate at Zn0.2 (Frame and Meering) also had the two lowest grain Zn concentrations (Table 1). The Zn concentrations of the kernels were low but not atypical of Zn concentrations commonly found in wheat crops in the region (McDonald 2006; Peck et al. 2008) and the plants were not showing visual symptoms of Zn deficiency. The responses observed in the current experiment suggest that adequate Zn nutrition of the developing grain can help to alleviate the detrimental effects of heat stress on grain growth and that this can occur in plants showing a marginal deficiency in Zn.
The reduction in kernel growth was observed some time after the stress had ended and it was not related to the observed changes in chlorophyll content of the flag leaf. This suggests the effects of the treatments on kernel weight were though changes within the grain rather that in the supply of photosynthate. This is supported by the data in Fig 5, which show that kernel growth rates were reduced by heat stress to a greater degree in Zn-deficient plants even when there were comparable reductions in chlorophyll fluorescence in the two Zn treatments. Moreover, the reduction in grain growth rates from heat stress at Zn0.2 was related to the grain Zn concentration among the four genotypes suggesting the Zn nutrition of the developing grain may influence its sensitivity to high temperatures. Previous studies on the effects of heat stress have concluded that the primary cause of low kernel weights from heat stress is a reduction in starch deposition due to fewer endosperm cell numbers and by heat inactivation of enzymes responsible for starch synthesis rather than due to changes in assimilate supply (Jenner et al. 1991, Stone and Nicolas 1995a). The responses observed in Experiment 1 are consistent with this. Heat stress was imposed early in grain development during cell division and the commencement of cell enlargement and starch synthesis. The nature of the response suggests that heat stress may have reduced kernel growth by limiting endosperm cell division (Stone and Nicolas 1995a) and that this effect was exacerbated by Zn deficiency. Without measurements of starch synthesis it is not known if the activity of key enzymes involved in starch synthesis were also affected by Zn treatment.
Despite the decline in the kernel growth rate from heat stress in Zn deficient plants, final kernel weight was not affected by heat stress in Frame and Halberd because of a decline in kernel weight as the grain matured. This phenomenon has been noted in a number of experiments but has not been satisfactorily explained (Jenner 1982). Nevertheless, the results suggest that the levels of grain Zn in crops marginally deficient in Zn may be inadequate to fully protect grain growth from heat stress.
There was also evidence that in vegetative tissue the level of Zn nutrition modified the sensitivity of PS II activity to heat stress. Zinc deficiency alone did not affect Fv/Fm significantly but it increased the reduction in Fv/Fm under heat stress. When exposed to high temperatures, there was an immediate reduction in Fv/Fm in both experiments and the decline was significantly greater in plants grown under marginally deficient levels of Zn (0.2 mg Zn kg−1 soil) than in plants adequately supplied with Zn (2 mg Zn kg−1 soil). The low values for Fv/Fm observed with Zn20 in Experiment 2 appeared to be caused by nutrient imbalances (particularly low Mg and S) associated with the high leaf Zn concentrations, rather than a direct effect of the high Zn concentrations. Interestingly, the reduction in Fv/Fm in the severely Zn deficient plants in Experiment 2 was significantly less than that observed in the moderately deficient plants and this was associated with a surprisingly high chlorophyll content at Zn0 (Table 3). The reason for the greater retention of chlorophyll and the smaller reduction in variable fluorescence in the severely Zn deficient plants is not immediately apparent.
Changes in Fv/Fm can reflect changes in chloroplast ultrastructure. Zinc deficiency damaged the structure of the chloroplasts and disrupted the structure of the thylakoids and greater damage occurred in the Zn-inefficient variety Goldmark. These changes are similar to those reported in leaves of Zn deficient plants previously (Brown et al. 1993; Chen et al. 2007). The severity of damage was increased with heat stress. Previous studies have examined the effects of Zn on chloroplast structure (eg Chen et al. 2007) or on chlorophyll fluorescence (eg Wang et al. 2009) but few studies have tried to link structural changes to changes in fluorescence. The remarkable observation from the present experiments was the apparent resilience in the activity of the PS II despite the significant amount of internal disruption that occurred within the chloroplasts. While Zn deficiency altered chloroplast ultrastructure, there was relatively little effect of Zn deficiency on Fv/Fm. Heat stress increased the deterioration of chloroplast structure and there was a significant reduction in Fv/Fm due to heat stress. However, chlorophyll fluorescence recovered quickly once the heat stress was removed. This occurred in leaves at two different stages of growth and when the length of the period of heat stress differed (3 days vs. 6 days). This indicates that plants may have effective repair mechanisms that allow them to cope with short periods of heat stress.
The ability to detoxify ROS generated during heat stress is an important mechanism to minimise membrane damage (Almeselmani et al. 2006; Cui et al. 2006). CuZnSOD is one member of a complex and coordinated array of enzymic and non-enzymic antioxidants that protect cells from oxidative stress. Production of SOD is the first response, converting the damaging superoxide radical to H2O2 (Cakmak 2000). However, the effect is transient: there is an initial increase in the activity of SOD upon first exposure to stress, followed by a decline because of reduced synthesis, enhanced degradation or inactivation of the enzymes (Cakmak and Marschner 1988; Yu et al. 1998, 1999; Cui et al. 2006). Based on the changes over time measured in plants exposed to heat stress, Zn deficiency compromised the ability to cope with heat stress in both these phases. Firstly, the activity of CuZnSOD in Zn-deficient plants was low immediately after exposure to heat stress (31 DAS) and secondly, the large decline in activity at 39 DAS suggests the ability to maintain CuZnSOD activity over a prolonged period was reduced in Zn-deficient plants. When grown at either Zn2 or Zn20, CuZnSOD activity was high and more stable over time during the period of heat stress and during the recovery.
The high SOD activity in leaves of Zn deficient plants in the Control treatment at 39 DAS was unexpected and is in stark contrast to the low SOD activity under heat stress. This apparent anomalous result may have been caused by the combined, but opposite, effects of the two stresses—heat stress and Zn deficiency—on SOD activity. While low CuZnSOD activity has been often reported under Zn deficiency (Cakmak and Marschner 1988; Cakmak 2000; Wang and Jin 2005; Chen et al. 2007), so have increases in CuZnSOD activity (Yu et al. 1999; Frei et al. 2010). The relative high value at Zn0 may provide another example of such an increase. There was also a trend for CuZnSOD activity in the Control plants to increase between 31 DAS and 39 DAS. This increase was not observed under heat stress. The photo-oxidative damage which results from heat stress causes an accumulation of H2O2, which can inactivate CuZnSOD (Cakmak 2000). The difference in the changes in activity in CuZnSOD between the Heat stress and Control may reflect differences in the severity of oxidative stress, the accumulation of H2O2 and the deactivation of CuZnSOD. Therefore the high values at Zn0 in the Control plants may reflect, firstly, an enhanced activity at low Zn and secondly an inactivation of CuZnSOD under heat stress.
Despite the significant changes in SOD activity there was not a consistent association with changes in chloroplast structure and chlorophyll fluorescence. Zinc deficiency damaged the structure of the chloroplast and greater damage was measured in Goldmark and after a period of heat stress. At 28 DAS the SOD activity of Goldmark was significantly less than that of Frame (12.8 vs. 21.1 units g−1 FW, P < 0.01) suggesting that it was potentially less able to detoxify ROS. Similarly, the initial decline in SOD activity under Zn deficiency may have also contributed to the observed damage to the chloroplasts. However, other measurements do not support a prolonged effect of SOD activity on chloroplast structure: the difference between Frame and Goldmark diminished over time and was not significantly at 31 DAS and 39 DAS, and there was an increase in SOD activity in the Control treatments after the end of the heat stress treatment, despite significant deterioration in chloroplast structure. These results suggest either (i) the structural damage measured at 39 DAS was affected by the Zn and heat treatments early (28–31 DAS) and this early protective role of Zn is critical to maintaining chloroplast structure over a longer period of time, or (ii) SOD activity alone does not play a critical role in maintaining chloroplast structure. Without earlier observations of chloroplasts structure (at 28 and 31 DAS) concurrent with measurements of SOD, this question can’t be resolved easily. However, based on observations in Zn efficient and inefficient genotypes of rice, Frei et al (2010) argued that enzymic detoxification of ROS is more a general stress response rather than a mechanism explaining differences in tolerance.
Similarly, chlorophyll fluorescence, which reflects chloroplast function and the integrity of the thylakoid membranes, was not consistently related to the Zn treatments. The fluorescence data for Zn20 in Experiment 2 were likely to have been affected by the low Mg and S concentrations and should be discounted in the discussion of the effect of Zn. In both experiments the parameters of chlorophyll fluorescence (F0, Fm and Fv/Fm) in plants grown at Zn0.2 were more sensitive to heat stress than those grown at Zn2. Under heat stress, plants grown at Zn2 had a higher Fv/Fm and a lower F0, which indicates reduced lipid fluidity of the thylakoid membrane (Armond et al 1980; Sundby et al. 1986), and a slightly higher Fm, which suggests less damage to the chlorophyll-protein complexes (Yamane et al. 1997). Both these results are consistent with the greater damage to chlorophyll structure at low Zn and thus supporting a protective role of adequate supplies of Zn. However, plants severely Zn deficient (Zn0) in Experiment 2 did not show the greatest sensitivity to heat stress with their fluorescence values being less affected than marginally-deficient plants (Zn0.2). Surprisingly, plants grown at Zn0 also had a high chlorophyll content (Table 3), which would enhance fluorescence characteristics, but the reason for this improvement in chlorophyll retention is not immediately apparent. Therefore, in both experiments plants marginally deficient in Zn (i.e. Zn0.2) showed greater sensitivity to heat than plants grown with adequate supplies of Zn (Zn2), which is consistent with previous reports of Zn deficiency on chlorophyll fluorescence(Wang et al. 2009) and also with the changes in chloroplast structure. The apparently anomalous result with Zn0 in the second experiment needs verification.
Varieties were selected that varied in their Zn efficiency to examine whether genetic differences in efficiency may affect tolerance to heat stress. The results were equivocal partly because there were only small differences in growth responses to Zn among the four varieties in both experiments. Goldmark, a Zn-inefficient genotype showed a greater loss in yield and kernel weight from heat stress in Experiment 1 and a greater loss in leaf chlorophyll content in both experiments than the more efficient genotypes Frame and Halberd (Tables 1, 2). However, the losses were similar to Meering which is also Zn efficient but is thermosensitive. Compared to the Zn efficient variety Frame, Goldmark had a similar CuZnSOD activity in Experiment 2 although there was a greater degree of chloroplast damage. Therefore, the initial difference in CuZnSOD between the two genotypes did not appear to afford different levels of protection to the chloroplasts from the effects of heat stress. Based on the results of the current experiments it would seem that Zn efficiency has little effect on thermotolerance.
In conclusion, the experiments provide evidence that plants poorly supplied with Zn may show greater sensitivity to heat stress than plants adequately supplied with Zn. The effect was observed in both PSII activity and in kernel growth but the responses in chlorophyll fluorescence were transitory. The responses in biomass production and yield to heat stress and to Zn deficiency were largely additive effects, but they nevertheless demonstrated that low supplies of Zn can exacerbate the adverse effects of heat stress. Significant effects occurred in plants not displaying visual symptoms of Zn deficiency and so crops suffering from ‘hidden hunger’ may be at risk from additional losses of productivity from heat stress. The ability of plants supplied with an adequate supply of Zn to maintain grain growth during heat stress appeared to be associated with factors within the grain rather than retention of chlorophyll and maintenance of the integrity of PSII Chloroplast ultrastructure was sensitive to both heat stress and Zn deficiency but chlorophyll fluorescence showed a high level of resilience to both stresses. The effect was not related to the Zn efficiency of the genotype.
References
Ainsworth EA, Rogers A, Leakey ADB (2008) Targets for crop biotechnology in a future high-CO2 and high-O3 world. Plant Physiol 147:13–19
Al-Khatib K, Paulsen GM (1990) Photosynthesis and productivity during high-temperature stress of wheat genotypes from major world regions. Crop Sci 30:1127–1132
Alloway BJ (2008) Zinc in soils and crop nutrition. International Zinc, Brussels
Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP (2006) Protective role of antioxidant enzymes under high temperature stress. Plant Sci 171:382–388
Armond PA, Björkman O, Staehelin LA (1980) Dissociation of supramolecular complexes in chloroplast membranes: a manifestation of heat damage to the photosynthetic apparatus. Biochim Biophys Acta 601:433–442
Barnabas B, Jager K, Feher A (2008) The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ 31:11–38
Berry J, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31:491–543
Brown PH, Cakmak I, Zhang Q (1993) Form and function of zinc in plants. In: Robson AD (ed) Zinc in soil and plants. Kluwer Academic, Dordrecht, pp 93–106
Cakmak I (2000) Tansley Review No. 111: possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol 146:185–205
Cakmak I, Marschner H (1988) Increase in membrane permeability and exudation in roots of zinc deficient plants. J Plant Physiol 132:356–361
Chen W, Yang X, He Z, Feng Y, Hu F (2007) Differential changes in photosynthetic capacity, 77K chlorophyll fluorescence and chloroplast ultrastructure between Zn-efficient and Zn-inefficient rice genotypes (Oryza sativa) under low zinc stress. Physiol Plant 132:89–101
Cui LJ, Li JL, Fan YM, Xu S, Zhang Z (2006) High temperature effects on photosynthesis, PSII functionality and antioxidant activity of two Festuca arundinacea cultivars with different heat susceptibility. Bot Stud 47:61–69
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Easterling WE, Aggarwal PK, Batima P, Brander KM, Erda L, Howden SM, Kirilenko A, Morton J, Soussana J-F, Schmidhuber J, Tubiello FN (2007) Food, fibre and forest products. In: Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE (eds) Climate change 2007: impacts, adaptation and vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK
Foyer CH, Harbinson J (1994) Oxygen metabolism and the regulation of photosynthetic electron transport. In: Foyer CH, Mullineaux P (eds) Causes of photooxidative stresses and amelioration of defense in plants. CRC, Boca Raton, pp 1–42
Frei M, Wang Y, Ismail AM, Wissuwa M (2010) Biochemical factors conferring shoot tolerance to oxidative stress in rice grown in low zinc soil. Funct Plant Biol 37:74–84
Giannopolitis CN, Ries SK (1977) Superoxide dismutases. I. Occurrence in higher plants. Plant Physiology 59:309–314
Graham RD (1984) Breeding for nutritional characteristics in cereals. Adv Plant Nutr 1:57–102
Graham AW (2004) Effects of zinc nutrition and high temperature on the growth, yield and grain quality of wheat (Triticum aestivum L.), PhD, School of Agriculture, Food and Wine, The University of Adelaide. 297 p
Jenner CF (1982) Storage of starch. In: Loewus FA, Tanner W (eds) Encyclopedia of plant physiology. Springer, Berlin, pp 700–747
Jenner CG, Ugalde TD, Aspinal D (1991) The physiology of starch and protein deposition in the endosperm of wheat. Aust J Plant Physiol 18:211–226
Liu X, Huang B (2000) Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci 40:503–510
McCain DC, Croxdale J, Markley JL (1989) Thermal damage to chloroplast envelope membranes. Plant Physiol 90:606–609
McDonald GK (2006) The effects of soil properties on variation in plant growth, grain yield and nutrient uptake. Aust J Exp Agric 46:93–105
McDonald GK, Graham RD, Lloyd J, Lewis J, Lonergan P, Khabas-Saberi H (2001) Breeding for improved zinc and manganese efficiency in wheat and barley. In: Rowe B (ed) 10th Australian Agronomy Conference, Hobart
McIntosh MS (1983) Analysis of combined experiments. Agron J 75:153–155
Paulsen GM (1994) High temperature response of crop plants. In: Boote KJ, Bennett JM, Sinclair TM, Paulsen GM (eds) Physiology and determination of crop yield. American Society of Agronomy, Crop science Society of America and Soil Science Society of America, Madison, pp 365–389
Peck AW, McDonald GK, Graham RD (2008) Zinc nutrition influences the protein composition of flour in bread wheat (Triticum aestivum L.). J Cereal Chem 47:266–274
Rashid A, Ryan J (2004) Micronutrient constraints to crop production in soils with Mediterranean-type characteristics: a review. J Plant Nutr 27:959–975
Reuter D, Robinson B (1997) Plant analysis: an interpretation manual. CSIRO, Melbourne
Reynolds ES (1963) The use of lead citrate at high pH as an electron opaque-stain in electron microscopy. J Cell Biol 17:208–212
Sayed OH (2003) Chlorophyll fluorescence as a tool in cereal crop research. Photosynthetica 41:321–330
Stone PJ, Nicolas ME (1994) Wheat cultivars vary widely in their responses to grain yield and quality to short periods of post-anthesis heat stress. Aust J Plant Physiol 21:887–900
Stone PJ, Nicolas ME (1995a) Effect of timing of heat stress during grain filling on two wheat varieties differing in heat tolerance. I. Grain growth. Aust J Plant Physiol 22:927–934
Stone PJ, Nicolas ME (1995b) A survey of the effects of high temperature during grain filling on yield and quality of 75 wheat cultivars. Aust J Agric Sci 46:475–492
Sundby C, Melis A, Mäenpää P, Andersson B (1986) Temperature-dependent changes in the antenna size of Photosystem II. Reversible conversion of Photosystem IIα to Photosystem IIβ. Biochim Biophys Acta 851:475–483
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wang H, Jin JY (2005) Photosynthetic rate, chlorophyll fluorescence parameters, and lipid peroxidation of maize leaves as affected by zinc deficiency. Photosynthetica 43:591–596
Wang H, Liu RL, Jin JY (2009) Effects of zinc and soil moisture on photosynthetic rate and chlorophyll fluorescence parameters of maize. Biologogia Plantarum 53:191–194
Weigel HJ (1983) The effect of high temperatures on leaf cells of Valerianella: relative heat stability of the tonoplast membrane of mesophyll vacuoles. Planta 159:398–403
Wrigley CW, Blumenthal CS, Gras PW, Barlow EWR (1994) Temperature variation during grainfilling and changes in wheat-grain quality. Aust J Plant Physiol 21:875–885
Yamane Y, Kashino Y, Koike H, Satoh K (1997) Increases in the fluorescence Fo level and reversible inhibition of Photosystem II reaction center by high-temperature treatments in higher plants. Photosynth Res 52:57–64
Yu Q, Osborne L, Rengel Z (1998) Micronutrient deficiency changes activities of superoxide dismutase and ascorbate peroxidase in tobacco plants. J Plant Nutr 21:1421–1437
Yu Q, Worth C, Rengel Z (1999) Using capillary electrophoresis to measure Cu/Zn superoxide dismutase concentration in leaves of wheat genotypes differing in tolerance to zinc deficiency. Plant Sci 143:231–239
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421
Zarcinas BA, Cartwright B, Spouncer LR (1987) Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Commun Soil Sci Plant Anal 18:131–146
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ismail Cakmak.
Rights and permissions
About this article
Cite this article
Peck, A.W., McDonald, G.K. Adequate zinc nutrition alleviates the adverse effects of heat stress in bread wheat. Plant Soil 337, 355–374 (2010). https://doi.org/10.1007/s11104-010-0532-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-010-0532-x