Abstract
Sevoflurane is a widely used anesthetic. A series of recent studies have shown that exposure to sevoflurane at an early stage is a risk factor for the development of learning and memory dysfunction. Euxanthone is a xanthone derivative obtained from Polygala caudata. This study was designed to investigate whether euxanthone can confer neuroprotective activities against sevoflurane-induced neurotoxicity and to determine the associated molecular mechanisms. Neonatal Sprague-Dawley (male) rats were exposed to sevoflurane with or without euxanthone treatment. The behavioral data of rats were collected at P41 (the beginning of the adult stage). The hippocampal tissue was obtained following exposure to sevoflurane. The reactive oxygen species (ROS) level in the hippocampal tissue was determined by a commercial kit. The expression of apoptotic markers and inflammatory cytokines was determined by western blot. The mRNA and protein expression of Nrf2 were determined by qRT-PCR and western blot, respectively. The rat in vitro model of neurotoxicity was established using isolated hippocampal neurons. Nrf2 expression was repressed by transfection of siRNA. The cell viability was assessed by the CCK-8 assay. The flow cytometry was performed to measure apoptotic cell death. Our data showed that euxanthone treatment at the neonatal stage protected against sevoflurane-induced neurotoxicity in adult rats. At the molecular level, our findings revealed that the neuroprotective activities of euxanthone were associated with decreased sevoflurane-induced apoptosis cell death and neuroinflammation. More importantly, our results provide the experimental evidence that euxanthone confers neuroprotection by upregulating Nrf2 expression. Euxanthone has a therapeutic potential for clinical prevention of sevoflurane-induced neurotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sevoflurane is an inhaled anesthetic that is widely used for pediatric patients, but it can cause agitation, delirium, and respiratory depression (Costi et al. 2014; Han et al. 2010). Recently, accumulating evidence suggests that exposure to sevoflurane at an early stage is a risk factor for the development of learning and memory dysfunction (Wilder et al. 2009). In fact, the safety of volatile anesthetics in clinical pediatric anesthesia has become a topic of interest among anesthesiologists and scientists (Sun 2010). In the last decade, extensive work has been performed to uncover the possible mechanisms associated with inhaled anesthesia-induced toxicity. Some of the mechanisms are thought to involve neuroapoptosis, neuroinflammation, reactive oxygen species accumulation, neurotransmitter disturbances, and changes in synaptic plasticity (Jevtovic-Todorovic 2012; Ji et al. 2015; Tao et al. 2014). Yet, there are few clinical interventions and treatments that prevent neuronal dysfunction presently (Tao et al. 2014). Hence, it is important to identify agents that can counteract sevoflurane-induced neurotoxicity in pediatric patients.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor expressed in a variety of cells and has been identified as a key mediator of cellular adaptation to redox stress (Sun et al. 2015). Several studies have shown that activation of Nrf2 may protect neuronal cells from apoptosis induced by oxidative stress (Su et al. 2016). Moreover, Nrf2 activation has been found to exert a beneficial effect against sevoflurane-induced neurotoxicity in neurons (Zhang et al. 2017a, b; Li et al. 2017). A recent study also showed that Nrf2 activation correlated with improved memory function post-sevoflurane treatment in aged rats (Huang et al. 2017). Collectively, these results show that activation of Nrf2 might provide a novel strategy for preventing sevoflurane-induced neurotoxicity.
Polygala caudata is a medicinal plant that mainly distributed in southwestern China. In the last decades, numerous evidence have demonstrated that euxanthone has various pharmacological activities (Pan and Mao 1984). It has been used as a folk medicine in China for hundreds of years to improve learning and memory function (Naidu et al. 2007). Additionally, multiple studies have confirmed Polygala caudata as safe and effective expectorant and sedative drug (Lin et al. 2005). Due to the complexity of Polygala caudata’s active components, the exact molecular mechanisms by which contribute to its pharmacological activities are still elusive. Recently, numerous studies were conducted to delineate the pharmacological effects and underlying mechanism of its ingredients (Yuan et al. 2018). Particularly, euxanthone, a xanthone derived from this plant, has been found to promote neurite outgrowth (Naidu et al. 2007; Ha et al. 2006), indicating that euxanthone may be used as a therapeutic agent in treating neurological conditions. Additionally, euxanthone is considered to be an anti-oxidant (Lin et al. 2005). However, the role of euxanthone in sevoflurane-induced neurotoxicity has not been explored. In this study, the primary neurons were used as the model cell line to investigate the effect of euxanthone on the sevoflurane-induced cell injury. Our results show that euxanthone significantly attenuates sevoflurane-induced apoptosis and neuroinflammation, suggesting that euxanthone might exert protective effects against sevoflurane-induced neurotoxicity.
Materials and Methods
Animals and Experimental Protocols
All protocols of animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of Shandong University. Animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals (8th edition, National Academies Press). Sprague-Dawley (male) rats (n = 72) were purchased from the Experimental Animal Center of Shandong University (Jinan, China). All rats were housed in a 12-h day-night cycle at a temperature of 23 °C. The pups were allowed free access to food and water. The sevoflurane was administered as previously described (Yu et al. 2016). In brief, on postnatal day 7 (P7), the pups were randomly divided into four groups (n = 18 animals per group): control group (received 20% oxygen), sevoflurane group (received 3% sevoflurane + 20% oxygen for 2 h), euxanthone group (EX, received i.p. injection of euxanthone at a dosage of 40 mg/kg), and a combination of sevoflurane- and euxanthone-treated group (injection of euxanthone at a dosage of 40 mg/kg 2 h prior to sevoflurane exposure). A gas monitor (Datex-Ohmeda, Tewksbury, MA) was utilized to monitor the gas concentration. The symptoms of apnea or hypoxia were examined by monitoring the respiratory frequency and skin color every 5 min. The pups were allowed to return to their maternal cage when the righting reflex was restored. All the administrations in each group were provided on 3 consecutive days (P7–P9). At the end of the last session of anesthesia (P9), hippocampal tissues were obtained from the brain of randomly selected 6 pups from each group and were prepared for ROS assay and western blot assay. Moreover, on postnatal day 41 (P41), the remaining 12 pups in each group were randomly allocated to two groups for the Morris water maze (n = 6) and step-through (n = 6), respectively. The detailed experimental procedures are presented in Fig. 1.
Behavioral and Cognitive Tests
Cognitive and memory functions were assessed by the Morris water maze and step-through test starting at P41 as described in the experimental protocol (Fig. 1). Both tests were conducted in the afternoon starting from 3 pm. The results were analyzed by a senior research fellow, who was blinded to the experimental grouping.
Morris Water Maze
The classic Morris water maze was conducted to evaluate the cognitive and memory function of pups as previously described (Yu et al. 2016). Briefly, a platform with a diameter of 10 cm was fixed 2 cm beneath the water surface in the pool. The water temperature was maintained at 23 °C. For 5 consecutive days, the probe training session was performed twice a day. During the training session, experimental animals were guided to approach and locate the hidden platform by swimming. The latency period the rats spent to find the hidden platform was recorded. The distance the rats had traveled before getting to the platform was also documented. At the end of the training sessions, the platform was removed. Then, the rats were put into water to swim without any interference for 2 min. Thereafter, the time spent on the number of crossing the previous platform by rats was recorded. The time spent in the target quadrant by rats was also documented. A video analysis system (Chengdu Instrument Ltd., Chengdu, China) was utilized to analyze the data.
Step-Through Test
The step-through test was conducted according to a previous protocol (Liu et al. 2013). The test was performed in an apparatus with six divided rooms. Briefly, every single room in the apparatus was separated into a light compartment and a dark compartment with an interconnecting semicircular door with a diameter of 3 cm. Beneath the dark compartment, a copper grid floor was placed and connected to a shocker maker (36 V). During the training sessions, rats were gently put in the light compartment with their backs facing the door. Then, the rats were allowed to move freely and enter the dark compartment for 3 min. Once the rat entered the dark compartment, it will be shocked by the electric current and retract back to the light compartment through the door. After an adapting period, a training session of 5 min was conducted. The tests were conducted at 24 and 48 h later.
During the retention trials, the number of mistakes and the latency period to the point of initial entry to the dark compartment were recorded within 5 min. If the rat did not receive a shock within 5 min, it was assigned a retention latency value of 300 s.
Measurement of ROS
The OxiSelectIn Vitro ROS/RNS Assay Kit (Cell Biolabs, San Diego, CA, USA) was utilized to determine the ROS level in the tissue and cell samples. The hippocampal tissue sample was homogenized on ice using 1% Triton-100 in PBS buffer supplemented with aprotinin, leupeptin, and pepstatin A. The cell sample was lysed with lysis buffer as previously described (Liu et al. 2018a). The total protein content in the lysate was determined by BCA assay (Thermo Scientific, Shanghai, China). The catalyst solution and dichlorodihydrofluorescein (DCFH) solution were added to the sample in a 96-well plate. After incubation for 20 min in darkness, the fluorescent signal was detected with a fluorometric plate reader at an excitation wavelength of 480 nm and emission wavelength of 530 nm.
Western Blotting
An equal amount of proteins per sample were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA). After blocking the membrane with 5% non-fat milk, the membranes were incubated overnight with the specific primary antibodies at 4 °C. The antibodies against cleaved caspase-3 (ab214430, 1:2000), Bcl-2 (ab196495, 1:1000), Bax (ab53154, 1:1000), and Nrf2 (ab137550, 1:1000) purchased from Abcam (Shanghai, China); IL-6 (sc-57315, 1:1000), TNF-α (sc-52746, 1:1000), and IL-1β (sc-57315, 1:1000) purchased from Santa Cruz (Santa Cruz, CA); and β-actin (AF0003, 1:1000) purchased from Beyotime (Shanghai, China) were used. Then, the membrane was incubated for 1 h with goat anti-mouse IgG-HRP (ab6789, 1:1000) as the secondary antibody. β-Actin was used as an internal control to normalize the relative expression levels of target genes. The positive bands were visualized using an enhanced chemiluminescent (ECL) detection reagent (Thermo Fisher, USA). The band intensities were analyzed and protein expression was quantified using Gel Doc 2000 (BioRad, USA).
Primary Neuronal Cell Culture and Treatment
All protocols of animal use were reviewed and approved by the Institutional Animal Care and Use Committee of Shandong University. Cultures of the primary hippocampal neurons were performed as described previously (Chaiprasongsuk et al. 2017). Briefly, Sprague-Dawley (male, postnatal D1) rats were purchased from the Experimental Animal Center of Shandong University (Jinan, China) and were used for cell isolation. Following the removal of meninges, cerebral hippocampi were isolated from rat brains in HBS. The tissue samples were added to a dissociation medium before mechanical dissociation. Then, the cell pellet was obtained by mild centrifugation (2000 rpm for 3 min at room temperature) followed by seeding in 3.5-cm culture dishes in the dissociation medium at a density of 3 × 105 cells per milliliter. Before cell plating, each cell culture dish was pretreated with 0.1% poly-d-lysine (Sigma, St. Louis, MI, USA) at room temperature for 2 h followed by rinsing twice with phosphate-buffered saline (PBS). Cells were kept in an incubator under 5% CO2 at 37 °C. The medium was replaced at 24 h with 48 ml serum-free medium (Neurobasal medium supplemented with 1 ml 2% B27, 0.5 ml penicillin-streptomycin, and 0.5 ml 0.25% Glumax). The percentage of neuronal cells was determined using indirect anti-NeuN IHC assay, which showed a purity of over 95% (Smothers et al. 2016). Seven days later, the isolated primary hippocampal neurons were randomly allocated into four groups. Sevoflurane was given in the atmosphere at a concentration of 4.1% using an anesthesia machine.
Cell Viability Assay
CCK-8 assay was performed as previously described (Chaiprasongsuk et al. 2017). Briefly, 5 × 103 cells were inoculated in 96-well plates and then the original medium was replaced with a fresh media supplemented with 10% (v/v) CCK-8 reaction solution followed by incubation for 2 h. The absorbance of cells was measured by a spectrophotometer (Tecan Group Ltd., Männedorf, Germany) at 450 nm.
Quantitative Real-Time PCR Assays for Nrf2
The TRIzol reagent (Invitrogen, USA) and PrimeScript™ RT Master Mix (TaKaRa, Dalian, China) were used to extract total RNA from cells and tumor tissues which were then reverse-transcribed into cDNA. The primers specific to Nrf2 and GAPDH were synthesized according to previously published sequences (Chaiprasongsuk et al. 2017)(Sangong, Shanghai, China). The PCR reaction was conducted using SYBR GREEN Master Mix (Solarbio Co., Beijing, China), and the relative expression of Nrf2 was calculated by the comparative ΔCt method (ABPrism software, Applied Biosystems, Foster City, CA).
Flow Cytometry Assay for Apoptosis
Apoptosis assay was measured using an apoptosis assay kit (Beyotime) following the manufacturer’s protocol. Briefly, the cells were incubated with Annexin V-FITC and propidium at a density of 5 × 105cells/ml in the dark for 15 min before detection using a flow cytometer (Merck Millipore, Germany).
Knockdown of Nrf2
Primary neuronal cells were transfected with siRNA targeting Nrf2 (OriGene Technologies, Beijing, China). Non-targeting sequence scramble siRNA was used as a control. The knockdown of Nrf2 expression was examined by qRT-PCR and western blot.
Luciferase Reporter Gene Assay
ARE luciferase plasmid was constructed by Pharma Gene (Shanghai, China) as described previously (Liu et al. 2018b). The ARE-luciferase activity was measured in a luminometer by the Dual-Luciferase Reporter Assay System according to the manufacturer’s instructions.
Statistical Analysis
Data are expressed as the mean ± SD. Data were analyzed by one-way ANOVA followed by Dunnett’s t test using SPSS17.0 and GraphPad Prism software (GraphPad Software Inc., La Jolla, CA). A difference with a P value less than 0.05 was defined as statistically significant.
Results
Euxanthone Treatment Ameliorates the Sevoflurane-Induced Impairment in Memory and Learning Functions
Given that P41–42 is considered as the beginning of adolescence in rats, the measurement of the behavioral tests was started at P41 to evaluate the effect of sevoflurane exposure on the cognitive impairment at the neonatal stage (Serlin and Torregrossa 2015). The effect of sevoflurane and euxanthone on spatial learning and memory was assessed using the Morris water maze. As shown in Fig. 2a and Table S1, sevoflurane markedly increased the escape latency of rats when compared with rats that received vehicle treatment. In contrast, euxanthone treatment significantly decreased the escape latency. Furthermore, the number of platforms crossed by the rats exposed to sevoflurane was also markedly decreased, but was reversed by treatment with euxanthone (Fig. 2b). In addition, sevoflurane exposure at the neonatal stage decreased the time spent on target quadrant. In contrast, rats that received euxanthone treatment and exposure to sevoflurane exhibited a marked increase in the time spent on target quadrant compared with rats exposed to sevoflurane only (Fig. 2c).
Effect of euxanthone on sevoflurane-induced cognitive and memory function of rats at adult stage. a Sevoflurane exposure at neonatal stage significantly increased the escape latency of rats at P41, which was reversed by euxanthone treatment. b Sevoflurane exposure at neonatal stage significantly decreased the platform crossing of rats at P41, which was attenuated by euxanthone treatment. c Sevoflurane exposure at neonatal stage significantly decreased the time spent in the target quadrant of rats at P41, which was attenuated by euxanthone treatment. d Sevoflurane exposure at neonatal stage significantly decreased the step-through latency at day 1 (no significant effect on day2 and day3), which was attenuated by euxanthone treatment. e Sevoflurane exposure at neonatal stage did not produce markedly change in the number of mistakes. ##P < 0.01 vs. vehicle, **P < 0.01 vs. sevoflurane
Euxanthone Treatment Ameliorates the Sevoflurane-Induced Impairment in Short-Term Learning and Memory
We further evaluated whether euxanthone exerts beneficial effects against sevoflurane-induced impairment in short-term learning and memory using the step-through test. As shown in Fig. 2d and Table S2, sevoflurane exposure at an early stage of life markedly reduced the latencies during the training and retention trials at day 1, while these effects diminished on day 2 and day 3. As evidenced by the step-through latency, the number of mistakes among sevoflurane treatment group and sevoflurane + EX group was not statistically significant (Fig. 2e and Table S3).
Euxanthone Exhibits Anti-apoptotic and Anti-inflammatory Effects in Rat Hippocampus
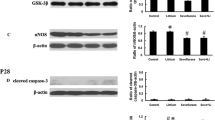
ROS production is considered to be one of the major causes of cell damage following sevoflurane exposure which promotes neuronal apoptotic cell death in the brain (Simon et al. 2000). In this study, we found that sevoflurane exposure significantly elevated the level of ROS in the hippocampus while treatment with euxanthone significantly reversed this effect (Fig. 3c). Additionally, the level of apoptosis of neurons in the hippocampus was evaluated by examining the expression levels of apoptosis-related molecules. As shown in Fig. 3b, sevoflurane exposure significantly increased the cleavage of caspase-3. Meanwhile, sevoflurane exposure markedly decreased Bcl-2 expression level and elevated Bax expression in the hippocampus. In contrast, treatment with euxanthone prevented sevoflurane-induced activation of caspase-3 (Fig. 3b). In addition, the sevoflurane-induced alteration in expression levels of Bcl-2 and Bax was markedly attenuated by euxanthone. These results indicated that euxanthone prevented sevoflurane-induced apoptotic neuronal cell death in the hippocampus. It is well established that inflammation of the neurological system contributes to sevoflurane-induced cognitive and memory dysfunction (Cui et al. 2018; Lv et al. 2017). In this study, the expression of inflammatory cytokines TNF-α, IL-6, and IL-1β in the hippocampal region of the rat model was increased after sevoflurane exposure (Fig. 3c). In contrast, the expression of these inflammatory cytokines was significantly repressed by euxanthone, demonstrating the anti-inflammatory activity of euxanthone (Fig. 3c).
Euxanthone attenuates sevoflurane-induced ROS generation, apoptosis, and neuroinflammation and upregulates Nrf2 in the hippocampus. a Euxanthone reduced sevoflurane-induced ROS generation in the hippocampus. b Euxanthone attenuated sevoflurane-induced increase in caspase-3 cleavage and Bax expression, and decrease in Bcl-2 expression. c Euxanthone attenuated sevoflurane-induced increase in production of inflammatory cytokines. d Euxanthone increased mRNA expression of Nrf2. e Euxanthone increased protein expression of Nrf2. ##P < 0.01 vs. vehicle, **P < 0.01 vs. sevoflurane
Euxanthone Upregulates Nrf2 Expression in the Hippocampal Region of a Rat Model
Nrf2 has been found to function as a crucial factor that modulates the redox and inflammatory responses of the neurological system upon external stimuli, including sevoflurane exposure (Tian et al. 2017). In this study, we measured its mRNA level in the hippocampal region of rats. As shown in Fig. 3d, sevoflurane did not cause any change in the mRNA level of Nrf2. However, euxanthone treatment significantly elevated the Nrf2 mRNA expression. Next, the protein level of Nrf2 was examined in the hippocampal tissue by western blot. In line with the mRNA level, the protein expression of Nrf2 was also elevated by euxanthone treatment. Collectively, these results showed that the beneficial effects of euxanthone on the cognitive and memory function were mediated by Nrf2 upregulation.
Euxanthone Protects Primary Neurons Against Sevoflurane-Induced Injury
To further examine the role of Nrf2 in the neuroprotective activities of euxanthone, primary neurons were isolated and used as the in vitro model. Firstly, the cytotoxic activities of euxanthone were examined by the CCK-8 assay. As shown in Fig. 4a, euxanthone at 10 and 20 μM did not affect the viability of neurons. However, euxanthone at 30 and 40 μM produced a significant change to the viability of neurons. As evidenced by the cell viabilities in 30 and 40 μM treatment, groups were reduced to 75% and 52%, respectively. Therefore, 20 μM was chosen as the optimal dosage in the subsequent experiments. Pretreatment with 20 μM euxanthone for 8 h before sevoflurane exposure significantly attenuated sevoflurane-induced reduction in the number of viable cells (Fig. 4b). Furthermore, the intracellular level of ROS was determined. As shown in Fig. 4c, sevoflurane markedly triggered the production of ROS in the neurons. In contrast, pretreatment with euxanthone abolished the production of ROS (Fig. 4c). Sevoflurane exposure produced about 20% apoptotic cell death whereas pretreatment with euxanthone could effectively decrease the pro-apoptotic activities of sevoflurane (Fig. 4d). In addition, sevoflurane exposure markedly activated caspase-3, decreased the expression of anti-apoptotic protein Bcl-2, and elevated the pro-apoptotic protein Bax (Fig. 4e). Next, we explored whether pretreatment with euxanthone could exert anti-inflammatory effects. In accordance with our findings in vivo, sevoflurane exposure remarkably increased the expression of inflammatory cytokines TNF-α, IL-6, and IL-1β (Fig. 4f). In contrast, pretreatment with euxanthone repressed the production of these inflammatory cytokines (Fig. 4f). These findings show that pretreatment with euxanthone can effectively prevent sevoflurane-induced apoptosis and neuronal inflammation.
Euxanthone attenuates sevoflurane-induced ROS generation, apoptosis, and neuroinflammation in primary hippocampal neurons. a Effect of euxanthone on cell viability of primary neurons. b Euxanthone protected primary neurons against sevoflurane-induced cytotoxicity. c Euxanthone markedly abolished sevoflurane-induced ROS generation. d Euxanthone significantly attenuated sevoflurane-induced apoptotic cell death. e Euxanthone attenuated sevoflurane-induced increase in caspase-3 cleavage and Bax expression, and decrease in Bcl-2 expression. f Euxanthone attenuated sevoflurane-induced increase in production of inflammatory cytokines. ##P < 0.01 vs. vehicle, **P < 0.01 vs. sevoflurane
Euxanthone Upregulates Nrf2 in Primary Neurons
The mRNA level of Nrf2 was determined by qRT-PCR. As shown in Fig. 5a, sevoflurane treatment did not alter the mRNA level of Nrf2 but pretreatment with euxanthone before sevoflurane exposure exhibited marked upregulation of Nrf2 mRNA level. Similarly, euxanthone increased the protein expression of Nrf2 (Fig. 5b). Since Nrf2 is a transcriptional factor, a luciferase plasmid was constructed to examine the transcriptional activities of Nrf2. As shown in Fig. 5c, euxanthone pretreatment increased the transcriptional activities of Nrf2 significantly. Taken together, our results show that euxanthone confers neuroprotection on primary neurons via activation of Nrf2.
Nrf2 Plays a Crucial Role in the Neuroprotective Activities of Euxanthone Against Sevoflurane-Induced Cell Injury
To further examine the role of Nrf2 in the neuroprotective activities of euxanthone, primary neurons were transfected with Nrf2 targeting shRNA. As shown in Fig. 6a, the expression of Nrf2 was markedly decreased following shRNA transfection. Given the crucial role of Nrf2 in the modulation of redox status of cells, we measured the intracellular level of ROS. As shown in Fig. 6b, Nrf2 knockdown almost completely abolished the inhibitory effect of euxanthone on the sevoflurane-induced production of ROS. Subsequently, the effect of Nrf2 knockdown on the anti-apoptotic activities of euxanthone was investigated. As shown in Fig. 6c, the pro-apoptotic effect of sevoflurane on neurons was notably diminished by euxanthone, while Nrf2 knockdown is capable to reverse the protective role of euxanthone on neurons. The suppressed anti-apoptotic effects of euxanthone were evidenced by increased levels of cleaved caspase-3 and Bax accompanied with decreased level of Bcl-2 (Fig. 6d). Additionally, the involvement of Nrf2 activation in the anti-inflammatory activities was explored. As shown in Fig. 6e, the sevoflurane-induced production of TNF-α, IL-1β, and IL-6, which was eliminated by euxanthone treatment, was restored by the Nrf2 knockdown. Altogether, these findings demonstrate that Nrf2 upregulation plays a key role in the neuroprotective activities of euxanthone against sevoflurane-induced cytotoxicity in primary neurons.
Nrf2 knockdown blocks the neuroprotective activities of euxanthone in primary neurons. a Protein expression of Nrf2 was significantly repressed by targeting siRNA. b Nrf2 knockdown abrogated the scavenging effect of euxanthone on sevoflurane-induced ROS generation. c Nrf2 knockdown abrogated the anti-apoptotic activities of euxanthone. d Nrf2 knockdown restored sevoflurane-induced caspase-3 cleavage, increase in Bax expression, and decrease in Bcl-2 expression. e Nrf2 knockdown restored sevoflurane-induced production of TNF-α, IL-1β, and IL-6. ##P < 0.01 vs. vehicle, **P < 0.01 vs. sevoflurane, ΔΔP < 0.01 vs. sevoflurane + EV (20)
Discussions
Sevoflurane, characterized by fast action, quick recovery, and low pungency, is widely applied as an inhaled anesthetic in pediatric surgery. However, a number of studies using animal models have shown that exposure to sevoflurane at neonatal stage results in neurotoxicity, reflected by long-term cognitive impairment and behavioral deficits (Zheng et al. 2013; Satomoto et al. 2009). Clinical data has also established that exposure of children under 4 years old to sevoflurane is a risk factor for the development of high cognitive disabilities and memory impairments in children (DiMaggio et al. 2011). Therefore, scientists are searching for novel agents that can provide protection against neurotoxicity caused by sevoflurane. Recently, a number of naturally occurring compounds have been found to provide health benefits in patients with neurodegenerative disorders, including AD, HD, and PD (Vauzour et al. 2008). Euxanthone, a derivative of Polygala caudata, has been found to promote neurite outgrowth (Naidu et al. 2007; Ha et al. 2006). However, the role of euxanthone in sevoflurane-induced neurotoxicity has not been explored so far. In this study, the neuroprotective activities of euxanthone against sevoflurane-induced neurotoxicity were examined in vitro and in vivo. Our findings indicate that euxanthone treatment in neonatal rats markedly attenuated sevoflurane-induced cognitive and memory dysfunction in adult rats. At the molecular level, our results show that euxanthone attenuated sevoflurane-induced apoptosis and neuroinflammation, demonstrating that euxanthone confers neuroprotection against sevoflurane-induced neurotoxicity.
A large body of literature has shown that the accumulation of ROS in neuronal cells is one of the major causes of apoptotic cell death following sevoflurane exposure. Hence, scavenging cellular ROS would lead to decreased apoptotic cell death (Chen et al. 2013). In fact, a few agents have been found to rescue sevoflurane-induced neuronal cell apoptosis by reducing ROS. For instance, anesthetic propofol has been reported to rescue sevoflurane-induced apoptosis in human neuroglioma cells by scavenging intracellular ROS (Tian et al. 2015). Recently, Zhao et al. have also reported that minocycline protects against sevoflurane-induced apoptosis in human neuroglioma cells by scavenging intracellular ROS (Tian et al. 2017). In line with these findings, we found that euxanthone reduced the ROS level in both brain tissue and cells after exposure to sevoflurane. Additionally, our findings revealed that euxanthone markedly attenuated sevoflurane-induced neuronal apoptosis, demonstrating its role as an anti-oxidant. The anti-oxidant property of euxanthone was also documented in skin cells (Thitilertdecha et al. 2014). In contrast to these findings, the anti-cancer activity of euxanthone has been attributed to its capability to increase ROS production in tumor cells (Kuete et al. 2016). This implies that the activity of euxanthone on the redox balance is cell-specific. Therefore, further studies are warranted to validate our findings.
In addition to apoptotic cell death, sevoflurane-induced neuroinflammation has also been considered to play a key role in cognitive impairments in neonatal rodents (Lu et al. 2010; Shen et al. 2013). Compared with control, sevoflurane exposure at the neonatal stage promoted excessive expression of pro-inflammatory cytokines including TNF-α and IL-1β in the hippocampus of mice (Shen et al. 2013). In vitro studies also showed that sevoflurane exposure led to the activation of NF-κB p65 and subsequent excessive production of TNF-α, IL-6, and IL-1β (Han et al. 2010; Wang et al. 2016; Bai et al. 2016). In this study, we determined whether the neuroprotective effect of euxanthone was also associated with the inhibition of neuroinflammation. Our findings show that sevoflurane exposure significantly elevated the expression of TNF-α, IL-6, and IL-1β, which were markedly suppressed by treatment with euxanthone. Similarly, in vitro studies using primary hippocampal neurons showed that euxanthone can protect against sevoflurane-induced inflammatory response. Taken together, these findings show that euxanthone plays anti-inflammatory roles against sevoflurane-induced inflammation in vivo and in vitro.
Transcription factor Nrf2 has been stated to be the key determinant of cellular responses to oxidative stress (Sun et al. 2015). It has been reported that Nrf2 activation can protect neuronal cells against apoptotic cell death induced by oxidative stress (Su et al. 2016). In addition, the role of Nrf2 activation in neuroinflammation has also been evidenced in neurological disorders (Qu et al. 2016). Particularly, upregulation and activation of Nrf2 have been found to confer protection against sevoflurane-induced neuronal cell apoptosis and neuroinflammation (Huang et al. 2017; Tian et al. 2017). In this study, our results show that the mRNA and protein expression of Nrf2 in the hippocampus of rat models were markedly elevated by euxanthone treatment. Similarly, euxanthone elevated the mRNA and protein expression of Nrf2 in primary neuronal cells. These findings point to the possibility that Nrf2 might orchestrate the neuroprotective activities of euxanthone. Indeed, knockdown of Nrf2 by siRNA significantly abrogated the neuroprotective effect of euxanthone in primary hippocampal neurons.
In summary, our results show that euxanthone treatment at the neonatal stage confers protection against sevoflurane-induced neurotoxicity in adult rats. At the molecular level, our findings reveal that these neuroprotective activities are associated with decreased sevoflurane-induced apoptotic cell death and neuroinflammation. More importantly, our results provide experimental evidence that euxanthone confers neuroprotection by upregulating Nrf2.
Change history
18 February 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s12031-021-01937-0
References
Bai S, Hu Z, Yang Y, Yin Y, Li W, Wu L, Fang M (2016) Anti-inflammatory and neuroprotective effects of triptolide via the NF-kappaB signaling pathway in a rat MCAO model. Anat Rec (Hoboken) 299(2):256–266
Chaiprasongsuk A, Lohakul J, Soontrapa K, Sampattavanich S, Akarasereenont P, Panich U (2017) Activation of Nrf2 reduces UVA-mediated MMP-1 upregulation via MAPK/AP-1 signaling cascades: the photoprotective effects of sulforaphane and hispidulin. J Pharmacol Exp Ther 360(3):388–398
Chen G, Gong M, Yan M, Zhang X (2013) Sevoflurane induces endoplasmic reticulum stress mediated apoptosis in hippocampal neurons of aging rats. PLoS One 8(2):e57870
Costi D et al (2014) Effects of sevoflurane versus other general anaesthesia on emergence agitation in children. Cochrane Database Syst Rev 9:CD007084
Cui RS, Wang K, Wang ZL (2018) Sevoflurane anesthesia alters cognitive function by activating inflammation and cell death in rats. Exp Ther Med 15(5):4127–4130
DiMaggio C, Sun LS, Li G (2011) Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg 113(5):1143–1151
Ha WY, Wu PK, Kok TW, Leung KW, Mak NK, Yue PYK, Ngai SM, Tsai SN, Wong RNS (2006) Involvement of protein kinase C and E2F-5 in euxanthone-induced neurite differentiation of neuroblastoma. Int J Biochem Cell Biol 38(8):1393–1401
Han LC, Zhang H, Wang W, Wei YY, Sun XX, Yanagawa Y, Li YQ, Xu LX, Wu SX (2010) The effect of sevoflurane inhalation on gabaergic neurons activation: observation on the GAD67-GFP knock-in mouse. Anat Rec (Hoboken) 293(12):2114–2122
Huang L, Huang K, Ning H (2017) Hispidulin prevents sevoflurane- induced memory dysfunction in aged rats. Biomed Pharmacother 97:412–422
Jevtovic-Todorovic V (2012) Developmental synaptogenesis and general anesthesia: a kiss of death? Curr Pharm Des 18(38):6225–6231
Ji MH, Qiu LL, Yang JJ, Zhang H, Sun XR, Zhu SH, Li WY, Yang JJ (2015) Pre-administration of curcumin prevents neonatal sevoflurane exposure-induced neurobehavioral abnormalities in mice. Neurotoxicology 46:155–164
Kuete V, Mbaveng AT, Nono ECN, Simo CC, Zeino M, Nkengfack AE, Efferth T (2016) Cytotoxicity of seven naturally occurring phenolic compounds towards multi-factorial drug-resistant cancer cells. Phytomedicine 23(8):856–863
Li R, Zhang LM, Sun WB (2017) Erythropoietin rescues primary rat cortical neurons from pyroptosis and apoptosis via Erk1/2-Nrf2/Bach1 signal pathway. Brain Res Bull 130:236–244
Lin LL, Huang F, Chen SB, Yang DJ, Chen SL, Yang JS, Xiao PG (2005) Xanthones from the roots of Polygala caudata and their antioxidation and vasodilatation activities in vitro. Planta Med 71(4):372–375
Liu Y, Zhuang X, Gou L, Ling X, Tian X, Liu L, Zheng Y, Zhang L, Yin X (2013) Protective effects of nizofenone administration on the cognitive impairments induced by chronic restraint stress in mice. Pharmacol Biochem Behav 103(3):474–480
Liu K, Gao H, Wang Q, Wang L, Zhang B, Han Z, Chen X, Han M, Gao M (2018a) Hispidulin suppresses cell growth and metastasis by targeting PIM1 through JAK2/STAT3 signaling in colorectal cancer. Cancer Sci 109(5):1369–1381
Liu MM, Huang KM, Qian L, Chatterjee P, Zhang S, Li R, Zhou S, Wang Z, Luo Y, Huang Y (2018b) Effects of bioactive constituents in the traditional Chinese medicinal formula Si-Wu-Tang on Nrf2 signaling and neoplastic cellular transformation. Phytomedicine 40:1–9
Lu Y, Wu X, Dong Y, Xu Z, Zhang Y, Xie Z (2010) Anesthetic sevoflurane causes neurotoxicity differently in neonatal naive and Alzheimer disease transgenic mice. Anesthesiology 112(6):1404–1416
Lv X, Yan J, Jiang J, Zhou X, Lu Y, Jiang H (2017) MicroRNA-27a-3p suppression of peroxisome proliferator-activated receptor-gamma contributes to cognitive impairments resulting from sevoflurane treatment. J Neurochem 143(3):306–319
Naidu M, Kuan CYK, Lo WL, Raza M, Tolkovsky A, Mak NK, Wong RNS, Keynes R (2007) Analysis of the action of euxanthone, a plant-derived compound that stimulates neurite outgrowth. Neuroscience 148(4):915–924
Pan MD, Mao Q (1984) Isolation and identification of wubangziside A and B from Polygala caudata Rehd et Wils. Yao Xue Xue Bao 19(12):899–903
Qu Z, Mossine VV, Cui J, Sun GY, Gu Z (2016) Protective effects of AGE and its components on neuroinflammation and neurodegeneration. NeuroMolecular Med 18(3):474–482
Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J (2009) Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology 110(3):628–637
Serlin H, Torregrossa MM (2015) Adolescent rats are resistant to forming ethanol seeking habits. Dev Cogn Neurosci 16:183–190
Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z (2013) Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology 118(3):502–515
Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5(5):415–418
Smothers CT, Szumlinski KK, Worley PF, Woodward JJ (2016) Altered NMDA receptor function in primary cultures of hippocampal neurons from mice lacking the Homer2 gene. Synapse 70(1):33–39
Su P, Zhang J, Wang S, Aschner M, Cao Z, Zhao F, Wang D, Chen J, Luo W (2016) Genistein alleviates lead-induced neurotoxicity in vitro and in vivo: involvement of multiple signaling pathways. Neurotoxicology 53:153–164
Sun L (2010) Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth 105(Suppl 1):i61–i68
Sun YX, Xu AH, Yang Y, Li J (2015) Role of Nrf2 in bone metabolism. J Biomed Sci 22:101
Tao G, Zhang J, Zhang L, Dong Y, Yu B, Crosby G, Culley DJ, Zhang Y, Xie Z (2014) Sevoflurane induces tau phosphorylation and glycogen synthase kinase 3beta activation in young mice. Anesthesiology 121(3):510–527
Thitilertdecha P, Guy RH, Rowan MG (2014) Characterisation of polyphenolic compounds in Clerodendrum petasites S. Moore and their potential for topical delivery through the skin. J Ethnopharmacol 154(2):400–407
Tian Y, Guo S, Guo Y, Jian L (2015) Anesthetic propofol attenuates apoptosis, Abeta accumulation, and inflammation induced by sevoflurane through NF-kappaB pathway in human neuroglioma cells. Cell Mol Neurobiol 35(6):891–898
Tian Y, Wu X, Guo S, Ma L, Huang W, Zhao X (2017) Minocycline attenuates sevoflurane-induced cell injury via activation of Nrf2. Int J Mol Med 39(4):869–878
Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JPE (2008) The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr 3(3–4):115–126
Wang W, Chen X, Zhang J, Zhao Y, Li S, Tan L, Gao J, Fang X, Luo A (2016) Glycyrrhizin attenuates isoflurane-induced cognitive deficits in neonatal rats via its anti-inflammatory activity. Neuroscience 316:328–336
Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO (2009) Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110(4):796–804
Yu Y, Zhang P, Yan J, Sun Y, Wu X, Xi S, Zhang L, Sun Y, Hu R, Jiang H (2016) Sevoflurane induces cognitive impairments via the MiR-27b/LIMK1-signaling pathway in developing rats. Inhal Toxicol 28(14):731–738
Yuan H, Jiang C, Zhao J, Zhao Y, Zhang Y, Xu Y, Gao X, Guo L, Liu Y, Liu K, Xu B, Sun G (2018) Euxanthone attenuates Abeta1-42-induced oxidative stress and apoptosis by triggering autophagy. J Mol Neurosci 66(4):512–523
Zhang DX, Zhang LM, Zhao XC, Sun W (2017a) Neuroprotective effects of erythropoietin against sevoflurane-induced neuronal apoptosis in primary rat cortical neurons involving the EPOR-Erk1/2-Nrf2/Bach1 signal pathway. Biomed Pharmacother 87:332–341
Zhang LM, Zhang DX, Zhao XC, Sun W (2017b) Erythropoietin rescues primary rat cortical neurons by altering the Nrf2:Bach1 ratio: roles of extracellular signal-regulated kinase 1/2. Neurochem Res
Zheng H, Dong Y, Xu Z, Crosby G, Culley DJ, Zhang Y, Xie Z (2013) Sevoflurane anesthesia in pregnant mice induces neurotoxicity in fetal and offspring mice. Anesthesiology 118(3):516–526
Funding
This study was funded by the National Natural Science Foundation of China No. 30872433.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All protocols of animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of Shandong University. Animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals (8th edition, National Academies Press).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s12031-021-01937-0
About this article
Cite this article
Zhou, H., Li, S. & Wang, G. RETRACTED ARTICLE: Euxanthone Ameliorates Sevoflurane-Induced Neurotoxicity in Neonatal Mice. J Mol Neurosci 68, 275–286 (2019). https://doi.org/10.1007/s12031-019-01303-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-019-01303-1









