Abstract
With the widespread use of metal oxide nanoparticles (MNPs), agricultural soil is gradually becoming a primary sink for MNPs. The effect of these nanoparticles on the fate and the toxicity of co-existing heavy metals is largely unknown. In this paper, pot experiments were conducted to evaluate the impact of ZnO nanoparticles (ZnO-NPs) on Cd toxicity and bioaccumulation in a soil-rice system. Different amounts of ZnO-NPs were added to three different levels of Cd-contaminated paddy soil (L-Cd, 1.0 mg kg−1; M-Cd, 2.5 mg kg−1; H-Cd, 5.0 mg kg−1). The results showed that the addition of ZnO-NPs significantly increased the soil pH value, and the soil pH value increased with the increase in ZnO-NP concentration. Reductions in plant height and biomass under Cd stress were recovered and increased after the addition of ZnO-NPs; the addition of ZnO-NP promoted rice biomass increased by 13~22% and 25~43% in the M-Cd and H-Cd groups, respectively, compared with that of the respective control treatment. A high concentration of ZnO-NPs could increase the concentration of bioavailable Cd in rhizosphere soil. In the L-Cd group, the Cd concentration of the rice in the L-Z500 treatment increased to 0.51 mg kg−1, exceeding the limit for acceptable Cd concentrations in rice of China (0.2 mg kg−1). This work revealed that ZnO-NPs could improve plant growth, especially in the early-growth stage, and alleviate the toxic effects of Cd. However, the addition of high-concentration (500 mg kg−1) ZnO-NPs in the lower Cd pollution soil could significantly facilitate the accumulation of Cd by Oryza sativa L.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid development of nanotechnology, the emission of nanoparticles (NPs) in environment is increasing. Nanoparticles will inevitably enter the soil and water in the process of scientific research, transportation, production, and waste treatment (Fan et al. 2017; Wang et al. 2016). In recent years, the environmental fate and ecotoxicity of nanoparticles have attracted extensive attention (Li et al. 2016; Gao et al. 2015). Previous studies have shown that nanoparticles could affect plant growth, soil enzyme activities, soil microbial biomass, and soil microbial community structure after nanoparticles enter the soil (Priester et al. 2012; Peng et al. 2017).
Zinc oxide nanoparticles (ZnO-NPs) were common metal oxide nanoparticles (MNPs) and widely used in personal care products, paints, and coating. Several previous studies have shown that ZnO-NPs have negative effects on plant growth (López-moreno et al. 2010; Lin and Xing 2008; Du et al. 2011). For example, ZnO-NP concentration of 1000 mg L−1 could reduce the root length of corn and cucumber by 17% and 51%, respectively (Zhang et al. 2015). In the seed incubation process, ZnO-NP concentration of 2000 mg L−1 could inhibit plant seed germination (radish, rape, ryegrass, lettuce, corn, and cucumber) and terminate root elongation (Lin and Xing 2007).
Due to their high reactivity, large specific surface area, and strong adsorption capacity, NPs can influence the fate and transport of co-existing contaminants (such as pesticides, heavy metals, and toxic organics), which may subsequently alter bioavailability and toxicity (Chai et al. 2013; Cai et al. 2017; Deng et al. 2017; Yang and Xing 2010; Glomstad et al. 2016). Several studies have shown that NPs could affect the co-existing contaminant uptake and accumulation in plants (like rice, wheat, cucumber, and collard greens) (Chai et al. 2013; Deng et al. 2017; Ma et al. 2017). However, most of these studies were conducted in the hydroponic condition or in a simple medium with short exposure and high doses (Chai et al. 2013; Cai et al. 2017; Hu et al. 2014; Ji et al. 2017); few studies have examined the soil condition for the whole life cycle of a plant. For example, a 10-day hydroponic experiment demonstrated that TiO2-NPs could alleviate Cd toxicity in terms of plant height, biomass, and root length and could lower the Cd content in rice roots and leaves (Ji et al. 2017). Similarly, Singh and Lee (2016) also found that the application of TiO2-NPs could increase photosynthetic rate and promote soybean plant growth, which indicated that the addition of TiO2-NPs could restricted the Cd toxicity in soybean plants in the 60-day pot experiment; however, the Cd concentration in soybean plants was also increased with the TiO2-NP concentration in soil. Thus, the investigation of the effect of NPs on co-existing contaminant behavior under soil conditions is urgently needed.
Heavy metal pollution in farmland is a worldwide environmental issue due to its harmful impacts on plants and food quality. Cd is considered to be the most toxicity heavy metal due to its high water solubility, bioavailability, and toxicity. The toxicity effects of Cd on plants have been extensively studied, and the symptoms of Cd toxicity in plants include growth inhibition and the disruption of physiological process (Nwugo and Huerta 2008). Several studies have shown that Cd could inhibit seed germination and root elongation and decrease the activities of some enzymes (Ali et al. 2014a). Cd could also disturb the metabolic process and uptake of nutrient elements in plants (Najeeb et al. 2011). Rice (Oryza sativa L.) is one of the major crops grown worldwide for human consumption, and Cd pollution in the paddy soil has become an increasing concern for food safety. After Cd was discharged into the paddy soil, it could easily uptake and bioaccumulate in rice, enters the food chains, and causes serious human health problem (Ali et al. 2015).
The current study focused on co-contamination between ZnO-NPs and Cd in a paddy soil. In this study, different doses of ZnO-NPs (50/100/500 mg kg−1) were added to three levels of the Cd-contaminated paddy soil (1.0/2.5/5.0 mg kg−1) in a pot experiment to assess (1) the impact of ZnO-NPs on Cd phytotoxicity during the whole life cycle and (2) the effect of ZnO-NPs on Cd uptake and bioaccumulation in soil-rice system. The results of this study will further our understanding on the influence of nanoparticles on co-existing contaminant behavior in agricultural soil.
Materials and methods
Soil and nanoparticle characterization
The soil used in this experiment was taken from a paddy field (0–30 cm) in Huizhou City, Guangdong Province, China. Soil samples were air-dried and passed through a 2-mm sieve, and the physical and chemical properties of the soil are shown in Table 1.
ZnO-NPs were purchased from Nanjing XFNANO Materials Tech Co., Ltd., with a purity of > 99%. The size of the ZnO-NPs (30–40 nm) was measured using field emission scanning electron microscope (ZEISS SUPRA® 55), and the specific surface area of ZnO-NPs (77.4 m2/g) was measured using Accelerated Surface Area and Porosimetry System (ASAP 2020 HD88).
Experimental design
There were three Cd stress treatments (L-Cd, 1.0 mg kg−1; M-Cd, 2.5 mg kg−1; H-Cd, 5.0 mg kg−1), as well as four ZnO-NP treatments (Z0, no ZnO-NP addition; Z50, 50 mg kg−1; Z100, 100 mg kg−1; Z500, 500 mg kg−1). The treatment concentrations were based on previous studies, in which different concentrations of ZnO-NPs, TiO2-NPs, and multi-walled carbon nanotubes were added in Cd-polluted soil. ZnO-NP concentrations of 50, 100, and 500 mg kg−1 significantly reduced the bioavailability of Cd in soil (Ye et al. 2018). A total of 12 treatments (Table 2) were conducted, and each treatment was conducted in triplicate.
CdCl2 (CdCl2·5H2O solution) was mixed thoroughly with the soil samples; then, the soil was equilibrated for 90 days and air-dried before being mixed with ZnO-NPs. ZnO-NPs were added as powder into the air-dried soil for each treatment, and the mixtures were stabilized for 24 h before planting (Xu et al. 2015; Priester et al. 2012). Each mixture was placed into a nylon net bag (5 cm diameter, 10 cm height, pore size 37 μm) and then placed in a polyvinyl chloride (PVC) container (15 cm diameter × 30 cm height).
Rice (Oryza sativa L.) seeds were obtained from the Rice Research Institute, Guangdong Academy of Agricultural Science, Guangzhou, China. The specific methods of seed culture were described in previous studies (Shi et al. 2014; Peng et al. 2015). Uniform rice seedlings (8 cm height) were transplanted into the nylon net bag. Two seedlings were planted per pot. The pot culture was conducted in a greenhouse with natural light. Plants were irrigated with deionized water every day to keep the depth of the water layer 3 cm above the soil surface. An alternating drying-wetting pattern was adopted following the booting stage (Peng et al. 2017). The fertilizers (200 mg CO (NH2)2 kg−1, 200 mg KH2PO4 kg−1) were added three times during the growing stage. The entire duration of the pot culture was 4 months.
Plant heights and tillering numbers were measured 30, 60, and 90 days after transplant, which was at the tillering stage, booting stage, and fruiting stage, respectively. After harvest, the rice plants were separated into their roots, shoots, and ears, and all plant samples were washed in tap water and then rinsed three times with distilled water. Samples were dried at 40 °C to a constant weight, and the plant biomasses were measured. Then, the dried plant samples were ground and passed through a 2-mm sieve. Rhizosphere soil and non-rhizosphere soil samples were collected from all treatments, air-dried, and passed through a 2-mm sieve.
Chemical analysis
Soil pH values were measured using a pH meter (PHS-3C) (water-to-solid ratio = 2.5:1). For determination of the total content of heavy metals, soil samples were digested with a mixture of HCl-HNO3-HF-HClO4 (10:15:10:5, v/v) (Zhang et al. 2016). Plant samples were digested with a mixture of HNO3-HClO4 (5:1, v/v) (Allen 1989). Bioavailable Cd was extracted with 0.11 ml L−1 of HOAc (Rauret et al. 1999). The concentration of heavy metals in the extractants was measured using inductively coupled plasma mass spectrometry (Agilent 7700x ICP-MS). All samples were measured in triplicate.
Statistical analysis
All the data were reported as the mean ± standard deviation (SD) of the triplicates for each treatment group. A one-way analysis of variance (ANOVA, IBM SPSS Statistics 20) followed by Tukey’s range test was applied to analyze the differences among various groups. Different letters indicate significant differences among the treatment means (P < 0.05).
Results
Soil pH
The pH values of the rhizosphere soil were significantly higher than those of the non-rhizosphere soil, the pH values of the non-rhizosphere soil ranged from 6.24 to 6.48, and the pH value of the rhizosphere soils ranged from 6.3–69 to 7.30 (Fig. 1).
As shown in Fig. 1, the addition of ZnO-NPs significantly increased the pH values of the rhizosphere and non-rhizosphere soil, and the pH values increased with the increasing ZnO-NP concentrations in the soil. The pH values of the rhizosphere soil in the L-Z500, M-Z500, and H-Z500 treatments increased by 0.59, 0.56, and 0.59, respectively, compared with those in the control treatment. The pH values of the non-rhizosphere soil in the L-Z500, M-Z500, and H-Z500 treatments increased by 0.19, 0.15, and 0.14, respectively, compared with those in the control treatment.
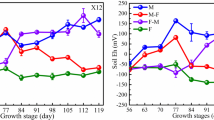
Plant height in different growth stages
As shown in Fig. 2a, plant heights in the M-Z0 and H-Z0 treatments were clearly lower than that in the L-Z0 treatment (P < 0.05), which indicated that Cd stress produced a significant inhibiting effect on plant height in the tillering stage.
In the tillering and booting stages, the plant heights increased with the increasing ZnO-NP concentrations in the soil. In the tillering stage, the plant heights in the L-Z50, L-Z100, and L-Z500 treatments increased by 4.22, 6.55, and 8.55 cm, respectively, compared with that in the L-Z0 treatment. In the M-Cd group, the plant heights in the M-Z50, M-Z100, and M-Z500 treatments increased by 8.17, 11.89, and 11.83 cm, respectively, compared with that in the M-Z0 treatment. In the H-Cd group, the plant heights in the H-Z50, H-Z100, and H-Z500 treatments increased by 11.61, 6.45, and 9.51 cm, respectively, compared with that in the H-Z0 treatment.
In the booting stage, the plant height in the L-Z500 treatment increased by 4.89 compared with that in the L-Z0 treatment; however, the plant height in the L-Z50 treatment decreased by 2.10 cm compared with that in the L-Z0 treatment (Fig. 2b). In the M-Cd group, the plant heights in the M-Z50, M-Z100, and M-Z500 treatments increased by 2.55, 7.11 and 5.56 cm, respectively, compared with that in the M-Z0 treatment. In the H-Cd group, the plant heights in the H-Z50, H-Z100, and H-Z500 treatments increased by 4.01, 6.01, and 4.67 cm, respectively, compared with that in the H-Z0 treatment.
With the ripe and harvest process, the differences of plant heights among the different ZnO-NP treatment concentrations gradually decreased and disappeared. In the fruiting stage, there was no clear difference in plant heights between the different ZnO-NP treatment concentrations (Fig. 2c).
Rice tillering
The plant tiller and productive tiller numbers across all the treatments are shown in Table 3. In the L-Cd group, there was no clear difference in the tiller number/productive tiller number between different ZnO-NP treatment concentrations.
In the M-Cd group, the tiller number and productive tiller number in the M-Z50 treatment increased by 19% and 28%, respectively, relative to those in the M-Z0 treatment, and the tiller number and productive tiller number in the M-Z500 treatment increased by 30% and 25%, respectively, compared with those in the M-Z0 treatment.
In the H-Cd group, the productive tiller number in the H-Z500 treatment increased by 44% compared with that in H-Z0 treatment.
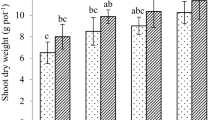
Plant biomass
Root biomass, shoot biomass, ear biomass, and total biomass across all the treatments are shown in Fig. 3. Root biomasses in the M-Z0 and H-Z0 treatments decreased by 22% and 27%, respectively, compared with that in the L-Z0 treatment (Fig. 3a), which indicated that Cd had a significant inhibiting effect on root biomass.
In the L-Cd group, there was no clear difference in the root biomasses between different ZnO-NP treatment concentrations. In the M-Cd group, the root biomasses in the M-Z50, M-Z100, and M-Z500 treatments increased by 51%, 18%, and 23%, respectively, compared with that in the M-Z0 treatment. In the H-Cd group, the root biomasses in the H-Z50, H-Z100, and H-Z500 treatments increased by 20%, 41%, and 12%, respectively, compared with that in the H-Z0 treatment. In the M-Cd and H-Cd groups, the addition of ZnO-NPs significantly increased the root biomass, which indicated that ZnO-NPs could alleviate Cd toxicity in terms of root biomass.
In the L-Cd group, there was no clear difference in the shoot biomasses between different ZnO-NP treatment concentrations (Fig. 3b). In the M-Cd group, the shoot biomass increased by 14~18% in the addition of ZnO-NP treatments compared with that in the M-Z0 treatment. In the H-Cd group, the shoot biomasses in the H-Z50, H-Z100, and H-Z500 treatments increased by 10%, 20%, and 32%, respectively, compared with that in the H-Z0 treatment, and the shoot biomasses increased with the increasing ZnO-NP concentrations in the soil.
As shown in Fig. 3c, the ear biomass in the H-Z0 treatment decreased by 31% compared with that in the L-Z0 treatment, which indicated that Cd significantly inhibited the ear biomass. In the L-Cd group, the ear biomass in the L-Z500 treatment increased by 32% compared with that in the L-Z0 treatment. In the H-Cd group, the ear biomasses in the H-Z50, H-Z100, and H-Z500 treatments increased by 50%, 81%, and 72%, respectively, compared with that in the H-Z0 treatment.
As shown in Fig. 3d, Cd significantly inhibited the total biomass; the total biomasses in the M-Z0 and H-Z0 treatments decreased by 10% and 23%, respectively, compared with that in the L-Z0 treatment. In the M-Cd group, the total biomasses in the M-Z50, M-Z100, and M-Z500 treatments increased by 22%, 18%, and 13%, respectively, compared with that in the H-Z0 treatment. In the H-Cd group, the total biomasses in the H-Z50, H-Z100, and H-Z500 treatments increased by 25%, 43%, and 42%, respectively, compared with that in the H-Z0 treatment. The positive effect of ZnO-NPs on rice biomass was more noticeable in the higher Cd stress group.
Bioavailable Cd concentration in the soil
The bioavailability of Cd in the soil had a large effect on the plant growth and Cd accumulation in plants. In the L-Cd group, the addition of ZnO-NPs had no significant influence on the concentration of bioavailable Cd in the non-rhizosphere soil. In the L-Z100 treatment, the concentration of bioavailable Cd in the rhizosphere soil increased by 10% relative to that in the L-Z0 treatment, and is obviously higher than that in the non-rhizosphere soil (Fig. 4a).
In the M-Cd group, the concentration of bioavailable Cd concentration in the rhizosphere soil increased by 9.0% and 14% for the M-Z50 and M-Z100 treatments, respectively, compared with that for the M-Z0 treatment (Fig. 4b). In the M-Z50 and M-Z100 treatments, the concentration of bioavailable Cd in the rhizosphere soil obviously higher than that of the non-rhizosphere soil.
In the H-Cd group, the concentration of bioavailable Cd in the rhizosphere soil increased by 6.8% for the H-Z100 treatment, compared with that for the M-Z0 treatment. However, the concentration of bioavailable Cd in the non-rhizosphere soil decreased by 6.6% for the H-Z50 treatment, compared with that for the M-Z0 treatment (Fig. 4c).
Cd concentrations in rice plants
As shown in Fig. 5, the Cd concentrations in the Oryza sativa L. tissues were ranked root > shoot > rice and increased as the Cd concentrations in the soil increased.
In the L-Cd and M-Cd groups, ZnO-NP concentrations of 50 and 100 mg kg−1 slightly decreased the Cd concentration in the roots (Fig. 5a). However, in the H-Cd group, the addition of ZnO-NPs increased the Cd concentration in the roots, a slightly increase in the concentration of Cd in the root was observed in the H-Z50 and H-Z100 treatments, and the Cd concentration in the root increased from 15.54 mg kg−1 for the H-Z0 treatment to 37.79 mg kg−1 for the H-Z500 treatment.
In the M-Cd group, ZnO-NP concentrations of 50 and 100 mg kg−1 significantly decreased the Cd concentration in the shoots (Fig. 5b). However, in the H-Cd group, the Cd concentration in the shoots increased by 1.96, 0.63, and 1.69 mg kg−1 for the H-Z50, H-Z100, and H-Z500 treatments, respectively, compared with that for the H-Z0 treatment.
The Cd concentration in rice in the ZnO-NP treatment concentration of 500 mg kg−1 was obviously higher than that in the ZnO-NP treatment concentrations of 50 and 100 mg kg−1 (Fig. 5c). In the L-Cd group, the Cd concentration of the rice in the L-Z500 treatment increased to 0.51 mg kg−1, exceeding the limit for acceptable Cd concentrations in rice of China (0.2 mg kg−1) (GB2762-2017). In the M-Cd group, the Cd concentration of the rice decreased to 0.13 and 0.16 mg kg−1 in the M-Z50 and M-Z100 treatments, below the limit for acceptable Cd concentrations in the rice of China. In the H-Cd group, the Cd concentration of the rice in the H-Z500 treatment increased by 0.08 mg kg−1 compared with that in the H-Z0 treatment.
Discussion
Cd could affect photosynthesis, inhibit enzymes, disrupt the uptake of nutrient elements, generate reactive oxygen species (ROS), and subsequently result in oxidative stress (Ji et al. 2017; Shah and Dubey 1995; Perfus-Barbeoch et al. 2002; Zhang et al. 2012; Ali et al. 2014b), all of which could decrease plant biomass (Ji et al. 2017). In the present study, Cd significantly inhibited rice growth; the plant height and biomass were significantly decreased with the increase in Cd concentration. In the tillering stage, plant height in the L-Z0 treatment was clearly higher than those in the M-Z0 and H-Z0 treatments.
The reduction of plant height and biomass under Cd stress was recovered and increased after the addition of ZnO-NPs, which indicated that ZnO-NPs could improve plant growth and alleviate the toxic effects of Cd. The addition of ZnO-NPs promoted rice biomass increased by 13~22% and 25~43% in the M-Cd and H-Cd groups, respectively. The positive effect was more noticeable in higher Cd stress treatments. Similarly, ZnO-NPs showed a positive effect on rice tillering, especially in high Cd stress. The ZnO nanoparticles would dissolve and release Zn2+ after addition into the soil (Wang et al. 2013; Laycock et al. 2017). As an essential trace element, adequate supplement of Zn2+ could improve plant growth. Higher concentration of ZnO-NPs was more effective than lower concentration in improving the plant height, biomass, and tillering. Cd and Zn were competing ions in soil-plant system due to their similar geochemical behavior in soil and the same uptake pathways in plants (Mo et al. 2017; De and Mark 2018). Zn played an important role in counteracting Cd toxicity in plants (Garg and Kaur 2013). Several studies have reported that Zn addition could reduce the uptake of Cd by root, consequently leading to a decrease of Cd accumulation in plants (Vasiliadou and Dordas 2009; Garg and Kaur 2013). The increase of Zn concentration in soil could inhibit Cd uptake at plasma membrane of root epidermal and cortical cells.
In this study, in the lower Cd stress (L-Cd and M-Cd treatment groups), ZnO-NP additions of 50 and 100 mg kg−1 slightly decreased the Cd concentration in roots and shoots. The Cd accumulation in shoots and grains of rice plants was determined by many physiological processes, such as root cell wall retention, extra transport of xylem, and phloem re-transport. Significant interactions could occur between Cd and Zn in their accumulation in plants. Cd and Zn may share a common transport system at root cell plasma membrane, and the competition for transport at the plasma membrane of root cells may be the basis for Zn inhibition of Cd uptake (Garg and Kaur 2013).
However, the addition of high-concentration (500 mg kg−1) ZnO-NPs obviously increased the Cd concentration in roots, shoots, and rice, and the effect was more noticeable in the higher Cd stress. Due to the same extranuclear electron structure, Zn and Cd compete with each other for the adsorption sites on the soil colloid, and this competition varied with the soil physical-chemical properties and the contents of Cd and Zn. The addition of high-concentration ZnO-NPs leads to a large amount of Zn2+ released to the soil, and Zn2+ could displace Cd2+ from soil absorption sites, which means that Zn might lead to increased Cd uptake by plants (Garg and Kaur 2013). In the present study, high concentration of ZnO-NPs increased the concentration of bioavailable Cd in the rhizosphere soil, and subsequently increased the Cd concentration in rice.
The bioconcentration factor (BCF) is used to calculate the distribution of heavy metals between the soil and biota, defined as (Fan et al. 2017; Mountouris et al. 2002):
where Cplant (mg kg−1) is the Cd concentration in the different tissues of Oryza sativa L., and Csoil (mg kg−1) is the Cd concentration in soil (1, 2.5, and 5 mg kg−1 in this study).
As shown in Fig. 6, the BCF was higher for the roots (3.11–10.68), followed by the shoots (0.27–0.67) and the rice (0.05–0.53), which indicated that Cd was enriched in the rice roots, and most of the Cd were retained in the roots. This is in accordance with many studies (De and Mark 2018; Garg and Kaur 2013); Cd retention in the roots is often regarded as a potential tolerance mechanism in minimizing Cd translocation to the shoots and grains. The BCF for the root was higher in the ZnO-NP treatment concentration of 500 mg kg−1 than in the treatment concentrations of 50 and 100 mg kg−1.
In the L-Cd group, the BCF for the rice in the L-Z500 treatment was obviously higher than that in the L-Z0 treatment, which also indicated that 500 mg kg−1 ZnO-NPs in the soil could significantly facilitate the accumulation of Cd by Oryza sativa L. In the H-Cd group, the BCF for the roots and shoots increased with the increasing ZnO-NP concentrations in the soil (Fig. 6c), while no clear change was observed for rice. The addition of high-concentration (500 mg kg−1) ZnO-NPs leads to the increase of Cd accumulation in plants, but did not result in higher toxicity. This was in accordance with the result of Kutrowska et al. (2017), which indicated that Zn addition had a protective effect on plant growth under Cd stress. Zn addition could restore the functional activities of antioxidant enzymes that were suppressed by Cd toxicity (De and Mark 2018).
Under different Cd stress conditions, the effect of ZnO-NPs had on the Cd toxicity and accumulation in the rice was different, which should be investigated further. Our results suggested that the scientific assessment of the potential environmental risks of ZnO-NPs is a requirement for understanding the impact on the soil-plant system and on human health.
Conclusions
-
1)
The addition of ZnO-NPs clearly increased the soil pH value, and the soil pH value increased with the increase in ZnO-NP concentration.
-
2)
Cd significantly inhibited rice growth; the plant height and biomass were significantly decreased with the increase in Cd concentration. The reduction of plant height and biomass under Cd stress was recovered and increased after the addition of ZnO-NPs, which indicated that ZnO-NPs could improve plant growth and alleviate the toxic effects of Cd. A high dose of ZnO-NPs significantly increased the rice height, tillering, and biomass, especially in the early-growth stage.
-
3)
High concentration of ZnO-NPs could increase the concentration of bioavailable Cd in rhizosphere soil, and subsequently increased the concentration of Cd in rice. In the L-Cd group, the Cd concentration of the rice in the L-Z500 treatment increased to 0.51 mg kg−1, exceeding the limit for acceptable Cd concentrations in rice of China (0.2 mg kg−1).
-
4)
In the L-Cd group, the bioaccumulation factor for rice in L-Z500 treatment was obviously higher than that in the L-Z0 treatment, which indicated that ZnO-NP concentration of 500 mg kg−1 in soil could significantly facilitate the accumulation of Cd by Oryza sativa L.
References
Ali B, Gill RA, Yang S, Gill MB, Ali S, Rafiq MT, Zhou W (2014a) Hydrogen sulfide alleviates cadmium-induced morpho-physiological and ultrastructural changes in Brassica napus. Ecotoxicol Environ Saf 110:197–207
Ali B, Qian P, Jin R, Ali S, Zhou W (2014b) Physiological and ultra-structural changes in Brassica napus seedlings induced by cadmium stress. Biol Plant 58(1):131–138
Ali B, Gill RA, Yang S, Gill MB, Farooq MA, Liu D, Daud MK, Ali S, Zhou W (2015) Regulation of cadmium-induced proteomic and metabolic changes by 5-aminolevulinic acid in leaves of Brassica napus L. PLoS One 10(4):e0123328
Allen SE (1989) Chemical analysis of ecological materials, 2nd edn. Blackwell Scientific Publications, Oxford
Cai F, Wu X, Zhang H, Shen X, Zhang M, Chen W, Gao Q, White JC, Tao S, Wang X (2017) Impact of TiO2 nanoparticles on lead uptake and bioaccumulation in rice (Oryza sativa L.). NanoImpact 5:101–108
Chai M, Shi F, Li R, Liu L, Liu Y, Liu F (2013) Interactive effects of cadmium and carbon nanotubes on the growth and metal accumulation in a halophyte spartina alterniflora, (Poaceae). Plant Growth Regul 71(2):171–179
De OVH, Mark T (2018) Tolerance, toxicity and transport of Cd and Zn in Populus trichocarpa. Environ Exp Bot 155:281–292
Deng Y, Eitzer B, White JC, Xing B (2017) Impact of multiwall carbon nanotubes on the accumulation and distribution of carbamazepine in collard greens (Brassica oleracea). Environ Sci Nano 4:149–159
Du W, Sun Y, Ji R, Zhu J, Wu J, Guo H (2011) TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J Environ Monit 13(4):822–828
Fan X, Wang P, Wang C, Hu B, Wang X (2017) Lead accumulation (adsorption and absorption) by the freshwater bivalve corbicula fluminea in sediments contaminated by TiO2 nanoparticles. Environ Pollut 231(Pt 1):712–721
Gao Y, Yang T, Jin J (2015) Nanoparticle pollution and associated increasing potential risks on environment and human health: a case study of China. Environ Sci Pollut Res 22:19297–19306
Garg N, Kaur H (2013) Impact of cadmium-zinc interactions on metal uptake, translocation and yield in pigeonpea genotypes colonized by arbuscular mycorrhizal fungi. J Plant Nutr 36(1):67–90
Glomstad B, Altin D, Sørensen L, Liu J, Jenssen BM, Booth AM (2016) Carbon nanotube properties influence adsorption of phenanthrene and subsequent bioavailability and toxicity to Pseudokirchneriella subcapitata. Environ Sci Technol 50:2660–2668
Hu X, Kang J, Lu K, Zhou R, Mu L, Zhou Q (2014) Graphene oxide amplifies the phytotoxicity of arsenic in wheat. Sci Rep 4:6122
Ji Y, Zhou Y, Ma C, Feng Y, Hao Y, Rui Y et al (2017) Jointed toxicity of TiO2 NPs and Cd to rice seedlings: NPs alleviated Cd toxicity and Cd promoted NPs uptake. Plant Physiol Biochem 110:82–93
Kutrowska A, Malecka A, Piechalak A, Masiakowski W, Hanc A, Baralkiewicz D et al (2017) Effects of binary metal combinations on zinc, copper, cadmium and lead uptake and distribution in Brassica juncea. J Trace Elem Med Biol 44:32–39
Laycock AJ, Romero-Freire A, Najorka J, Svendsen C, Van Gestel CAM, Rehkaemper M (2017) A novel multi-isotope tracer approach to test ZnO nanoparticle and soluble Zn bioavailability in joint soil exposures. Environ Sci Technol 51(21):12756–12763
Li M, Luo Z, Yan Y, Wang Z, Chi Q, Yan C, Xing B (2016) Arsenate accumulation, distribution, and toxicity associated with titanium dioxide nanoparticles in daphnia magna. Environ Sci Technol 50(17):9636–9643
Lin D, Xing B (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150(2):243–250
Lin D, Xing B (2008) Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Technol 42(15):5580–5585
López-moreno ML, Rosa GDL, Hernándezviezcas JÁ, Castillomichel H, Botez CE, Peraltavidea JR et al (2010) Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ Sci Technol 44(19):7315–7320
Ma C, Liu H, Chen G, Zhao Q, Eitzer B, Wang Z, Cai W, Newman LA, White JC, Dhankher OP, Xing B (2017) Effects of titanium oxide nanoparticles on tetracycline accumulation and toxicity in Oryza sativa (L.). Environ Sci Nano 4:1827–1839
Mo LY, Zhao DN, Qin M, Qin LT, Zeng HH, Liang YP (2017) Joint toxicity of six common heavy metals to Chlorella pyrenoidosa. Environ Sci Pollut Res 5:1–7
Mountouris A, Voutsas E, Tassios D (2002) Bioconcentration of heavy metals in aquatic environments: the importance of bioavailability. Mar Pollut Bull 44(10):1136–1141
Najeeb U, Jilani G, Ali S, Sarwar M, Xu L, Zhou WJ (2011) Insight into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J Hazard Mater 186:565–574
Nwugo CC, Huerta AJ (2008) Effects of silicon nutrition on cadmium uptake, growth and photosynthesis of rice (Oryza sativa L.) seedlings exposed to long-term low level cadmium. Plant Soil 311:73–86
Peng C, Duan D, Xu C, Chen Y, Sun L, Zhang H, Yuan X, Zheng L, Yang Y, Yang J, Zhen X, Chen Y, Shi J (2015) Translocation and biotransformation of CuO nanoparticles in rice (Oryza sativa L.) plants. Environ Pollut 197:99–107
Peng C, Xu C, Liu Q, Sun L, Luo Y, Shi J (2017) Fate and transformation of CuO nanoparticles in the soil-rice system during the life cycle of rice plants. Environ Sci Technol 51(9):4907–4917
Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C (2002) Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J 32:539–548
Priester JH, Ge Y, Mielke RE, Horst AM, Moritz SC, Espinosa K et al (2012) Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. PNAS 109(37):14734–14735
Rauret G, López-Sánchez JF, Sahuquillo A, Rubio R, Davidson CM, Ure AM, Quevauviller P (1999) Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monit 1:57–61
Shah K, Dubey K (1995) Effect of cadmium on RNA level as well as activity and molecular forms of ribonuclease in growing rice seedlings. Plant Physiol Biochem 3:577–584
Shi J, Peng C, Yang Y, Yang J, Zhang H, Yuan X, Chen Y, Hu T (2014) Phytotoxicity and accumulation of copper oxide nanoparticles to the Cu-tolerant plant Elsholtzia splendens. Nanotoxicology 8:179–188
Singh J, Lee BK (2016) Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): a possible mechanism for the removal of Cd from the contaminated soil. J Environ Manag 170:88–96
Vasiliadou S, Dordas C (2009) Increased concentration of soil cadmium affects on plant growth, dry matter accumulation, Cd, and Zn uptake of different tobacco cultivars (Nicotiana tabacum L.). Int J Phytoremediation 11(2):115–130
Wang P, Menzies NW, Lombi E, Mckenna BA, Johannessen B, Glover CJ, Kappen P, Kopittke PM (2013) Fate of ZnO nanoparticles in soils and cowpea (Vigna unguiculata). Environ Sci Technol 47(23):13822–13830
Wang Y, Zhu X, Lao Y, Lv X, Tao Y, Huang B, Wang J, Zhou J, Cai Z (2016) TiO2 nanoparticles in the marine environment: physical effects responsible for the toxicity on algae Phaeodactylum tricornutum. Sci Total Environ 565:818–826
Xu C, Peng C, Sun L, Zhang S, Huang H, Chen Y, Shi J (2015) Distinctive effects of TiO2, and CuO nanoparticles on soil microbes and their community structures in flooded paddy soil. Soil Biol Biochem 86:24–33
Yang K, Xing B (2010) Adsorption of organic compounds by carbon nanomaterials in aqueous phase: Polanyi theory and its application. Chem Rev 110(10):5989–6008
Ye XY, Zhang W, Long JH, Chang WJ, Zeng H (2018) Impact of engineered nanoparticles on Cd chemical speciation and bioavailability in paddy soil. Chin J Environ Eng 12:3426–3432
Zhang M, Liu XC, Yuan LY, Wu KQ, Duan J, Wang XL, Yang LX (2012) Transcriptional profiling in Cd-treated rice seedling roots using suppressive subtractive hybridization. Plant Physiol Biochem 50:79–86
Zhang R, Zhang H, Tu C, Hu X, Li L, Luo Y, Christie P (2015) Phytotoxicity of ZnO nanoparticles and the released Zn (ii) ion to corn (Zea mays L.) and cucumber (Cucumis sativus L.) during germination. Environ Sci Pollut Res 22(14):11109–11117
Zhang W, Alakangas L, Wei Z, Long J (2016) Geochemical evaluation of heavy metal migration in Pb-Zn tailings covered by different topsoils. J Geochem Explor 165:134–142
Funding
This study is financially supported by the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (31621091) and the General Financial Grant from the China Postdoctoral Science Foundation (2017M620504).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, W., Long, J., Li, J. et al. Impact of ZnO nanoparticles on Cd toxicity and bioaccumulation in rice (Oryza sativa L.). Environ Sci Pollut Res 26, 23119–23128 (2019). https://doi.org/10.1007/s11356-019-05551-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05551-x