Abstract
Postoperative cognitive dysfunction (POCD) is a common complication of central nervous system after anesthesia or surgery. Sevoflurane, an inhalation anesthetic, may inhibit cholinergic pathway that induce neuronal death and neuroinflammation, ultimately leading to POCD. Transauricular vagus nerve stimulation (taVNS) has neuroprotective effects in POCD rats, but the mechanisms related to cholinergic system have not been revealed. Sprague–Dawley rats were anesthetized with sevoflurane to construct the POCD model. The immunotoxin 192-IgG-saporin (192-sap) selectively lesioned cholinergic neurons in the basal forebrain, which is the major source of cholinergic projections to hippocampus. After lesion, rats received 5 days of taVNS treatment (30 min per day) starting 24 h before anesthesia. Open field test and Morris water maze were used to test the cognitive function. In this study, rats exposed to sevoflurane exhibited cognitive impairment that was attenuated by taVNS. In addition, taVNS treatment activated cholinergic system in the basal forebrain and hippocampus, and downregulated the expression of apoptosis- and necroptosis-related proteins, such as cleaved Caspase-3 and p-MLKL, in the hippocampus. Meanwhile, the activation of Iba1+ microglial by sevoflurane was reduced by taVNS. 192-sap blocked the cholinergic system activation in the basal forebrain and hippocampus and inhibited taVNS-mediated neuroprotection and anti-inflammation effects in the hippocampus. Generally, our study indicated that taVNS might alleviate sevoflurane-induced hippocampal neuronal apoptosis, necroptosis and microglial activation though activating cholinergic system in the basal forebrain.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative cognitive dysfunction (POCD), a common complication of central nervous system in older patients undergoing anesthesia and surgery, is defined as a decline in cognitive function, including memory, information processing, and executive function[1]. POCD is associated with an heightened risk of impaired quality of life, long-term cognitive decline, and mortality within 1 year after surgery[2]. In addition, POCD may be a precursor to further cognitive deterioration or even Alzheimer’s disease[3]. With the increase in number of surgical procedures in the older population, POCD has attracted more attention.
Sevoflurane, a commonly used inhalation anesthetic, could significantly increase the incidence of POCD in elderly patients[4]. The underlying mechanisms of sevoflurane-induced cognitive decline remain unclear, but a series of animal studies suggest that it may be associated with neuronal loss, neuroinflammation and changes in neurotransmitters[5,6,7]. Apoptosis and necroptosis are important pathways of neuronal loss in neurodegenerative diseases[8]. Sevoflurane could induce mitochondrial injury, activate caspase 3, and eventually lead to hippocampal neuronal apoptosis in aged rats[9]. Our previous study suggested that sevoflurane could induce RIPK1/RIPK3/MLKL-mediated necroptosis in hippocampal neurons of aged rats[6]. In addition, sevoflurane could activate microglia to induce neuroinflammation, and inhibit the release of acetylcholine to reduce its neuroexcitatory effect[4, 10]. The above-mentioned mechanisms of sevoflurane-induced cognitive decline are closely related and interact. Suppression of neuronal loss, neuroinflammation, and neurotransmitters changes could ameliorate sevoflurane-induced cognitive dysfunction.
Vagus nerve stimulation (VNS), a novel neuromodulation therapy refractory, has been approved for the treatment of epilepsy[11] and depression[12]. The transauricular VNS (taVNS) stimulates the auricular branch of the VN in a completely non-invasive method and can achieve the same effect as invasive VNS[13]. Our previous clinical study demonstrated that taVNS reduced the incidence of delayed neurocognitive recovery in the elderly, which might be related to inhibition of inflammatory cytokine production and reduction of peripheral cholinesterase activity[14]. Animal studies have showed that taVNS reduces anesthesia- and surgery-induced neuronal apoptosis and neuroinflammation in the hippocampus of rats[15]. However, the central mechanism of taVNS on neuroprotection remains unclear.
Cholinergic system may be directly involved in preventing cell death and inflammatory response[16]. The VNS projects to nucleus of the solitary tract (NST) and then to basal forebrain, which is the major source of cholinergic projections in brain[17]. Several studies have shown that VNS activates cholinergic nucleus basalis and cholinergic circuitry[18]. The basal forebrain is the primary source of cholinergic projection neurons in the hippocampus[19]. taVNS has also been proved to activate cholinergic system in hippocampus in stress model rats [20]. Activation of cholinergic system suggests the increased ACh level, decreased AChE level and activation of AChR. Both activation of cholinergic receptors and inhibition of cholinesterase activity inhibit sevoflurane-induced neuroinflammation and neurotoxicity[21, 22]. However, it is not completely clear whether taVNS-mediated anti-apoptosis and anti-inflammation effects in the hippocampus are related to activation of basal forebrain cholinergic system. In addition, few studies have investigated the effects of taVNS on neuronal necroptosis. In AD rats, ACh was significantly negative correlated with hippocampal necroptosis executive protein P-MLKL[23]. Therefore, we also hypothesized that taVNS could inhibit sevoflurane-induced neuronal necroptosis by activating cholinergic pathways.
In this study, we investigated the neuroprotection of taVNS on sevoflurane-induced POCD in aged rats and its potential mechanisms. We hypothesized that taVNS attenuates hippocampal neuronal apoptosis and necroptosis, as well as neuroinflammation, by activating the basal forebrain cholinergic system.
Materials and Methods
All experiments involving animals were performed in accordance with the Health Guidelines for the Care and Use of Laboratory Animals (National Institutes of Technology). All animal procedures were approved by the Ethics Committee for Animal of the Third Hospital of Hebei Medical University.
Animal
Aged male Sprague–Dawley rats (18 months-old, weighing 500–600 g) were provided by Liaoning Changsheng Biotechnology Co., Ltd.(Liaoning, China). All rats were housed in in plastic cage(3 rats per cage) under 12 light-dark cycles with ad libitum access to food and water. Rats were acclimated for at least 7 days before initiating any procedure.
Experimental Protocols
Sprague–Dawley rats were randomly assigned to five groups (n = 20): (1) control; (2) POCD; (3) POCD + taVNS; (4) POCD + taVNS + 192 immunoglobulin G-saporin (192-sap); and (5) POCD + 192-sap. rats in the POCD, POCD + taVNS, POCD + taVNS + 192-sap, and POCD + 192-sap groups were exposed to 2% of sevoflurane delivered by humidified 30% O2 carrier gas for 5 h. Control animals were expose to humidified 30% O2 without sevoflurane for the same period. Rats in the POCD + taVNS and POCD + taVNS + 192-sap groups received taVNS for 5 consecutive days starting 24 h before anesthesia. In addition, rats in the POCD + taVNS + 192-sap and POCD + 192-sap groups were administrated 192-sap via intraventricular injections 7 days prior to Morris Water Maze (MWM) training. Rats in the POCD + taVNS and POCD + taVNS + 192-sap groups received taVNS after the last MWM training. After the last taVNS, ten animals were randomly selected from each group for Open Field Test (OFT) and MWM test. After MWM test, the ten rats from each group were sacrificed under anesthetized with sodium pentobarbital (50 mg/kg, i.p.) for analysis. Five animals in each group were used for the Nissl staining and immunofluorescence analysis, and the remaining five animals were used for the western blot, flow cytometry and cholinergic function analysis. Finally, the remaining 10 rats in each group underwent MWM test on the 7th day after anesthesia to evaluate cognitive function. The experimental process was shown in Fig. 1.
The experimental schematic diagram. 192-sap was administrated to selectively damage cholinergic neurons in the basal forebrain 1 week before Morris water maze (MWM) training that was performed 1–5 days before sevoflurane inhalation anesthesia. Aged rats received taVNS starting 24 h before anesthesia for 30 min per day for 5 consecutive days. On the 3th day after anesthesia, Open field test (OFT) and MWM test were evaluated, and then rats were sacrificed for a series of testing. Another batch of rats was tested for MWM test on the 7th day after anesthesia
Basal Forebrain Cholinergic Depletion
192-sap can selectively deplete basal forebrain cholinergic neurons. Rats in the POCD + taVNS + 192-sap and POCD + 192-sap groups were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and received stereotaxic injections of 192-sap (Advanced Targeting Systems, San Diego, CA). 10 µl of 192-sap (0.125 mg/ml) was injected into the lateral ventricles using a 10 µl Hamilton micro syringe in a volume of 5 µl per side. Injections were performed at the following coordinates (in mm): AP: −0.9, ML: +/− 1.5, DV:4. In order to prevent backflow, the needle remained in place for 5 min after injection. Rats in the control, POCD and POCD + taVNS groups were injected with vehicle (PBS) alone in a similar manner.
taVNS
After WMW training, rats in the POCD + taVNS and POCD + taVNS + 192-sap groups received taVNS starting 24 h before anesthesia. Rats were grabbed and fixed with a special fixator to prevent movement. The positive and negative electrodes containing magnets were adsorbed on the left concha auricularis of the rats. The taVNS was administered to the left ear by a commercial transcutaneous electrical nerve stimulation unit (Nanjing Hanshi Electroacupuncture Instrument Equipment Co. Ltd., Nanjing, China) at 10 Hz/1mA. Stimulation was performed for 30 min once per day for 5 consecutive days. Rats in the control, POCD, and POCD + 192-sap groups were fixed in the same manner without stimulation.
Open Field Test (OFT)
We assessed the spontaneous locomotor activity (SLA) using the OFT test. After 30 min of acclimatization, rats were allowed to freely move in an empty black box (100 × 100 × 40 cm) for 5 min. Before each use, 75% ethanol was use to wipe the chamber to eliminate residual odor left by the previous rat. The EthoVision™ 7.0 automatic camera system (Noldus, Netherlands) was used for recorded the distance, speed and duration of movement.
Morris Water Maze (MWM) Test
We test the ability of memory and learning using the MWM test. The water maze was a circular tank (180 cm in diameter and 50 cm in depth) with an invisible 12 cm diameter round platform 1.5 cm beneath the surface, filled with water (23 °C–25 °C). The MWM was divided into four quadrants I– IV. Rats were trained to find the hidden platform from quadrants I– IV for four times every day for 5 days. If the rat fails to climb onto the platform within 60 s, the rat is guided to the platform and stay on the platform for 30 s. Probe test was performed on the 3th and 7th days after anesthesia. During the probe test, the platform was removed and the rats were allowed to swim freely for 60s. The latency to the platform, the frequency of crossing the original platform, and the amount of time spent in the target quadrant were recorded using JLBehv-MWM (Shanghai Ji’liang Software Technology Co., Ltd.
China).
Western Blot Assays
The hippocampus was separated from the brain, homogenized in lysis buffer, and centrifuged at 12,000 × g for 10 min at 4 °C. The supernatant was collected and the concentration of protein in the supernatant was determined using the BCA Protein Assay Kit (Beyotime, Shanghai, China). Protein samples was dissolved in 5×sample loading buffer and heated for 10 min at 100 °C. Proteins (30 µg) were electrophoresed by SDS-PAGE gel (4%–20%) and transferred onto polyvinylidene fluoride (PVDF) membrane (IPVH00010, Millipore, USA). The Membranes were blocked with 5% nonfat dry milk in TBST buffer at room temperature for 2 h. The membranes were incubated with the following primary antibodies at 4 °C reaction of the night: rabbit anti-RIPK1 antibody (1:1000, ABclonal,A7414), rabbit anti-RIPK3 antibody (1:1000, ABclonal, A5431), rabbit anti-MLKL antibody (1:1000, ABclonal, A19685), rabbit anti-phospho-MLKL (Ser345) (1:1000, abcam, EPR9515(2)). rabbit anti-Bax antibody (1:1000, Proteintech, 50599-2-Ig), rabbit anti- BCL-2 antibody (1:10000, Proteintech, 12789-1-AP), rabbit anti-Caspase-3 antibody (1:10000, Immunoway, YC0026), rabbit anti- cleaved Caspase-3 antibody (1:1000,abcom, ab32499), rabbit anti-ChAT antibody (1:1000, Proteintech, 20747-1-AP) and rabbit anti-GAPDH antibody(1:1000, Proteintech, 10494-1-AP). After incubation, the membranes were rinsed three times with TBST at room temperature and incubated with HRP-conjugated IgG antibody (Proteintech, 1:4000, HRP-20,758 or HRP-67,760) for 1 h at room temperature. Membranes were rinsed three times with TBST buffer, and then then detected by digital scanning (EPSONV 300, China) with enhanced chemiluminescence reagent (Abbkine, K22030, China). Immunoblots was analyzed with Image J software.
Nissl Staining
The rats were harvested and the brain tissues were perfused and fixed with 4% paraformaldehyde. The tissues were dehydrated and embedded in paraffin wax, and cut into coronal hippocampal brain sections (4 μm). Sections were stained with Nissl staining solution (C0117; Beyotime, Shanghai, China). The pathological changes in the hippocampus were analyzed by a pathologist using a light microscope (BX51; Olympus, Tokyo, Japan).
Immunofluorescence
Brain slices containing the basal forebrain and hippocampal were prepared for immunofluorescence staining. The slices were incubated with primary antibodies diluted in PBS overnight: mouse anti-NeuN antibody (1:100, Proteintech, YC0006), rabbit anti-phospho-MLKL (Ser345) (1:150, abcam, EPR9515(2)), rabbit anti- cleaved Caspase-3 antibody (1:200,abcom, ab32499), rabbit anti-ChAT antibody (1:100, Proteintech, 20747-1-AP), mouse anti-c-Fos antibody(1:200, Servicebio, GB12069), mouse anti-Iba1 antibody (1:500, Servicebio, GB12105). After washing with PBS three times, sections were incubated with Alexa Fluor-555 donkey anti-mouse IgG (H + L) (1:500, Beyotime, China) and Alexa Fluor-488 goat anti-rabbit IgG (H + L)(1:500, Beyotime, China) for 90 min at 37 °C in the dark. After washing, the 4ʹ,6-diamidino-2-phenylindole (DAPI) fluoromount (Southernbiotech, 0100-20) were used to stain sections for 8 min at room temperature. The branch tips in a given microglia of Iba1-positive cells were performed using Sholl analysis. Images were acquired using a Nikon Eclipse CI fluorescence microscope.
Flow Cytometry Detection
Fluorescence-activated cell sorting (FACS) analysis in hippocampus tissue was conducted as previously described. Briefly, the hippocampus weas dissected from rat after anesthesia. The equal amount of hippocampus tissue (1 g) was chopped into small pieces and filtered through a 200-mesh nylon net. The filtrated cells were centrifuged at 300×g at 4 °C for 10 min. Remove the supernatant and add 100 µl PBS to prepare a single cell suspension (1 × 106/L). Cell apoptotic and necroptotic status was identified according to the manufacture protocol of PE Annexin V Apoptosis Detection Kit I (559,763, BD Biosciences). The cells were analyzed by FACS Calibur (Becton, Dickinson Company, USA). The PI + AnnexinV−, PI + AnnexinV+, PI−AnnexinV−, and PI−AnnexinV + cells express necroptotic, viable, late apoptotic, and early apoptotic cells, respectively.
Statistical Analysis
Data were reported as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test were performed to compare mean values between multiple groups. The escape latency during training days were analyzed by Two-way repeated-measures ANOVA followed by Tukey’s post hoc test. All statistical analyses were conducted using SPSS (version 22.0) and GraphPad Prism (version 8.3.0) software. A P value < 0.05 was considered statistically significant.
Results
192-sap Weakened taVNS-Induced Improvement in Cognitive Tests After Sevoflurane Inhalation Anesthesia
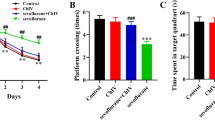
To eliminate the impacts of spontaneous activity on the assessment of cognitive function, we performed an open-field test on the 3th day after anesthesia (Fig. 2A). There were no significant differences in duration in center, traveling speed, and distance traveled among the five groups (Fig. 2B–D ), indicating that surgery did not cause a decrease in spontaneous locomotor activity. Then, we carried out WMW test to detect the cognitive function after anesthesia (Fig. 2E). During WMW training session, no significant differences were observed in escape latency and average swimming speed among the five groups (Fig. 2F, G ), indicating that all experimental rats had learned to seek the platform location after practice. On the 3th and 7th days, the time spent in the target quadrant and the times of crossing the platform in POCD group were significantly lower than those in the control group. The taVNS significantly increased the time spent in the target quadrant and the times of crossing the platform compare with the POCD group, while 192-sap reversed these changes. The time spent in the target quadrant and the times of crossing the platform were not significantly different between the control and POCD + taVNS groups. In addition, these were also not significantly different among the POCD, POCD + taVNS + 192-sap and POCD + 192-sap groups.(Fig. 2 H, I).
Effects of taVNS on sevoflurane-induced learning and memory impairment in aged rats. Trajectory (A), duration in center (B), traveling speed (C), distance traveled (D) of open field test on the 3th day after anesthesia. Representative swimming trace of rats during in MWM test with hidden platform (E). Escape latency (F) and average swimming speed (G) in the training phase of MWM with visible platform. Time spent in the target quadrant (H) and times of crossing the platform (I) in the probe test of MWM on the 3th and 7th day after anesthesia. These data are presented as the mean ± SD, n = 10 rats per group. *p < 0.05 vs. the control and POCD + taVNS groups
192-sap Blocked taVNS Induced- Cholinergic Activation in Basal Forebrain and hippocampus After Sevoflurane Inhalation Anesthesia
To test the effect of taVNS on cholinergic neurons, we quantified ChAT and c-Fos immunoreactivity in the cholinergic basal forebrain after the last taVNS (Fig. 3A). There was no significant difference in the number of ChAT cells and the number of c-Fos/ChAT-double positive cells between the control and the POCD groups. taVNS significantly increased the number of ChAT cells and the number of c-Fos/ChAT-double positive cells in cholinergic basal forebrain (Fig. 3B, C). Therefore, taVNS significantly activated the cholinergic neurons in basal forebrain. Previous study had shown that 192-sap could cause substantial cholinergic denervation[24]. We found that 192-sap significantly decreased the number of ChAT cells and suppressed the c-Fos expression in ChAT cells, suggesting that 192-sap inhibited the activation of cholinergic neurons of taVNS. In addition, we also investigated the expression of ChAT protein in cholinergic basal forebrain and hippocampus. The taVNS significantly increased the expression of ChAT in basal forebrain (Fig. 4A) and hippocampus (Fig. 4B), while 192-sap reversed this effect. Compared with the control group, sevoflurane increased AChE activity and decreased the level of ACh in hippocampus (Fig. 4C, D). Compared with the POCD group, taVNS significantly decreased AChE activity and increased the level of ACh in hippocampus, while 192-sap inhibited the effect of taVNS.
Effects of taVNS on c-Fos and ChAT activation of basal forebrain in aged rats. Representative images of ChAT (green) and c-Fos (red) in the basal forebrain, scale bar = 50 μm (A). Quantification of ChAT+ cells (B) and ChAT+/c-Fos+ double labeled cells (C) in the basal forebrain. These data are presented as the mean ± SD, n = 5 rats per group. ap < 0.05 vs. control group, bp < 0.05 vs. POCD group, cp < 0.05 vs. POCD + taVNS group, dp < 0.05 vs. POCD + taVNS + 192-sap group. DAPI = 4′,6-diamidino-2-phenylindole
Effects of taVNS on ChAT protein expression levels in the basal forebrain and hippocampus, as well as AChE activity levels and ACh levels in the hippocampus of aged rats. The protein levels of ChAT in the basal forebrain (A) and hippocampus (B). The AChE activity levels (C) and ACh levels (D) in hippocampus. These data are presented as the mean ± SD, n = 5 rats per group. ap < 0.05 vs. control, POCD + taVNS, POCD + taVNS + 192-sap, POCD + 192-sap groups, bp < 0.05 vs. control and POCD + taVNS groups
192-sap Prevented taVNS-Mediated Neuroprotection in Hippocampus After Sevoflurane Inhalation Anesthesia
Nissl staining was used to evaluate the loss and death of neuron in the CA1 region of hippocampus. The neurons in control group were regular in cell shape and evenly stained. In the POCD group, the number of cells was decreased; the arrangement was disordered; the nuclei were hyperchromatic and pyknotic; the Nissl bodies were unclear and fewer (Fig. 5A). taVNS significantly restored the above pathological changes and increased the number of Nissl bodies, while 192-sap reversed the protective effects (Fig. 5C).
Effects of taVNS on sevoflurane-induced pathological changes in the hippocampus of aged rats. Representative images of Nissl staining in the hippocampal CA1 region, scale bar = 50 μm (A). Representative flow cytometry results in hippocampus (B1: necroptosis cells, B2 and B4: apoptosis cells, B3: live cells) (B). Quantification of number of CA1 neurons from Nissl staining in the hippocampal CA1 region (C). The percentage of apoptosis cells (E) and necroptosis cells (F) and live cells (D) analyzed by flow cytometry. Data are presented as mean ± SD, n = 5 rats per group. *P < 0.05 vs. Control group and POCD + taVNS group
Figure 5B showed the ratio of apoptotic and necroptotic cells in five groups: B1:necroptotic cells, B2 and B4: apoptotic cells, and B3: live cells. Compared with the control group, the percentage of apoptotic and necroptotic cells was significantly increased in POCD group (Fig. 5D). taVNS significantly reduced the percentage of apoptosis and necroptosis in hippocampus, while 192-sap reversed the protective effects (Fig. 5E, F).
192-sap Attenuated taVNS-Induced Anti-Apoptosis in Hippocampus After Sevoflurane Inhalation Anesthesia
To evaluate the hippocampal neuronal apoptosis, we examined the expressions of apoptosis-related proteins (BCL-2, Bax, Caspase-3 and cleaved Caspase-3) in five groups (Fig. 6B). Compared with the C group, the protein expression levels of Bax, Caspase-3 and cleaved Caspase-3 increased, and the protein expression levels of BCL-2 decreased in POCD group. Compared with the POCD group, the protein expression level of BCL-2 increased, and the proteins expression levels of Bax, Caspase-3 and cleaved Caspase-3 decreased in POCD + taVNS group, indicating that taVNS ameliorated neuronal apoptosis. However, 192-sap administration decreased the expression level of BCL-2 and increased the expression levels of Bax, Caspase-3 and cleaved Caspase-3 in the POCD + taVNS + 192-sap group compared with the POCD + taVNS group. In addition, there were no differences in the expression levels of BCL-2, Bax, Caspase-3 and cleaved Caspase-3 in the POCD, POCD + taVNS + 192-SAP, and POCD + 192-sap groups.
Effects of taVNS on sevoflurane-induced apoptosis of hippocampus in aged rats. The representative neuronal apoptosis images of CA1 from cleaved Caspase-3/NeuN/DAPI immunofluorescence staining, scale bar = 50 μm (A). The representative western blot images of BCL-2, Bax, Caspase-3, cleaved Caspase-3 and GAPDH in the hippocampus (B). The percentages of Caspase 3+NeuN+DAPI+/NeuN+DAPI+ from immunofluorescence staining (C). These data are presented as the mean ± SD, n = 5 rats per group. ap < 0.05 vs. control group, bp < 0.05 vs. POCD group, cp < 0.05 vs. POCD + taVNS group
To further explore neuronal apoptosis in hippocampal CA1, cleaved Caspase-3 was double-stained with NeuN (Fig. 6A). Compared with the control group, the percentage of cleaved Caspase-3+ NeuN+ / NeuN+ cells was increased in the POCD group. taVNS significantly decreased the percentage of cleaved Caspase-3+ NeuN+ / NeuN+ cells, while 192-sap partially reversed the beneficial effect. There was no difference in the percentage of cleaved Caspase-3+ NeuN+ / NeuN+ cells among the POCD, POCD + taVNS + 192-SAP, and POCD + 192-sap groups (Fig. 6C).
192-sap Attenuated taVNS-Induced Anti-Necroptosis in Hippocampus After Sevoflurane Inhalation Anesthesia
To evaluate the hippocampal necroptosis, we examined the expressions of necroptosis-related proteins (RIPK1, RIPK3, MLKL and p-MLKL) (Fig. 7B). Compared with the C group, the protein expression levels of RIPK1, RIPK3, MLKL and p-MLKL were increased in POCD group. Compared with the POCD group, the expression levels of RIPK1, RIPK3, MLKL and p-MLKL were decreased in the POCD + taVNS group, indicating that taVNS ameliorated necroptosis. However, 192-sap administration increased the expression levels of RIPK1, RIPK3, MLKL and p-MLKL in the POCD + taVNS + 192-sap group compared with the POCD + taVNS group (Fig. 7B). In addition, there were no differences in the expression levels of RIPK1, RIPK3, MLKL and p-MLKL among the POCD, POCD + taVNS + 192-sap, and POCD + 192-sap groups.
Effects of taVNS on sevoflurane-induced necroptosis in hippocampus. Representative neuronal necroptosis images of CA1 from p-MLKL/NeuN/DAPI immunofluorescence staining, scale bar = 50 μm (A). The representative western blot images of RIPK1, RIPK3, MLKL, p-MLKL, GAPDH in the hippocampus (B). The Percentages of p-MLKL+NeuN+DAPI+/NeuN+DAPI+ from immunofluorescence staining (C). These data are presented as the mean ± SD, n = 5 rats per group. ap < 0.05 vs. control group, bp < 0.05 vs. POCD group, cp < 0.05 vs. POCD + taVNS group
To further explore neuronal necroptosis in hippocampal CA1 region, p-MLKL was double-stained with NeuN (Fig. 7A). Compared with the control group, the percentage of cleaved p-MLKL + NeuN+ / NeuN+ cells was increased in the POCD group. taVNS significantly decreased the percentage of p-MLKL + NeuN+ in NeuN+ cells, while 192-sap partially reversed the beneficial effect. In addition, there was no difference in the percentage of p-MLKL+ NeuN+ in NeuN+ cells in the POCD, POCD + taVNS + 192-SAP, and POCD + 192-sap groups (Fig. 7C).
192-sap Attenuated taVNS-Induced Anti-Inflammation in Hippocampus After Sevoflurane Inhalation Anesthesia
We assessed neuroinflammation by quantifying changes in microglial morphology on 3 days after anesthesia (Fig. 8A). The de-ramified morphology with larger cell bodies, thicker processes, and fewer branches, represents microglial activation associated with an active inflammatory response. The number of Iba1+ cells and the percentage of de-ramified Iba+ cells were increased in the POCD group compared with the control group. The taVNS treatment significantly decreased the number of Iba-1+ cells and the percentage of de-ramified Iba+ cells in the POCD + taVNS group compared with the POCD group, while 192-sap reversed these changes in microglia. In addition, there was no difference in the number of Iba1+ cells and the percentage of de-ramified Iba1+ cells among the POCD, POCD + taVNS + 192-SAP, and POCD + 192-sap groups (Fig. 8B, C).
Effects of taVNS on sevoflurane-induced microglial activation of hippocampus in aged rats. The representative photomicrographs of Iba1 staining of hippocampus, scale bar = 50 μm (A). The number of Iba1+ cells/0.2mm2 (B) and the percentage of de-ramified Iba1+/Iba1+ (C) from immunofluorescence staining. These data are presented as the mean ± SD, n = 5 rats per group. ap < 0.05 vs. control group, bp < 0.05 vs. POCD group, cp < 0.05 vs. POCD + taVNS group
Discussion
Our study found that taVNS improved the learning and memory ability of aged rats after sevoflurane inhalation anesthesia, activated cholinergic pathways in the basal forebrain and hippocampus, and attenuated neuronal apoptosis, neuronal necroptosis and microglial activation in hippocampus in aged rats. Cholinergic basal forebrain lesions prevented VNS-mediated improvement of cognitive function, anti-apoptosis and anti-necroptosis in hippocampal neuron and microglial activation. Overall, our results suggested that the cholinergic basal forebrain may be involved in taVNS-mediated protective effects in hippocampus of aged rats after sevoflurane inhalation anesthesia.
In the present study, sevoflurane was selected to induce POCD in aged rats. The MWM test, a classic hippocampal-dependent learning test, was chosen to detect the memory and learning ability of rats[25]. We found that sevoflurane prolonged escape latency and decreased crossing platform times, suggesting that sevoflurane inhalation anesthesia successfully established a POCD model in aged rats. Furthermore, taVNS attenuated the sevoflurane-induced memory deficits. Cai et al. and Xiong et al. have also demonstrated that VNS alleviated postoperative memory impairment after surgery, which might be relate to the reduction of apoptosis and the inhibition of neuroinflammation in hippocampus[15, 26]. Next, we also examined the anti-inflammatory and anti-apoptosis effects mediated by taVNS.
In this study, the results of FACS analysis showed an increased rates of apoptosis and necroptosis of hippocampus after sevoflurane inhalation anesthesia. Bax/BCL-2, Caspase 3 and cleaved-Caspase3 were increased, and the mitochondrial apoptosis pathway was activated by sevoflurane in this study. VNS treatment has been proved to attenuate brain mitochondrial dysfunction and cell apoptosis and ameliorate cognitive dysfunction in obese-insulin resistant rats[27]. Our results showed that taVNS inhibited mitochondrial apoptosis pathway in aged rats after sevoflurane inhalation anesthesia. Necroptosis is also driven by a well-defined molecular pathway in which RIPK1 forms an intracellular complex with RIPK3 to assemble necrosomes to activate the mixed lineage kinase domain (MLKL), ultimately leading to cell death[28]. The expression levels of RIPK1/RIPK3/MLKL and p-MLKL were significantly increased, indicating the necroptosis pathway was activated by sevoflurane, which was consistent with our previous study[6]. Furthermore, we also demonstrated that taVNS decreased the expression of RIPK1/RIPK3/MLKL and p-MLKL. Before us, the relationship between taVNS and necroptosis have not been reported. Most studies focus on the inflammation induced necroptosis in hippocampus[29]. It is well known that TNF triggers its receptor (TNFR1) and recruits RIP1 to mediate necroptosis signaling pathway[30]. Our results also demonstrate that taVNS inhibited surgery-induced activation of microglia, which decreased the inflammation level of hippocampus[31]. The anti-necroptosis effect of taVNS might be related to the inhibition of inflammatory response. Nevertheless, recent studies had also shown that necroptosis might precede and trigger the inflammatory response[32], and that RIPK3 deficiency prevented epithelial cell necroptosis and inflammation in both the intestinal and skin of these mice[33]. The relationship between inflammation and necroptosis is complex, and the anti-inflammatory and anti-necroptosis effects mediated by taVNS may occur simultaneously or sequentially.
Sevoflurane-induced neuroinflammation and neuroapoptosis may be related to the inhibition of cholinergic pathway in hippocampus[34]. Recent studies showed that cholinergic system by itself could be directly involved in preventing cell death and inflammatory response[35, 36]. The neuroprotective mechanisms engaged by VNS are not unknown. Previous study showed that VNS increased the expression of ChAT in the NST [37]and the dorsal motor nucleus of vagus (DMV)[38], indicating activation of cholinergic circuits in brain. Choline acetyltransferase (ChAT), a biosynthetic enzyme that produces ACh, mainly present in the cytoplasm of cholinergic nerve ending[38]. The protective mechanism of taVNS may be related to the activation of cholinergic system. We found that taVNS increased the number of ChAT expressing neurons that also exhibited enhanced c-Fos immunoreactivity in the basal forebrain nuclei. The c-Fos is a functional anatomical marker of neuronal activation[39]. Our results suggested that taVNS activated cholinergic neurons in basal forebrain nuclei, which is consistent with a previous study[18]. It is known that hippocampal cholinergic neurons originate from the basal forebrain nuclei[40]. Namgung et al. showed that VNS increased the expression of ChAT in hippocampus of an animal model of continuous stress[41]. Our data showed increased ChAT expression and ACh levels and decreased AChE activity in hippocampus, which may be linked to taVNS-mediate activation of basal forebrain nuclei. ACh, through activation of the acetylcholine receptors (AChRs), exerts an anti-apoptotic effect by inducing the activation of anti-apoptotic PI3-kinase—Akt pathways[42] and reducing apoptotic mediators such as FAS and p38 MAPK[43]. The hippocampus is a region with a high density of nicotinic AChRs. ACh could activate α7nAchR that significantly reduces caspase-3 expression and neuroinflammation in the hippocampus[44]. Increased activity of ACHE induces RIPK1-mediated necroptosis in SH-SY5Y cells[10]. In addition, Motawi et al. showed that hippocampal p-MLKL was significantly negatively correlated with ACh levels[23]. Cholinergic stimulation of the hippocampus has direct effects not only on neurons but also on microglia [45]. Basal forebrain cholinergic degeneration leads to increased number and lasting activation of microglia in the hippocampus[46, 47]. Therefore, the alleviation of neuronal apoptosis, necroptosis and inflammation in hippocampus of taVNS may be due to the activation of cholinergic system. Given that basal forebrain nuclei act as the major factor affecting changes in the hippocampal cholinergic system, we further examined whether the neuroprotection of taVNS on POCD is related to the activation of basal forebrain cholinergic neurons.
In the basal forebrain, the medial septal nucleus (MS) and the vertical limb of the diagonal band nucleus (VDB) project cholinergic fibers to the hippocampus[48]. In this study, an immunotoxin, 192-sap was used to selectively deplete basal forebrain cholinergic neurons that provide cholinergic input to the hippocampus. The use of 192-sap is known to model Alzheimer-like learning and memory deficits in rats and to verify cholinergic hypothesis in cognitive impairment[49]. The effect of 192-sap is time-dependence and dose-dependence. The low doses (1.25ug and 2.5ug) of 192-sap did not affect the learning and memory in aged rats[50]. We administrated 1.25ug of 192-sap to produce basal forebrain nuclei lesions, which did not impair the learning and memory ability of POCD rats. The depletion of cholinergic neurons in basal forebrain nuclei reversed the taVNS-mediated increased expression of ChAT in hippocampus. In addition, neuroprotective and anti-inflammatory effects of taVNS in hippocampus of POCD rats were largely diminished by 192-sap. This suggested that the hippocampal cholinergic innervation from the basal forebrain nuclei was required for VNS-mediated neuroprotective effects and anti-inflammatory effects in hippocampus. Notably, we assumed that taVNS decreased the activity of AChE to exert protective effects. Paradoxically, 192-sap reduced AChE activity and reversed the protective effect of taVNS. This phenomenon may be analogous to that AChE inhibitor reduces AChE activity to ameliorate POCD[51], whereas cholinergic depletion reduces AChE activity to exacerbate POCD[52]. The effect of AChE activity on cognition may be mediated through ACh. taVNS decreased the activity of AChE which resulted in increased level of ACh. 192-sap decreased the activity of AChE and the level of ACh. 192-sap induced the basal forebrain cholinergic neurons damage and prevented antiapoptosis, antinecroptosis and anti-inflammation effects of taVNS in hippocampus, indicating the importance of cholinergic signaling in taVNS-treated POCD rats.
Conclusion
Overall, taVNS could ameliorate sevoflurane-induced cognitive impairment and improve neuronal apoptosis, necroptosis and microglial activation in hippocampus. The underlying mechanism of taVNS protective effects may be attributable to the activation of cholinergic system in basal forebrain and hippocampus. Further study should evaluate the mechanism of cholinergic lesions prevent the neuroprotection of VNS therapy in POCD.
Data Availability
The data are available on reasonable request from corresponding author.
References
Evered LA, BS Silbert (2018) Postoperative cognitive dysfunction and noncardiac surgery. Anesth Analg 127:496–505
Needham MJ, Webb CE, Bryden DC (2017) Postoperative cognitive dysfunction and dementia: what we need to know and do. Br J Anaesth 119:i115–i125
Seitz DP, Reimer CL, Siddiqui N (2013) A review of epidemiological evidence for general anesthesia as a risk factor for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 47:122–127
Wang CM, Chen WC, Zhang Y, Lin S, He HF (2021) Update on the mechanism and treatment of sevoflurane-induced postoperative cognitive dysfunction. Front Aging Neurosci 13:702231
Ling Y, Li X, Yu L, Sun Y, Yang D, Li Z (2021) Sevoflurane induces neuronal apoptosis via enhancing DNMT3L expression and promoting methylation of PSD95 promoter in postoperative cognitive dysfunction. Mol Cell Toxicol 17:287–295
Yin C, Zhang Q, Zhao J, Li Y, Yu J, Li W, Wang Q (2022) Necrostatin-1 against sevoflurane-induced cognitive dysfunction involves activation of BDNF/TrkB pathway and inhibition of necroptosis in aged rats. Neurochem Res 47:1060–1072
Huang C, Chu JMT, Liu Y, Kwong VSW, Chang RCC, Wong GTC (2022) Sevoflurane induces neurotoxicity in the animal model with Alzheimer’s disease neuropathology via modulating glutamate transporter and neuronal apoptosis. Int J Mol Sci 23(11):6250
Xu C, Wu J, Wu Y, Ren Z, Yao Y, Chen G, Fang EF, Noh JH, Liu YU, Wei L, Chen X, Sima J (2021) TNF-α-dependent neuronal necroptosis regulated in Alzheimer’s disease by coordination of RIPK1-p62 complex with autophagic UVRAG. Theranostics 11:9452–9469
Liu X, Song X, Yuan T, He J, Wang X, Wang Q (2016) Effects of calpain on sevoflurane-induced aged rats hippocampal neuronal apoptosis. Aging Clin Exp Res 28:633–639
Ayazgök B, Tüylü Küçükkılınç T (2018) Low-dose bisphenol A induces RIPK1-mediated necroptosis in SH-SY5Y cells: effects on TNF-α and acetylcholinesterase. J Biochem Mol Toxicol e22233
González HFJ, Yengo-Kahn A, Englot DJ (2019) Vagus nerve stimulation for the treatment of Epilepsy. Neurosurg Clin N Am 30:219–230
Bottomley JM, LeReun C, Diamantopoulos A, Mitchell S, Gaynes BN (2019) Vagus nerve stimulation (VNS) therapy in patients with treatment resistant depression: a systematic review and meta-analysis. Compr Psychiatry 98:152156
Farmer AD, Strzelczyk A, Finisguerra A, Gourine AV, Gharabaghi A, Hasan A, Burger AM, Jaramillo AM, Mertens A, Majid A (2021) International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (version 2020). Front Hum Neurosci 14:568051
Zhou Q, Yu L, Yin C, Zhang Q, Wang X, Kang K, Shao D, Wang Q (2022) Effect of transcutaneous auricular vagus nerve stimulation on delayed neurocognitive recovery in elderly patients. Aging Clin Exp Res 1–9
Cai L, Lu K, Chen X, Huang JY, Zhang BP, Zhang H (2019) Auricular vagus nerve stimulation protects against postoperative cognitive dysfunction by attenuating neuroinflammation and neurodegeneration in aged rats. Neurosci Lett 703:104–110
Resende RR, Adhikari A (2009) Cholinergic receptor pathways involved in apoptosis, cell proliferation and neuronal differentiation. Cell Commun Signal 7:20
Groves DA, VJ Brown (2005) Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev 29:493–500
Hulsey DR, Hays SA, Khodaparast N, Ruiz A, Das P, Rennaker RL II (2016) Reorganization of motor cortex by vagus nerve stimulation requires cholinergic innervation. Brain Stimul 9:174–181
Dannenberg H, Pabst M, Braganza O, Schoch S, Niediek J, Bayraktar M, Mormann F, Beck H (2015) Synergy of direct and indirect cholinergic septo-hippocampal pathways coordinates firing in hippocampal networks. J Neurosci 35:8394–8410
Dong Y, Zhang G, Zhang B, Moir RD, Xia W, Marcantonio ER, Culley DJ, Crosby G, Tanzi RE, Xie Z (2009) The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol 66:620–631
Wang T, Zhu H, Hou Y, Gu W, Wu H, Luan Y, Xiao C, C Zhou (2019) Galantamine reversed early postoperative cognitive deficit via alleviating inflammation and enhancing synaptic transmission in mouse hippocampus. Eur J Pharmacol 846:63–72
Yin J, Zhao X, Wang L, Xie X, Geng H, Zhan X, J Teng (2019) Sevoflurane-induced inflammation development: involvement of cholinergic anti-inflammatory pathway. Behav Pharmacol 30:730–737
Motawi TMK, Abdel-Nasser ZM, Shahin NN (2020) Ameliorative effect of necrosulfonamide in a rat model of Alzheimer’s disease: targeting mixed lineage kinase domain-like protein-mediated necroptosis. ACS Chem Neurosci 11:3386–3397
Conner JM, Kulczycki M, Tuszynski MH (2010) Unique contributions of distinct cholinergic projections to motor cortical plasticity and learning. Cereb Cortex 20:2739–2748
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858
Xiong J, Wang H, Bao Y, Guo Y, Sun Y (2019) Electric vagal nerve stimulation inhibits inflammation and improves early postoperation cognitive dysfunction in aged rats. BMC Anesthesiol 19:217
Chunchai T, Samniang B, Sripetchwandee J, Pintana H, Pongkan W, Kumfu S, Shinlapawittayatorn K, KenKnight BH, Chattipakorn N (2016) Vagus nerve stimulation exerts the neuroprotective effects in obese-insulin resistant rats, leading to the improvement of cognitive function. Sci Rep 6:26866
Linkermann A, DR Green (2014) Necroptosis. N Engl J Med 370:455–465
Jayaraman A, Htike TT, James R, Picon C, Reynolds R (2021) TNF-mediated neuroinflammation is linked to neuronal necroptosis in Alzheimer’s disease hippocampus. Acta Neuropathol Commun 9:159
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11:700–714
Feng X, Valdearcos M, Uchida Y, Lutrin D, Maze M, Koliwad SK (2017) Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight 2:e91229
Pasparakis M, P Vandenabeele (2015) Necroptosis and its role in inflammation. Nature 517:311–320
Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, Eftychi C, Lin J, Corona T, Hermance N, Zelic M, Kirsch P, Basic M, Bleich A, Kelliher M, Pasparakis M (2014) RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 513:90–94
Lv G, Li C, Wang W, Li N, Wang K (2020) Silencing SP1 alleviated sevoflurane-induced POCD development via cholinergic anti-inflammatory pathway. Neurochem Res 45:2082–2090
Resende RR, Adhikari A (2009) Cholinergic receptor pathways involved in apoptosis, cell proliferation and neuronal differentiation. Cell Commun Signal 7:1–20
Benfante R, Di Lascio S, Cardani S, Fornasari D (2021) Acetylcholinesterase inhibitors targeting the cholinergic anti-inflammatory pathway: a new therapeutic perspective in aging-related disorders. Aging Clin Exp Res 33:823–834
Huffman WJ, Subramaniyan S, Rodriguiz RM, Wetsel WC, Grill WM, Terrando N (2019) Modulation of neuroinflammation and memory dysfunction using percutaneous vagus nerve stimulation in mice. Brain Stimul 12:19–29
Benishin CG, Carroll PT (1981) Acetylation of choline and homocholine by membrane-bound choline-O-acetyltransferase in mouse forebrain nerve endings. J Neurochem 36:732–740
Kovács KJ (2008) Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol 20:665–672
Solari N, Hangya B (2018) Cholinergic modulation of spatial learning, memory and navigation. Eur J Neurosci 48:2199–2230
Namgung U, Kim K-J, Jo B-G, Park JM (2022) Vagus nerve stimulation modulates hippocampal inflammation caused by continuous stress in rats. J Neuroinflamm 19:1–15
Huang X, Cheng Z, Su Q, Zhu X, Wang Q, Chen R, Wang X (2012) Neuroprotection by nicotine against colchicine-induced apoptosis is mediated by PI3-kinase–akt pathways. Int J Neurosci 122:324–332
Kim MH, Kim MO, Heo JS, Kim JS, Han HJ (2008) Acetylcholine inhibits long-term hypoxia-induced apoptosis by suppressing the oxidative stress-mediated MAPKs activation as well as regulation of Bcl-2, c-IAPs, and caspase-3 in mouse embryonic stem cells. Apoptosis 13:295–304
Sun Y, Song D, Wang M, Chen K, Zhang T (2017) α7 nicotinic acetylcholine receptor agonist attenuates the cerebral injury in a rat model of cardiopulmonary bypass by activating the Akt/GSK3β pathway. Mol Med Rep 16:7979–7986
Maurer SV, Williams CL (2017) The Cholinergic system modulates memory and hippocampal plasticity via its interactions with non-neuronal cells. Front Immunol 8:1489
Dobryakova YV, Volobueva MN, Manolova AO, Medvedeva TM, Kvichansky AA, Gulyaeva NV, Markevich VA, Stepanichev MY, Bolshakov AP (2019) Cholinergic Deficit Induced by Central Administration of 192IgG-Saporin is Associated with activation of Microglia and Cell loss in the dorsal Hippocampus of rats. Front Neurosci 13:146
Nazmi A, Griffin EW, Field RH, Doyle S, Hennessy E, O’Donnell M, Rehill A, McCarthy A, Healy D, Doran MM (2021) Cholinergic signalling in the forebrain controls microglial phenotype and responses to systemic inflammation. bioRxiv
Ballinger EC, Ananth M, Talmage DA, Role LW (2016) Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 91:1199–1218
Petrosini L, De Bartolo P, D Cutuli (2021) Neurotoxic effects, mechanisms, and outcome of 192 IgG-Saporin lesions. Handbook of Neurotoxicity 1–23
Leanza G, Nilsson OG, Wiley RG, A Björklund (1995) Selective lesioning of the basal forebrain cholinergic system by intraventricular 192 IgG-saporin: behavioural, biochemical and stereological studies in the rat. Eur J Neurosci 7:329–343
Kalb A, C von Haefen M, Sifringer A, Tegethoff N, Paeschke M, Kostova A, Feldheiser CD, Spies (2013) Acetylcholinesterase inhibitors reduce neuroinflammation and -degeneration in the cortex and hippocampus of a surgery stress rat model. PLoS ONE 8:e62679
Xu H, Chen L, Zhang X, Jiang X, Tian W, Yu W, Wang X, Tian J, Su D (2019) Central cholinergic neuronal degeneration promotes the development of postoperative cognitive dysfunction. Lab Invest 99:1078–1088
Funding
This work was supported by the The key project of the Precision Medicine Joint Fund of the Natural Science Foundation of Hebei Province (Grant No. H2021206021) and 2022 Government-funded Provincial Medical Outstanding Talent Project.
Author information
Authors and Affiliations
Contributions
QZ designed and wrote original draft, ZZL curated data curation and review original draft, XPW and LL curated data and analyze formal, LQW, CPY and QZ curated data and software, QJW review original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Third Hospital of Hebei Medical University.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Q., Zheng, Z., Wang, X. et al. taVNS Alleviates Sevoflurane-Induced Cognitive Dysfunction in Aged Rats Via Activating Basal Forebrain Cholinergic Neurons. Neurochem Res 48, 1848–1863 (2023). https://doi.org/10.1007/s11064-023-03871-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03871-6