Abstract
Background
Neuroinflammation plays a dominant role in the progression of postoperative cognitive dysfunction (POCD). This study was carried out to explore the neuroprotective effect of Chikusetsu saponin IVa (ChIV) against sevoflurane-induced neuroinflammation and cognitive impairment.
Methods
The neuroprotective activity of ChIV against sevoflurane-induced cognitive dysfunction in aged rats was evaluated by Morris water maze, NOR test and Y-maze test, respectively. The expression of NLRP3, ASC and caspase-1, pro-inflammatory cytokines and apoptotic-related protein were detected in the hippocampus and primary neurons using western blot. TUNEL assay and immunohistochemistry staining were applied to assess the apoptotic cell and number of NLRP3-positive cells in the hippocampus. The oxiSelectIn Vitro ROS/RNS assay kit was used to detect the ROS level. The CCK-8 assay was applied to measure the viability of primary neurons. Flow cytometry was carried out to determine cell apoptosis.
Results
Pretreatment with ChIV significantly alleviated neurological dysfunction in aged rat exposure to sevoflurane. Mechanistically, ChIV treatment significantly alleviated sevoflurane-induced apoptotic cell and neuroinflammation. Of note, the neuroprotective effect of ChIV against sevoflurane-induced neurotoxicity through blocking NLRP3/caspase-1 pathway. In consistent with in vivo studies, ChIV was also able to repress sevoflurane-induced apoptosis and neuroinflammation in primary neurons. Furthermore, pretreatment with NLRP3/caspase-1 pathway inhibitor (MCC950) significantly augmented the neuroprotective effect of ChIV.
Conclusion
Our finding confirmed that ChIV provides a neuroprotective effect against sevoflurane-induced neuroinflammation and cognitive impairment by blocking the NLRP3/caspase-1 pathway, which may be an effective strategy for the clinical treatment of elderly patients with POCD induced by anesthesia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative cognitive dysfunction (POCD) is one of the important complications following major surgery and anesthesia in elderly patients, presenting cognitive function impairment such as learning and memorizing, which could increase complications and mortality [1, 2]. According to the latest research, the pathogenesis of POCD includes increased neuron apoptosis, neurogenesis decline, synaptic plasticity impairment, and neurodegeneration caused by neuroinflammation, oxidative stress, and cholinergic system disorder in the central nervous system [3, 4]. In addition, several studies have shown that anesthetics such as sevoflurane and isoflurane exposure induced neuroinflammation and neuronal cell death in the hippocampus, resulted in cognitive dysfunction in aged rats [5, 6]. Of note, neuroinflammation plays a key role in the onset of POCD in elderly patients [7, 8].
The inflammation-related mechanisms of POCD include peripheral inflammation and central inflammation. Sterile surgery results in the release of systemic pro-inflammatory factors, destroying the integrity of the blood–brain barrier (BBB), and promoting macrophage migration into the brain parenchyma, and then causes related neuroinflammation [8]. Studies have shown that surgery could lead to inflammation and activation of glial cells in rat hippocampus, and ultimately lead to POCD [9, 10]. As the center of learning and memory, the hippocampus extensively expresses IL-1β and TNF-α receptors. These pro-inflammatory factor receptors play an important role in normal learning and memory, but in a pathological state, the hippocampus was more vulnerable to pro-inflammatory factor damage, which ultimately leads to POCD [11, 12]. Interestingly, inflammasomes are a set of intracellular protein complexes, which drive host and immune response by releasing cytokines and inducing pyroptosis. The NOD-like receptor protein 3 (NLRP3) was an essential inflammasome in immune response and could be activated by anesthesia administration [13]. NLRP3 inflammasome contains NLRP3, apoptotic speck protein (ASC) and procaspase-1. The NLRP3 inflammasome regulated maturation and release of pro-inflammatory cytokines, such as IL-1 and IL-18 through cleaving caspase-1, which results in a large number of inflammatory factors secretion and over-induces inflammatory cytokines to form an “inflammatory waterfall effect” and cause the activation of immune/inflammatory response [14,15,16]. Recent studies confirmed that anesthesia-induced the secretion of IL-1β and IL-18 to promote neuroinflammation and neurotoxicity by activating the NLRP3 inflammasome in the hippocampus of aged rats [13, 17]. Besides, in vivo experiments showed that anti-TNF-α antibody cholinesterase inhibitors, IL-1β receptor antagonists, non-steroidal anti-inflammatory drugs and cholinergic agonists could reduce the expression of inflammatory factors in peripheral and hippocampus, and improve the cognitive function of animals after operation [18]. Inflammation was a defensive response of the body to external noxious stimuli, but the inappropriate dosage of anti-inflammation drugs affects the body’s physiological function. At present, most of the anti-inflammation drugs are non-tissue-specific and have large side effects [19, 20]. Of note, the role of POCD alleviation was mainly focused on animal experiments, and the safety of its application in clinical research is still unclear [3, 21]. Therefore, it is particularly important to find alternative active drugs with fewer side effects in the clinical treatment of POCD.
Chikusetsu saponin IVa (ChIV), a triterpenoid saponins derived from Chinese medicine Rhizoma Panacis japonica, which exhibited antioxidant, antitumor, cardiovascular protection, antivirus, neuroprotective, anti-inflammation, and nervous system protection [22,23,24]. For example, Wang et al. confirmed that ChIV attenuates isoprenaline-induced myocardial fibrosis by activating autophagy [25]. Yuan et al. [26] found that ChIV ameliorates high fat diet-induced inflammation by inhibition of NLRP3 inflammasome activation. In vitro studies found that ChIV significantly inhibited cancer cell migration, invasion and induced cell apoptosis [24, 27]. Recently, accumulating evidence has shown that ChIV plays an important in regulating isoflurane-induced neurotoxicity [28, 29]. Besides, the neuroprotective effect of ChIV on methyl-4-phenylpyridinium ion (MPP+)-induced cytotoxicity and H2O2-induced oxidative stress have also been confirmed in vitro studies [30]. However, the role of ChIV in sevoflurane-induced neuroinflammation and cognitive impairment and the underlying mechanism have not been explored. In this study, the effect of ChIV on sevoflurane-induced neuroinflammation and cognitive impairment through regulating the NLRP3/caspase-1 pathway was investigated using rat and cellular models. Our data confirmed that ChIV attenuates sevoflurane-induced apoptosis and inflammation by blocking the NLRP3/caspase-1 pathway, which suggested that ChIV exerted neuroprotective effects against anesthesia-induced neurotoxicity.
Materials and methods
Animals and treatment
Male Sprague–Dawley rat (80 weeks old) were purchased from the Trophic Animal Feed High-tech Co., Ltd (Jiangsu, China) and maintained in a pathogen-free facility. All animal experiments were reviewed and approved by the Animal Ethics Committee of Kunshan Traditional Chinese Medicine Hospital. After one week of adaptive feeding, a total of 108 rats were randomly divided into four groups (n = 27 rats/group): control, sevoflurane, ChIV (Sigma, USA), and sevoflurane + ChIV. Rats in the control group were received to 20% oxygen for 3 h, while rats in the sevoflurane group were exposed to 4% sevoflurane + 20% oxygen for 3 h. Rats in ChIV group were injected intraperitoneally with 30 mg/kg ChIV for 12 h. Rats in sevoflurane + ChIV group were injected intraperitoneally with 30 mg/kg ChIV for 12 h prior to 4% sevoflurane + 20% oxygen for 3 h. And the detailed description of the experimental protocol as shown in Fig. S1.
Behavioral and cognitive tests
Morris water maze
After pretreatment with ChIV and a combination of sevoflurane exposure, rats in each group were feed to the 31 day (D31). The Morris water maze was used to assess the cognitive and memory function of rats according to the previous studies [31]. Briefly, the acquisition tests were carried out for four consecutive days. Each quadrant (N, S, E, and W) was trained alternately according to the animal number. For five consecutive days, the probe training session was performed twice a day. During the training session, experimental animals were guided to approach and locate the hidden platform by swimming. The latency period the rats spent to find the hidden platform was recorded. The distance the rats had traveled before getting to the platform was also documented. At the end of the training sessions, the platform was removed. Then, the rats were put into water to swim without any interference for 2 min. Thereafter, the time spent on the number of crossing the previous platform by rats was recorded. The time spent in the target quadrant by rats was also documented.
NOR test
A novel object recognition test (NOR test) was used to evaluate memory performance, which was conducted following the protocols described in previous studies [32]. An open field apparatus with a size of 60 × 60 × 30 cm was utilized as the test box. The act of rubbing or touching objects with the nose was considered exploratory. The identification index was carried out according to the following equation: (the time spent in the new object—the time spent in the familiar objects)/(the total time spent by exploring two objects).
Y-maze test
Y-maze test was used to evaluate the immediate spatial working memory according to the previous studies [32]. Briefly, rats were accustomed to the apparatus for 0.5 h before the test. Then a rat was placed at the end of an arm and allowed to explore the maze for 10 min. When rats were continuedly entered into three arms in alternating order was defined as successive spontaneous alternation (SA). The percentage of SA was calculated using the formula: the number of successive SA/the total number of times to explore the maze.
Cell culture and treatment
Cultures of the primary hippocampal neurons were performed as described previously [31]. Briefly, Sprague–Dawley (male, postnatal D1) rats were purchased from Trophic Animal Feed High-tech Co., Ltd (Jiangsu, China) and were used for cell isolation. Following the removal of meninges, cerebral hippocampi were isolated from rat brains. The tissue samples were added to a dissociation medium before mechanical dissociation. Then, the cell pellet was obtained by mild centrifugation (2000 rpm for 3 min at room temperature) followed by seeding in 3.5 cm culture dishes in the dissociation medium at a density of 3 × 105 cells per milliliter. Before cell plating, each cell culture dish was pretreated with 0.1% poly-d-lysine (Sigma, USA) at room temperature for 2 h followed by rinsing twice with PBS. Cells were kept in an incubator under 5% CO2 at 37 °C. The medium was replaced at 24 h with a 48 mL serum-free medium. Sevoflurane was given in the atmosphere at a concentration of 4.0% using an anesthesia machine.
Western blot
Total protein was extracted for western blotting analysis. The PVDF (polyvinylidene fluoride) was incubated overnight at 4 °C with the primary TNF-α antibody (1:1000, ab6671, Abcam, UK), IL-6 antibody (1:1000, ab9324, Abcam, UK), IL-1β antibody (1:1000, ab9722, Abcam, UK), Bax antibody (1:1000, ab32503, Abcam, UK), Bcl-2 antibody (1:1000, ab196495, Abcam, UK), Cleaved-caspase-3 antibody (1:2000, ab49822, Abcam, UK), NLRP3 antibody (1:1000, ab232401, Abcam, UK), ASC antibody (1:500, sc-271054, Santa Cruz, USA) and caspase-1 antibody (1:500, sc-56036, Santa Cruz, USA), and then with horseradish peroxidase-coupled secondary antibody (IgG-HRP, 1:1000, #7076, Cell Signaling Technology, USA). Signa was detected with chemiluminescence using an ECL kit (Bio-Rad, USA).
TUNEL assay
The apoptotic cell in the hippocampus tissues of rats in each group was determined by the TUNEL assay. In short, the pretreated samples were counterstained by the Anti-NeuN antibody (1:1000, ab128886, Abcam, UK) for 5 min at room temperature. Subsequently, samples were washed three times with PBS and placed on the microscopic glass to further analyze the number of TUNEL-positive cells.
Determination of ROS level
The OxiSelectIn Vitro ROS/RNS Assay Kit (Cell Biolabs, USA) was applied to detect the level of ROS in the hippocampus tissues and cell samples according to the previously described [33]. And the ROS level in each group was analyzed in triplicate using the commercial kits according to the manufacturer’s instructions.
CCK-8 assay
Cell Counting Kit-8 (CCK-8, Sigma, Japan) was used to detect the proliferation of primary neurons. Cells were seeded in 96-well plates at 5000 cells per well and cultured in 5% CO2 at 37 °C incubators for 2 h to adhere cells. Added 10 μL of the cell proliferation reagent CCK-8 to each well and mixed then incubated for 2 h in the incubator. The dual-wavelength microplate reader was used to measure the detection wavelength of 450–490 nm and reference wavelength 600–650 nm (Beckman Coulter, USA). Each experiment was set up with three parallel repeats.
Flow cytometry
Collected the samples mentioned above, washed with PBS, centrifuged at 800 × g for 6 min, suspended in ice-cold 70% ethanol/PBS, centrifuged at 800 × g for another 6 min, and suspended with PBS. Resuspended cells with 100 μL medium and added 5 μL of annexin V and 1 μL of propidium iodide according to the manufacturer’s instructions of Alexa Fluor 488 Annexin V/Dead Cell Apoptosis Kit (Thermo Fisher, USA), and incubated for 15 min at room temperature. BD LSR II flow cytometry was used to detect cell apoptosis (BD Biosciences, USA).
Immunohistochemistry staining
The hippocampus tissues were collected and fixed with formalin neutral solution of 10% volume fraction, paraffin-embedded and then sectioned. Subsequently, the DAB horseradish peroxidase color development Kit (Beyotime, China) was applied to the conjugated NLRP3 antibody (1:1000, ab214185, Abcam, UK) staining at room temperature. The slides were dyed with hematoxylin for 30 s, dehydrated and fixed, and then sealed with neutral glue. In addition, all stained images were observed and photographed with a fluorescence microscope (Olympus, Japan) at 400 × magnification.
Statistical analysis
The experimental data and image preprocessing were analyzed by SPSS 20 statistical software (IBM, USA) and GraphPad Prism7.0 software (La Jolla, USA), respectively. Differences in the escape latency in MWM was analyzed using Two-way ANOVA with repeated measurements. Besides, Student’s t-test was used to analyze the significant differences between the two groups, and the differences between multiple groups were compared by one-way ANOVA and followed by Tukey’s post hoc test. Moreover, p < 0.05 was identified as statistically significant.
Results
ChIV alleviates sevoflurane-induced neurological dysfunction in memory and learning
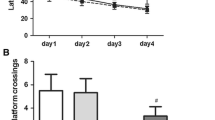
Morris water maze test showed that sevoflurane exposure significantly enhanced the escape latency of rats compared with the control group and only ChIV treatment group (p < 0.01, Fig. 1a), while ChIV treatment significantly decreased the escape latency (p < 0.001, Fig. 1a). Moreover, sevoflurane significantly decreased the number of platforms crossed by the aged rats, but alleviated by ChIV administration (p < 0.001, Fig. 1b). Furthermore, compared with rats treated with sevoflurane or ChIV only, the time spent in the target quadrant of rats treated with ChIV before sevoflurane exposure was increased (p < 0.01, Fig. 1c). Taken together, ChIV reversed the inhibitory effect of sevoflurane exposure on neurological dysfunction in memory and learning.
ChIV alleviates sevoflurane-induced neurological dysfunction in memory and learning. a Effect of ChIV on the escape latency of rats exposure to sevoflurane; b Effect of ChIV on the platform crossing of rats exposure to sevoflurane; c Effect of ChIV on the time spent in the target quadrant of rats exposed to sevoflurane. Data represent the mean ± SEM of n = 6 rat per group. **p < 0.01, ***p < 0.001, compared with the control group; ##p < 0.01, ###p < 0.001, compared with the sevoflurane group
ChIV alleviates sevoflurane-induced impairment in memory recognition and spatial working memory
To further expand our findings, we attempt to assess the effect of ChIV on the memory recognition and spatial working memory of rats exposed to sevoflurane. As shown in Fig. 2a, b, sevoflurane exposure significantly decreased the discrimination index of short- or long-term memory compared with the control group and ChIV treatment only (p < 0.001). However, ChIV pretreatment significantly elevated memory recognition in sevoflurane exposure to rats (p < 0.001, Fig. 2a, b). Besides, sevoflurane exposure notably decreased the spatial working memory of rats compared with the control group (p < 0.001, Fig. 2c), but ChIV treatment was restored the downregulation effect of sevoflurane on spontaneous alterations (p < 0.001, Fig. 2c). Whereas, there was no significant difference between the control group and ChIV treatment only group (p > 0.05). Taken together, ChIV alleviates sevoflurane-induced memory impairment.
ChIV alleviates sevoflurane-induced impairment in memory recognition and spatial working memory. a Effect of ChIV on the short-term memory of rats exposure to sevoflurane was determined by NOR test; b Effect of ChIV on the long-term memory of rats exposure to sevoflurane was determined by NOR test; c Effect of ChIV on the spatial working memory of rats exposure to sevoflurane was determined by Y-maze test. Data represent the mean ± SEM of n = 6 rat per group. ***p < 0.001, compared with the control group; ###p < 0.001, compared with the sevoflurane group
ChIV decreases sevoflurane-induced neuroinflammation and apoptosis in rat hippocampus
Neuroinflammation and apoptotic cells play an important role in regulating anesthesia-induced neurotoxicity [32]. In this study, our data revealed that sevoflurane exposure significantly promoted the expression of pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α) in the hippocampal region of rats compared with the control group by western blot (all p < 0.001, Fig. 3a). In contrast, ChIV treatment decreased the expression levels of IL-6, IL-1β and TNF-α in the hippocampal region of rats treated with sevoflurane exposure (all p < 0.01, Fig. 3a). Meanwhile, TUNEL analysis results showed that the number of apoptotic cells in the hippocampus of rat exposure to sevoflurane was significantly increased compared with the control group (p < 0.001, Fig. 3b, c). However, ChIV pretreatment significantly decreased cell apoptosis in the hippocampus of rat exposure to sevoflurane (p < 0.01, Fig. 3b, c). In addition, we found that compared with the control group and ChIV treatment only group, sevoflurane exposure significantly increased the ROS levels in the hippocampus of rats (p < 0.001, Fig. 3d), but this upregulation effect was diminished by ChIV pretreatment (p < 0.01, Fig. 3d). Furthermore, western blot showed that sevoflurane exposure significantly decreased anti-apoptotic related protein Bcl-2 expression in the hippocampus of rats (p < 0.001, Fig. 3e), and increased the levels of the pro-apoptotic related protein cleaved-caspase-3 and Bax (all p < 0.001), while treatment with ChIV significantly decreased sevoflurane-induced cell apoptosis in the hippocampus (all p < 0.001). Taken together, ChIV rescued sevoflurane-induced neuroinflammation and apoptosis in the rat hippocampus.
ChIV decreases sevoflurane-induced neuroinflammation and apoptosis in rat hippocampus. a The expression of proinflammatory cytokines in the hippocampus of rats were measured by western blot; b, c The number of TUNEL positive cells in the hippocampus were detected by TUNEL fluorescent assay; d The level of ROS in the hippocampus was detected by oxiSelect In Vitro ROS/RNS assay kit; e: Western blot was applied to determine the expression of apoptotic related proteins in the hippocampus. Data represent the mean ± SEM of n = 3 rat per group. ***p < 0.001, compared with the control group; ##p < 0.01, ###p < 0.001, compared with the sevoflurane group
ChIV inhibits sevoflurane-induced NLRP3 inflammasome activation
As we knew, NLRP3 inflammasome was upregulated in the development of several inflammatories, ischemia–reperfusion injury and metabolic diseases, etc. [34, 35]. As expected, our experiment confirmed that the expression of NLRP3, ASC and caspase-1 were upregulated in the hippocampus of rats exposed to sevoflurane compared with the control group (all p < 0.001, Fig. 4a). Meanwhile, the expression of IL-1β and IL-18 was upregulated in the hippocampus of rat exposure to sevoflurane (both p < 0.001). In contrast, the increases were abolished by ChIV pretreatment (all p < 0.001, Fig. 4a). In addition, immunohistochemical staining showed that the number of NLRP3-positive cells in the hippocampal of aged rats' exposure to sevoflurane was higher than in the control group and ChIV treatment only group (p < 0.001, Fig. 4b, c). However, ChIV pretreatment repressed this effect (p < 0.01). Taken together, ChIV prevented sevoflurane-induced NLRP3 inflammasome activation.
ChIV inhibits sevoflurane-induced NLRP3 inflammasome activation. a Western blot was used to detect the expression of NLRP3, ASC, caspase-1, IL-1β and IL-18 in the hippocampus of rats exposed to sevoflurane; b, c Immunohistochemical staining was applied to evaluate the number of NLRP3 positive cells in the hippocampus. Data represent the mean ± SEM of n = 3 rat per group. ***p < 0.001, compared with the control group; ###p < 0.001, compared with the sevoflurane group
ChIV reverses sevoflurane-induced apoptotic cell and inflammation activation in vitro
To further explore the protective effect of ChIV on primary neurons against sevoflurane-induced neurotoxicity, we attempt to examine the role of ChIV in the primary neurons treated with sevoflurane. CCK-8 analysis results showed that ChIV at both concentrations of 40 and 50 μM notably decreased the viability of the primary neurons (p < 0.01, Fig. 5a), but ChIV at 10, 20 and 30 μM did not affect the cell viability. Meanwhile, and the concentration of 30 μM of ChIV was chosen for the follow-up experiments. Pretreatment with ChIV at 30 μM for 6 h significantly ameliorated sevoflurane-induced the death of neurons (p < 0.001, Fig. 5b). However, no significant difference was observed in both control group and ChIV treatment only group (p > 0.05). In addition, ChIV markedly blocked sevoflurane-induced apoptotic cell (p < 0.001, Fig. 5c, d), which was consistent with the results of western blot (Fig. 5e). Furthermore, sevoflurane treatment significantly increased the expression of IL-1β, IL-18, NLRP3, ASC, caspase-1 and proinflammatory cytokines secretion (all p < 0.001, Fig. 5f), while pretreatment with ChIV reverses sevoflurane-induced neuronal inflammation (p < 0.001). Similarly, in accordance with our findings in vivo, the ROS generation was significantly upregulated in primary neurons treated with sevoflurane (p < 0.001, Fig. 5g), but ChIV pretreatment decreased the ROS production. Collectively, ChIV exerts neuroprotective against sevoflurane-induced injury.
ChIV reverses sevoflurane-induced apoptotic cell and inflammation activation in vitro. a, b CCK-8 was used to detect the viability of primary neurons; c, d Flow cytometry was applied to detect the apoptotic cells; e, f Western blot was applied to detect the protein level; g The level of ROS in the hippocampus was detected by oxiSelect In Vitro ROS/RNS assay kit. ***p < 0.001, compared with treatment with ChIV at 0 μM; ###p < 0.001, compared with the control group; ▲▲p < 0.01, ▲▲▲p < 0.001, compared with the sevoflurane group
ChIV exerts neuroprotective activities against sevoflurane-induced neuroinflammation by blocking NLRP3/caspase-1 pathway
To further determine the role of the NLRP3/caspase-1 pathway in ChIV alleviates sevoflurane-induced neuroinflammation and apoptotic cell, we examined the primary neuron viability, death and apoptosis, inflammatory cytokines secretion and ROS production. As shown in Fig. 6a, MCC950, a highly potent specific NLRP3/caspase-1 pathway inhibitor, was augmented the inhibitory effect of ChIV on sevoflurane-induced the death of primary neurons (p < 0.05). Flow cytometry analysis results showed that the anti-apoptotic activity of ChIV in primary neurons treated with sevoflurane was further enhanced by MCC950 administration (p < 0.05, Fig. 6b, c). In addition, the expression of cleaved-caspase-3 and Bax were decreased in ChIV pretreatment primary neurons (p < 0.001, Fig. 6d, f), as well as upregulated the level of Bcl-2 protein (p < 0.001). Similarly, MCC950 addition upregulated the anti-apoptotic activity of ChIV (p < 0.05). Furthermore, sevoflurane significantly increased pro-inflammatory cytokine secretion (p < 0.001, Fig. 6e, g, h) and ROS production (p < 0.001, Fig. 6i), while MCC950 and ChIV strongly repressed the expression of proinflammatory cytokines and ROS production (all p < 0.05, Fig. 6e, g–i). In conclusion, these results confirmed that ChIV ameliorates sevoflurane-induced neuroinflammation and neurotoxicity in primary neurons by blocking the NLRP3/caspase-3 pathway.
ChIV exerts neuroprotective activities against sevoflurane-induced neuroinflammation by blocking NLRP3/caspase-1 pathway. a The viability of primary neurons was detected by CCK-8 assay; b, c The apoptosis of primary neurons was determined by flow cytometry; d, f western blot was applied to measure the expression of cleaved caspase-3, Bax, and Bcl-2; g, h Western blot was used to detect the expression of inflammatory-related proteins; i The level of ROS in the hippocampus was detected by oxiSelect In Vitro ROS/RNS assay kit. ***p < 0.001, compared with the control group; ##p < 0.01, ###p < 0.001, compared with the sevoflurane group; ▲p < 0.05, compared with the sevoflurane + ChIV group
Discussion
POCD refers to the persistent impairment of memory, abstract thinking, and orientation in patients undergoing anesthesia surgery, accompanied by decreased social activity, such as changes in personality, social ability, cognitive ability, and skills after surgery [36, 37]. POCD has become a major health problem for the elderly [38, 39]. Therefore, it is an urgent need to clarify the correlation relationship between anesthetic exposure and the onset and progression of cognitive impairment after surgery. In this study, our results showed that ChIV pretreatment significantly alleviated sevoflurane-induced the dysfunctions of memory and cognition in aged rats, which was related to the regulation of neuroinflammation and apoptosis. In accordance with previous studies, the expression of proinflammation cytokines was overexpressed in sevoflurane-induced neurocognitive impairment in aged rats hippocampus [40]. These findings suggested that hyper-inflammatory response may be associated with sevoflurane-induced cognitive impairment and neurotoxicity in aged rats.
In the current study, the active ingredients of Chinese herbal medicine have significant therapeutic effects on anesthesia-induced neurotoxicity and cognitive dysfunction [41, 42]. For example, ChIV was extracted from Chinese medicine Rhizoma Panacis japonica, which possessed pharmacological functions including antitumor [43], antioxidant [28] and immunomodulatory [44], cardio-protection [45] and anti-obesity [46]. Interestingly, previous studies have been shown that the neuroprotective effect of ChIV against isoflurane-induced neurotoxicity and cognitive deficits [29]. In our present study, we found that ChIV not only exerted neuroprotective activity against sevoflurane exposure induced cognitive deficit of aged rats but also decreased neuroinflammation in the hippocampus. In addition, ChIV decreased lipopolysaccharide (LPS)-induced oxidative stress and proinflammatory cytokines secretion [44, 47]. Meanwhile, increasing evidence indicates that oxidative stress plays an important role in anesthesia-induced neurotoxicity [48, 49]. In our experiments, ChIV decreased the ROS production and the expression of IL-1β, IL-6, and TNF-α during aged rats exposure to sevoflurane, which revealed that ChIV provides protection resistant to sevoflurane-induced oxidative stress and inflammatory response. Furthermore, treatment with ChIV alleviates PC12 cell injury and apoptosis [50]. Neuronal apoptosis could be aggravated the deterioration of neuropathic diseases, such as cerebral ischemia–reperfusion injury [51], neurotoxicity [52], Parkinson’s disease [53], and stroke [54], etc. Of note, neuronal apoptosis in the hippocampus can lead to neuronal damage, cognitive impairment and even death [55, 56]. Our study showed that pretreatment with ChIV decreased the expression of cleaved-caspase-3 and Bax, as well as upregulated Bax protein level in the hippocampus of aged rats exposure to sevoflurane, and these results are consistent with the expression of the apoptotic-related protein in vitro.
Accumulating evidence has been shown that NLRP3 inflammasome activated was a critical initiator of the inflammatory response, which was interacted with apoptosis-associated speck-like protein (ASC) to induce caspase-1 cleavage and maturation and secretion of IL-1β and IL-18 [57, 58]. Of note, previous studies confirmed that NLRP3/caspase-1 pathway activation was involved in anesthesia-induced neurotoxicity [59]. In this study, we also found that sevoflurane exposure was significantly increased the expression of NLRP3, ASC, caspase-1, and secretion of IL-1β and IL-18 in the hippocampus of aged rats and primary neurons. Meanwhile, several studies in the last few years indicate that pretreatment with MCC950 (an NLRP3/caspase-1 pathway inhibitor) ameliorated isoflurane-induced cognitive dysfunction and neuroinflammation [60]. Caspase 1, the effector protease of the inflammasome, is activated during pyroptosis and cleaves the proinflammatory cytokines interleukin-1β (IL-1β) and IL-18 [61]. This proinflammatory microenvironment is favorable for tumor initiation and progression, as increased serum levels of proinflammatory ILs such as IL-1β and IL-18 have been observed in several types of cancer [62, 63]. Recently, inhibition of pyroptosis has been considered as a novel strategy to eradicate neurotoxicity induced by anesthesia [60, 64]. In addition, clinical studies have shown that hyper-inflammatory cytokines triggered neuronal injury and promoted microglia activation in the central nervous system, leading to impairment of memory and learning functions in elderly patients [65]. Similarly, our experiments showed that sevoflurane exposure induced memory and learning impairment and increased the number of NLRP3-positive cells in the hippocampus of aged rats. Importantly, MCC950 addition was exerted a protective effect against sevoflurane-induced primary neuron damage. These findings suggested that the NLRP3/caspase-1 pathway may be a critical therapeutic target for POCD. However, some limitations still exist in the current study. First, the underlying molecular mechanisms that contribute the regulatory role of ChIV on NLRP3/caspase-1 pathway are still poorly understood and need to be addressed in future studies. Secondly, the pharmacokinetic characteristics and safety of ChIV in vivo also have not yet fully determined. Moreover, due to practical constraints, the number of animals used for behavioral experiments was small. To make our results more convincing, we should validate our current findings in a large sample.
Conclusion
In summary, our studies revealed that ChIV pre-treatment confers neuroprotective effect against sevoflurane-induced neuroinflammation and cognitive dysfunction through blocking the NLRP3/caspase-1 pathway, which provides a new therapeutic method for POCD by anesthesia.
References
Feinkohl I, Winterer G, Spies CD, Pischon T. Cognitive reserve and the risk of postoperative cognitive dysfunction. Dtsch Arztebl Int. 2017;114(7):110–7. https://doi.org/10.3238/arztebl.2017.0110.
Lachmann G, Kant I, Lammers F, Windmann V, Spies C, Speidel S, Borchers F, Hadzidiakos D, Hendrikse J, Winterer G, de Bresser J. Cerebral microbleeds are not associated with postoperative delirium and postoperative cognitive dysfunction in older individuals. PLoS One. 2019;14(6):e0218411. https://doi.org/10.1371/journal.pone.0218411.
Yang W, Kong LS, Zhu XX, Wang RX, Liu Y, Chen LR. Effect of dexmedetomidine on postoperative cognitive dysfunction and inflammation in patients after general anaesthesia: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2019;98(18):e15383. https://doi.org/10.1097/md.0000000000015383.
Quan C, Chen J, Luo Y, Zhou L, He X, Liao Y, Chou J, Guo Q, Chen AF, Wen O. BIS-guided deep anesthesia decreases short-term postoperative cognitive dysfunction and peripheral inflammation in elderly patients undergoing abdominal surgery. Brain Behav. 2019;9(4):e01238. https://doi.org/10.1002/brb3.1238.
Yang F, Shan Y, Tang Z, Wu X, Bi C, Zhang Y, Gao Y, Liu H. The neuroprotective effect of hemin and the related mechanism in sevoflurane exposed neonatal rats. Front Neurosci. 2019;13:537. https://doi.org/10.3389/fnins.2019.00537.
Cao Y, Li Z, Ma L, Yang N, Guo X. Isoflurane-induced postoperative neurovascular and cognitive dysfunction is associated with VEGF overexpression in aged rats. J Mol Neurosci. 2019. https://doi.org/10.1007/s12031-019-01350-8.
Nishigaki A, Kawano T, Iwata H, Aoyama B, Yamanaka D, Tateiwa H, Shigematsu-Locatelli M, Eguchi S, Locatelli FM, Yokoyama M. Acute and long-term effects of haloperidol on surgery-induced neuroinflammation and cognitive deficits in aged rats. J Anesth. 2019;33(3):416–25. https://doi.org/10.1007/s00540-019-02646-0.
Li N, Zhang X, Dong H, Hu Y, Qian Y. Bidirectional relationship of mast cells-neurovascular unit communication in neuroinflammation and its involvement in POCD. Behav Brain Res. 2017;322(Pt A):60–9. https://doi.org/10.1016/j.bbr.2017.01.006.
Kok WF, Koerts J, Tucha O, Scheeren TW, Absalom AR. Neuronal damage biomarkers in the identification of patients at risk of long-term postoperative cognitive dysfunction after cardiac surgery. Anaesthesia. 2017;72(3):359–69. https://doi.org/10.1111/anae.13712.
Hovens IB, van Leeuwen BL, Mariani MA, Kraneveld AD, Schoemaker RG. Postoperative cognitive dysfunction and neuroinflammation; cardiac surgery and abdominal surgery are not the same. Brain Behav Immun. 2016;54:178–93. https://doi.org/10.1016/j.bbi.2016.02.003.
Zhang Z, Li X, Li F, An L. Berberine alleviates postoperative cognitive dysfunction by suppressing neuroinflammation in aged mice. Int Immunopharmacol. 2016;38:426–33. https://doi.org/10.1016/j.intimp.2016.06.031.
Sha HY, Zhao JB, Sha MX, Guo SM. Effects of Vitamin B12 on postoperative cognitive dysfunction induced by isoflurane anesthesia in rats. Eur Rev Med Pharmacol Sci. 2017;21(8):1959–66.
Ye JS, Chen L, Lu YY, Lei SQ, Peng M, Xia ZY. Honokiol-mediated mitophagy ameliorates postoperative cognitive impairment induced by surgery/sevoflurane via inhibiting the activation of NLRP3 inflammasome in the hippocampus. Oxid Med Cell Longev. 2019;2019:8639618. https://doi.org/10.1155/2019/8639618.
Wei P, Yang F, Zheng Q, Tang W, Li J. The potential role of the NLRP3 inflammasome activation as a link between mitochondria ROS generation and neuroinflammation in postoperative cognitive dysfunction. Front Cell Neurosci. 2019;13:73. https://doi.org/10.3389/fncel.2019.00073.
Ashraf A, Mahmoud PA, Reda H, Mansour S, Helal MH, Michel HE, Nasr M. Silymarin and silymarin nanoparticles guard against chronic unpredictable mild stress induced depressive-like behavior in mice: involvement of neurogenesis and NLRP3 inflammasome. J Psychopharmacol. 2019;33(5):615–31. https://doi.org/10.1177/0269881119836221.
Ystgaard MB, Scheffler K, Suganthan R, Bjoras M, Ranheim T, Sagen EL, Halvorsen B, Saugstad OD, Yndestad A. Neuromodulatory effect of NLRP3 and ASC in neonatal hypoxic ischemic encephalopathy. Neonatology. 2019;115(4):355–62. https://doi.org/10.1159/000497200.
Peng J, Zhang P, Zheng H, Ren YQ, Yan H. Dexmedetomidine reduces hippocampal microglia inflammatory response induced by surgical injury through inhibiting NLRP3. Chin J Traumatol. 2019;22(3):161–5. https://doi.org/10.1016/j.cjtee.2019.03.002.
Detrait ER, Danis B, Lamberty Y, Foerch P. Peripheral administration of an anti-TNF-alpha receptor fusion protein counteracts the amyloid induced elevation of hippocampal TNF-alpha levels and memory deficits in mice. Neurochem Int. 2014;72:10–3. https://doi.org/10.1016/j.neuint.2014.04.001.
Alam A, Hana Z, Jin Z, Suen KC, Ma D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–56. https://doi.org/10.1016/j.ebiom.2018.10.021.
Skvarc DR, Berk M, Byrne LK, Dean OM, Dodd S, Lewis M, Marriott A, Moore EM, Morris G, Page RS, Gray L. Post-operative cognitive dysfunction: an exploration of the inflammatory hypothesis and novel therapies. Neurosci Biobehav Rev. 2018;84:116–33. https://doi.org/10.1016/j.neubiorev.2017.11.011.
Locatelli FM, Kawano T, Iwata H, Aoyama B, Eguchi S, Nishigaki A, Yamanaka D, Tateiwa H, Shigematsu-Locatelli M, Yokoyama M. Resveratrol-loaded nanoemulsion prevents cognitive decline after abdominal surgery in aged rats. J Pharmacol Sci. 2018;137(4):395–402. https://doi.org/10.1016/j.jphs.2018.08.006.
Qi D, Yang X, Chen J, Li F, Shi X, Zhang C, Yang Z. Determination of chikusetsusaponin V and chikusetsusaponin IV in rat plasma by liquid chromatography-mass spectrometry and its application to a preliminary pharmacokinetic study. Biomed Chromatogr. 2013;27(11):1568–73. https://doi.org/10.1002/bmc.2961.
Li Y, Zhang T, Cui J, Jia N, Wu Y, Xi M, Wen A. Chikusetsu saponin IVa regulates glucose uptake and fatty acid oxidation: implications in antihyperglycemic and hypolipidemic effects. J Pharm Pharmacol. 2015;67(7):997–1007. https://doi.org/10.1111/jphp.12392.
Yang J, Qian S, Cai X, Lu W, Hu C, Sun X, Yang Y, Yu Q, Gao SP, Cao P. Chikusetsusaponin IVa butyl ester (CS-IVa-Be), a novel IL6R antagonist, inhibits IL6/STAT3 signaling pathway and induces cancer cell apoptosis. Mol Cancer Ther. 2016;15(6):1190–200. https://doi.org/10.1158/1535-7163.mct-15-0551.
Wang L, Yuan D, Zheng J, Wu X, Wang J, Liu X, He Y, Zhang C, Liu C, Wang T, Zhou Z. Chikusetsu saponin IVa attenuates isoprenaline-induced myocardial fibrosis in mice through activation autophagy mediated by AMPK/mTOR/ULK1 signaling. Phytomedicine. 2019;58:152764. https://doi.org/10.1016/j.phymed.2018.11.024.
Yuan C, Liu C, Wang T, He Y, Zhou Z, Dun Y, Zhao H, Ren D, Wang J, Zhang C, Yuan D. Chikusetsu saponin IVa ameliorates high fat diet-induced inflammation in adipose tissue of mice through inhibition of NLRP3 inflammasome activation and NF-kappaB signaling. Oncotarget. 2017;8(19):31023–40. https://doi.org/10.18632/oncotarget.16052.
Chen X, Wu QS, Meng FC, Tang ZH, Chen X, Lin LG, Chen P, Qiang WA, Wang YT, Zhang QW, Lu JJ. Chikusetsusaponin IVa methyl ester induces G1 cell cycle arrest, triggers apoptosis and inhibits migration and invasion in ovarian cancer cells. Phytomedicine. 2016;23(13):1555–65. https://doi.org/10.1016/j.phymed.2016.09.002.
Yuan D, Wan JZ, Deng LL, Zhang CC, Dun YY, Dai YW, Zhou ZY, Liu CQ, Wang T. Chikusetsu saponin V attenuates MPP+-induced neurotoxicity in SH-SY5Y cells via regulation of Sirt1/Mn-SOD and GRP78/caspase-12 pathways. Int J Mol Sci. 2014;15(8):13209–22. https://doi.org/10.3390/ijms150813209.
Fang X, Han Q, Li S, Zhao Y, Luo A. Chikusetsu saponin IVa attenuates isoflurane-induced neurotoxicity and cognitive deficits via SIRT1/ERK1/2 in developmental rats. Am J Transl Res. 2017;9(9):4288–99.
Wan J, Deng L, Zhang C, Yuan Q, Liu J, Dun Y, Zhou Z, Zhao H, Liu C, Yuan D, Wang T. Chikusetsu saponin V attenuates H2O2-induced oxidative stress in human neuroblastoma SH-SY5Y cells through Sirt1/PGC-1alpha/Mn-SOD signaling pathways. Can J Physiol Pharmacol. 2016;94(9):919–28. https://doi.org/10.1139/cjpp-2015-0262.
Zhou H, Li S, Wang G. Euxanthone ameliorates sevoflurane-induced neurotoxicity in neonatal mice. J Mol Neurosci. 2019;68(2):275–86. https://doi.org/10.1007/s12031-019-01303-1.
Huang L, Huang K, Ning H. Hispidulin prevents sevoflurane- Induced memory dysfunction in aged rats. Biomed Pharmacother. 2018;97:412–22. https://doi.org/10.1016/j.biopha.2017.10.142.
Han M, Gao H, Xie J, Yuan YP, Yuan Q, Gao MQ, Liu KL, Chen XH, Han YT, Han ZW. Hispidulin induces ER stress-mediated apoptosis in human hepatocellular carcinoma cells in vitro and in vivo by activating AMPK signaling pathway. Acta Pharmacol Sin. 2019;40(5):666–76. https://doi.org/10.1038/s41401-018-0159-7.
Pellegrini C, Fornai M, Antonioli L, Blandizzi C, Calderone V. Phytochemicals as novel therapeutic strategies for nlrp3 inflammasome-related neurological, metabolic, and inflammatory diseases. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20122876.
Ai QD, Chen C, Chu S, Zhang Z, Luo Y, Guan F, Lin M, Liu D, Wang S, Chen N. IMM-H004 therapy for permanent focal ischemic cerebral injury via CKLF1/CCR4-mediated NLRP3 inflammasome activation. Transl Res. 2019. https://doi.org/10.1016/j.trsl.2019.05.007.
Nakao S, Yamamoto T, Kimura S, Mino T, Iwamoto T. Brain white matter lesions and postoperative cognitive dysfunction: a review. J Anesth. 2019;33(2):336–40. https://doi.org/10.1007/s00540-019-02613-9.
Safavynia SA, Goldstein PA. The role of neuroinflammation in postoperative cognitive dysfunction: moving from hypothesis to treatment. Front Psychiatry. 2018;9:752. https://doi.org/10.3389/fpsyt.2018.00752.
Kotekar N, Shenkar A, Nagaraj R. Postoperative cognitive dysfunction—current preventive strategies. Clin Interv Aging. 2018;13:2267–73. https://doi.org/10.2147/cia.s133896.
Luo A, Yan J, Tang X, Zhao Y, Zhou B, Li S. Postoperative cognitive dysfunction in the aged: the collision of neuroinflammaging with perioperative neuroinflammation. Inflammopharmacology. 2019;27(1):27–37. https://doi.org/10.1007/s10787-018-00559-0.
Li D, Liu L, Li L, Li X, Huang B, Zhou C, Zhang Z, Wang C, Dong P, Zhang X, Yang B, Zhang L. Sevoflurane induces exaggerated and persistent cognitive decline in a type II diabetic rat model by aggregating hippocampal inflammation. Front Pharmacol. 2017;8:886. https://doi.org/10.3389/fphar.2017.00886.
Lu WI, Lu DP. Impact of chinese herbal medicine on american society and health care system: perspective and concern. Evid Based Complement Alternat Med. 2014;2014:251891. https://doi.org/10.1155/2014/251891.
Chen PJ, Shang AQ, Wang WW, Yang JP. Astragaloside suppresses tumor necrosis factor receptor-associated factor 5 signaling pathway and alleviates neurodegenerative changes in retinal pigment epithelial cells induced by isoflurane. J Cell Biochem. 2019;120(1):1028–37. https://doi.org/10.1002/jcb.27599.
Song X, Wang W, Zhang X, Jiang Y, Yang X, Deng C, Yue Z, Tang Z. Deglucose chikusetsusaponin IVa isolated from rhizoma panacis majoris induces apoptosis in human HepG2 hepatoma cells. Mol Med Rep. 2015;12(4):5494–500. https://doi.org/10.3892/mmr.2015.4035.
Wang H, Qi J, Li L, Wu T, Wang Y, Wang X, Ning Q. Inhibitory effects of chikusetsusaponin IVa on lipopolysaccharide-induced pro-inflammatory responses in THP-1 cells. Int J Immunopathol Pharmacol. 2015;28(3):308–17. https://doi.org/10.1177/0394632015589519.
Duan J, Yin Y, Wei G, Cui J, Zhang E, Guan Y, Yan J, Guo C, Zhu Y, Mu F, Weng Y, Wang Y, Wu X, Xi M, Wen A. Chikusetsu saponin IVa confers cardioprotection via SIRT1/ERK1/2 and homer1a pathway. Sci Rep. 2015;5:18123. https://doi.org/10.1038/srep18123.
Yin J, Seo CS, Hwang IH, Lee MW, Song KH. Anti-obesity activities of chikusetsusaponin IVa and Dolichos lablab L. Seeds. Nutrients. 2018. https://doi.org/10.3390/nu10091221.
Lee HJ, Shin JS, Lee WS, Shim HY, Park JM, Jang DS, Lee KT. Chikusetsusaponin IVa methyl ester isolated from the roots of achyranthes japonica suppresses LPS-induced iNOS, TNF-alpha, IL-6, and IL-1beta expression by NF-kappaB and AP-1 inactivation. Biol Pharm Bull. 2016;39(5):657–64. https://doi.org/10.1248/bpb.b15-00572.
Li L, Yu Q, Liang W. Molecular pathways of mitochondrial dysfunctions: possible cause of cell death in anesthesia-induced developmental neurotoxicity. Brain Res Bull. 2015;110:14–9. https://doi.org/10.1016/j.brainresbull.2014.10.011.
Li B, Feng XJ, Hu XY, Chen YP, Sha JC, Zhang HY, Fan HG. Effect of melatonin on attenuating the isoflurane-induced oxidative damage is related to PKCalpha/Nrf2 signaling pathway in developing rats. Brain Res Bull. 2018;143:9–18. https://doi.org/10.1016/j.brainresbull.2018.09.018.
Duan J, Yin Y, Cui J, Yan J, Zhu Y, Guan Y, Wei G, Weng Y, Wu X, Guo C, Wang Y, Xi M, Wen A. Chikusetsu saponin IVa ameliorates cerebral ischemia reperfusion injury in diabetic mice via adiponectin-mediated AMPK/GSK-3beta pathway in vivo and in vitro. Mol Neurobiol. 2016;53(1):728–43. https://doi.org/10.1007/s12035-014-9033-x.
Chen X, Zhang X, Xue L, Hao C, Liao W, Wan Q. Treatment with enriched environment reduces neuronal apoptosis in the periinfarct cortex after cerebral ischemia/reperfusion injury. Cell Physiol Biochem. 2017;41(4):1445–566. https://doi.org/10.1159/000468368.
Hu X, Hu X, Huang G. LncRNA MALAT1 is involved in sevoflurane-induced neurotoxicity in developing rats. J Cell Biochem. 2019. https://doi.org/10.1002/jcb.29127.
Chen Z, Wang E, Hu R, Sun Y, Zhang L, Jiang J, Zhang Y, Jiang H. Tetranectin gene deletion induces Parkinson's disease by enhancing neuronal apoptosis. Biochem Biophys Res Commun. 2015;468(1–2):400–7. https://doi.org/10.1016/j.bbrc.2015.10.118.
Ye X, Shen T, Hu J, Zhang L, Zhang Y, Bao L, Cui C, Jin G, Zan K, Zhang Z, Yang X, Shi H, Zu J, Yu M, Song C, Wang Y, Qi S, Cui G. Purinergic 2X7 receptor/NLRP3 pathway triggers neuronal apoptosis after ischemic stroke in the mouse. Exp Neurol. 2017;292:46–55. https://doi.org/10.1016/j.expneurol.2017.03.002.
Zhang B, Zhang JW, Wang WP, Dong RF, Tian S, Zhang C. Effect of lamotrigine on epilepsy-induced cognitive impairment and hippocampal neuronal apoptosis in pentylenetetrazole-kindled animal model. Synapse. 2017. https://doi.org/10.1002/syn.21945.
Xu J, Huai Y, Meng N, Dong Y, Liu Z, Qi Q, Hu M, Fan M, Jin W, Lv P. L-3-n-butylphthalide activates Akt/mTOR signaling, inhibits neuronal apoptosis and autophagy and improves cognitive impairment in mice with repeated cerebral ischemia-reperfusion injury. Neurochem Res. 2017;42(10):2968–81. https://doi.org/10.1007/s11064-017-2328-3.
Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev. 2015;265(1):35–52. https://doi.org/10.1111/imr.12286.
Liu Q, Zhang D, Hu D, Zhou X, Zhou Y. The role of mitochondria in NLRP3 inflammasome activation. Mol Immunol. 2018;103:115–24. https://doi.org/10.1016/j.molimm.2018.09.010.
Wang Z, Meng S, Cao L, Chen Y, Zuo Z, Peng S. Critical role of NLRP3-caspase-1 pathway in age-dependent isoflurane-induced microglial inflammatory response and cognitive impairment. J Neuroinflammation. 2018;15(1):109. https://doi.org/10.1186/s12974-018-1137-1.
Fan Y, Du L, Fu Q, Zhou Z, Zhang J, Li G, Wu J. Inhibiting the NLRP3 inflammasome with MCC950 ameliorates isoflurane-induced pyroptosis and cognitive impairment in aged mice. Front Cell Neurosci. 2018;12:426. https://doi.org/10.3389/fncel.2018.00426.
Cieniewicz B, Dong Q, Li G, Forrest JC, Mounce BC, Tarakanova VL, van der Velden A, Krug LT. Murine gammaherpesvirus 68 pathogenesis is independent of caspase-1 and caspase-11 in mice and impairs interleukin-1beta production upon extrinsic stimulation in culture. J Virol. 2015;89(13):6562–74. https://doi.org/10.1128/jvi.00658-15.
Xi G, Gao J, Wan B, Zhan P, Xu W, Lv T, Song Y. GSDMD is required for effector CD8(+) T cell responses to lung cancer cells. Int Immunopharmacol. 2019;74:105713. https://doi.org/10.1016/j.intimp.2019.105713.
Lee C, Do HTT, Her J, Kim Y, Seo D, Rhee I. Inflammasome as a promising therapeutic target for cancer. Life Sci. 2019. https://doi.org/10.1016/j.lfs.2019.116593.
Sun L, Ma W, Gao W, Xing Y, Chen L, Xia Z, Zhang Z, Dai Z. Propofol directly induces caspase-1-dependent macrophage pyroptosis through the NLRP3-ASC inflammasome. Cell Death Dis. 2019;10(8):542. https://doi.org/10.1038/s41419-019-1761-4.
Wilson KD, Ochoa LF, Solomon OD, Pal R, Cardona SM, Carpio VH, Keiser PH, Cardona AE, Vargas G, Stephens R. Elimination of intravascular thrombi prevents early mortality and reduces gliosis in hyper-inflammatory experimental cerebral malaria. J Neuroinflammation. 2018;15(1):173. https://doi.org/10.1186/s12974-018-1207-4.
Funding
The study is supported by funding from hospital to young physicians.
Author information
Authors and Affiliations
Contributions
AS: designed the study, performed the experiments and wrote the paper. JF and SF: performed the experiments and collected the data. JW: analyzed the data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shao, A., Fei, J., Feng, S. et al. Chikusetsu saponin IVa alleviated sevoflurane-induced neuroinflammation and cognitive impairment by blocking NLRP3/caspase-1 pathway. Pharmacol. Rep 72, 833–845 (2020). https://doi.org/10.1007/s43440-020-00078-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-020-00078-2