Abstract
Nanotechnology is among the most innovative fields of twenty-first century. Nanoparticles (NPs) are organic or inorganic materials having sizes ranging from 1 to 100 nm; in recent years NPs have come into extensive use worldwide. The dramatic increase in use of NPs in numerous applications has greatly increased the likelihood of their release to the environment. Zinc oxide nanoparticles (ZnO-NPs) are considered a ‘biosafe material’ for organisms. Earlier studies have demonstrated the potential of ZnO-NPs for stimulation of seed germination and plant growth as well as disease suppression and plant protection by virtue of their antimicrobial activity. Both positive and negative effects of ZnO NPs on plant growth and metabolism at various developmental periods have been documented. Uptake, translocation and accumulation of ZnO-NPs by plants depend upon the distinct features of the NPs as well as on the physiology of the host plant. This review will contribute to current understanding the fate and behavior of ZnO-NPs in plants, their uptake, translocation and impacts on mitigating several negative plant growth conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
The “Nano-Era” that emerged in the 1990s is now a progressive field of research and technology. Nanotechnology is endowed with far-ranging applications in various sectors such as- cancer therapy, targeted drug delivery, biomedicines , waste-water treatment, cosmetic industries, electronics and biosensors (Nel et al. 2006; Peralta-Videa et al. 2011). The development of nanotechnology in conjunction with biotechnology has significantly expanded the application of nanomaterials in the above fields.
Materials with a particle size less than 100 nm in at least one dimension are generally classified as nanoparticles (NPs) (Khot et al. 2012). NPs divided into three main classes: natural, incidental, and engineered. Since the earth’s formation, natural NPs have been present from sources such as volcanic ejecta and soil dispersal (Handy et al. 2008). The second class, incidental NPs, results from human industrial activities, for example- burning coal, welding and from diesel exhaust (Monica and Cremonini 2009). Of immediate interest is the third category which represents engineered nanoparticles (ENPs). These are designed and synthesized to possess unique physicochemical properties such as conductivity, reactivity, and optical sensitivity which may differ markedly compared to their bulk form (Lin and Xing 2007). Engineered nanoparticles are further divided into five subclasses: carbonaceous nanoparticles, metal oxide nanoparticles, semiconductors, metal nanoparticles, and nanopolymers (Handy et al. 2008; Monica and Cremonini 2009; Ma et al. 2010; Bhatt and Tripathi 2011).
Based on core material, NPs can be broadly divided into inorganic and organic forms. Inorganic NPs includes metals (Al, Bi, Co, Cu, Au, Fe, In, Mo, Ni, Ag, Sn, Ti, W, Zn), metal oxides (Al2O3, CeO2, CuO, Cu2O, In2O3, La2O3, MgO, NiO, TiO2, SnO2, ZnO, ZrO2) and quantum dots, while fullerenes and carbon nanotubes comprise-organic NPs.
Metal-based NPs are widely used and presumably released to the environment; they must, therefore, be monitored for their potential toxic effects on activity, abundance and diversity among flora and fauna. To illustrate, some NPs are estimated to be absorbed 15–20 times more than their bulk particles by plants (Srivastav et al. 2016). It is estimated that 260,000–309,000 metric tons of NPs were produced globally in 2010 (Yadav et al. 2014). As per another estimate, worldwide consumption of NPs is likely to grow from 225,060 metric tons to nearly 585,000 metric tons between 2014–2019 (BCC Research 2014).
ZnO-NPs, with an estimated global annual production between 550 and 33,400 tons, are the third most commonly used metal-containing nanomaterial (Bondarenko et al. 2013; Connolly et al. 2016; Peng et al. 2017). Environmental levels of ZnO-NPs were reported to be in the range of 3.1–31 μg/kg soil and 76–760 μg/L water (Boxall et al. 2007; Ghosh et al. 2016). ZnO is a bio-safe material that possesses photo-oxidizing and photocatalytic capabilities for both chemicals and biota (Sirelkhatim et al. 2015; Vaseem et al. 2010).
The use of NPs in agriculture is a promising area which could potentially improve prevailing crop management over the long-term. For example, use of nano-encapsulated pesticides have been successfully applied for the release of chemicals in a controlled and specifically targeted manner which provides for a safer and easier pest control system (Beddington 2010; Nair et al. 2010). NPs are generally believed to increase profitability and sustainability, both of which are essential requirements for improved agricultural production (Som et al. 2010).
Zinc (Zn) is an essential micronutrient for normal growth in animals, humans, and plants. Numerous studies have focused on the effect of zinc on plant growth and metabolism (Auld 2001). It is vital to crop nutrition, as it is required in various enzymatic reactions, metabolic processes, and oxidation-reduction reactions. Zinc is essential for proper activity of enzymes such as dehydrogenases, aldolases, iso-merases, transphosphorylases, and RNA and DNA polymerases, which are required for a range of critical physiologic functions (Lacerda et al. 2018). Zinc is also involved in synthesis of tryptophan, cell division, and maintenance of membrane structure and photosynthesis. It acts as a regulatory cofactor in protein synthesis (Lacerda et al. 2018; Marschner 2011). Thus, adequate Zn fertilization supports increase cereal, vegetable and forage production (Prasad et al. 2012).
Zinc insufficiency is characterized by reduced leaf size with interveinal necrosis and rippled leaf margins. Low values of leaf area, SPAD values, and total N and NO3 concentration were observed under conditions of severe zinc deficiency (Castillo-Gonzalez et al. 2019). With worsening deficiency, activities of superoxide dismutase, catalase and glutathione peroxidase increased. Under severe Zn deficiency, decreases in trunk cross-sectional area, yield and percentage kernel are observed. Increased activity of superoxide dismutase, catalase and peroxidase enzymes is associated with detoxification of reactive oxygen species.
Zinc deficiency not only retards growth and yield of plants, but also imparts adverse effects to humans (Singh 2009; Shukla et al. 2014). More than 3 billion people worldwide suffer from Fe and Zn deficiencies. This condition is particularly widespread in areas where population is heavily dependent on a regular diet of cereal-based foods, in which Fe and Zn are stored almost exclusively in the husk and are therefore lost during milling and polishing (Cakmak 2002; Graham et al. 2001).
Zinc oxide (ZnO) is an amphoteric oxide which is almost insoluble in water and alcohol, but is soluble in most acids (Spero et al. 2000). ZnO crystallizes into two main forms, hexagonal wurtzite and cubic zincblende. The wurtzite structure is the most stable form at ambient conditions. Due to its unique properties including high thermal conductivity, refractive index, binding energy, UV protection and antibacterial capabilities, ZnO-NPs are widely applied in numerous products and materials including medicine, cosmetics, solar cells, rubber and concrete, and-foods (Uikey and Vishwakarma 2016). ZnO-NPs are the most common Zn-NPs used as a UV protector (e.g., in personal care products, coatings and paints), biosensors, electronics, and in rubber manufacture (Brayner et al. 2010; Kool et al. 2011). The wide range of industrial applications for ZnO-NPs can be used to predict future increases, in their production. The economical application of ZnO-NPs as Zn fertilizers can eventually become practical in large-scale agriculture globally.
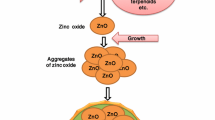
4.2 Synthesis of ZnO-NPs
Several methods have been reported for synthesis of ZnO-NPs. The primary objective of each method is development of stable and uniform NPs.
4.2.1 Chemical Synthesis
NPs can be chemically synthesized by a number of techniques including spray pyrolysis, thermal decomposition, molecular beam epitaxy, chemical vapor deposition, and laser ablation. Chemical synthesis methods are among the most commonly used techniques, and can be performed using a range of precursors and variations of temperature, time and concentration of reactants. Modification of one or more parameters results in morphological differences in size and structures of the resulting NPs. Several popular chemical methods for synthesis of ZnO-NPs are described below.
4.2.1.1 Reaction of Metabolic Zinc with Alcohol
Several types of alcoholic media such as ethanol, methanol, or propanol are used for chemical synthesis of ZnO-NPs. At the laboratory scale, synthesis typically involves addition of 5 mg of zinc metal powder to 10 mL of ethanol. The reaction mixture is sonicated for 20 min and transferred to a stainless steel autoclave and sealed under an inert gas. The reaction mixture is heated slowly (2–200 °C per minute) and maintained at this temperature for 24–48 h. The resulting suspension is then centrifuged to retrieve the product, washed, and finally vacuum-dried. In alcoholic media the growth of oxide particles is slow and controllable (Koch et al. 2000).
4.2.1.2 Vapor Transport Synthesis
The vapor transport process is the most common method for synthesis of ZnO-NPs. In this process, zinc and oxygen (or oxygen mixtures) are reacted resulting in formation of ZnO-NPs. Numerous methods are available for generation of Zn and oxygen vapor. Decomposition of ZnO is an easier, direct, and simple method; however, it is limited to very high temperatures (e.g., 1400 °C).
4.2.1.3 Hydrothermal Technique
The hydrothermal technique is an efficient synthetic method because of the low process temperature required; furthermore, it is easier to control particle size.
Particle morphology and size are carefully controlled by adjusting reaction temperature, time, and concentrations of precursor’-s.
As stock solution of Zn (CH3COO)2.-2H2O (0.1 M) is first prepared. To this stock solution, 25 mL of NaOH (from 0.2 M to 0.5 M) prepared in methanol, is added under stirring to attain a pH value between 8 and 11. The solutions is transferred to a Teflon-lined sealed stainless steel autoclave and maintained at temperatures in the range of 100–200 °C for 6–12 h under autogenous pressure. The resulting white solid product is washed with methanol, filtered, and dried in a laboratory oven at 60 °C. This is followed by structural characterization of synthesized samples via X-ray diffraction (Lee et al. 2006).
The hydrothermal technique offers several advantages such as utilization of sample equipment, catalyst-free growth, low cost, uniform production, ecofriendliness, and being less hazardous compared with other synthesis methods. The technique is attractive for microelectronics and plastic electronics manufacture due to low reaction temperatures needed. This hydrothermal technique has been successfully employed for preparation of ZnO-NPs and other luminescent materials.
4.2.1.4 Precipitation Method
In this method ZnO-NPs are synthesized using zinc nitrate and urea as precursors. In a typical synthesis, 4.735 g zinc nitrate (Zn [NO3]2-6H2O) is dissolved in 50 mL of distilled water and kept under constant stirring for 30 min for complete dissolution. In a separate container, 3.002 g urea is added to 50 mL distilled water under constant stirring for 30 min. The urea solution acts as precipitating agent. The urea solution is added drop-wise into the zinc nitrate solution with vigorous stirring at 70 °C for 2 h to allow formation of NPs. The solution eventually turns cloudy white. This precursor product is centrifuged at 8000 rpm for 10 min and washed with distilled water for removal of any impurities or absorbed ions. Calcination of the product is carried out at 500 °C in air for 3 h using a muffle furnace (Chen et al. 2008).
4.2.2 Green Synthesis
In recent years, green synthesis of metal NPs has become appealing to nanoscience and nanobiotechnology. There is a growing interest in biosynthesis of metal NPs using plants, which appear to be the optimal candidate for large-scale biosynthesis of NPs. Products of green synthesized NPs tend to be stable.
Synthesis using plants and their extracts are advantageous over other biological synthesis processes, for example using microorganism, which involves complex procedures for maintaining viable cultures (Sastry et al. 2003). Experimentals synthesis of metal NPs have used fungi like Fusarium oxysporum (Nelson et al. 2005), Penicillium sp. (Hemanth et al. 2010) and bacteria such as Bacillus subtilis (Natarajan et al. 2010; Elumalai et al. 2010). However, synthesis using plant extracts is the most widely accepted method of green, eco-friendly production of NPs. It has the advantage that plants are widely distributed, readily available, and safe to handle (Ankamwar et al. 2005). Moreover, the NPs produced are more varied in shape and size in comparison with those produced by other organisms (Korbekandi et al. 2009).
4.3 Uptake of ZnO-NPs in Plants
Several possible mechanisms exist for entry of ZnO-NPs into plants (Capaldi Arruda et al. 2015). NPs may dissolve in soil water and produce ions which are incorporated into the plant. Alternatively, upon exposure to the root, NPs will penetrates the cell wall and cell membrane of the epidermis accompanied by a complex series of processes for entry into the vascular bundle (xylem) and movement to the stele. Due to their size, many NPs may not be capable of passing through the cell wall of an intact plant cell. Solute exclusion techniques provide data on restrictive pore sizes of cell walls. The largest pore size of a plant cell wall is typically in the range of a few nanometers; for example, 3.5–3.8 nm is common in root hairs and 4.5–5.2 nm in palisade parenchyma cells (Carpita et al. 1979). NP sizes less than 5 nm in diameter are capable of transversing the cell wall of undamaged cells e (Fleischer et al. 1999). The xylem serves as the most important vehicle for distribution and translocation of NPs to leaves. The epidermis, cortex, endodermis, cambium and xylem are known to accumulate higher concentrations of NPs than other plant tissues.
The mechanism of NP uptake is generally considered active transport that includes cellular processes such as signaling, and regulation of the plasma membrane (Etxeberria et al. 2006). NPs can move through tissues via: apoplastic and symplastic routes. Apoplastic transport occurs outside the plasma membrane and through the extracellular spaces, cell walls of adjacent cells, and xylem vessels (Sattelmacher 2001); in contrast, symplastic transport involves movement of water and substances between the cytoplasm of adjacent cells through specialized structures called plasmodesmata (Roberts and Oparka 2003) and sieve plates. The apoplastic pathway is important for radial movement within plant tissues; it allows nanomaterials to reach the root central cylinder and vascular tissue, for further movement upward to aerial parts (Sun et al. 2014). Once inside the central cylinder, NPs are translocated upward though the xylem, following the transpiration stream (Sun et al. 2014).
Uptake, translocation and accumulation of NPs depend on plant species and the size, charge, chemical configuration, stability and concentration of the NPs. The mobility of NPs is also determined by van der Waals forces, Brownian motion (diffusion), gravity, and double-layer forces (Handy et al. 2008; Biswas and Wu 2005).
4.4 Distinguishing Properties of ZnO-NPs
ZnO-NPs are a new type of cost-effective and low-toxicity NPs which have attracted substantial interest in different fields as they possess a number of distinct and useful properties.
4.4.1 Physical Properties
ZnO-NPs possess valuable physical properties. As the dimensions of semiconductor materials shrinks continuously to nanometer or even smaller scales, some of their physical properties undergo changes known as “quantum size effects.”
4.4.2 Antibacterial Properties
The antibacterial activity of ZnO-NPs lies in their ability to induce oxidative stress. Zn2+ ions, released by dissolution of ZnO, interact with thiol groups of respiratory enzymes, thus inhibiting their action. It has been demonstrated that ZnO-NPs affect the properties of the cell membrane and lead to ROS formation. When bacterial cells come into contact with ZnO-NPs, they absorb Zn2+, which inhibits the action of respiratory enzymes, generates ROS, and produces free radicals, causing oxidative stress. ROS irreversibly damage bacterial membranes, DNA, and mitochondria, resulting in cell death (Dwivedi et al. 2014).
Ghasemi and Jalal (2016) investigated the effect of ZnO-NPs on the efficiency of the conventional antibiotics ciprofloxacin and cefta-zidime as well as their mechanisms of action against resistant Acinetobacter baumannii, an opportunistic pathogen that causes a range of diseases including pneumonia and meningitis. The antibacterial activity of both anti-biotics increased in the presence of a sub-inhibitory concentration of ZnO-NPs. Combining ZnO-NPs with antibiotics increased uptake of antibiotics and changed bacterial cells from rod to cocci. ZnO-NPs used against Vibrio cholerae (a causative agent of severe diarrhea) were investigated by Sarwar et al. (2016). ZnO-NPs deformed cellular architecture, increased fluidity and caused depolarization of cell membranes and protein leakage. ROS production and DNA damage were also observed. These results suggest the synergistic action of ZnO-NPs and anti-biotics as an alternative treatment for certain bacterial diseases.
4.5 The Role of ZnO-NPs in Agriculture
Limited studies have been carried out to date to determine the effects of ZnO-NPs on plant growth and productivity (Lin and Xing 2007; Stampoulis et al. 2009). It is well recognized that ZnO-NPs affect crop development and yield and accumulate in plant tissue, including edible portions. The behavior of ZnO-NPs in plants is not completely clear; however, the optoelectrical, physical and antimicrobial activities of ZnO-NPs offer several positive effects to plants (Table 4.1) (Liu and Lal 2015; Hussain et al. 2016). ZnO-NPs up to a certain concentration have the capability to enhance growth (Faizan et al. 2018), where they provide Zn2+ as a micronutrient (Liu and Lal 2015). Several reports suggest that ZnO-NPs improve growth and development in soybean (Priester et al. 2012), cucumber (Zhao et al. 2013), peanut (Prasad et al. 2012) and green pea (Mukherjee et al. 2014) (Table 4.1). Prasad et al. (2012) report that peanut seeds, when treated with 1000 mg/kg of ZnO NPs (average size ~25 nm), exhibited enhanced germination rate and improved seedling vigor, along with early flowering and higher leaf chlorophyll content. Similar inductive effects of ZnO-NPs resulted in increase stem and root growth. Pod yield per plant was 34% higher with ZnO-NPs compared to chelated bulk ZnSO4-exposed plants.
ZnO-NPs enhanced growth and biomass production of alfalfa, tomato, and cucumber plants (de la Rosa et al. 2013; Panwar et al. 2012). Application of ZnO-NPs increased photosynthetic pigment levels in pearl millet (Tarafdar et al. 2014). Ramesh et al. (2014a, b) reported that low concentrations of ZnO-NPs imparted a beneficial effect on seed germination in wheat.
Significant increases in root growth and dry weight in onion was observed after ZnO-NP application (Raskar and Laware 2014). In contrast, Zhao et al. (2013) observed that ZnO-NPs had no impact on growth of cucumber plants, gas exchange, or chlorophyll content. Lower concentrations of ZnO-NPs were not harmful to cell division and early seedling growth in onion (Raskar and Laware 2014).
4.6 Zinc Oxide Nanoparticles and Plants Under Abiotic Stress
ZnO-NPs play important roles in plants for minimizing the harmful effects of ROS to cell organelles. Apart from its well-documented damaging effects, ROS are also known to trigger various defense systems by activating a cell signaling cascade and inducing or suppressing expression of many genes (Hancock et al. 2001). Nonetheless, plants are equipped with enzymatic and nonenzymatic systems of antioxidants generation, which continuously scavenge harmful ROS. ZnO-NPs play an important role in the protection of plants against various abiotic stresses by stimulating the activities of antioxidant enzymes and accumulating osmolytes, free amino acids and nutrients (Fig. 4.1; Table 4.2) (Taran et al. 2017; Venkatachalam et al. 2017; Wang et al. 2018). Application of ZnO-NPs at low concentrations was found effective in alleviating various abiotic stresses and enhanced plant growth and development (Table 4.2) (Mahajan et al. 2011; Soliman et al. 2015).
In contrast, however, a significant number of studies show toxic effects of ZnO-NPs (Miralles et al. 2012; Husen and Siddiqi 2014), and application of ZnO-NPs to stressed plants could galvanize the process of ROS generation leading to oxidative damage (Lin et al. 2010; Wang et al. 2014; Chichiricco and Poma 2015).
4.6.1 Drought Stress
Drought stress is both a natural event, and also a consequence of anthropogenic climate change that limits crop production and distribution. Among various strategies adopted to counter drought-induced damage to plants, the use of NPs has proved promising.
Under conditions of water scarcity stomatal closure is the primary response that plants adopt to preserve water. When stomata are open, CO2 is taken up at the cost of water transpired; when stomata are closed, water is conserved but uptake of CO2 is compromised. Perception of drought stress triggers the activation of signal transduction cascades. This leads to stomatal closure and, in consequence, conservation of water through reduced transpiration coupled with reduced CO2 uptake. According to Taran et al. (2017), foliar application of ZnO-NPs to wheat reduced the adverse effects of drought stress and improved yield components of wheat.
4.6.2 Metal Stress
Cadmium is considered quite toxic to plants (Garg and Kaur 2013). Cadmium induces phytotoxicity by disturbing many Zn-dependent physiological processes via displacement of Zn from the active sites of enzymes (Asmub et al. 2000). Venkatachalam et al. (2017) found that ZnO-NPs alleviated toxicity induced by Cd and Pb in Leucaena leucocephala seedlings. This finding is similar to results reported by Garg and Kaur (2013), who determined that the presence of Zn decreased Cd content in both roots and leaves of Cajanus cajan, thereby enhancing plant survival and growth. Zinc supplementation can protect plants against Cd-induced oxidative stress via modulating the redox status of the plant (Aravind and Prasad 2005).
4.6.3 Salinity Stress
The application of ZnO-NPs has led to mitigation of some adverse effects of soil salinity. According to Soliman et al. (2015), foliar application of 60 mg/L ZnO-NPs proved optimal for alleviating the effects of salt stress on Moringa peregrina plants (Table 4.2). Torabian et al. (2016) observed that alleviation of salt stress was greater in sunflower plants supplied with ZnO-NPs compared to plants treated with dissolved ZnO. The salt-alleviating effects of NPs was further confirmed when Almutairi (2016) reported that nano-Si differentially regulated the expression of salt stress genes. There was a positive response of SOD activity to foliar application of ZnO, particularly nanoparticles, under salt stress. Because Zn is present within the molecular structure of SOD, foliar application of ZnO promotes the formation and activity of this enzyme. Zinc deficiency probably increases ROS levels and, thus, requires higher SOD activity. Although salinity increased SOD activity, foliar application of ZnO contributed to its enhanced production. This may explain the role of Zn in salinity alleviation. In experiment by Sanaeiostovar et al. (2012), applied zinc increased the SOD activity of wheat cultivars. Foliar spray of ZnO-NPs reduced the negative effects of salinity on sunflower growth (Torabian et al. 2016).
4.7 Conclusion
The findings reported herein reveal that ZnO-NPs serve as a natural regulator for plants under both stressed and stress-free conditions. ZnO-NPs have the potential to enhance plant growth and development. ZnO-NPs play a pivotal role in modulating key plant physiological parameters under stressfull conditions such as lipid peroxidation, and production of proline and various antioxidant enzymes. ZnO-NPs can be efficiently synthesized by chemical and green synthesis methods.
The small size of ZnO-NPs facilitates easy penetration into plant cells and regulates water channels that assist seed germination and growth of plants. Moreover, different modes of ZnO-NP application successfully counter the adverse affects of ROS under abiotic stress through increased activity of CAT, POX and SOD. The role of ZnO-NPs in plants needs further investigation at both sub-cellular and molecular levels.
References
Alharby HF, Metwali EMR, Fuller MP, Aldhebiani AY (2016) Impact of zinc oxide nanoparticle application on callus induction, plant regeneration, element content and antioxidant enzyme activity in tomato (solanum lycopersicum mill.) under salt stress. Arch Biol Sci 68:723–735
Almutairi ZM (2016) Effect of nano-silicon application on the expression of salt tolerance genes in germinating tomato (Solanum lycopersicum L.) seedlings under salt stress. Plant Omics J 9:106–114
Ankamwar B, Damle C, Ahmad A, Sastry M (2005) Biosynthesis of gold and silver nanoparticles using Emblics Officinalis fruit extract and their phase transfer and transmetallation in an organic solution. J Nanosci Nanotechnol 5:1665–1671
Aravind P, Prasad MNV (2005) Cadmium–zinc interactions in a hydroponic system using Ceratophyllum demersum L.: adaptive ecophysiology, biochemistryand molecular toxicology. Braz J Plant Physiol 17:3–20
Asmub M, Mullenders LHF, Hartwig A (2000) Interference by toxic metal compounds with isolated zinc finger DNA repair proteins. Toxico Lett 15(112–113):227–231
Auld DS (2001) Zinc coordination sphere in biochemical zinc sites. Biometals 14:271–313
BCC Research (2014) Global markets for nanocomposites, nanoparticles, nanoclays, and nanotubes. https://www.bccresearch.com/market-research/nanotechnology/nanocomposites-market-nan021f.html?vsmaid=203/. Accessed 19 Oct 2017
Beddington J (2010) Food security: contributions from science to a new and greener revolution. Philos Trans R Soc B Biol Sci 365(1537):61–71
Bhatt I, Tripathi BN (2011) Interaction of engineered nanoparticles with various components of the environment and possible strategies for their risk assessment. Chemosphere 82:308–317
Biswas P, Wu CY (2005) Critical review: nanoparticles and the environment. J Air Waste Manage Assoc 55:708–746
Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A (2013) Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol 87:1181–1200
Boxall A, Chaudhry Q, Sinclair C, Jones A, Aitken R, Jefferson B, Watts C (2007) Current and future predicted environmental exposure to engineered nanoparticles. Technical Report, Central Science Laboratory, York
Brayner R, Dahoumane SA, Yepremian C, Djediat C, Meyer M, Coute A, Fievet F (2010) Zinc oxide nanoparticles: synthesis, characterization and ecotoxicological studies. Langmuir 26:6522–6528
Burman U, Saini M, Kumar P (2013) Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol Environ Chem 95:605–612
Cakmak I (2002) Plant nutrition research: priorities to meet human needs for food in sustainable ways. Plant Soil 247:3–24
Capaldi Arruda SC, Silva A, D L, Galazzi RM, Azevedo RA, Arruda MAZ (2015) Nanoparticles applied to plant science: a review. Talanta 131:693–705
Carpita N, Sabularse D, Montezinos D, Delmer DP (1979) Determination of the pore size of cell walls of living plant cells. Science 2059(4411):1144–1147
Castillo RR, Lozano D, Gonzalez B, Manzano M, Izquierdo-Barba I, Vallet-Regi M (2019) Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: an update. Expert Opin Drug Deliv 16(4):415–439
Chaudhary D, Basanti B, Singh DJ, Subhash K, Anil P (2018) An insight into in vitro micropropagation studies for banana- review. Int J Agri Sci 10(5):5346–5349
Chen C, Liu P, Lu C (2008) Investigation of photocatalytic degradation using nano-sized ZnO catalysts. Chem Eng J 144:509–513
Chichiricco G, Poma A (2015) Penetration and toxicity of nanomaterial in higher plants. Nanomaterials 5:851–873
Connolly M, Fernandez M, Conde E, Torrent F, Navas JM, Fernandez-Cruz ML (2016) Tissue distribution of zinc and subtle oxidative stress effects after dietary administration of ZnO nanoparticles to rainbow trout. Sci Total Environ 1(551–552):334–343
de la Rosa G, Lopez-Moreno ML, de Haro D, Botez CE, Peralta-Videa JR, Gardea- Torresdey JL (2013) Effects of ZnO nanoparticles in alfalfa, tomato, and cucumber at the germination stage: root development and X-ray absorption spectroscopy studies. Pure Appl Chem 85:2161–2174
Dhoke SK, Mahajan P, Kamble R, Khanna A (2013) Effect of nanoparticles suspension on the growth of mung (Vigna radiata) seedlings by foliar spray method. Nanotechnol Dev 3:e1
Dwivedi S, Wahab R, Khan F, Mishra YK, Musarrat J, Al-Khedhairy A (2014) Reactive oxygen species mediated bacterial biofilm inhibition via zinc oxide nanoparticles and their statistical determination. PLoS One 9:e111289
Elumalai EK, Prasad TNVKV, Hemachandran J, Vivivan Therasa S, Thirumalai T, David E (2010) Extracellular synthesis of silver nanoparticles using leaves of Euphorbia hirta and their antibacterial activities. J Pharm Sci Res 2:549–554
Etxeberria E, Gonzalez P, Baroja-Fernandez E, Romero JP (2006) Fluid phase endocytic uptake of artificial nano-spheres and fluorescent quantum dots by sycamore cultured cells: evidence for the distribution of solutes to different intracellular compartments. Plant Signal Behav 1:196–200
Faizan M, Faraz A, Yusuf M, Khan ST, Hayat S (2018) Zinc oxide nanoparticles-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 56:678–686
Fleischer A, O’Neill MA, Ehwald R (1999) The pore size of non-graminaceous plant cell wall is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturon II. Plant Physiol 121:829–838
Garg N, Kaur H (2013) Impact of cadmium-zinc interactions on metal uptake, translocation and yield in pigeonpea genotypes colonized by arbuscular mycorrhizal fungi. J Plant Nutr 36:67–90
Ghasemi F, Jalal R (2016) Antimicrobial action of zinc oxide nanoparticles in combination with ciprofloxacin and ceftazidime against multidrug-resistant Acinetobacter baumannii. J Glob Antimicrob Resist 6:118–122
Ghosh M, Jana A, Sinha S, Jothiramajayam M, Nag A, Chakraborty A, Mukherjee A, Mukherjee A (2016) Effects of ZnO nanoparticles in plants: cytotoxicity, genotoxicity, deregulation of antioxidant defenses, and cell-cycle arrest. Mutat Res Genet Toxicol Environ Mutagen 807:25–32
Graham RD, Welch RM, Bouis HE (2001) Addressing micronutrients malnutrition through enhancing the nutritional quality of staple foods principles, perspectives and knowledge gaps. Adv Agron 70:77–142
Hancock JT, Desikan R, Neill SJ (2001) Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans 29:345–350
Handy RD, Owen R, Valsami-Jones E (2008) The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, and future needs. Ecotoxicology 17:315–325
Hassan N, Salah T, Hendawey MH, Borai IH, Mahdi AA (2018) Magnetite and zinc oxide nanoparticles alleviated heat stress in wheat plants. Curr Nanomaterials 3:32–43
Hemanth NKS, Kumar G, Karthik L, Bhaskara RKV (2010) Extracellular biosynthesis of silver nanoparticles using the filamentous fungus Penicillium sp. Arch Appl Sci Res 2:161–167
Husen A, Siddiqi KS (2014) Phytosynthesis of nanoparticles: concept, controversy and application. Nanoscale Res Lett 9:229–252
Hussain I, Singh NB, Singh A, Singh H, Singh SC (2016) Green synthesis of nanoparticles and its potential application. Biotechnol Lett 38:545–560
Hussain A, Ali S, Rizwan M, Rehman MZ, Javed MR, Imran M, Chatha SA, Nazir R (2018) Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ Pollut 242:1518–1526
Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW (2012) Applications of nanomaterials in agricultural production and crop protection: a review. Crop Protec 35:64–70
Kool PL, Ortiz MD, van Gestel CAM (2011) Chronic toxicity of ZnO nanoparticles, non-nano ZnO and ZnCL2 to Folsomia candida (Collembola) in relation to bioavailability in soil. Environ Pollut 159:2713–2719
Koch U, Fojtik A, Weller H, Henglein A (2000) Photochemistry of semiconductor colloids. Preparation of extremely small ZnO particles, fluorescence phenomena and size quantization effects. Chem Phys Lett 122:507–510
Korbekandi H, Iravani S, Abbasi S (2009) Production of nanoparticles using organisms production of nanoparticles using organisms. Crit Rev Biotechnol 29:279–306
Lacerda JS, Martinez HE, Pedrosa AW, Clemente JM, Santos RH, Oliveira GL, Jifon JL (2018) Importance of zinc for arabica coffee and its effects on the chemical composition of raw grain and beverage quality. Crop Sci 58:1360–1370
Latef AAHA, Alhmad MFA, Abdelfattah KE (2017) The possible roles of priming with ZnO nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J Plant Growth Regul 36:60–70
Lawre S, Raskar S (2014) Influence of zinc oxide nanoparticles on growth, flowering and seed productivity in onion. Int J Curr Microbiol App Sci 3:874–881
Lee CY, Tseng TY, Li SY, Lin P (2006) Effect of phosphorus dopant on photoluminescence and field-emission characteristics of Mg0.1Zn0.9O nanowires. J Appl Phys 99:024303
Lin D, Xing B (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150:243–250
Lin C, Su YB, Takahiro M, Fugetsu B (2010) Multi-walled carbon nanotubes induce oxidative stress and vacuolar structure changes to Arabidopsis T87 suspension cells. Nano Biomed 2:170–181
Liu R, Lal R (2015) Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci Total Environ 514:131–139
Lopez-Moreno ML, De La Rosa G, Hernandez-Viezcas JA, Castillo-Michel H, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL (2010) Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 NPs on soybean (Glycine max) plants. Environ Sci Technol 44:7315–7320
Ma X, Geiser-Lee J, Deng Y, Kolmakov A (2010) Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ 408:3053–3061
Mahajan P, Dhoke SK, Khanna AS, Tarafdar JC (2011) Effect of nano-ZnO on growth of mung bean (Vigna radiata) and chickpea (Cicer arietinum) seedlings using plant agar method. Appl Biol Res 13:54–61
Marschner H (2011) Marschner’s mineral nutrition of higher plants. Academic Press, San Diego, p 651
Miralles P, Johnson E, Church TL, Harris AT (2012) Multiwalled carbon nanotubes in alfalfa and wheat: toxicology and uptake. J R Soc Interface 9:3514–3527
Monica RC, Cremonini R (2009) Nanoparticles and higher plants. Caryol 62:161–165
Mukherjee A, Peralta-Videa JR, Bandyopadhyay S, Rico CM, Zhao L, Gardea- Torresdey JL (2014) Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics 6:132–138
Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Kumar DS (2010) Nanoparticulate material delivery to plants. Plant Sci 179(3):154–163
Natarajan K, Subbalaxmi SV, Ramchandra M (2010) Microbial production of silver nanoparticles. Dig J Nanomater Biostruct 5:135–140
Nel A, Xia T, Madler L, Li N (2006) Toxic potential of materials at the nano level. Science 311:622–627
Nelson D, Priscyla DM, Oswaldo LA, Gabriel IHDS, Elisa E (2005) Mechanical aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nanobiotechnol 3:8
Panwar J, Jain N, Bhargaya A, Akhtar M, Yun Y (2012) Positive effect of zinc oxide nanoparticles on tomato plants: a step towards developing nano-fertilizers. International Conference on Environmental Research and Technology (ICERT), Malaysia https://doi.org/10.13140/2.1.2697.8889
Peng YH, Tsai YC, Hsiung CE, Lin YH, Shih Y (2017) Influence of water chemistry on the environmental behaviors of commercial ZnO nanoparticles in various water and wastewater samples. J Hazard Mater 322:348–356
Peralta-Videa JR, Zhao L, Lopez-Morena ML, de la Rosa G, Hong J, Gardea-Torresdey JL (2011) Nanomaterials and the environment: a review for the biennium 2008–2010. J Hazard Mater 186:1–15
Pradhan S, Patra P, Das S, Chandra S, Mitra S, Dey KK, Akbar S, Palit P, Goswami A (2013) Photochemical modulation of biosafe manganese nanoparticles on vigna radiata: a detailed molecular, biochemical and biophysical study. Environ Sci Technol 47:13122–13131
Prasad TNVKV, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Raja Reddy K, Sreeprasad TS, Sajanlal PR, Pradeep T (2012) Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J Plant Nutr 35:905–927
Priester JH, Ge Y, Mielke RE, Horst AM, Moritz SC, Espinosa K, Gelb J, Walker SL, Nisbet RM, An YJ (2012) Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc Natl Acad Sci U S A 109:E2451–E2456
Raliya R, Tarafdar JC (2013) ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in cluster bean (Cyamopsis tetragonoloba L.). Agric Res 2:48–57
Ramesh M, Palanisamy K, Babu K, Sharma NK (2014a) Effects of bulk & nano-titanium dioxide and zinc oxide on physio-morphological changes in Triticum aestivum Linn. J Glob Biosci 3:415–422
Ramesh P, Rajendran A, Meenakshisundaram M (2014b) Green synthesis of zinc oxide nanoparticles using flower extract Cassia auriculatas. J Nanosci Nanotechnol 1:41–45
Raskar S, Laware S (2014) Effect of zinc oxide nanoparticles on cytology and seed germination in onion. Int J Curr Microbiol App Sci 3:467–473
Rizwan M, Ali S, Ali B, Adrees M, Arshad M, Hussain A, Zia ur Rehman M, Waris AA (2019) Zinc iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214:269–277
Roberts AG, Oparka KJ (2003) Plasmodesmata and the control of symplastic transport. Plant Cell Environ 26:103–124
Sanaeiostovar A, Khoshgoftarmanesh AH, Shariatmadari H, Afyuni M, Schulin R (2012) Combined effect of zinc and cadmium levels on root antioxidative responses in three different zinc-efficient wheat genotypes. J Agron Crop Sci 198:276–285
Sarwar S, Chakraborti S, Bera S, Sheikh IA, Hoque KM, Chakrabarti P (2016) The antimicrobial activity of ZnO nanoparticles against Vibrio cholerae: variation in response depends on biotype. Nanomedicine 12:1499–1509
Sastry M, Ahmad A, Islam NI, Kumar R (2003) Biosynthesis of metal nanoparticles using fungi and actinomycetes. Curr Sci 85:162–170
Sattelmacher B (2001) The apoplast and its significance for plant mineral nutrition. New Phytol 149:167–192
Sedghi M, Hadi M, Toluie SG (2013) Effect of nano zinc oxide on the germination parameters of soybean seeds under drought stress. Ann West Univ Timişoara Ser Biol XVI(2):73–78
Shukla AK, Tiwari PK, Prakash C (2014) Micronutrients deficiencies vis-avis food and nutritional security of India. Indian J Fertil 10:94–112
Sirelkhatim A, Shahrom M, Azman S, Noor HMK, Chuo AL, Siti KMB, Habsah H, Dasmawati M (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Micro Nano Lett 7(3):219–242
Singh MV (2009) Micronutrient nutritional problems in soils of India and improvement for human and animal’s health. Indian J Fertil 5(4):11–26
Singh NB, Amist N, Yadav K, Singh D, Pandey JK, Singh SC (2013) Zinc oxide nanoparticles as fertilizer for the germination, growth and metabolism of vegetable crops. J Nanoeng Nanomanuf 3:1–12
Soliman AS, El-feky SA, Darwish E (2015) Alleviation of salt stress on Moringa peregrina using foliar application of nanofertilizers. J Hortic For 7:36–47
Som C, Berges M, Chaudhry Q, Dusinska M, Fernandes TF, Olsen SI, Nowack B (2010) Toxicology 269:160–169
Spero JM, Devito B, Theodore L (2000) Regulatory chemical handbook. CRC press, Boca Raton
Srivastav AK, Kumar M, Ansari NG, Jain AK, Shankar J, Arjaria N, Jagdale P, Singh D (2016) A comprehensive toxicity study of zinc oxide nanoparticles versus their bulk in wistar rats: toxicity study of zinc oxide nanoparticles. Hum Exp Toxicol 35(12):1286–1304
Stampoulis D, Sinha SK, White JC (2009) Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol 43:9473–9479
Sun D, Hussain HI, Yi Z, Siegele R, Cresswell T, Kong L, Cahill DM (2014) Uptake and cellular distribution, in four plant species, of fluorescently labeled mesoporous silica nanoparticles. Plant Cell Rep 33:1389–1402
Taheri M, Qarache HA, Qarache AA, Yoosefi M (2015) The effects of zinc-oxide nanoparticles on growth parameters of corn (SC704). STEM Fellowship J 1:17–20
Tarafdar JC, Raliya R, Mahawar H, Rathore I (2014) Development of zinc nanofertilizer to enhance crop production in pearl millet (Pennisetum americanum). Agric Res 3:257–262
Taran N, Storozhenko V, Svietlova N, Batsmanova L, Shvartau V, Kovalenko M (2017) Effect of zinc and copper nanoparticles on drought resistance of wheat seedlings. Nanoscale Res Lett 12:60
Torabian S, Zahedi M, Khoshgoftarmanesh A (2016) Effect of foliar spray of zinc oxide on some antioxidant enzymes activity of sunflower under salt stress. J Agric Sci Technol 18:1013–1025
Uikey P, Vishwakarma K (2016) Review of zinc oxide (ZnO) nanoparticles applications and properties. Int J Emerg Tech Com Sci Elec 21(2):239
Vaseem M, Umar A, Hahn YB (2010) ZnO Nanoparticles: growth, properties and applications. In: Umar A, Hahn Y-B (eds) Metal oxide nanostructures and their applications, 4th edn. American Scientific Publishers, Los Angeles, pp 1–36
Venkatachalam P, Jayaraj M, Manikandan R, Geetha N, Rene ER, Sharma NC, Sahi SV (2017) Zinc oxide nanoparticles (ZnONPs) alleviate heavy metalinduced toxicity in Leucaena leucocephala seedlings: a physiochemical analysis. Plant Physiol Biochem 110:59–69
Wang C, Liu H, Chen J, Tian Y, Shi J, Li D, Guo C, Ma Q (2014) Carboxylated multi-walled carbon nanotubes aggravated biochemical and subcellular damages in leaves of broad bean (Vicia faba L.) seedlings under combined stress of lead and cadmium. J Hazard Mater 274:404–412
Wang F, Jin X, Adams CA, Shi Z, Sun Y (2018) Decreased ZnO nanoparticles phytotoxicity to maize by arbuscular mycorrhizal fungus and organic phosphorus. Environ Sci Pollut Res 25:23736–23747
Yadav T, Mungray AA, Mungray AK (2014) Fabricated nanoparticles. Rev Environ Contam Toxicol 230:83–110
Zhao L, Sun Y, Hernandez-Viezcas JA, Servin AD, Hong J, Niu G, Peralta-Videa JR, Duarte-Gardea M, Gardea-Torresdey JL (2013) Influence of CeO2and ZnO nanoparticles on cucumber physiological markers and bioaccumulation of Ce and Zn: a life cycle study. J Agric Food Chem 61:11945–11951
Zhao L, Peralta-Videa JR, Rico CM, Hernandez-Viezcas JA, Sun Y, Niu G, Duarte-Gardea M, Gardea-Torresdey JL (2014) CeO2 and ZnO nanoparticles change the nutritional qualities of cucumber (Cucumis sativus). J Agric Food Chem 62:2752–2759
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Faizan, M., Hayat, S., Pichtel, J. (2020). Effects of Zinc Oxide Nanoparticles on Crop Plants: A Perspective Analysis. In: Hayat, S., Pichtel, J., Faizan, M., Fariduddin, Q. (eds) Sustainable Agriculture Reviews 41. Sustainable Agriculture Reviews, vol 41. Springer, Cham. https://doi.org/10.1007/978-3-030-33996-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-33996-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-33995-1
Online ISBN: 978-3-030-33996-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)