Abstract
The last decade has witnessed significant progress in nanotechnology and its application in diverse fields. This growing interest has also been intensified in agriculture sector, which is evident from the publication of interesting scientific articles and lodging of patent applications, showing the relevance of nanotechnology in sustainable crop production. Crop losses due to plant pathogens, insects and weeds are a considerable challenge that the current agricultural production system faces worldwide. This article depicts the use of nanoparticles that paves the way for developing novel crop protection products in near future. Owing to the fact that nanoparticles have been used in many economically important applications, it is highly desirable to ascertain the possible deleterious effects as well. Moreover, intentional application of nanoparticle-based pesticides and fertilizers is an issue of great concern, as it may pose serious hazards. From this perspective, the authors discussed the recent research aimed at defining effects of nanoparticles on various environments and human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Attempts to boost food production with ever increasing population growth, limited availability of land and water resources, climate change, high incidence of diseases and insect pests, and bioaccumulation of agrochemicals are the biggest global challenges. To overcome these massive threats, different technological innovations like high yielding varieties, fertilizers, synthetic pesticides, hybrid seeds and transgenic crops have been developed that have contributed to enhancing global agricultural sustainability. Unfortunately, extreme dependency on these innovations has contributed many ill effects. Micronutrient imbalance, nitrate pollution and eutrophication are the serious consequences of excessively or improperly applied fertilizers. Similarly, heavy pesticide use poses greatest risk to non-target organisms including humans, beneficial microorganisms, beneficial insects, birds, earthworms and aquatic life forms. Furthermore, bio-magnification, development of pesticide resistance and resurgence of pest populations are the serious problems associated with pesticide use. The sensitivity of high yielding crops to diseases, insects and abiotic factors intensified the use of fertilizers and pesticides. Genetically modified (GM) crops are one of the greatest attempts to minimize chemical treatments. Unfortunately, pests may also develop resistance against GM crops, as they have already developed resistance to many pesticides. For instance, field-evolved resistance against Bt crops for some populations of 5 of 13 major pest species has recently reviewed, compared with resistant development in only one pest species in 2005 [1]. Moreover, novel genes may trigger risk of horizontal gene transfer with more severe, long-lasting ecological and economic consequences. Except new strains of plants, each of these modern agriculture innovations is totally reliant on the energy resources, especially petroleum. Global petroleum production is predicted to arrive at a maximum in the coming decades and to decline thereafter, a phenomenon known as peak petroleum [2]. Moreover, the world’s population is projected to reach 9.7 billion by 2050, placing an unprecedented pressure on global food security [3]. This alarming situation calls the production of an additional 1 billion tonnes of cereals and 200 million tonnes of meat annually [4]. Additionally, the growing demand for meat will put enormous burden on agricultural land and farmers need to grow crops to produce animal feed. Unfortunately, insect pests, plant pathogens, and weeds account for 14, 13 and 13 % crop losses respectively, causing an annual loss of $2000 billion worldwide [5]. Worldwide, about 3 million metric tons of pesticides, costing around $40 billion are applied annually [5]. This situation calls an alarming condition of more than 26 million cases of non-fatal pesticide poisonings [6]. In addition, deleterious effect on non-target beneficial microorganisms, risk to humans and other life forms, ground and surface water contamination and development of pesticide resistance are some major risks associated with heavy pesticide use. Moreover, climate change will affect the occurrence and distribution of insect pests and diseases that may cause unpredictable negative impacts on agricultural production worldwide [7]. This inevitable risk to food security and agriculture will invite more troubles in developing countries. For instance, a recent study by The Associated Chambers of Commerce and Industry of India said that annual crop losses due to pest and disease infestation results in crop losses worth Rs. 50,000 crore ($500 billion) that is significant in a country like India where at least 200 million people go to bed on an empty stomach each night [8]. Given the background, nanopesticides or nano-plant protection products represent a hopeful scientific development that offer a variety of benefits including increased effectiveness, durability, and a reduction in the amounts of active ingredients (AIs) that is being used in protecting crops against diseases, insects and weeds [9]. The last decade has witnessed the development of a variety of nanoformulation types including nanoemulsions, microemulsion, nanocapsules, nanospheres, solid lipid nanoparticles, products containing metals and metal oxides nanoparticles, porous hollow silica nanoparticles, layered double hydroxides and nanoclays. Excellent reviews were published that provide knowledge on these latest developments in nanopesticide research [9–14]. More than 3000 patent applications have been lodged in the last decade for nanopesticides alone [15]. Polymer-based formulations, inorganic metal/metal oxide, and nanoemulsions have received the greatest attention [15]. Increased efficacy of the nanoformulations have not yet been fully characterized perhaps, increase in the apparent solubility of weakly soluble AIs, slow or targeted release of AIs and protection of AIs from premature degradation makes nanoformulations superior in comparison to commercial formulations [9, 13]. Moreover, nanoformulations are more efficacious and required in lower doses than the conventional formulations that result in lower environmental burdens. The value of nanoformulations in crop protection is therefore huge to enhance the crop production. But, there is still no consensus among researchers, where the formulation and material scientists are in agreement with this emerging field, environmental scientists communicate on the notion of possible hazards [16]. These issues through different sets of eyes can make large differences in overall framing of nanopesticides among the general public. The aim of this review is to summarize nanopesticide research in crop protection that has been made over the last decade. Moreover, the benefits and possible hazards associated with nanoparticles are discussed. The information gathered herein identifies important directions for future research.
Definitions
International organization for standardization (ISO), the world’s largest developer of standards, has defined nanomaterial as a material with any external dimension in the nanoscale or having internal structure or surface structure in the nanoscale, where length range from approximately 1–100 nm is considered as nanoscale [17]. Materials with one, two or three external dimensions in the nanoscale are nano-objects, and nanoparticles are those nano-objects in which all three external dimensions lie in the nanoscale range where the lengths of the longest and the shortest axes of the nano-object do not differ significantly [17]. Several countries and international organizations including Organization for Economic Cooperation and Development (OECD), Scientific Committee on Emerging and Newly-Identified Health Risks (SCENIHR), European Commission (EU), United States Food and Drug Administration (US FDA), United States Environmental Protection Agency (US EPA), Health Canada, National industrial Chemical Notification and Assessment Scheme (NICNAS), Danish Ministry of the Environment etc. have presented their own definitions but there is no universal agreement regarding the definition of nanomaterials [15, 18]. Unfortunately, the proposed definitions vary considerably in their breadth of understanding nanomaterials that may lead to conflicting classification and assessment of nanomaterials, which might have unpleasant impacts on commerce and public perceptions of nanotechnology [18]. Given the background, the products that should be considered as nanopesticides under one definition may not be considered as nanopesticide in another. Further, at this stage, it is difficult to answer that nanopesticides are already in the market or not, until one arrives at a universally agreed definition [14, 16]. Researchers from the Department of Environmental Geosciences of the University of Vienna discussed nanopesticides with a broader definition, where all plant-protection products that (1) intensionally include entities covering a size range up to 1000 nm (2) designated with nano-prefix (3) claimed to exhibit novel properties associated with their small size, are considered as nanopesticides [9, 14]. For this review, the authors have used the same broader definition, in order to offer a summary of the nano-plant protection products discussed in peer-reviewed papers and reports. Further, the role of nanofertilizers for balanced crop nutrition has also been discussed.
Plant Disease Management
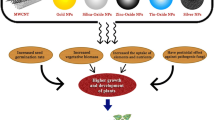
Nanoparticles of metals and metal oxides attracted a great scientific attention in plant disease management, and were found promising against serious plant pathogens that are responsible for huge economic losses. Moreover, recent studies also indicate the great potential of polymers and Ag-based nanocomposites as alternatives to commercial fungicides. The potential benefits of nanoparticles in plant disease management and better crop protection are summarized in Fig. 1.
Siver Nanoparticles (AgNPs)
AgNPs are the most commonly used anti-bacterial agents in health industry, food storage, textile industry and a number of environmental applications. Studies conducted during the last decade have also demonstrated the efficacy of AgNPs against major plant pathogens causing huge losses worldwide. Nanosized silica-silver (1–5 nm) prepared by combining AgNO3, sodium silicate and water soluble polymer, successfully controlled powdery mildews of pumpkin in both field and greenhouse conditions at 0.3 ppm [19]. Moreover, at 3.0 ppm, the developed product significantly inhibited a variety of plant pathogenic fungi in vitro, and found better than 20 nm AgNPs and 100 nm AgNPs. Interestingly, higher concentration (10 ppm) did not cause any adverse effect on a number of beneficial bacteria or plant pathogenic bacteria but complete inhibition was observed at 100 ppm [19]. Colletotrichum gloeosporioides induces anthracnose in a wide range of fruits, such as apple, avocado, mango and papaya, is controlled primarily by the application of synthetic fungicides during the postharvest period. Unfortunately, the pathogen has established cross resistance against benzimidazole fungicides. Although, prochloraz showed strong toxicity to C. gloeosporioides, but a recent study indicates the residue levels of this fungicide found above the maximum residue limit [20], which may represent serious harm to human health. AgNPs (5–24 nm) at a minimum concentration (56 μg Ag/mL PDA) showed 90 % growth inhibition of C. gloeosporioides [21]. Although the results are encouraging but the study was done only in Petri dishes where AgNPs exhibited a dose-dependent fungistatic activity. AgNPs synthesized using a novel bacterial strain BHU-S4 (Serratia sp.), designated as bsAgNPs (10–20 nm), showed complete inhibition of conidial germination of Bipolaris sorokiniana [22], the cause of one of the most dreadful diseases as spot blotch of wheat. Detached leaf assays indicated the obstruction in conidial germination. Interestingly, glass house experiments proved the beneficial effect of bsAgNPs as indicated by significant increase in root length, shoot length and shoot dry weight to withstand pathogen harmful effects. It is important to note here that histochemical staining revealed maximum lignifications of vascular bundles, which plays a key role in plant development as well as disease resistance, and SDS-PAGE profile revealed no protein damage in bsAgNPs treated plants [22].
Sclerotium-forming phytopathogenic fungi are responsible for many economically important diseases worldwide. For instance, Rhizoctonia solani, the causal organism of sheath blight of rice (Oryza sativa L.), is responsible for significant yield losses in all rice growing countries. Application of fungicides (azoxystrobin and flutolanil) appeared to be less efficient in increasing yields of moderately susceptible rice cultivars than susceptible and very susceptible cultivars. Moreover, fungicides are suggested only when the disease outbreaks cause significant losses, because azoxystrobin and flutolanil cost approximately $118 and $102 per ha, respectively [23]. Similarly, basal drop caused by Sclerotinia sclerotiorum is one of the most destructive diseases of horticultural crops worldwide. In a lab based study, AgNPs (4–8 nm) significantly inhibited the hyphal growth of R. solani, S. sclerotiorum and S. minor in a dose-dependent manner where hyphae exposed to AgNPs were severely damaged that ultimately resulted in plasmolysis and collapse of hyphae [24]. Moreover, AgNPs (7 ppm) were found to completely inhibit the sclerotial germination of S. sclerotiorum, while that of R. solani and S. minor were suppressed by more than 75 % [24]. Although the results are laboratory based but have significant practical implications because sclerotia play a key role in disease cycle; contributes to an increase in inoculum density and resists unfavorable abiotic factors such as heat, drought, and fungicides. Interestingly, in addition to R. solani and S. sclerotiorum, AgNPs (10–50 nm) prepared using Acalypha indica leaf extract also showed tremendous inhibitory action against Alternaria alternata, Macrophomina phaseolina, Botrytis cinerea and Curvularia lunata at a concentration of 15 mg/10 μL [25]. AgNPs (7–21 nm, 8 μg/mL) synthesized using cell free culture supernatant of a Bacillus strain (GP-23), showed inhibitory activity against Fusarium oxysporum by fragmenting and distorting the hyphae and disrupting the spores [26].
Seed-borne diseases are primarily controlled by fungicides, hot water, or chlorine treatment. Since, certain level of risk is associated with all the fungicides, therefore treated seeds should be handled cautiously and regulated in an effective way to avoid their use for human consumption or livestock feed. Bakanae disease of rice, caused by Gibberella fujikuroi, is one of the most important seed-borne fungal diseases causing significant yield losses worldwide. A recent study demonstrated the potential of AgNPs to manage this menace effectively. AgNPs (7.5 nm), at a concentrations ranging from 0.015 to 1.5 µg/mL reduced the conidial viability of G. fujikuroi by 50 %, when exposed for 1–20 min [27]. Further, significant reduction in colony-forming units (CFUs) of the pathogen was observed by the treatment of infested rice seeds with 150 µg/mL of AgNPs for 10 min and up to 24 h. Interestingly, seed treatment with 150 µg/mL AgNPs for 12 or 24 h significantly improved seedling emergence and plant height, without showing any adverse effects on germination rate and seedling growth even up to 48 h exposure [27]. This study is of great interest to develop novel seed disinfection methods that not only minimize inoculum build-up on seeds but also contributes to improve seedling emergence and plant height. Two conidia-producing fungi that are economically important pathogens of grasses are B. sorokiniana, which causes seedling blight, root rot, crown rot, and spot blotch on various gramineous species, and Magnaporthe oryzae, the cause of blast on rice and gray leaf spot on turfgrass. Silver (Ag) ions and AgNPs (20–30 nm) were demonstrated to produce a significant effect on the colony formation of B. sorokiniana and M. oryzae. Moreover, both ionic and nanoparticle Ag significantly reduced disease severity when applied 3 h before spore inoculation on perennial ryegrass (Lolium perenne) [28]. Surprisingly, their effectiveness significantly reduced when applied at 24 h after inoculation. The pathogens reproduce by means of asexual conidia, infection is initiated at high humidity (~100 % relative humidity) and warm temperature (25 °C) and germ tubes penetrate plant surfaces within 24 h [29]. Reduction in antifungal activity of Ag after 24 h of inoculation indicates that direct contact of Ag with spores or germ tubes is critical in disease inhibition [28].
Fusarium culmorum is an ubiquitous soil-borne plant pathogen which infects different small-grain cereals, in particular wheat and barley, and is the culprit behind foot rot, root rot and head blight. Significant yield reductions and deterioration of the quality by contamination of the grains with the fungal mycotoxins is a matter of concern. Relative to the control, a significant reduction in mycelial growth, in the number of germinating fragments and sprout length was observed for fungal spores incubated for 24 h with 2.5 ppm solution of AgNPs (5–65 nm) [30]. The results are only lab based but signify the importance of AgNPs to hinder spore germination that play a key role in pathogenesis. White rot caused by Sclerotium cepivorum is a serious disease of onion (Allium cepa L.) and other Allium spp., causing significant yield reductions prior to harvest or causing rot in storage. Laboratory and field testing of three different types of nanosilver liquid (WA-CV-WA13B, WA-AT-WB13R, and WA-PR-WB13R) having average particle size of 7–25 nm, found very promising to tackle this menace and provided 90 % inhibition at a concentration of 7 ppm, increased the biomass and dry weights, without affecting the soil microbial population [31]. In a follow-up study, these nanosilver formulations were further tested against eighteen fungal pathogens that cause economically important diseases on crops, vegetables and fruits. Significant inhibition of fungal pathogens was observed at 100 ppm and maximum inhibition of most fungi was recorded with WA-CV-WB13R [32]. AgNPs synthesized using the crude extracts of two brown seaweeds (Spatoglossum asperum and Hedophyllum sessile) showed extremely high antimicrobial activity against Xanthomonas axonopodis pv. citri, X. oryzae pv. oryzae and Ustilaginoidea virens [33]. Moreover, antimicrobial activity of AgNPs has also been reported against an important tree pathogen [34]. AgNPs (4–8 nm) were reported to cause significant growth reduction inhibiting conidial germination of Raffaelea sp., that has become a destructive pathogen in Korea, causing oak wilt and damaging both forest and landscape oaks [34]. All these studies suggest the possibility of AgNPs as an alternative to pesticides for the management of a variety of phytopathogenic fungi.
Recently, Ag-based nanocomposites (Ag@dsDNA@GO) were developed using dsDNA as a template for growing AgNPs on graphene oxide (GO), and tested against X. perfonans, the cause of one of the most dreadful diseases of tomatoes that leads to reduction in production by 10–50 % [35]. Excellent antibacterial activity was achieved with 20 ppm Ag (18 nm)@dsDNA@GO and 16 ppm Ag (5 nm)@dsDNA@GO composites in vitro after an incubation of only 1 h. More importantly, in a greenhouse experiment, Ag (18 nm) @dsDNA@GO at 100 ppm significantly reduced the disease severity as compared to untreated control and provided similar results to those of the current grower standard treatment (copper + mancozeb), without showing any phytotoxicity [35]. Further, within 15 min of exposure in vitro, Ag-dsDNA-GO (10 μg/mL) killed both copper tolerant and sensitive X. perforans strains in suspensions containing approximately 103 CFU/mL, whereas copper alone (10, 25, 50 μg/mL) did not reduce bacterial population significantly, as compared to the untreated control even after 24 h of exposure [36]. Interestingly, Ag-dsDNA-GO also showed antibacterial activity against copper-tolerant X. vesicatoria, X. euvesicatoria, and X. gardneri strains. More importantly, significant reduction in disease severity was observed when the plants were treated with Ag-dsDNA-GO (75 or 100 μg/mL) prior to artificial inoculation, as compared to copper-mancozeb and negative controls [36]. Both the studies highlight the possibility of Ag-dsDNA-GO as an efficient alternative to copper in tomato production.
Titanium Dioxide Nanoparticles (TiO2NPs)
Besides Ag-based nanocomposites [35], TiO2NPs were also found effective against X. perfonans [37]. A significant reduction in the CFUs was observed with nanoscale TiO2(<8 nm) doped with either Zn or Ag, whereas undoped TiO2NPs were not found effective within 10 min of light exposure [37]. Moreover, a preliminary greenhouse study herein showed a significant reduction in bacterial spot lesions with all the nanoformulations as compared to control. Interestingly, significant reduction in bacterial spot lesions with nanoscale TiO2/Ag as compared to TiO2NPs indicate that doping with Ag improved the bactericidal activity of nanosized TIO2 [37]. Nanoscale TiO2/Ag was numerically better than nanoscale TiO2/Zn but both are statistically similar. It is important to mention here that further testing of nanoscale TiO2/Zn in greenhouse and growth-chamber experiments significantly reduced disease severity as compared to the control, and no significant difference was observed between control and industry standard copper + mancozeb treatment [37]. Moreover, nanoscale TiO2/Zn (500–800 ppm) significantly reduced bacterial spot disease incidence as compared with copper, copper + mancozeb and the untreated control in field trials. More importantly, nanoparticle treatments did not cause any undesirable effects on tomato yield in any of the field trials [37]. Results of this study has significant practical implications as the Environmental Protection Agency (EPA) classifies zinc as a minimum-risk pesticide, and application of zinc doped nanoparticles may offer a more practical and reliable choice than Ag [37]. Laboratory and field testing of TIO2NPs/Zn (7 nm) was also found successful against a new Xanthomonas sp., causing bacterial leaf spot of rose [38].
Zinc Oxide Nanoparticles (ZnONPs)
The effect and mode of action of ZnONPs on the growth of plant pathogenic fungi is in the preliminary stage. B. cinerea, the causal agent of grey mold, severely affects stored table grapes causing heavy loss while Penicillium expansum is the main culprit behind the rotting of stored apples and pears. Unfortunately, both the pathogens are accountable for heavy postharvest fruit loss, even after the application of most superior postharvest technologies [39]. More alarmingly, B. cinerea has also developed multiple fungicide resistance due to repeated and incorrect use of fungicides [40]. ZnONPs with size of 70 ± 15 nm significantly suppressed the growth of B. cinerea by disturbing cellular functions and causing hyphal deformity, while inhibited conidiophores and conidia development of P. expansum that ultimately resulted in the death of fungal hyphae [41]. This is the first study which demonstrated the antifungal nature of ZnONPs against two major post harvest pathogens and suggests the potential role of ZnONPs to minimize postharvest losses, ensuring food safety. Research on ZnONPs has also been focused on remediation, where the use of nanoparticles not only inhibit the target pathogen but also contribute in the breakdown of pesticides. For instance, ZnO-thiram composite antifungal system (0.25 g/L of 20 nm ZnONPs with 0.01 g/L thiram) was demonstrated to inhibit Phytophthora capsici growth in a synergistic mode, where thiram enhanced the ZnO-induced oxidative damage [42]. Interestingly, 0.25 g/L ZnONPs completely degraded 0.01 g/L thiram under simulated sunlight irradiation within 6 h [42]. Thiram, a widely used dithiocarbamate fungicide, is known to cause necrotic type death of human skin fibroblasts cells and recently demonstrated to have adverse effect on reproduction and immune functions of birds [43]. In view of this, these kinds of residue-free green synergistic antifungal nanotechnology developments have the potential to manage plant disease economically; more significantly and greatly benefit the human society by minimizing health and environmental burdens.
Copper Nanoparticles (CuNPs)
Cost is one of the most important factors in the selection of protection measures, and copper based fungicides remain inexpensive and efficient relative to modern strobilurin fungicides, viz., azoxystrobin and fenbuconazole, as strobirulins have potential for resistance development [44]. Rotation of these compounds frequently leads to the inclusion of copper in disease management programs. The problems associated with copper fungicides are the adverse effects on entomopathogenic fungi, phytotoxicity, allergic reactions, itching, and eczema. Studies on the effect of CuNPs highlight their potential in managing some important diseases at a relatively low dose. For instance, bacterial blight of pomegranate (Punica granatum), caused by X. axonopodis pv. punicae (Xap), has been reported to cause huge economic losses in India. CuNPs was found to suppress Xap growth only at 0.2 ppm that is 10,000 times lower than that of commercial copper-oxychloride [45]. In addition, nanocopper treated bacterial cell walls were degraded, unable to colonize the plant tissues and did not produce intense water soaking [45]. Recently, CuNPs (3–10 nm) were found superior than the commercially available fungicide bavistin and demonstrated to produce a significant antifungal effect against Phoma destructiva, C. lunata, A. alternata and F. oxysporum [46].
Nano Sulphur
Sulfur alone and in combination with lime has incontestably long been the most popular and certainly one of the oldest substances having fungicidal, miticidal and insecticidal properties. But, lime sulfur is not necessarily the best choice in all situations, especially for controlling apple scab because of its high phytotoxicity values, and had a tendency to reduce leaf size and fruit quality [47]. Moreover, as an acaricide, its application resulted in the death of the natural enemies, besides showing severe phytotoxicity symptoms in physic nut [48]. A nanosulphur formulation (IARI nano-S, 50–90 nm) significantly inhibited the conidial germination of Erysiphe cichoracearum (Powdery mildew fungi) at 1000 ppm and found superior than commercial sulphur products, viz., sulphur 80WP, merck sulphur and canadian nano-S [49]. Moreover, IARI nano-S was found to cause the shredding of the myceloid appendages and the affected cleistothecia were failed to release ascospores [49]. These results are of great importance as appendages help the cleistothecia to adhere with the host plant and the ascospores are responsible to cause new infections. Similarly, as compared to commercial sulphur, significantly higher bioactivity of nanosulphur (1–100 nm) was reported against E. cichoracearum and Tetranychus urticae (Red spider mite) [50].
Chitosan Nanoparticles (CNPs)
Chitosan, a natural polymer, is reported to induce systemic resistance in plants, acts as a yield enhancer, and has potential role in induction of cell death and stomatal closing [51]. Bulk chitosan has drawn less attention as an antifungal agent due to its insolubility in aqueous media and lower antifungal activity [52]. However, in recent times, use of chitosan in nanoform has also paved the way for its entry as a successful plant protection agent. Copper–CNPs (196.4 ± 2.2 nm) at 0.1 % concentration has been demonstrated to inhibit the growth of A. alternata, M. phaseolina and R. solani by 89.5, 63.0 and 60.1 %, respectively [52]. In a follow up study, a significant increase in tomato seed germination percentage, seedling length, fresh weight and dry weight was observed with Copper–CNPs (374.3 ± 8.2 nm) as compared to control [53]. Moreover, a significant reduction in mycelial growth and spore germination of A. solani and F. oxysporum was observed at 0.10 and 0.12 % of Copper–CSNPs. In pot experiments, Copper–CNPs treated plants appeared healthy without showing any Fusarium wilt symptom and showed significantly lower Alternaria blight disease severity that was significantly superior to 0.2 % mancozeb and control [53]. More importantly, a recent study first time demonstrated the relatively high effectiveness of CNPs (90 ± 5 nm) in triggering innate immune plant responses by inducing defense related enzyme, up regulating the genes involved in plant defense including that of several antioxidant enzymes as well as elevating the levels of total phenolics and nitric oxide, even at almost ten times low dose regimens, as compared to that required for natural chitosan, therefore introducing CNPs as a potential candidate for next generation organic cultivation [54].
Insect Pest Management
The development of nanoformulations against insect pests is particularly aimed at minimizing the quantity of AIs, controlled release and protection from degradation (Fig. 2). To this end, a variety of formulations have been proposed for insecticidal compounds. Results of some of the studies are encouraging and may have significant positive impact to fight notorious insect pests.
Nanoemulsions
Nanoemulsions are promising candidates for the delivery of water-insoluble AIs and typically contain 5–10 % of surfactant, as compared to 20 % in microemulsions [14]. An efficient two-step process for the preparation of oil-in-water nanoemulsions at constant temperature (25 °C) was earlier described by Wang et al. [55]. Further, β-cypermethrin (β-CP) loaded nanoemulsions were compared with β-CP microemulsion. It is important to mention here that precipitation of commercial β-CP microemulsion occured in the sprayed solution within 24 h of dilution, whereas excellent stability with no precipitation of sprayed solution diluted from the nanoemulsion was observed [55]. Recently, a promising green oil-in-water nanoemulsion for β-CP delivery was developed using methyl laurate as oil phase, and alkyl polyglycoside (APG) and polyoxyethylene 3-lauryl ether (C12E3) as mixed surfactants [56]. Further, β-CP loaded nanoemulsion was demonstrated to have excellent spreading performance and was found homogeneous after dilution [56]. In both the studies, incorporation of β-CP did not cause any noticeable effect on the size and stability of the nanoemulsions and the solubility; hence the bioavailability of β-CP has increased in these nanoemulsion systems. Besides nanoemulsions, microemulsion of nanosized permethrin (131 ± 9 nm), a pyrethroid pesticide, was found safe to various plant species and soil microbes, as compared to permethrin [57]. It is important to mention here that there is substantial uncertainty about the use of these two terms in scientific literature, but nanoemulsions and microemulsion are distinguished by their thermodynamic stability [58]. Microemulsions are thermodynamically stable, whereas nanoemulsions are thermodynamically unstable [58]. Nanoemulsions are also developed for the efficient delivery of poorly-water soluble/insoluble naturally occurring compounds having insecticidal properties. For instance, nanoemulsion (155.2 ± 3.8 nm) containing apolar fraction from fruits of Manilkara subsericea (5 %), octyldodecyl myristate as oil (5 %), sorbitan monooleate/polysorbate 80 as surfactants (5 %) and water (85 %) exhibited significantly higher levels of mortality of cotton pest Dysdercus peruvianus [59]. Further, no significant inhibition of acetylcholinesterase (fish origin) and non-toxicity in mice indicated the safe nature of the developed product [59]. Nanoemulsion (43.31 nm) developed using Simmondsia chinensis (Jojoba) seed-oil and Tween-20 as a nonionic surfactant and water was found more promising against Sitophilus oryzae, as compared to the bulk jojoba oil [60]. Insect mortality bioassays recorded LC50 of 0.31 and LC90 of 0.66 mL/kg for nanoemulsion, compared to LC50 of 3.12 and LC90 of 10.16 mL/kg for bulk jojoba oil [60]. The loss of eucalyptus oil by volatilization was stabilized by the addition of aqueous filtrate of de-oiled karanja (Pongamia glabra) and jatropha (Jatropha curcas) in eucalyptus essential oil nanoemulsion (77 nm) that showed LC50 values of 0.1646 mg/L against Tribolium castaneum, a secondary pest of stored grains, whereas LC50 value of 5.4872 mg/L was recorded without the aqueous filtrate [61]. The sum of all these studies suggest the greater efficacy and better biosafety of nanoemulsions that makes them promising from both environmental and economical points of view.
Polymer-Based Nanoformulations
Recent research is focused exclusively on the controlled release of herbicides and insecticides using polymer-based nanoformulation. These studies demonstrated the wide range of polymer-based nanosystems for the controlled release and protecting of photo-labile compounds in pest management programmes. In this context, controlled release of azadirachtin-A was achieved by encapsulation in nano-micelles of polyethylene glycol (PEG)-based amphiphilic copolymers and various dimethyl esters [62]. Moreover, an increase in the molecular weight of PEG during polymerization further reduced the release rate of azadirachtin-A, which increased the shelf-life of this photo-liable pesticide [62]. Nano-micelles of PEG-based amphiphilic polymers were also reported for the controlled release of carbofuran, β-cyfluthrin and imidacloprid [63–65]. The developed β-cyfluthrin nanoformulation showed prolonged activity and found more effective against Callosobruchus maculatus, as compared to commercial formulation [64]. In all the above studies, the release of AIs in water was significantly slower than the commercial formulations and followed first-order kinetics. In sandy loam soil, encapsulation of thiamethoxam with nano-ranged PEG-based amphiphilic polymers resulted in much slower release than commercial formulation [66]. Higher efficacy and stability of these formulations may be attributed to the high solubilization power and low critical micelle concentration (CMC) of amphiphilic polymers [65]. Nanoacephate (80–120 nm), prepared by encapsulation of acephate in PEG, was found more promising against Spodoptera litura and did not induce any cytotoxicity in human fibroblast cell line [67]. Interestingly, PEG-based amphiphilic polymers not only found suitable for the controlled delivery of thiram, a contact fungicide, but also acted as moisture barriers, reduced the seed deterioration rate and checked thiram degradation [68]. These findings can further be explored for the targeted and single application of PEG-based nanoformulations for effective pest control in field conditions.
Chitosan, a biodegradable polymer obtained from the deacetylation of chitin, has attracted considerable interest to achieve effective and controlled release. Nano types of pyrifluquinazon prepared using chitosan as a carrier, showed best lethal efficiency against green peach aphid, Myzus persicae at 14 days after treatment, whereas the non-nanoformulation had the best lethal efficiency at 2 days after treatment [69]. Efforts were also made to develop controlled release hybrid nanoformulation using polymer coated liposomes. For instance, controlled release of etofenprox was achieved by encapsulation in chitosan coated lecithin molecules. The developed nanoformulation (100–800 nm) showed prolonged activity and higher efficacy against S. litura than pure etofenprox [70]. Moreover, chitosan-coated beeswax solid lipid nanoparticles (CH-BSLNs) were also found successful in reducing the photolysis of deltamethrin, achieving highest encapsulation efficiency (95 %) and higher payload (approximately 12.5 %) [71]. Further, compared to BSLNs, higher payload (approximately 1.79 times), higher encapsulation efficiency (83.6 %) and higher photo-protection of deltamethrin were achieved by a novel nano-structured lipid carriers (NLC) system with corn oil as liquid lipid [72]. Recently, neem-based nanocapsules prepared using biodegradable polymers, viz., poly-ε-caprolactone (PCL) and poly-β-hydroxybutyrate (PHB), caused higher mortality of Bemisia tabaci, a serious pest of many crops [73]. These findings suggest the wide range of novel nanocarrier systems in protecting photo-labile compounds used in crop protection.
Pheromones are highly specific chemical compounds that are used to disrupt the mating and trapping of targeted insect pests. Volatile pheromones are extremely unstable due to their chemical structure; and it is highly desirable to protect them from decomposition and the formulation must ensure a controlled release [74]. Nanofiber webs obtained by incorporating pheromones in Polyamide 6 as well as cellulose acetate polymer carriers via electrospinning was demonstrated to release pheromones in a nearly linear fashion over several weeks [75]. Therefore, nanofiber webs can further be explored to reduce the frequency of pheromone recharging in field conditions.
Nanogels and Nanohybrids
The problems associated with the use of pheromone hydrogels are swelling and shrinking with the changes in temperature and humidity, limits their use under adverse environmental conditions [76]. Pheromone nanogels having the fiber diameters ranging from 500 nm to 1 um was developed by immobilizing methyl eugenol (ME) into nanosized low-molecular mass gelators (LMMGs), i.e. all-trans tri (p-phenylenevinylene) bis-aldoxime, showed high pheromone retention capacity and enhanced the shelf-life of ME by protection from evaporation. More importantly, under adverse rainy season, this controlled release system showed excellent efficacy in a fruit orchard against Bactrocera dorsalis, one of the harmful pests affecting a number of fruits including guava [76]. Recently, zinc-layered hydroxide (ZLH) nanohybrid intercalated with insect pheromone hexenoic acid (HE) was developed using a simple co-precipitation technique [77]. It is important to note here that the controlled released of HE was successfully achieved and the developed formulation did not cause any adverse affect on the germination of wheat and green bean seeds. Interestingly, higher percentage of seed germination and higher radical seed growth was observed with this nanohybrid, as compared to HE alone [77]. These findings are of strong practical significance and can be best utilized against insect pests of various crops.
Nanoherbicides
Weed control becomes one of the biggest challenges in the agriculture sector. Single herbicide use or lasting exposure of weeds with diverse herbicides results in the development of herbicide resistance. In addition, longer persistence time of toxic herbicidal residues can create germination problem for the next crop. The most important aspect in controlling the multiplication of any weed is the destruction of seed bank in soil, to restrict their germination. Nanotechnology provides a solution by developing target specific herbicides, which may enter the roots and get transported in all plant parts. Their interaction with metabolic pathways restricts the glycolysis, thus ultimately causing the death of the target due to starvation [78]. Moreover, due to their tiny size, nanoherbicides are being able to mix easily in soil and eradicate the target species without leaving any contradictory effects [79]. Nanoencapsulation ensures better herbicide application by avoiding phytotoxicity, provides improved penetration through cuticle and tissue, and allows slow and constant release of the active ingredients (Fig. 2) [11]. For instance, alginate/chitosan and chitosan/tripolyphosphate (CS/TPP) nanoparticles (400 nm) as carriers for the combined herbicides (imazapic and imazapyr) showed efficiencies exceeding 60 %, improved their mode of action and reduced their toxicity [80]. Moreover, after 300 min, free imazapic and imazapyr showed release percentages of 55 and 97 %, respectively, while a 30 and 20 % decrease was observed for the nanopreparation of imazapic and imazapyr [80]. An earlier study also demonstrated good encapsulation efficiency (62 %) of CS/TPP nanoparticles (300 nm) for paraquat that was found to be stable for at least 60 days [81]. Moreover, MTT test revealed reduction in the cytotoxicity of encapsulated herbicide on CHO cells, and nanoparticle-bound paraquat showed less chromosome damage on A. cepa, as compared to the free herbicide [81]. Similarly, encapsulation percentages of 64–84 % were achieved with nanocapsules of poly (epsilon-caprolactone) containing herbicide atrazine, which offers efficient delivery, as well as improved adhesion of herbicides to the target plants [82]. Additionally, this system was found to be effective for the control of the target species (Brassica sp.) where the use of poly (epsilon-caprolactone) nanoparticles reduced the mobility of atrazine in soil, and reduced the genotoxicity that is evident from A. cepa chromosome aberration assay [83]. It is also important to note that encapsulation of ametryn and atrazine with poly (epsilon-caprolactone) nanocapsules lower the toxicity to both microalgae Pseudokirchneriella subcapitata and human lymphocyte cultures, as compared to the herbicides alone [84]. Moreover, the association efficiencies of ametryn, atrazine, and simazine in poly (epsilon-caprolactone) nanocapsules were better than 84 % and the controlled release was governed mainly by relaxation of the polymer chains [85]. Similarly, solid lipid nanoparticles (SLN) showed good physicochemical stability and high encapsulation efficiencies for atrazine and simazine and reduced their toxicity [86]. These reports are in general agreement that the nanoencapsulation of herbicides provides a valuable means of weed control in agriculture while minimizing human and environment burdens. Investigations aimed to determine the environmental fate of nanoherbicides is still scare. To determine the fate of atrazine nanoformulation in soil, pioneering study by applying regulatory protocols defined in the OECD guidelines indicates quick discharge of atrazine from the polymer nanocarriers relative to the degradation kinetics, and atrazine coupled with the nanocarriers was subject to biotic or abiotic degradation [87].
Nanofertilizers
Fertilizers play foremost role to boost the production across the spectrum of crops. The nutrient use efficiencies (NUE) of nitrogen (N), phosphorus (P), and potassium (K) had remained constant for the past several decades and hardly exceed 30–35, 18–20 and 35–40 %, respectively [88]. Improvement of NUE is must required to enhance the crop production in marginal lands with low nutrient availability. But, little success has been achieved to increase the NUE in conventional fertilizer formulations so far [89]. Fortunately, the emerging nanofertilizer based approaches are predicted to be more proficient than even polymer-coated conventional slow-release fertilizers, due to their high surface area to volume ratio [90]. Nanofertilizers are expected to improve the NUE by preventing the nutrient ions from either getting fixed or lost in the environment [91]. These smart delivery systems of encapsulated nanofertilizers restrict the interaction of nutrients with soil, water and microorganisms, and release nutrients after their internalization in the plant system [90].
Nitrogen fertilizers are one of the most important crop boosters, when judged with regard to the energy required for their synthesis, tonnage used and monetary value. However, as compared with amounts of N applied to soil, the nitrogen use efficiency by crops is very low [90]. Urea is one of the commercially available, fast-release N fertilizers, which is easy to use in agricultural fields. Global urea demand is anticipated to reach 120 Mt by the year 2018–2019. Efforts to reduce the release rate of urea can increase its efficiency of use and further prevent groundwater contamination. Given the context, a novel urea slow-release nanocomposite, based on urea intercalation into montmorillonite clay showed slow-release behavior for urea dissolution, even in low montmorillonite amounts (20 % in weight) [92]. Similarly, urea blended with nanoporous zeolite (1:1) and conventional zeolite (1:1) was shown to release N up to 48 and 34 days respectively, while N release from conventional urea was found to cease within 4 days under ambient conditions [93]. In a follow up study using two different soil types (inceptisol and alfisols) and maize as a model crop, the grain N content of nanozeolite + urea on both the soils were found to be consistently higher than conventional urea but the response was more pronounced in alfisol than inceptisol [94]. An early burst followed by a slow-release even on the 60th day was observed in a urea-modified hydroxyapatite nanoformulation encapsulated under pressure into cavities of the soft wood of Gliricidia sepium, while commercial fertilizer showed a heavily early burst and subsequent release of low and non-uniform quantities until around day 30 [89]. The major advantages of using slow-release nanoformulation of fertilizer is improved NUE efficiency, higher crop yield, less environmental burdens from leaching of N, as compared to the conventional water-soluble fertilizers [89].
Surface water eutrophication is the major problem associated with phosphate salts that are heavily used in agriculture. Synthetic apatite nanoparticles (15.8 ± 7.4) developed using a one-step wet chemical method, demonstrated to increase the growth rate and seed yield of soybean by 32.6 and 20.4 %, respectively, as compared to (Ca(H2PO4)2), a regular P fertilizer [95]. In addition, above ground and below ground biomass production was enhanced by 18.2 and 41.2 %, respectively. Apatite nanoparticles may act as a novel class of P fertilizer that can potentially enhance crops’ yields and biomass production, while minimizing risks of water eutrophication [95]. Recent research also highlighted the importance of the nanofertilizers in improving yield. Cucumber leaves sprayed with nanofertilizers (nanonat and ferbanat) significantly improved the yield as compared to control [96]. Similarly, iron (Fe), P and K nanofertilizers were demonstrated to significantly increase yield, flower number, fresh stigma weight, dry stigma weight, stigma length, fresh flower weight and dry flower weight of saffron [97]. Application of Fe nano-oxide solution as a foliar spray in wheat was found to be promising, as highest values of spike weight, 1000 grain weight, biologic yield, grain yield and protein content were achieved [98]. Iron deficiency is a widespread problem, mainly in high pH and calcareous soils; therefore, combining Fe compounds with nanotechnology may be a solution to combat Fe deficiency. Moreover, besides enhancing growth parameters, foliar applications of nanofertilizers (ZnO and Fe3O4 NPs-containing Hoagland solution) alleviate salt stress in Moringa peregrina [99]. Similarly, a significant increase in rice grain yield and nitrogen use efficiency was observed after applying slow-released fertilizer added nanosized carbon. For instance, in comparison to Jingzhengda (JSCU), a slow-released fertilizer having 42 % N, the total N concentration in surface water of paddy soil under JSCU+ nanocarbon was declined by 31.0 %, and the period of N runoff loss due to rainfall was shortened by 2.2 days. Similarly, treatment with Stanley slow-released compound fertilizer (SSRF, N-P2O5-K2O = 20:9:11) + nanocarbon, the average total N concentration was decreased by 29.8 % and the time of N runoff loss was shortened by 1.8 day [100]. Recently, nanocomposites based on polyacrylamide (PAAm), methyl cellulose (MC), and calcic montmorillonite (MMt) hydrogels showed excellent controlled desorption of urea and demonstrated to release higher amounts of nutrient that is almost 200 times slower than pure urea [101]. Moreover, nitrous oxide (N2O) emissions in the field were reduced substantially for urea nanocomposites associated with an exfoliated clay mineral prepared using PAAm hydrogel or polycaprolactone [102]. Comparison in the release profiles of a urea based controlled release fertilizer indicated that 100 % release of urea takes place in the first 5 h, 100 % of urea was released in 97 h, and 87 % was released in 150 h from pure urea, urea intercalated kaolinite nanocomposite, and the encapsulated nanocomposite, respectively [103]. Moreover, swelling and release of urea was dependent on the type and concentration of salt solution added to the medium, pH levels of the solutions, and temperature, where the release of urea was predominated by a Case II release mechanism with skeleton erosion [104]. A novel nanocomposite based on urea–formaldehyde in the presence of MMt may act as a new controlled release system for N fertilizers, where nanocomposites offered excellent mechanical resistance and urea release was evidently controlled by the extent of polymerization [105]. These results are of strong practical significance as showing the efficacy of novel nanosomposites for slow-release of fertilizers that increases the nutrient use efficiency and yield, whereas reduces pollution.
Risks of Nanoparticles
Nanoparticles have raised concern of environmental risks due to their widespread applications. Despite growing scientific facts of possible hazards, hundreds of thousands of tones of nanomaterials are already being released into the environment. The most reliable data on environmental contamination indicates that 260,000–309,000 metric tonnes of global engineered nanomaterials (ENMs) produced during 2010, ended up in landfills (63–91 %), soils (8–28 %), water bodies (0.4–7 %), and the atmosphere (0.1–1.5 %) [106]. Ecotoxicology and environmental safety issues focused on the interactions of nanoparticles in different environmental systems, including their bioavailability, assimilation in beneficial organisms, damage mechanisms, biological responses of non-target organisms and subsequent fate is a serious issue to be addressed. The following section will shed light on the recent studies in determining the effect of nanoparticles in different systems.
Effects on Beneficial Soil Microorganisms
Microbial population in the rhizosphere may play a key role in plant reaction to nanoparticles, thus must be taken into consideration in effect assessment. For instance, a significant increase in the biomass of maize plants was observed with the application of 100 mg/kg of AgNPs (20 nm), which could be correlated with a significant alteration in rhizosphere associated bacterial community that significantly altered carbon use and community composition profiles [107]. Comparatively, fungal communities were less susceptible, and the bulk and AgNPs treated rhizospheric soil were significantly different with respect to the observed microbial changes [107]. By contrast, application of 350 and 790 mg/kg AgNPs (20 nm) in soil significantly reduced lateral and fine root development and shoot biomass of pine after 4 months [108]. Further, at highest concentration, no ectomycorrhizal fungi (EMF) was found to be associated with AgNPs contaminated roots, whereas 5 EMF genera were observed in control [108]. Similarly, application of 10 nm AgNPs (1 and 3 mg Ag/L) in sand was found toxic to beneficial soil bacterium, Pseudomonas chlororaphis O6, as evident from the loss in bacterial culturability, whereas no cell death was observed in loam soil. Interestingly, addition of humic acid in AgNPs amended sand maintained the culturability [109]. The high toxicity of AgNPs was also demonstrated on arctic soil microbial communitity, where Bradyrhizobium canariense, a community-identified plant-associating bacterium showed noticeable sensitivity to AgNPs [110]. Moreover, mixtures of nanoparticles (Ag, copper, and silica) even at very small concentrations can disrupt microbial communities [111] and some AgNPs can act as a continuous source of bioaccessible Ag fraction over time [112]. The toxicity of nanoparticles depends on the morphology and surface properties of nanoparticles. For example, the first comparative study on the bactericidal properties of AgNPs on Escherichia coli indicates shape dependent biocidal action as truncated triangular Ag nanoparticles with a {111} lattice plane as the basal plane were found to be more biocidal, compared to spherical and rod-shaped nanoparticles [113]. Moreover, the toxicity of four AgNPs representing various surface charging scenarios suggest that surface charge is one of the most important factors that govern the toxicity [114]. Thus, risk evaluation for each nanosilver product should consider the physical, chemical, and possibly toxic characteristics unique to that product [115]. Therefore, there is an urgent need to evaluate Ag-based nanomaterials on a case-by-case basis. Bar-coded pyrosequencing, to explore the responses of diverse bacterial taxa to TIO2NPs (15–20 nm) and ZnONPs (20–30 nm) in incubated soil microcosms indicates the susceptibility of symbiotic nitrogen-fixing bacteria under the order Rhizobiales, the family Bradyrhizobiaceae, and the genus Bradyrhizobium [116]. Moreover, members of the family Methylobacteriaceae, which contain methanotrophs, that metabolize methane as their only source of carbon and energy, declined by both the nanoparticles [116]. Therefore, application of these nanoparticles as agrochemicals may have serious consequences by hampering symbiotic nitrogen-fixation in major legume crops and preventing methane emissions from soil. In addition, maximum toxicity of ZnONPs on soil microorganisms was observed in acidic soil, followed by the neutral soil. Relatively, the toxicity of ZnONPs was less in alkaline soil [117]. Nanoparticles of Ag, CuO and ZnO showed toxicity to P. putida KT2440, a beneficial environmental microbe, suggesting that the NP (≤1 mg Ag/L, ≈10 mg Cu, Zn/L) application may put negative impact on soil microbial processes [118]. Effects of CuONPs and AgNPs were accompanied by cell death whereas ZnONPs were bacteriostatic. Further, bulk materials of these nanoparticles lacked inhibitory activity suggesting the aggregation of nanoparticles into larger particles may reduce their non-target effects [118]. Therefore, studies on the soil environment that can promote aggregation of nanoparticles may lessen their toxic activity. In contrast to the above mentioned studies showing the harmful effect of metal based nanoparticles on soil beneficial organisms, nanoformulations of commercial fungicide hexaconazole showed no adverse effect. For instance, nitrogen-fixation, nitrogen-assimilation, indole acetic acid (IAA) production and phosphate solubilization of nitrogen-fixing blue green algae (BGA) and bacteria was found to be significantly inhibited in the presence of commercial hexaconazole, whereas stimulatory to slightly inhibitory effect on the growth measurable parameters of the organisms were observed with nanohexaconazole [119]. Nanohexaconazole was also found to be more stable, causing no adverse significant effect on soil nitrifiers and was found superior in comparison to conventional hexaconazole [120].
All these findings suggest a complex interaction of nanoparticles with soil organisms where different soil types, soil factors and nanoparticle size and type may play significant roles in determining the environmental fate of nanoparticles. Beneficial microbial populations are one of the most important key factors that influence soil ecosystem processes, especially by decomposing soil organic matter, nutrient recycling, disease suppression and growth enhancement etc. Therefore, any factor that induces significant negative effects on soil microbial populations would certainly deteriorate soil quality and sustainability. Thus, intentional application of nanoparticles, particularly in crop protection is a prime concern and identification of safe nanoparticles, procedures for their safe application, follow up mitigation and remediation strategies, if found hazardous, should be a priority. Estimation of beneficial microbial communities in soil must be considered as a crucial factor while investigating the impacts of nanoparticle exposure in soil. Evidences also support the inhibitory effect of AgNPs on soil exoenzyme activities that are crucial to support the soil biochemical processes [121].
Effects on Terrestrial Ecosystem
Sophisticated exposure models and ecotoxicological literature explain that AgNPs concentration below the current and future predicted environmental concentrations (PECs) in diverse ecological compartments can affect prokaryotes, invertebrates and fish, is an indicative of noteworthy environmental hazards [122]. In terrestrial ecosystems, earthworms are considered as soil engineers, dominating invertebrate biomass, and good indicators of soil health. The first ecotoxicological life history trait data on earthworms using the limit-test design for Ag, Cu, Ni, Al2O3, SiO2, TiO2 and ZnO2 nanoparticles and their corresponding metal salt confirmed the toxicity of AgNPs, CuNPs and TiO2NPs, and total reproductive failure was observed with both the Ag treatments [123]. The first evidence on the capacity of earthworms (Eisenia fetida) to sense and consistently avoid soils containing AgNPs suggests that nanoparticle-organism interactions may be unpredictable and may result in ecologically significant effects on the behavior at environmentally relevant concentrations [124]. Moreover, significant decrease in the reproduction of E. fetida was observed with AgNO3 (94.21 mg/kg), AgNPs coated with oleic acid (50.60 + 1.02, 727.6 mg/kg), and AgNPs coated with polyvinylpyrrolidone (56.35 +1.16 nm, 773.3 mg/kg), where more Ag accumulating was observed with AgNO3 as compared to earthworms exposed to equivalent concentrations of AgNPs [125]. Toxicity mechanisms are still poorly characterized, apparently in some cases nanoscale specific properties may cause significant bio-uptake and toxicity than that caused by Ag ions [122]. For instance, reduced bioavailability and toxicity of ionic Ag with time, whereas increase in toxicity of AgNPs (50–80 nm) with time suggests that long term soil aging experiments are required to properly assess the environmental risk of AgNPs [126]. Similarly, AuNPs (20 nm) were more bioavailable and demonstrated to be taken up by E. fetida from soil and distributed among tissues that may cause undesirable effects on reproduction and have the potential to enter terrestrial food webs [127]. Moreover, trophic transfer of AuNPs from soil along a simulated terrestrial food chain using earthworms (E. fetida) and juvenile bullfrogs (Rana catesbeiana) indicates the efficient transfer and more bioavailability of nanoparticles to higher order consumers through trophic exposure, as compared to direct exposure [128]. Study conducted on the effect of CuNPs on E. fetida suggests that oxidized CuNPs may enter the food chain but earthworms are adversely affected only at relatively high concentrations (>65 mg Cu/kg soil) [129]. Study on the exposure of earthworms (E. andrei and E. fetida) to TIO2NPs showed the safe nature of TIO2NPs [130]. Juvenile survival and growth, adult survival, cocoon production, cocoon viability, or total number of juveniles hatched from cocoons was not significantly affected in field and artificial soil containing between 200 and 10,000 mg TIO2NPs per kilogram of dry soil. However, earthworms avoided nano-TiO2 amended artificial soils indicate the capability of earthworms to detect TIO2NPs in soil [130]. Following a 14 day exposure on filter paper test, ZnONPs showed acute toxicity to E. fetida, while TIO2NPs was found safe [131]. In contrast, both metal oxide nanoparticles were found safe without showing any acute toxicity in sand. In particular, ZnONPs showed greater toxicity than TIO2NPs in E. fetida [131]. In contrast to the safe nature of TIO2NPs [131], 1H NMR-based metabolomics provides evidences of significant changes in the metabolic profile, and oxidative stress in E. fetida, when exposed to TiO2 nanomaterial (5 nm) [132]. Hundred percent mortality after 96 h exposure to the highest concentration (1000 mg ZnONPs/kg agar) and highest mortality at the lowest exposure concentration (50 mg ZnO/L) were observed in earthworms exposed in agar and filter paper assay, respectively. Interestingly, significant reduction in the toxicity of ZnONPs on filter paper by applying soil extracts instead of deionized water (DW) to simulate a realistic exposure system, makes filter paper toxicity tests a good choice for the environmental risk assessment of nanoparticles [133]. Reproduction of E. veneta was found to be declined by 50 % when exposed to ZnONPs (<100 nm), but was almost totally inhibited by ZnCl2 [134]. Further, ZnONPs did not cause any adverse effect on immune activity, but that was suppressed by 20 % when exposed to ZnCl2 [134]. Based on the above mentioned studies, it is obvious that risk assessment approaches to test individual nanoparticles are required to separate the beneficial from that of hazardous. Moreover, targeted assays that can precisely sense damage at cellular or molecular level are required.
Phytotoxicity
Environmental impacts of nanoparticles can be understood, taking into consideration their toxic effects in different plant systems. The phytotoxicity of TiO2NPs (27 nm) and AgNPs (10–15 nm) was compared. A significant decrease in the root elongation was observed with AgNPs even at the lowest (50 mg/L) concentrations, while TiO2NPs showed no phytotoxicity on tomatoes (Lycopersicon esculentum) [135]. Both nanoforms were taken up by the plants and deposited in the stems, leaves and fruits. Moreover, lower chlorophyll contents, higher superoxide dismutase activity and less fruit productivity was observed with AgNPs, whereas TiO2NPs only resulted in higher superoxide dismutase activity at the highest concentration (5000 mg/kg) in green house trials [135]. By contrast, rice seed germination and seedlings growth decreased with an increase in size and concentrations of AgNPs. Smaller AgNPs (20 nm) was taken in higher amounts, showed less negative effects and only trapped in the roots, while deformity in the leaf cells was observed when seeds were treated with 150 nm AgNPs at the concentration of 10 or 100 mg/L [136]. Similarly, AgNPs treatments up to 30 μg/mL accelerated root growth but 60 μg/mL restricted the root growth in rice [137]. Transmission electron microscopy (TEM) revealed the penetration of AgNPs through plant cell wall and damaging effect on cell morphology and its structural features. Moreover, AgNPs caused a significant decline in total soluble carbohydrates in rice roots and damaged rhizosphere associated bacteria by the leakage of reducing sugars and protein through the bacterial membrane [137]. In contrast to the non-phytotoxic effect of TiO2NPs (27 nm) in tomatoes [135], TiO2NPs (30 nm) inhibited root hydraulic conductivity, leaf growth and transpiration in maize seedlings [138]. Interestingly, TiO2NPs (42.8 nm) did not produce any significant effect on plant growth and nutrient content in maize and soybean [139]. Although, arbuscular mycorrhizal fungal (AMF) communities were affected but the composition of rhizosphere associated bacterial communities was not influenced [139]. Fascinatingly, TiO2NPs (29 ± 9 nm), multi-walled carbon nano tubes (MWCNTs, 20–30 nm) and CeO2 NPs (>50 nm) did not produce any negative effect on red clover plant biomass and AMF root colonization [140]. Further, as compared to control, 8 % increase in the nitrogen-fixation levels and decrease in the number of flowers was observed with 3000 and 3 mg/kg MWCNTs treatments, respectively [140]. By contrast, soil supplemented with MWCNTs (125–250 nm) induced 2 times more flowers and fruit in tomato, as compared to plant grown in normal soil [141]. Further, Raman spectroscopy confirmed the presence of MWCNTs in flowers, and comparative metagenomic analysis of microbial communities revealed an increase in the relative abundance of Bacteroidetes and Firmicutes, whereas a sharp decline in Proteobacteria and Verrucomicrobia communities was observed with increasing concentration of MWCNTs [141]. As compared to control, ZnONPs (10 nm) did not cause any noticeable effect in the stem but significantly increased root elongation in peas (Pisum sativum L.). Interestingly, at a concentration of 500 mg/kg, H2O2 in the leaves increased by 61 % with a twofold lipid peroxidation, wheras bulk treatments showed no effect in H2O2 and lipid peroxidation [142]. By contrast, root elongation was significantly inhibited by CuONPs (95.73 % for maize and 97.28 % for rice) and ZnONPs (50.45 % for maize and 66.75 % for rice) at a concentration of 2000 mg/L [143]. Further, higher toxicity in both maize and rice was observed with ZnONPs (<50 nm), as compared to ZnO microparticles (<5 μm). No negative effects were observed in the corresponding solutions (Cu++ and Zn++), suggesting the phytotoxic nature of nanoparticles alone [143]. Similarly, CuONPs exposure to Brassica juncea L. resulted in shoot-growth suppression, reduction in total chlorophyll, carotenoids contents and shortening of primary and lateral roots [144]. Application of foliar spray of Fe nanoparticles was not found to induce any toxic effect on Physcomitrella patens (bryophyte) as reactive oxygen species (ROS) production, malondialdehyde (MDA) production and glutathione regulation were not disturbed significantly [145]. Similarly, no significant phytotoxicity was observed in macrophytes, viz., Lepidium sativum, Sinapis alba and Sorghum saccharatum for nanosized zerovalent iron (nZVI) tested at 2340 and 33,560 mg/L [146]. Interestingly, seedling length and biomass production was increased at the highest concentrations tested [146]. By contrast, no visible toxic effects were observed on rice seedlings growth with freshly added nZVI (250 mg/kg), whereas noticeable toxic symptoms were observed at the highest concentration (1000 mg/kg) tested [147]. Surprisingly, nZVI toxicity was reduced after ageing with 2 and 4 weeks in soils as compared to fresh nZVI [147]. It is therefore clear that both positive and negative effects have been reported about the same metal/metal oxide nanoparticles in different plant species. Further, some studies differed in their findings about the effect of same nanoparticles on similar plant species. These contradictory findings may be due to the differences in the concentration, size and methods of preparation of nanoparticles used by different workers. Further, different plant species may also differ in their interactions with nanoparticles. For instance, 4–10 times lower Cu uptake was observed in two salt marsh plants, viz., Halimione portulacoides and Phragmites australis, after exposure of CuNPs. Interestingly, P. australis was able to translocate ionic Cu as well as CuNPs but H. portulacoides failed to translocate CuNPs [148]. Similarly, a high and extended exposure of TiO2NPs (5 mg/L for 14 days), did not cause any adverse effect on the growth rate and chlorophyll a content of Lemna minor, even TiO2NPs attached on the cell walls, but no cellular uptake was observed [149].These findings are of great importance and shed light on the effective utilization of appropriate plant species for phytoremediation purposes.
Human Toxicity
One of the most critical issues for the acceptability of nanopesticides is to ensure that the nano-AIs are tested in the same way humans would be exposed in the real world. The chances of pulmonary and dermal exposure to nanoparticles are more; therefore it is essential to evaluate toxicity in dermal and pulmonary cells. In view of this, two cytotoxic assays (MTT and Neutral Red) performed in two independent laboratories to test the toxicity of 24 nanaoparticles on two human pulmonary cell lines, namely A549 and THP-1. Different copper based nanorticles with size ranging from a minimum of 22.9 nm to a maximum of 94 nm and ZnONPs (53.6 nm) showed most toxicity in MTT assays, which was found to be a reliable and sensitive method to test the toxicity on THP-1 cells [150]. MTT assay was also found reliable to test the toxicity of AgNPs on four different mammalian cell lines. In comparison to Ag ions, the toxicity of AgNPs (70 ± 5 nm) for PC-12 cells and NIH-3T3 cells was 5 and 18 times higher, respectively. Further, electric-cell-substrate-impedance-sensing (ECIS), an automated method to monitor cellular behavior in real-time confirmed that AgNPs confer toxicity with an immediate effect [151]. These findings unveil the sensitivity difference between cell types and cytotoxicity assays that has to be taken very carefully while assessing nanoparticle toxicity. Recent findings on the toxicity of AgNPs on human cell lines revealed size dependent toxicity of AgNPs that could be related to oxidative stress. For instance, testing of AgNPs (47 and 42 nm) and AuNPs (30, 50 and 90 nm) in human pulmonary fibroblasts (HPF) revealed the more toxic nature of 4.7 nm AgNPs than the large AgNPs and AuNPs, whereas similar cytotoxicity was observed for all the AuNPs. Generation of oxidative stress was evident from a significant increase in ROS production, reduction of glutathione level and slight inactivation of superoxide dismutase [152]. Moreover, AgNPs of the same size (4.7 nm) were also found to cause more toxicity than the large AgNPs (42 nm) in human dermal fibroblasts and generated oxidative stress [153]. Interestingly, both AgNPs sizes induced oxidative stress related genotoxicity (DNA damage) in 4 human cell lines, where 4.7 nm AgNPs was more genotoxic and pulmonary fibroblasts showed the highest sensitivity [154]. Inductively coupled plasma mass spectrometry (ICP-MS) revealed that smaller AgNPs (5 nm) enter cells more easily in A549, SGC-7901, HepG2 and MCF-7 cell lines than larger ones, which may be the reason of higher toxicity [155]. Surprisingly, instead of any oxidative stress, AgNPs (20.4 nm) were found to induce mitochondrial injury related cytotoxicity in human liver HepG2 cells, which showed more sensitivity than human colon Caco2 cells [156]. Perhaps, these differences in the toxicity mechanisms may be due to the differential behavior of different cell types that may play an important role in toxicity mechanisms. Besides these variations in cytotoxicity, consensus on the driving force behind the toxicity of AgNPs is still lacking. For instance, toxicity of AgNPs on A549 lung cells was found to be dependent on the Ag ion fraction. AgNPs suspension was found to be more toxic than its supernatant at low Ag ion fraction (≤2.6 %), whereas significant additional toxicity was not observed at high Ag ion fraction (≥5.5 %) [157]. Interestingly, cellular transcriptome analysis of the same cell line revealed alteration in the regulation of more than 1000 genes in response to AgNPs exposure for 24 h, whereas only 133 genes responded to Ag ions [158]. Further, comparison of AgNPs and CuONPs with their metal ions (CuCl2 and AgNO3) revealed the toxicity of CuONPs and AgNO3, whereas AgNPs and CuCl2 did not produce any toxicity on human lung cell lines A549 and BEAS-2B. Non-toxicity of AgNPs was explained due to low release of Ag ions within short time periods [159]. Moreover, exposure of the same cell line (BEAS-2B cells) to different sized (10, 40 and 75 nm) and surface coated AgNPs showed cytotoxicity of only 10 nm particles that is independent of surface coating and associated with the intracellular Ag release rate, an emerging evidence of ‘Trojan horse’ effect [160]. Furthermore, AgNPs (10 nm) had a greater ability to induce cyctotoxic effects in the MC3T3-E1 cells than the larger sized (50 and 100 nm) AgNPs [161]. AgNPs, mainly due to their antimicrobial properties, extensively used in health industry, food storage, textile coatings and a number of environmental applications and manufactured at a rate of 320 tons each year [162]. Moreover, out of 1814 nanoproducts listed in consumer products inventory (CPI), AgNPs are the most popular advertised nanomaterial, present in 438 products (24 %) [163]. At present, AgNPs are emerging as a viable option for the management of many plant pathogens and should be used with caution. Most of the studies demonstrated that AgNPs-induced cytotoxic effects are mainly size-dependent, and thus the particle size needs careful consideration while developing AgNPs based nanopesticides.
TiO2NPs have increasingly been used in pharmaceuticals and cosmetics due to the unique properties derived from their small size. Interestingly, nanosized TiO2 and ZnO are used in cosmetics or sunscreens and regarded safe for human [164]. Unfortunately, based on sufficient evidence in experimental animals and inadequate evidence from epidemiological studies, a report by the International Agency for Research on Cancer (IARC) of the World Health Organization declared TiO2 as a possible Group 2B carcinogenic to humans [165]. Recent studies indicate the dysfunctioning of central nervous system (CNS), ROS production induced genotoxicity and cell damage by TiO2NPs. For instance, TiO2NPs (anatase isoform), even at a lower concentration (≥0.1 μg/mL) produced cytotoxicity not only after acute but even after prolonged exposure in two CNS lines, namely human neuronal (SH-SY5Y) and human glial (D384) [166]. The underlying mechanism of strong cytotoxic effect could be related to a constitutive actin reorganization-dependent endocytic uptake and internalization of TiO2NPs by glial cells [167]. Because cancer is linked with mutation, large genotoxicity data has been generated using standard genotoxicity assays, indicate oxidative stress mediated DNA damage [168]. Although in case of AgNPs, several studies have revealed that nanoparticle size is a critical factor which can influence the toxicity, less and contradictory data is available for TiO2NPs. For instance, study on lung adenocarcinoma epithelial cell line (A549) indicates that smaller TiO2NPs were easily incorporated into the cells and cause phosphorylation of histone H2AX (γ-H2AX) that is regarded as a sensitive marker for genotoxicity [169]. By contrast, a recent study revealed that the genotoxicity is independent of TiO2NPs size. Two different sized TiO2NPs (21 and 50 nm) produced statistically significant but similar genotoxic effects in human embryonic kidney cell line (HEK293) only at a higher dose (1000 µg/mL) [170]. Perhaps, this may be due to the difference in sensitivity of different cell types to TiO2NPs. Fortunately, nearly all tests for measuring the mutagenicity of TiO2NPs were found negative in different organisms including humans [168]. Moreover, no significant absorption of TiO2 in human gut was observed after an oral dose, regardless of particle size [171]. Although the toxicity of TiO2NPs has been demonstrated by in vivo and in vitro studies, but the conclusions are not comparable and even some of them might be conflicting due to differences in the experimental parameters used by different workers [172]. Moreover, little is known about the interaction of TiO2NPs with CNS yet. TIO2NPs are used in many commercial products and the potential for human exposure is increasing. Therefore, investigations dealing with the effects of nanoparticles on the CNS with special regard to TIO2NPs are urgently needed.
There is still insufficient information regarding the particle size related toxicity of ZnONPs, including their transportation in cells and interaction with cell membrane and cell organelles [173]. ZnONPS induces oxidative stress, decreases viability, and increases cell death in Caco2 cells. The cytotoxicity of ZnONPs was found to be time and dose dependent, where 26 nm ZnONPs showed highest toxicity on Caco2 cells, and ZNONPs of 62 nm appeared less toxic than ZnONPS of 90 nm in diameter [173].Similarly, 50 nm ZnONPs showed toxicity in human lung epithelial cells (L-132) at approximately 25 μg/mL concentration, possibly through oxidative stress-induced apoptosis [174]. After 4 h of exposure, ZnO NPs (30 + 5 nm, ≥0.01 μg/mL) significantly inhibited proliferation and induced substantial apoptosis in human pulmonary adenocarcinoma cell line (LTEP-a-2) [175]. Further, an increase in intracellular ROS level up to 30–40 % coincided with approximately 70–80 % depletion of GSH content in LTEP-a-2 cells, indicating that ZnONPs induced apoptosis mainly through increased ROS production [175]. Therefore, with increasing evidences of ZnONPs mediated toxicity, more detailed investigations in the underlying toxicity mechanisms are required.
Conclusion
Global pesticide demand is predicted to increase significantly in the years to come. The application of nanopesticides and nanofertilizers in agriculture aims in particular to reduce pesticide applications and enhance yields through pest suppression and optimized nutrient management. Nanoparticles of ZnO, TiO2 and especially Ag are intentionally used in many applications, and recent studies also highlighted their potential in plant disease management. Moreover, nanoherbicides and nanofertilizers have been demonstrated to have a great future in the coming years, but a vast knowledge regarding their potential risk is required before products are brought to market. Scientific studies have established toxicity of some nanoparticles to different environments including humans; therefore there should be a strict regulation for the manufacturers to correctly represent the composition and the characteristics of their developed nanopesticide formulation. Current scientific knowledge does not offer a trustworthy measurement to be made of the associated benefits and risk of nanopesticides [14] and lack of satisfactory information and low adequacy of experimental protocols impede inclusive risk assessment. In view of this, quantitative structure–toxicity relationship (QSTR) models for prediction of cytotoxicity of metal oxide nanoparticles are developed by periodic table-based descriptors that can powerfully encode cytotoxicity of metal oxides leading to models with high statistical quality as well as interpretability. Further, the information required for descriptor calculation is independent of nanoparticles size, thus nullifying a noteworthy dilemma that various physical properties of nanoparticles change for different size ranges [176]. Recently, nano quantitative toxicity–toxicity relationship (nano-QTTR) models for interspecies cytotoxicity correlation are developed that can be employed for the extrapolation of the cytotoxicity data of one species to another [177]. Moreover, surface-enhanced Raman spectroscopy (SERS) based on 1, 2-di (4-pyridyl) ethylene (BPE), crystal violet and ferric dimethyl-dithiocarbamate (ferbam) indicator molecules were found promising in rapid detection of AgNPs in environmental and biological samples. It is important to note that ferbam showed the maximum ability to bind AgNPs and was demonstrated to detect a low as 0.1 mg/L AgNPs in genuine surface water and 0.57 mg/L in spinach juice [178]. The knowledge on the behavior of nanomaterials in different environment is rapidly evolving, but is still limited by the lack of robust and integrative research. Collaborative research among different laboratories on the effect of a particular nanoparticle type on different biological systems can make significant impact for developing safer nanopesticides. Each biological system provides a unique setting to examine the fate of nanoparticles, therefore combining the understanding on the behavior of nanoparticles across diverse ecosystem is a critical step in determining the safety of mankind in future.
References
Tabashnik BE, Brevault T, Carriere Y (2013) Insect resistance to Bt crops: lessons from the first billion acres. Nat Biotechnol 31:510–521. doi:10.1038/nbt.2597
Frumkin H, Hess J, Vindigni S (2009) Energy and public health: the challenge of peak petroleum. Public Health Rep 124(1):5–19
Department of Economic and Social Affairs of the United Nations Secretariat (2015) World population prospects: the 2015 revision, key findings and advance tables. http://esa.un.org/unpd/wpp/publications/files/key_findings_wpp_2015.pdf
Food and Agriculture Organization (2009) Global agriculture towards 2050. http://www.fao.org/fileadmin/templates/wsfs/docs/Issues_papers/HLEF2050_Global_Agriculture.pdf
Pimentel D (2009) Pesticide and pest control. In: Peshin R, Dhawan AK (eds) Integrated pest management: innovation-development process, vol 1. Springer, Dordrecht, pp 83–87. doi:10.1007/978-1-4020-8992-3
Richter ED (2002) Acute human pesticide poisonings. In: Pimentel D (ed) Encyclopedia of pest management. Dekker, New York, pp 3–6
Food and Agriculture Organization (2016) Climate change and food security: risks and responses. http://www.fao.org/3/a-i5188e.pdf
Food Quality & Nutrition (2015) India’s Farmers Fighting Pests. https://croplife.org/news/keeping-indias-pests-in-line/
Kah M, Beulke S, Tiede K, Hofmann T (2013) Nanopesticides: state of knowledge, environmental fate and exposure modelling. Crit Rev Environ Sci Technol 43:1823–1867. doi:10.1080/10643389.2012.671750
Kookana RS, Boxall ABA, Reeves PT, Ashauer R, Beulke S, Chaudhry Q, Cornelis G, Fernandes TF, Gan J, Kah M, Lynch I, Ranville J, Sinclar C, Spurgeon D, Tiede K, Van den Brink PJ (2014) Nanopesticides: guiding principles for regulatory evaluation of environmental risks. J Agric Food Chem 62:4227–4240. doi:10.1021/jf500232f
Perez-de-Luque A, Rubiales D (2009) Nanotechnology for parasitic plant control. Pest Manag Sci 65:540–545. doi:10.1002/ps.1732
Gogos A, Knauer K, Bucheli T (2012) Nanomaterials in plant protection and fertilization: current state, foreseen applications, and research priorities. J Agric Food Chem 60:9781–9792. doi:10.1021/jf302154y
Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster E (2012) Application of nanomaterials in agricultural production and crop protection: a review. Crop Prot 35:64–70. doi:10.1016/j.cropro.2012.01.007
Kah M, Hofmann T (2014) Nanopesticide research: current trends and future priorities. Environ Int 63:224–235. doi:10.1016/j.envint.2013.11.015
Australian Pesticides and Veterinary Medicines Authority (2015) Nanotechnologies for pesticides and veterinary medicines: regulatory considerations-final report. http://apvma.gov.au/node/15626
Kah M (2015) Nanopesticides and nanofertilizers: emerging contaminants or opportunities for risk mitigation. Front Chem. doi:10.3389/fchem.2015.00064
ISO/TS80004-2:2015(en) Nanotechnologies-Vocabulary-Part2: Nano-objects. https://www.iso.org/obp/ui/#iso:std:iso:ts:80004:-2:ed-1:v1:en
Boverhof DR, Bramante CM, Butala JH, Clancy SF, Lafranconi M, West J, Gordon SC (2015) Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul Toxicol Pharmacol 73(1):137–150. doi:10.1016/j.yrtph.2015.06.001
Park HJ, Kim SH, Kim HJ, Choi SH (2006) A new composition of nanosized silica-silver for control of various plant diseases. Plant Pathol J 22(3):295–302. doi:10.5423/PPJ.2006.22.3.295
Ciscato C, Barbosa C, Gebara A (2015) Analysis of Pesticide Residues in Mango by GC/MS/MS With Bond Elut QuEChERS EN Kits. http://www.agilent.com/cs/library/applications/5991-6054EN.pdf
Aguilar-Méndez MA, Martín-Martinez ES, Ortega-Arroyo L, Cobián-Portillo G, Sánchez-Espíndola E (2011) Synthesis and characterization of silver nanoparticles: effect on phytopathogen Colletotrichum gloesporioides. J Nanopart Res 13:2525–2532. doi:10.1007/s11051-010-0145-6
Mishra S, Singh BR, Singh A, Keswani C, Naqvi AH, Singh HB (2014) Biofabricated silver nanoparticles act as a strong fungicide against Bipolaris sorokiniana causing spot blotch disease in wheat. PLoS One 9(5):e97881. doi:10.1371/journal.pone.0097881
Groth DE, Bond JA (2007) Effects of cultivars and fungicides on rice sheath blight, yield, and quality. Plant Dis 91:1647–1650. doi:10.1094/PDIS-91-12-1647
Min JS, Kim KS, Kim SW, Jung JH, Lamsal K, Kim SB, Jung M, Lee YS (2009) Effects of colloidal silver nanoparticles on sclerotium-forming phytopathogenic fungi. Plant Pathol J 25(4):376–380
Krishnaraj C, Ramachandran R, Mohan K, Kalaichelvan PT (2012) Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim Acta Part A Mol Biomol Spectrosc 93:95–99. doi:10.1016/j.saa.2012.03.002
Gopinath V, Velusamy P (2013) Extracellular biosynthesis of silver nanoparticles using Bacillus sp. GP-23 and evaluation of their antifungal activity towards Fusarium oxysporum. Spectrochim Acta Part A Mol Biomol Spectrosc 106:170–174. doi:10.1016/j.saa.2012.12.087
Jo YK, Cromwell W, Jeong HK, Thorkelson J, Roh JH, Shin DB (2015) Use of silver nanoparticles for managing Gibberella fujikuroi on rice seedlings. Crop Prot 74:65–69. doi:10.1016/j.cropro.2015.04.003
Jo YK, Kim BH, Jung G (2009) Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis 93:1037–1043. doi:10.1094/PDIS-93-10-1037
Howard R, Ferrari M (1989) Role of melanin in appressorium function. Exp Mycol 13(4):403–418. doi:10.1016/0147-5975(89)90036-4
Kasprowicz MJ, Koziol M, Gorczyca A (2010) The effect of silver nanoparticles on phytopathogenic spores of Fusarium culmorum. Can J Microbiol 56(3):247–253. doi:10.1139/w10-012
Jung JH, Kim SW, Min JS, Kim YJ, Lamsal K, Kim SU, Lee YS (2010) The effect of nano-silver liquid against the white rot of the green onion caused by Sclerotium cepivorum. Mycobiology 38(1):39–45. doi:10.4489/MYCO.2010.38.1.039
Kim SW, Jung JH, Lamsal K, Kim YS, Min JS, Lee YS (2012) Antifungal effects of silver nanoparticles (AgNPs) against various plant pathogenic fungi. Mycobiology 40(1):53–58. doi:10.5941/MYCO.2012.40.1.053
Jothirethinam A, Prathiba S, Shanthi N, Arunkumar K (2015) Green synthesized silver nanoparticles prepared from the antimicrobial crude extracts of two brown seaweeds against plant pathogens. Am J Nanotechnol 6(2):31–39. doi:10.3844/ajntsp.2015.31.39
Woo KS, Kim KS, Lamsal K, Kim YJ, Kim SB, Jung M, Sim SJ, Kim HS, Chang SJ, Kim JK, Le YS (2009) An in vitro study of the antifungal effect of silver nanoparticles on oak wilt pathogen Raffaelea sp. J Microbiol Biotechnol 19(8):760–764. doi:10.4014/jmb.0812.649
Ocsoy I, Paret ML, Ocsoy MA, Kunwar S, Chen T, You M, Tan W (2013) Nanotechnology in plant disease management: DNA-directed silver nanoparticles on graphene oxide as an antibacterial against Xanthomonas perforans. ACS Nano 7(10):8972–8980. doi:10.1021/nn4034794
Strayer A, Ocsoy I, Tan W, Jones JB, Paret ML (2016) Low concentrations of a silver-based nanocomposite to manage bacterial spot of tomato in the greenhouse. Plant Dis. doi:10.1094/PDIS-05-15-0580-RE
Paret ML, Vallad GE, Averett DR, Jones JB, Olson SM (2013) Photocatalysis: effect of light-activated nanoscale formulations of TiO2 on Xanthomonas perforans and control of bacterial spot of tomato. Phytopathology 103(3):228–236. doi:10.1094/PHYTO-08-12-0183-R
Paret ML, Palmateer AJ, Knox GW (2013) Evaluation of a light-activated nanoparticle formulation of titanium dioxide with zinc for management of bacterial leaf spot on rosa ‘Noare’. HortScience 48(2):189–192
Spadaro D, Garibaldi A, Martines GF (2004) Control of Penicillium expansum and Botrytis cinerea on apple combining a biocontrol agent with hot water dipping and acibenzolar-S-methyl, baking soda, or ethanol application. Postharvest Biol Technol 33(2):141–151. doi:10.1016/j.postharvbio.2004.02.002
Miccolis De, Angelini RM, Rotolo C, Masiello M, Gerin D, Pollastro S, Faretra F (2014) Occurrence of fungicide resistance in populations of Botryotinia fuckeliana (Botrytis cinerea) on table grape and strawberry in southern Italy. Pest Manag Sci 70(12):1785–1796. doi:10.1002/ps.3711
He L, Liu Y, Mustapha A, Lin M (2011) Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol Res 166(3):207–215. doi:10.1016/j.micres.2010.03.003
Xue J, Luo Z, Li P, Ding Y, Cui Y, Wu Q (2014) A residue-free green synergistic antifungal nanotechnology for pesticide thiram by ZnO nanoparticles. Sci Rep. doi:10.1038/srep05408
Lopez-Antia A, Ortiz-Santaliestra ME, Blas EG, Camarero PR, Mougeot F, Mateo R (2015) Adverse effects of thiram-treated seed ingestion on the reproductive performance and the offspring immune function of the red-legged partridge. Environ Toxicol Chem 34(6):1320–1329. doi:10.1002/etc.2925
Timmer LW, Dewdney MM, Chung KR (2009) Florida citrus pest management guide: Melanose. In: Rogers ME, Dewdney MM, Spann TM (ed) 2009 Florida Citrus Pest Management Guide. University of Florida, IFAS, Gainesville, pp 97–98. http://polk.ifas.ufl.edu/citrus/Polk_Citrus_10-13-09/Pest_Management_files/2009%20Pest%20Management%20Guide.pdf
Mondal KK, Mani C (2012) Investigation of the antibacterial properties of nanocopper against Xanthomonas axonopodis pv. punicae, the incitant of pomegranate bacterial blight. Ann Microbiol 62(2):889–893. doi:10.1007/s13213-011-0382-7
Kanhed P, Birla S, Gaikwad S, Gade A, Seabra AB, Rubilar O, Duran N, Rai M (2014) In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi. Mater Lett 115:13–17. doi:10.1016/j.matlet.2013.10.011
Holb IJ, de Jong PF, Heijne B (2003) Efficacy and phytotoxicity of lime sulphur in organic apple production. Ann Appl Biol 142(2):225–233. doi:10.1111/j.1744-7348.2003.tb00245.x
Venzon M, Oliveira RM, Perez AL, Rodríguez-Cruz FA, Martins Filho S (2013) Lime sulfur toxicity to broad mite, to its host plants and to natural enemies. Pest Manag Sci 69(6):738–743. doi:10.1002/ps.3431
Gogoi R, Singh PK, Kumar R, Nair KK, Alam I, Srivastava C, Yadav S, Gopal M, Choudhury SR, Goswami A (2013) Suitability of nano-sulphur for biorational management of powdery mildew of Okra (Abelmoschus esculentus Moench) caused by Erysiphe cichoracearum. J Plant Pathol Microbiol 4(4):171. doi:10.4172/2157-7471.1000171
Gopal M, Kumar R, Goswami A (2012) Nano-pesticides—a recent approach for pest control. J Plant Prot Sci 4(2):1–7
Hadwiger LA (2013) Multiple effects of chitosan on plant systems: solid science or hype. Plant Sci 208:42–49. doi:10.1016/j.plantsci.2013.03.007
Saharan V, Mehrotra A, Khatik R, Rawal P, Sharma SS, Pal A (2013) Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int J Biol Macromol 62:677–683. doi:10.1016/j.ijbiomac.2013.10.012
Saharan V, Sharma G, Yadav M, Choudhary MK, Sharma SS, Pal A, Raliya R, Biswas P (2015) Synthesis and in vitro antifungal efficacy of Cu–chitosan nanoparticles against pathogenic fungi of tomato. Int J Biol Macromol 75:346–353. doi:10.1016/j.ijbiomac.2015.01.027
Chandra S, Chakraborty N, Dasgupta A, Sarkar J, Panda K, Acharya K (2015) Chitosan nanoparticles: a positive modulator of innate immune responses in plants. Sci Rep. doi:10.1038/srep15195
Wang L, Li X, Zhang G, Dong J, Eastoe J (2007) Oil-in-water nanoemulsions for pesticide formulations. J Colloid Interface Sci 314(1):220–235. doi:10.1016/j.jcis.2007.04.079
Du Z, Wang C, Tai X, Wang G, Liu X (2016) Optimization and characterization of biocompatible oil-in-water nanoemulsion for pesticide delivery. ACS Sustain Chem Eng 4(3):983–991. doi:10.1021/acssuschemeng.5b01058
Kumar RSS, Shiny PJ, Anjali CH, Jerobin J, Goshen KM, Magdassi S, Mukherjee A, Chandrasekaran N (2013) Distinctive effects of nano-sized permethrin in the environment. Environ Sci Pollut Res 20(4):2593–2602. doi:10.1007/s11356-012-1161-0
McClements DJ (2012) Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter 8(6):1719–1729. doi:10.1039/C2SM06903B
Fernandes CP, de Almeida FB, Silveira AN, Gonzalez MS, Mello CB, Feder D, Apolinário R, Santos MG, Carvalho JCT, Tietbohl LAC, Rocha L, Falcão DQ (2014) Development of an insecticidal nanoemulsion with Manilkara subsericea (Sapotaceae) extract. J Nanobiotechnol 12(22):1–9. doi:10.1186/1477-3155-12-22
Abouelkassem SH, Abdelrazeik AB, Rakha OM (2015) Nanoemulsion of jojoba oil, preparation, characterization and insecticidal activity against Sitophilus oryzae (Coleoptera: Curculionidae) on wheat. Int J Agric Innov Res 4(1):72–75
Pant M, Dubey S, Patanjali PK, Naik SN, Sharma S (2014) Insecticidal activity of eucalyptus oil nanoemulsion with karanja and jatropha aqueous filtrates. Int Biodeterior Biodegrad 91:119–127. doi:10.1016/j.ibiod.2013.11.019
Kumar J, Shakil NA, Singh MK, Singh MK, Pandey A, Pandey RP (2010) Development of controlled release formulations of azadirachtin-A employing poly (ethylene glycol) based amphiphilic copolymers. J Environ Sci Health Part B 45:310–314. doi:10.1080/03601231003704457
Shakil NA, Singh MK, Pandey A, Kumar J, Parmar VS, Singh MK, Pandey RP, Watterson AC (2010) Development of poly(ethylene glycol) based amphiphilic copolymers for controlled release delivery of carbofuran. J Macromol Sci Part A Pure Appl Chem 47(3):241–247. doi:10.1080/10601320903527038
Loha KM, Shakil NA, Kumar J, Singh MK, Adak T, Jain S (2011) Release kinetics of beta-cyfluthrin from its encapsulated formulations in water. J Environ Sci Health Part B 46(3):201–206. doi:10.1080/03601234.2011.540200
Adak T, Kumar J, Shakil NA, Walia S (2012) Development of controlled release formulations of imidacloprid employing novel nano-ranged amphiphilic polymers. J Environ Sci Health Part B 47(3):217–225. doi:10.1080/03601234.2012.634365
Sarkar DJ, Kumar J, Shakil NA, Walia S (2012) Release kinetics of controlled release formulations of thiamethoxam employing nano-ranged amphiphilic PEG and diacid based block polymers in soil. J Environ Sci Health Part A Toxic Hazard Subst Environ Eng 47(11):1701–1712. doi:10.1080/10934529.2012.687294
Pradhan S, Roy I, Lodh G, Patra P, Choudhury SR, Samanta A, Goswami A (2013) Entomotoxicity and biosafety assessment of PEGylated acephate nanoparticles: a biologically safe alternative to neurotoxic pesticides. J Environ Sci Health B 48(7):559–569. doi:10.1080/03601234.2013.774891
Kaushik P, Shakil NA, Kumar J, Singh MK, Yadav SK (2013) Development of controlled release formulations of thiram employing amphiphilic polymers and their bioefficacy evaluation in seed quality enhancement studies. J Environ Sci Health B 48(8):677–685. doi:10.1080/03601234.2013.778614
Kang MA, Seo MJ, Hwang IC, Jang C, Park HJ, Yu YM, Youn YN (2012) Insecticidal activity and feeding behavior of the green peach aphid, Myzus persicae, after treatment with nano types of pyrifluquinazon. J Asia Pac Entomol 15(4):533–541. doi:10.1016/j.aspen.2012.05.015
Hwang IC, Kim TH, Bang SH, Kim KS, Kwon HR, Seo MJ, Youn YM, Park HJ, Aoki CY, Yu YM (2011) Insecticidal effect of controlled release formulations of etofenprox based on nano-bio technique. J Fac Agric Kyushu Univ 56(1):33–40
Nguyen HM, Hwang IC, Park JW, Park HJ (2012) Photoprotection for deltamethrin using chitosan-coated beeswax solid lipid nanoparticles. Pest Manag Sci 68(7):1062–1068. doi:10.1002/ps.3268
Nguyen HM, Hwang IC, Park JW, Park HJ (2012) Enhanced payload and photo-protection for pesticides using nanostructured lipid carriers with corn oil as liquid lipid. J Microencapsul 29(6):596–604. doi:10.3109/02652048.2012.668960
de Carvalho S, Vendramim JD, de Sá ICG, da Silva MFDGF, Ribeiro LP, Forim MR (2015) Systemic insecticidal effect of neem-based nanoformulations against Bemisia tabaci (Hemiptera: Aleyrodidae) biotype B in tomato. Bragantia 74(3):298–306. doi:10.1590/1678-4499.0404
Heuskin S, Verheggen FJ, Haubruge E, Wathelet JP, Lognay G (2011) The use of semiochemical slow-release devices in integrated pest management strategies. Biotechnol Agron Soc Environ 15(3):459–470
Hellmann H, Greiner A, Wendorff JH (2011) Design of pheromone releasing nanofibers for plant protection. Polym Adv Technol 22(4):407–413. doi:10.1002/pat.1532
Bhagat D, Samanta SK, Bhattacharya S (2013) Efficient management of fruit pests by pheromone nanogels. Sci Rep. doi:10.1038/srep01294
Ahmad R, Hussein MZ, Kadir WRWA, Sarijo SH, Hin TYY (2015) Evaluation of controlled-release property and phytotoxicity effect of insect pheromone zinc-layered hydroxide nanohybrid intercalated with hexenoic acid. J Agric Food Chem 63(51):10893–10902. doi:10.1021/acs.jafc.5b03102
Ali MA, Rehman I, Iqbal A, Din S, Rao AQ, Latif A, Samiullah TR, Azam S, Husnain T (2014) Nanotechnology: a new frontier in Agriculture. Adv Life Sci 1(3):129–138
Prasad R, Kumar V, Prasad KS (2014) Nanotechnology in sustainable agriculture: present concerns and future aspects. Afr J Biotechnol 13(6):705–713. doi:10.5897/AJBX2013.13554
Maruyama CR, Guilger M, Pascoli M, Bileshy-José M, Abhilash PC, Fraceto LF, de Lima R (2016) Nanoparticles based on chitosan as carriers for the combined herbicides imazapic and imazapyr. Sci Rep. doi:10.1038/srep19768
Grillo R, Pereiraa AES, Nishisaka CS, de Lima R, Oehlke K, Greiner R, Fraceto LF (2014) Chitosan/tripolyphosphate nanoparticles loaded with paraquat herbicide: an environmentally safer alternative for weed control. J Hazard Mater 278:163–171. doi:10.1016/j.jhazmat.2014.05.079
Grillo R, Rosa AH, Fraceto LF (2014) Poly(ε-caprolactone) nanocapsules carrying the herbicide atrazine: effect of chitosan-coating agent on physico-chemical stability and herbicide release profile. Int J Environ Sci Technol 11(6):1691–1700. doi:10.1007/s13762-013-0358-1
Pereira AE, Grillo R, Mello NF, Rosa AH, Fraceto LF (2014) Application of poly (epsilon-caprolactone) nanoparticles containing atrazine herbicide as an alternative technique to control weeds and reduce damage to the environment. J Hazard Mater 268:207–215. doi:10.1016/j.jhazmat.2014.01.025
Clemente Z, Grillo R, Jonsson M, Santos NZ, Feitosa LO, Lima R, Fraceto LF (2014) Ecotoxicological evaluation of poly(epsilon-caprolactone) nanocapsules containing triazine herbicides. J Nanosci Nanotechnol 14(7):4911–4917. doi:10.1166/jnn.2013.8681
Grillo R, dos Santos NZ, Maruyama CR, Rosa AH, de Lima R, Fraceto LF (2012) Poly (ε-caprolactone)nanocapsules as carrier systems for herbicides: physico-chemical characterization and genotoxicity evaluation. J Hazard Mater 231–232:1–9. doi:10.1016/j.jhazmat.2012.06.019
de Oliveira JL, Campos EV, Gonçalves da Silva CM, Pasquoto T, Lima R, Fraceto LF (2015) Solid lipid nanoparticles co-loaded with simazine and atrazine: preparation, characterization, and evaluation of herbicidal activity. J Agric Food Chem 63(2):422–432. doi:10.1021/jf5059045
Kah M, Machinski P, Koerner P, Tiede K, Grillo R, Fraceto LF, Hofmann T (2014) Analysing the fate of nanopesticides in soil and the applicability of regulatory protocols using a polymer-based nanoformulation of atrazine. Environ Sci Pollut Res 21(20):11699–11707. doi:10.1007/s11356-014-2523-6
Subramanian KS, Manikandan A, Thirunavukkarasu M, Rahale CS (2015) Nano-fertilizers for balanced crop nutrition. In: Rai M, Ribeiro C, Mattoso L, Duran N (ed) Nanotechnologies in food and agriculture. Springer, pp 69–80. doi:10.1007/978-3-319-14024-7_3
Kottegoda N, Munaweera I, Madusanka N, Karunaratne V (2011) A green slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsulated wood. Curr Sci 101(1):73–78
Derosa MC, Monreal C, Schnitzer M, Walsh RP, Sultan Y (2010) Nanotechnology in fertilizers. Nat Nanotechnol 5:91. doi:10.1038/nnano.2010.2
Naderi MR, Danesh-Shahraki A (2013) Nanofertilizers and their roles in sustainable agriculture. Int J Agric Crop Sci 5(19):2229–2232
Pereira EI, Minussi FB, da Cruz CCT, Bernardi ACC, Ribeiro C (2012) Urea–montmorillonite-extruded nanocomposites: a novel slow-release material. J Agric Food Chem 60(21):5267–5272. doi:10.1021/jf3001229
Manikandan A, Subramanian KS (2014) Fabrication and characterisation of nanoporous zeolite based N fertilizer. Afr J Agric Res 9(2):276–284. doi:10.5897/AJAR2013.8236
Manikandan A, Subramanian KS (2016) Evaluation of zeolite based nitrogen nano-fertilizers on maize growth, yield and quality on inceptisols and alfisols. Int J Plant Soil Sci 9(4):1–9. doi:10.9734/IJPSS/2016/22103
Liu R, Lal R (2014) Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci Rep. doi:10.1038/srep05686
Ekinci M, Dursun A, Yildirim E, Parlakova F (2014) Effects of nanotechnology liquid fertilizers on the plant growth and yield of cucumber (Cucumis sativus L.). Acta Sci Pol Hortic 13(3):135–141
Amirnia R, Bayat M, Tajbakhsh M (2014) Effects of nano fertilizer application and maternal corm weight on flowering at some saffron (Crocus sativus L.) ecotypes. Turk J Field Crops 19(2):158–168
Bakhtiari M, Moaveni P, Sani B (2015) The effect of iron nanoparticles spraying time and concentration on wheat. Biol Forum Int J 7(1):679–683
Sh Soliman A, El-feky SA, Darwish E (2015) Alleviation of salt stress on Moringa peregrina using foliar application of nanofertilizers. J Hortic For 7(2):36–47. doi:10.5897/JHF2014.0379
Wu MY (2013) Effects of incorporation of nano-carbon into slow-released fertilizer on rice yield and nitrogen loss in surface water of paddy soil. In: Intelligent system design and engineering applications (ISDEA), 2013 third international conference on, Hong Kong, pp–676-681. doi: 10.1109/ISDEA.2012.161
Bortolin A, Aouada FA, Mattoso LHC, Ribeiro C (2013) Nanocomposite PAAm/methyl cellulose/montmorillonite hydrogel: evidence of synergistic effects for the slow release of fertilizers. J Agric Food Chem 61(31):7431–7439. doi:10.1021/jf401273n
Pereira EI, da Cruz CCT, Solomon A, Le A, Cavigelli MA, Ribeiro C (2015) Novel slow-release nanocomposite nitrogen fertilizers: the impact of polymers on nanocomposite properties and function. Ind Eng Chem Res 54(14):3717–3725. doi:10.1021/acs.iecr.5b00176
Sempeho SI, Kim HT, Mubofu E, Pogrebnoi E, Shao G, Hilonga A (2015) Encapsulated urea-kaolinite nanocomposite for controlled release fertilizer formulations. J Chem. doi:10.1155/2015/237397
Wen P, Wu Z, He Y, Han Y, Tong Y (2016) Characterization of p(AA-co-AM)/bent/urea and its swelling and slow release behavior in a simulative soil environment. J Appl Polym Sci 133(12):43082. doi:10.1002/app.43082
Yamamotoa CF, Pereirab EI, Mattosob LHC, Matsunakac T, Ribeiro C (2016) Slow release fertilizers based on urea/urea–formaldehyde polymer nanocomposites. Chem Eng J 287:390–397. doi:10.1016/j.cej.2015.11.023
Keller AA, McFerran S, Lazareva A, Suh S (2013) Global life cycle releases of engineered nanomaterials. J Nanopart Res 15:1692. doi:10.1007/s11051-013-1692-4
Sillen WMA, Thijs S, Abbamondi GR, Janssen J, Weyens N, White JC, Vangronsveld J (2015) Effects of silver nanoparticles on soil microorganisms and maize biomass are linked in the rhizosphere. Soil Biol Biochem 91:14–22. doi:10.1016/j.soilbio.2015.08.019
Sweet MJ, Singleton I (2015) Soil contamination with silver nanoparticles reduces Bishop pine growth and ectomycorrhizal diversity on pine roots. J Nanopart Res 17:448. doi:10.1007/s11051-015-3246-4
Calder AJ, Dimkpa CO, McLean JE, Britt DW, Johnson W, Anderson AJ (2012) Soil components mitigate the antimicrobial effects of silver nanoparticles towards a beneficial soil bacterium, Pseudomonas chlororaphis O6. Sci Total Environ 429:215–222. doi:10.1016/j.scitotenv.2012.04.049
Kumar N, Shah V, Walker VK (2011) Perturbation of an arctic soil microbial community by metal nanoparticles. J Hazard Mater 190(1–3):816–822. doi:10.1016/j.jhazmat.2011.04.005
Kumar N, Shah V, Walker VK (2012) Influence of a nanoparticle mixture on an arctic soil community. Environ Toxicol Chem 31(1):131–135. doi:10.1002/etc.721
Coutris C, Joner EJ, Oughton DH (2012) Aging and soil organic matter content affect the fate of silver nanoparticles in soil. Sci Total Environ 420:327–333. doi:10.1016/j.scitotenv.2012.01.027
Pal S, Tak YK, Song J (2007) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol 73(6):1712–1720. doi:10.1128/AEM.02218-06
El Badawy AM, Silva RG, Morris B, Scheckel KG, Suidan MT, Tolaymat TM (2011) Surface charge-dependent toxicity of silver nanoparticles. Environ Sci Technol 45(1):283–287. doi:10.1021/es1034188
Costanza J, El Badawy AM, Tolaymat TM (2011) Comment on “120 years of nanosilver history: implications for policy makers”. Environ Sci Technol 45(17):7591–7592. doi:10.1021/es200666n
Ge Y, Schimel JP, Holden PA (2012) Identification of soil bacteria susceptible to TiO2 and ZnO nanoparticles. Appl Environ Microbiol 78(18):6749–6758. doi:10.1128/AEM.00941-12
Shen Z, Chen Z, Hou Z, Li T, Lu X (2015) Ecotoxicological effect of zinc oxide nanoparticles on soil microorganisms. Front Environ Sci Eng 9(5):912–918. doi:10.1007/s11783-015-0789-7
Gajjar P, Pettee B, Britt DW, Huang W, JohnsonWP Anderson AJ (2009) Antimicrobial activities of commercial nanoparticles against an environmental soil microbe, Pseudomonas putida KT2440. J Biol Eng 3(9):1–13. doi:10.1186/1754-1611-3-9
Kumar R, Gopal M, Pabbi S, Paul S, Imteyaz A, Yadav S, Nair KK, Chauhan N, Srivastava C, Gogoi R, Singh PK, Goswami A (2016) Effect of nanohexaconazole on nitrogen fixing blue green algae and bacteria. J Nanosci Nanotechnol 16(1):643–647. doi:10.1166/jnn.2016.10901
Kumar R, Nair KK, Imteyaz A, Gogoi R, Singh PK, Srivastava C, Gopal M, Goswami A (2015) Development and quality control of nanohexaconazole as an effective fungicide and its biosafety studies on soil nitifiers. J Nanosci Nanotechnol 15(2):1350–1356. doi:10.1166/jnn.2015.9088
Shin YJ, Kwak J, An YJ (2012) Evidence for the inhibitory effects of silver nanoparticles on the activities of soil exoenzymes. Chemosphere 88(4):524–529. doi:10.1016/j.chemosphere.2012.03.010
Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR (2011) Silver nanoparticles: behaviour and effects in the aquatic environment. Environ Int 37(2):517–531. doi:10.1016/j.envint.2010.10.012
Heckmann LH, Hovgaard MB, Sutherland DS, Autrup H, Besenbacher F, Scott-Fordsmand JJ (2011) Limit-test toxicity screening of selected inorganic nanoparticles to the earthworm Eisenia fetida. Ecotoxicology 20(1):226–233. doi:10.1007/s10646-010-0574-0
Shoults-Wilson WA, Zhurbich OI, McNear DH, Tsyusko OV, Bertsch PM, Unrine JM (2011) Evidence for avoidance of Ag nanoparticles by earthworms (Eisenia fetida). Ecotoxicology 20(2):385–396. doi:10.1007/s10646-010-0590-0
Shoults-Wilson WA, Reinsch BC, Tsyusko OV, Bertsch PM, Lowry GV, Unrine JM (2011) Effect of silver nanoparticle surface coating on bioaccumulation and reproductive toxicity in earthworms (Eisenia fetida). Nanotoxicology 5(3):432–444. doi:10.3109/17435390.2010.537382
Diez-Ortiz M, Lahive E, George S, Ter Schure A, Van Gestel CA, Jurkschat K, Svendsen C, Spurgeon DJ (2015) Short-term soil bioassays may not reveal the full toxicity potential for nanomaterials; bioavailability and toxicity of silver ions (AgNO3) and silver nanoparticles to earthworm Eisenia fetida in long-term aged soils. Environ Pollut 203:191–198. doi:10.1016/j.envpol.2015.03.033
Unrine JM, Hunyadi SE, Tsyusko OV, Rao W, Shoults-Wilson WA, Bertsch PM (2010) Evidence for bioavailability of Au nanoparticles from soil and biodistribution within earthworms (Eisenia fetida). Environ Sci Technol 44(21):8308–8313. doi:10.1021/es101885w
Unrine JM, Shoults-Wilson WA, Zhurbich O, Bertsch PM, Tsyusko OV (2012) Trophic transfer of Au nanoparticles from soil along a simulated terrestrial food chain. Environ Sci Technol 46(17):9753–9760. doi:10.1021/es3025325
Unrine JM, Tsyusko OV, Hunyadi SE, Judy JD, Bertsch PM (2010) Effects of particle size on chemical speciation and bioavailability of copper to earthworms (Eisenia fetida) exposed to copper nanoparticles. J Environ Qual 39(6):1942–1953. doi:10.2134/jeq2009.0387
McShane H, Sarrazin M, Whalen JK, Hendershot WH, Sunahara GI (2012) Reproductive and behavioral responses of earthworms exposed to nano-sized titanium dioxide in soil. Environ Toxicol Chem 31(1):184–193. doi:10.1002/etc.714
Cañas JE, Qi B, Li S, Maul JD, Cox SB, Das S, Green MJ (2011) Acute and reproductive toxicity of nano-sized metal oxides (ZnO and TiO2) to earthworms (Eisenia fetida). J Environ Monit 13(12):3351–3357. doi:10.1039/c1em10497g
Whitfield Åslund ML, McShane H, Simpson MJ, Simpson AJ, Whalen JK, Hendershot WH, Sunahara GI (2012) Earthworm sublethal responses to titanium dioxide nanomaterial in soil detected by 1H NMR metabolomics. Environ Sci Technol 46(2):1111–1118. doi:10.1021/es202327k
Li L-Z, Zhou DM, Peijnenburg WJ, Van Gestel CA, Jin SY, Wang YJ, Wang P (2011) Toxicity of zinc oxide nanoparticles in the earthworm, Eisenia fetida and subcellular fractionation of Zn. Environ Int 37(6):1098–1104. doi:10.1016/j.envint.2011.01.008
Hooper HL, Jurkschat K, Morgan AJ, Bailey J, Lawlor AJ, Spurgeon DJ, Svendsen C (2011) Comparative chronic toxicity of nanoparticulate and ionic zinc to the earthworm Eisenia veneta in a soil matrix. Environ Int 37(6):1111–1117. doi:10.1016/j.envint.2011.02.019
Song U, Jun H, Waldman B, Roh J, Kim Y, Yi J, Ju Lee E (2013) Functional analyses of nanoparticle toxicity: A comparative study of the effects of TiO2 and Ag on tomatoes (Lycopersicon esculentum). Ecotoxicol Environ Saf 93:60–67. doi:10.1016/j.ecoenv.2013.03.033
Thuesombata P, Hannongbuab S, Akasitb S, Chadchawanc S (2014) Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol Environ Saf 104:302–309. doi:10.1016/j.ecoenv.2014.03.022
Mirzajani F, Askari H, Hamzelou S, Farzaneh M, Ghassempour A (2013) Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicol Environ Saf 88:48–54. doi:10.1016/j.ecoenv.2012.10.018
Asli S, Neumann PM (2009) Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ 32:577–584. doi:10.1111/j.1365-3040.2009.01952.x
Burke DJ, Zhu S, Pablico-Lansigan MP, Hewins CR, Samia ACS (2014) Titanium oxide nanoparticle effects on composition of soil microbial communities and plant performance. Biol Fertil Soils 50(7):1169–1173. doi:10.1007/s00374-014-0938-3
Moll J, Gogos A, Bucheli TD, Widmer F, Van-der Heijden MGA (2016) Effect of nanoparticles on red clover and its symbiotic microorganisms. J Nanobiotechnol 14(36):1–8. doi:10.1186/s12951-016-0188-7
Khodakovskaya MV, Kim B-S, Kim JN, Alimohammadi M, Dervishi E, Mustafa T, Cernigla CE (2013) Carbon nanotubes as plant growth regulators: effects on tomato growth, reproductive system, and soil microbial community. Small 9(1):115–123. doi:10.1002/smll.201201225(COMPLETE)
Mukherjee A, Peralta-Videa JR, Bandyopadhyay S, Rico CM, Zhao L, Gardea-Torresdey JL (2014) Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics 6:132–138. doi:10.1039/C3MT00064H
Yang Z, Chen J, Dou R, Gao X, Mao C, Wang L (2015) Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants, maize (Zea mays L.) and rice (Oryza sativa L.). Int J Environ Res Public Health 12(12):15100–15109. doi:10.3390/ijerph121214963
Prakash M, Nair G, Chung IM (2015) Study on the correlation between copper oxide nanoparticles induced growth suppression and enhanced lignification in Indian mustard (Brassica juncea L.). Ecotoxicol Environ Saf 113:302–313. doi:10.1016/j.ecoenv.2014.12.013
Caniveta L, Dubotc P, Garçonb G, Denayera FO (2015) Effects of engineered iron nanoparticles on the bryophyte, Physcomitrella patens (Hedw.) Bruch & Schimp, after foliar exposure. Ecotoxicol Environ Saf 113:499–505. doi:10.1016/j.ecoenv.2014.12.035
Libralato G, Devoti AC, Zanella M, Sabbioni E, Mičetić I, Manodor L, Pigozzo A, Manenti S, Groppi F, Ghirardini AV (2016) Phytotoxicity of ionic, micro- and nano-sized iron in three plant species. Ecotoxicol Environ Saf 123:81–88. doi:10.1016/j.ecoenv.2015.07.024
Wang J, Fang Z, Cheng W, Tsang EP, Zhao D (2016) Ageing decreases the phytotoxicity of zero-valent iron nanoparticles in soil cultivated with Oryza sativa. Ecotoxicology. doi:10.1007/s10646-016-1674-2
Andreotti F, Mucha AP, Caetano C, Rodrigues P, Gomes CR, Almeida CMR (2015) Interactions between salt marsh plants and Cu nanoparticles—effects on metal uptake and phytoremediation processes. Ecotoxicol Environ Saf 120:303–309. doi:10.1016/j.ecoenv.2015.06.017
Li L, Sillanpa M, Tuominen M, Lounatmaa K, Schultz E (2013) Behavior of titanium dioxide nanoparticles in Lemna minor growth test conditions. Ecotoxicol Environ Saf 88:89–94. doi:10.1016/j.ecoenv.2012.10.024
Lanone S, Rogerieux F, Geys J, Dupont A, Maillot-Marechal E, Boczkowski J, Lacroix G, Hoet P (2009) Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Part Fibre Toxicol 6(14):1–12. doi:10.1186/1743-8977-6-14
Sambale F, Wagner S, Stahl F, Khaydarov RR, Scheper T, Bahnemann D (2015) Investigations of the toxic effect of silver nanoparticles on mammalian cell lines. J Nanomater. doi:10.1155/2015/136765
Avalos A, Haza AI, Morales P (2015) Manufactured silver nanoparticles of different sizes induced DNA strand breaks and oxidative DNA damage in hepatoma and leukaemia cells and in dermal and pulmonary fibroblasts. Folia Biol (Praha) 61(1):33–42
Avalos A, Haza AI, Mateo D, Morales P (2016) Interactions of manufactured silver nanoparticles of different sizes with normal human dermal fibroblasts. Int Wound J 13(1):101–109. doi:10.1111/iwj.12244
Avalos A, Haza AI, Mateo D, Morales P (2015) Effects of silver and gold nanoparticles of different sizes in human pulmonary fibroblasts. Toxicol Mech Methods 25(4):287–295. doi:10.3109/15376516.2015.1025347
Liu W, Wu Y, Wang C, Li HC, Wang T, Liao CY, Cui L, Zhou QF, Yan B, Jiang GB (2010) Impact of silver nanoparticles on human cells: effect of particle size. Nanotoxicology 4(3):319–330. doi:10.3109/17435390.2010.483745
Sahu SC, Zheng J, Graham L, Chen L, Ihrie J, Yourick JJ, Sprando RL (2014) Comparative cytotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells in culture. J Appl Toxicol 34(11):1155–1166. doi:10.1002/jat.2994
Beer C, Foldbjerg R, Hayashi Y, Sutherland DS, Autrup H (2012) Toxicity of silver nanoparticles—nanoparticle or silver ion? Toxicol Lett 208(3):286–292. doi:10.1016/j.toxlet.2011.11.002
Foldbjerg R, Irving ES, Hayashi Y, Sutherland DS, Thorsen K, Autrup H, Beer C (2012) Global gene expression profiling of human lung epithelial cells after exposure to nanosilver. Toxicol Sci 130(1):145–157. doi:10.1093/toxsci/kfs225
Cronholm P, Karlsson HL, Hedberg J, Lowe TA, Winnberg L, Elihn K, Wallinder IO, Moller L (2013) Intracellular uptake and toxicity of Ag and CuO nanoparticles: a comparison between nanoparticles and their corresponding metal ions. Small 9(7):970–982. doi:10.1002/smll.201201069
Gliga AR, Skoglund S, Wallinder IO, Fadeel B, Karlsson HL (2014) Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part Fibre Toxicol 11(11):1–17. doi:10.1186/1743-8977-11-11
Kim TH, Kim M, Park HS, Shin US, Gong MS, Kim HW (2012) Size-dependent cellular toxicity of silver nanoparticles. J Biomed Mater Res Part A 100(4):1033–1043. doi:10.1002/jbm.a.34053
Nowack B, Krug HF, Height M (2011) 120 years of nanosilver history: implications for policy makers. Environ Sci Technol 45(4):1177–1183. doi:10.1021/es103316q
Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MF Jr, Rejeski D, Hull MS (2015) Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol 6:1769–1780. doi:10.3762/bjnano.6.181
Schilling K, Bradford B, Castelli D, Dufour E, Nash JF, Pape W, Schulte S, Tooley I, van den Bosch J, Schellauf F (2010) Human safety review of “nano” titanium dioxide and zinc oxide. Photochem Photobiol Sci 9(4):495–509. doi:10.1039/b9pp00180h
IARC (2010) IARC monographs on the evaluation of carcinogenic risks to humans: carbon black, titanium dioxide, and talc. http://monographs.iarc.fr/ENG/Monographs/vol93/mono93.pdf
Coccini T, Grandi S, Lonati D, Locatelli C, De Simone U (2015) Comparative cellular toxicity of titanium dioxide nanoparticles on human astrocyte and neuronal cells after acute and prolonged exposure. Neurotoxicology 48:77–89. doi:10.1016/j.neuro.2015.03.006
Huerta-García E, Márquez-Ramírez SG, Ramos-Godinez MP, López-Saavedra A, Herrera LA, Parra A, Alfaro-Moreno E, Gomez EO, López-Marure R (2015) Internalization of titanium dioxide nanoparticles by glial cells is given at short times and is mainly mediated by actin reorganization-dependent endocytosis. Neurotoxicology 51:27–37. doi:10.1016/j.neuro.2015.08.013
Chen T, Yan J, Li Y (2014) Genotoxicity of titanium dioxide nanoparticles. J Food Drug Anal 22(1):95–104. doi:10.1016/j.jfda.2014.01.008
Toyooka T, Amano T, Ibuki Y (2012) Titanium dioxide particles phosphorylate histone H2AX independent of ROS production. Mutat Res Genet Toxicol Environ Mutagen 742(1–2):84–91. doi:10.1016/j.mrgentox.2011.12.015
Demir E, Akça H, Turna F, Aksakal S, Burgucu D, Kaya B, Tokgün O, Vales G, Creus A, Marcos R (2015) Genotoxic and cell-transforming effects of titanium dioxide nanoparticles. Environ Res 136:300–308. doi:10.1016/j.envres.2014.10.032
Jones K, Mortona J, Smith I, Jurkschat K, Harding A-H, Evans G (2015) Human in vivo and in vitro studies on gastrointestinal absorption of titanium dioxide nanoparticles. Toxicol Lett 233(2):95–101. doi:10.1016/j.toxlet.2014.12.005
Song B, Liu J, Feng X, Wei L, Shao L (2015) A review on potential neurotoxicity of titanium dioxide nanoparticles. Nanoscale Res Lett 10(342):1–17. doi:10.1186/s11671-015-1042-9
Kang T, Guan R, Chen X, Song Y, Jiang H, Zhao J (2013) In vitro toxicity of different-sized ZnO nanoparticles in Caco-2 cells. Nanoscale Res Lett 8(496):1–8. doi:10.1186/1556-276X-8-496
Sahu D, Kannan GM, Vijayaraghavan R, Anand T, Khanum F (2013) Nanosized zinc oxide induces toxicity in human lung cells. ISRN Toxicol. doi:10.1155/2013/316075->
Wang C, Hu X, Gao Y, Ji Y (2015) ZnO nanoparticles treatment induces apoptosis by increasing intracellular ROS levels in LTEP-a-2 cells. Biomed Res Int. doi:10.1155/2015/423287
Kar S, Gajewiczb A, Puzynb T, Roy K, Leszczynskic J (2014) Periodic table-based descriptors to encode cytotoxicity profile of metal oxide nanoparticles: a mechanistic QSTR approach. Ecotoxicol Environ Saf 107:162–169. doi:10.1016/j.ecoenv.2014.05.026
Kar S, Gajewiczb A, Roy K, Leszczynskic J, Puzyn T (2016) Extrapolating between toxicity endpoints of metal oxide nanoparticles: predicting toxicity to Escherichia coli and human keratinocyte cell line (HaCaT) with Nano-QTTR. Ecotoxicol Environ Saf 126:238–244. doi:10.1016/j.ecoenv.2015.12.033
Guo H, Xing B, Hamlet LC, Chica A, He L (2016) Surface-enhanced Raman scattering detection of silver nanoparticles in environmental and biological samples. Sci Total Environ. doi:10.1016/j.scitotenv.2016.02.084
Acknowledgments
The first and the last authors acknowledge the support of the training programmes, “Specialized Training Programme on Nanotechnology” and “Specialized Training Programme on Nanotechnology: Production, Estimation and Characterization of Nanoparticles”, organized by Institute of Wood Science and Technology, Bengaluru, India, during 2012 and 2013. They wish to acknowledge https://mindthegraph.com/ for giving permission to use their icons in the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pandey, S., Giri, K., Kumar, R. et al. Nanopesticides: Opportunities in Crop Protection and Associated Environmental Risks. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 88, 1287–1308 (2018). https://doi.org/10.1007/s40011-016-0791-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-016-0791-2