Abstract
Purpose
Neuroinflammation may contribute to the pathogenesis of the cognitive symptoms of postoperative delirium (POD) and its subsequent long-term cognitive impairment. Haloperidol (HAL), a dopamine receptor antagonist, is widely used to treat POD, whereas the effects of HAL on postoperative neuroinflammation and related cognitive deficits have been underdetermined.
Methods
Aged rats underwent sham or abdominal surgery and were subcutaneously treated with either vehicle, low-dose (0.5 mg/kg bolus, then 0.5 mg/kg/day infusion), or high-dose (2.0 mg/kg bolus, then 2.0 mg/kg/day infusion) HAL. All treatments were initiated immediately after surgery and continued for 48 h. On either postoperative day 2 (early) or 7 (late), all rats were tested for trace and context fear memory retention after acquisition of trace fear conditioning. Following the cognitive testing, the levels of pro-inflammatory cytokines, as well as dopamine and its metabolite, in hippocampus and medial prefrontal cortex (mPFC) were measured.
Results
In the early postoperative period, surgery induced acute neuroinflammation along with related trace and context memory dysfunction. Dopamine turnover was increased in both hippocampus and mPFC, whereas no relationship with memory functions was observed. However, HAL even at high-dose failed to restore the surgery-induced neuroinflammation and related cognitive deficits. In the late postoperative period, chronic neuroinflammation was detected only in hippocampus, which was associated with context, but not trace memory dysfunction. Neither low- nor high-dose HAL could prevent the development of these late-phase neurocognitive deficits.
Conclusion
Our findings indicate that perioperative administration with HAL may have no effects on postoperative neuroinflammation and related cognitive impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative delirium (POD) is a common but potentially serious surgical complication associated with healthcare burden, particularly in elderly patients [1, 2]. Specifically, emerging evidence suggests a close relationship between the development of POD and increasing risk of subsequent persistent cognitive decline, including dementia [3]. Although the pathogenesis of POD remains elusive, comprehensive clinical and laboratory investigations indicate that the maladaptive inflammatory responses within the brain, i.e., neuroinflammation, play a key role [4]. Recently, we further showed that the postoperative neuroinflammation may transit from acute to chronic in an age- and hippocampal-specific manner, likely resulting in the development of POD-associated long-lasting cognitive deficits [5]. However, there is still no clinically proven pharmacotherapy targeting neuroinflammation.
Haloperidol (HAL), a typical anti-psychotic, is the most widely used medication for reducing hyperactive symptoms of POD via dopamine (DA) D2 antagonism [6, 7]. The decreasing excessive dopaminergic activity by HAL may reduce schizophrenia-like symptoms overlapping with delirium, e.g., agitation, psychomotor activity, combativeness, hallucination, and depression. However, there is ongoing controversy regarding the net efficacy of HAL for the long-term consequences of POD, mostly due to lack of high-quality research evidence [1, 2, 7]. Specifically, it remains to be elucidated whether HAL influences postoperative neuroinflammation and related cognitive impairment.
We earlier reported that trace fear-conditioning task could be applied to assess delirious-like behaviors in aged rats [5]. The previous studies have shown that trace and context memory retention following trace fear conditioning are representative of attentional and cognitive abilities, respectively [8, 9]. Since these deficits are the core symptoms of clinical delirium, our behavioral paradigm may be clinically relevant to evaluate the state of delirium in rodents. In this study, using this approach, we examined the effects of HAL treatment and the role of DA signaling on the postoperative neuroinflammatory trajectory and related neurocognitive behaviors in aged rats.
Materials and methods
Animals and experimental designs
All experiments were approved by the Institutional Animal Care and Use Committee of Kochi Medical School. Male Wistar rats aged 18–21 months and weighing 570–620 g were used in this study. One week before the surgical procedure, all randomized rats were subjected to a standard open-field test to assess baseline spontaneous activity in novel environment. HAL (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.3% tartaric acid, diluted with saline. Either 0.5 mg/kg bolus followed by an infusion of 0.5 mg/kg/day (Low-HAL group), 2.0 mg/kg bolus followed by an infusion of 2.0 mg/kg/day (High-HAL group) HAL, or vehicle alone was administered subcutaneously using osmotic mini-pumps (Alzet Model 2ML4; Alzet, Cupertino, CA, USA). Control animals received an equivalent volume of 0.3% tartaric acid. All treatments were initiated immediately after surgery and continued for 48 h. The doses were selected based on the previous study [10]. Mini-pumps filled with drug or vehicle solutions were inserted subcutaneously on the back flank during surgical or sham surgical procedure.
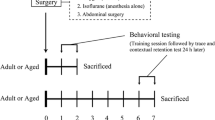
The study consisted of two experiments, Experiment-1 and Experiment-2 that were performed to explore acute and late postoperative phases, respectively (Fig. 1). In Experiment-1, cognitive function of all rats was assessed 2 days after surgery or sham procedure, followed by brain sampling and analysis. Since our previous study with the same animal model showed that systemic inflammation manifested during postoperative days 1–3 [11], we defined this period as the early postoperative phase that is potentially relevant for the development of POD. In Experiment-2, an identical behavioral and neurochemical analysis was performed 7 days after surgery under the same experimental conditions. In both experiments, each group consisted of 8 animals.
Experimental protocol and time course. All animals underwent either a sham procedure (only anesthesia and analgesia) or an abdominal surgery. Behavioral testing, bar test, and trace fear-conditioning (TFC) task were performed either early (2 days; Experiment-1) or late (7 days; Experiment-2) after surgery, followed by quantification of hippocampal cytokines. The rats in each group were randomly divided into 3 groups, either control, Low-haloperidol (HAL), and High-HAL treatment. All treatments were initiated immediately after surgery and continued for 48 h. Each treatment group consisted of 8 animals
Anesthesia and surgery
Abdominal surgery was performed as our previous reports [5, 12]. Briefly, the animals were anesthetized with isoflurane (1.5–2.0%) in oxygen at 0.5 L/min through a nose cone connected to a vaporizer. After a 2-cm vertical midline incision, peritoneal cavity was carefully explored and the small intestine was exposed. The exteriorized bowel was manipulated for 3 min. Postsurgical analgesia was provided with wound infiltration of 0.2% ropivacaine (300 µl; AstraZeneca, Osaka, Japan). Body temperature was continuously monitored by rectal probe and maintained at 36.5 ± 0.3 °C by a heating lamp. Sham control animals were only anesthetized, shaved, and given analgesia in the same manner as the experimental rats. The mean arterial oxygen saturation, pulse rate, and arterial pressure were non-invasively. Postoperative pain was quantified 2 h after surgery, based on facial expression by the rat grimace scale (RGS) from 0 (no pain) to 2 (severe pain) based on the previous study [13].
Behavioral testing
HAL-induced catalepsy was also determined using a standard bar test. A 1.6-cm-diameter bar was fixed horizontally 10 cm above the floor of a Plexiglas cage. Rats were placed in the test cage and allowed to acclimatize for 5 min before performing the test. Both forepaws were then placed on the bar, and measured the length of time during which each rats maintained the initial position (maximum cut-off time, 180 s).
Inattention and cognitive deficits were assessed after a 2-day (Experiment-1) or 7-day (Experiment-2) postsurgical recovery period with a trace fear-conditioning task as previously described [5]. Briefly, the conditioning chamber had a standard grid floor connected to a shock generator–scrambler. The animals were placed and habituated to the conditioning chamber for a 2 min period, posteriorly receiving one training session of 8 trials of trace fear conditioning. The trace fear-conditioning trial comprised a 10-s, 4 kHz, 75 dB tone conditioned stimulus (CS) and a 0.7-s, 0.8 mA foot shock unconditioned stimulus (US) separated by an empty 30-s trace interval. The total seconds of freezing throughout the 30-s trace interval were calculated for each rat and shown as a percentage of freezing time during the total duration of the 30-s trace interval. Freezing behavior was defined as the absence of all movement, excluding respiratory movements. The trace and context memory retention tests were performed 4 h after the training session. During the trace memory retention testing, the animal was placed in a novel context consisting in the conditioning chamber. After a 2-min baseline period, a 10-s CS tone was given, followed by a 4-min post-CS period in the absence of foot shock. During the context memory retention testing, the animals were placed into the original conditioning chamber for a 4-min period without receiving the CS or US. The behavior of rats was videotaped and subsequently analyzed by a researcher blinded to the treatment groups. The freezing behavior was expressed as a percentage of freezing time during the 4-min observational period.
Tissue collection and neurochemical analysis
The animals were sacrificed by a lethal dose of sodium pentobarbital (60 mg/kg i.p.) followed by decapitation, either at 2 or 7 days after surgery and completion of behavioral testing. Subsequently, tissues and plasma were collected. Plasma concentration of HAL was quantified using a liquid–liquid extraction followed by a liquid chromatography–mass spectrometry analysis conducted with an HP 1100 LCDAD–MSD system (Hewlett-Packard Company, USA). The whole brain was rapidly harvested, and hippocampus and medial prefrontal cortex (mPFC) were dissected. The levels of tumor necrosis factor-α (TNF-α) and interleukin-1 β (IL-1β) were analyzed by ELISA kits 438207 (Biolegend, USA) and ER2IL1B (Thermo Scientific, USA), respectively, following manufacturers’ instructions. The concentration of DA and its metabolite, 3,4-dihydroxyphenylacetic acid (DOPAC), was measured by high-performance liquid chromatography (HPLC) with electrochemical detection, as described in detail in Supplementary data 1. DA turnover was calculated by the ratio DOPAC/DA, and used as an index of DA metabolism.
Statistical analysis
All the results were expressed as mean ± standard deviation (SD). Difference between the study groups was assessed using the Kruskal–Wallis test, and differences between individual groups by the Wilcoxon–Mann–Whitney test with Bonferroni correction as a post hoc analysis. Correlations between variables were evaluated with Pearson’s correlation test. All data were analyzed using the statistical software SAS (version 9.3; SAS Institute Inc., Cary, NC), and p < 0.05 was considered statistically significant.
Results
There were no significant differences in the parameters measured in the preoperative open-field test (Supplementary data 2), hemodynamics during anesthesia, and RGS 2 h after surgery (Supplementary data 3) among experimental groups. The plasma HAL concentrations of Experiment-1 were within the human pharmacological active range of 7.1 ± 1.2 ng/ml (Low-HAL groups) and 20.5 ± 3.7 ng/ml (High-HAL groups) [14]. Consistently, the results of bar test in Experiment-1 showed that HAL caused a significant dose-dependent cataleptic effect in both sham-surgery (p < 0.05) and surgery (p < 0.05) groups (Fig. 2a). The HAL-induced catalepsy was not observed 7 days after surgery (Fig. 2b, p = 0.57). No other adverse effects were detected through the experimental period.
Effect of haloperidol treatment on the cataleptic behavior of bar test in aged rats. The elapsed time (s) in Experiment-1 (a) and Experiment-2 (b) is shown. Each vertical bar represents the mean ± SD. The three study groups of both sham and surgery animals were indicated in “Materials and methods (n = 8 in each group). *Significant (p < 0.05), ns non-significant (p > 0.05)
Trace fear-conditioning task at 2-day after surgery
In Experiment-1, significant effects of the factor trial number during the training session were comparably observed in both sham surgery (Fig. 3a) and surgery (Fig. 3b) groups. In addition, there were no group differences in the freezing responses (p = 0.74), indicating neither surgery nor HAL influenced acquisition of fear memory in the conditioning. When trace memory retention was tested 4 h later in a novel chamber, freezing levels were comparably low during the 2-min baseline (before CS) in all groups (Supplementary data 4). All groups exhibited greater freezing in the post-CS period compared with baseline (all p < 0.05), and a significant group effect was observed during the 30-s trace interval following the CS offset (Fig. 3c). Subsequent pairwise comparisons demonstrated that surgical rats froze significantly less than the animals in corresponding sham-surgery groups (all p < 0.05). On the other hand, HAL in both Low and High regimens showed no effects within the sham-surgery groups. Notably, HAL also had no effects on the surgery-induced impairments of trace memory retention; the freezing percentage was not different within surgery groups (p = 0.79).
Trace fear-conditioning task in early postoperative period (Experiment-1). Behavioral responses during the training session in sham (a) and surgery (b) animals. Freezing behavior is expressed as a percentage of total time (mean ± SD) across the 8 trials of the conditioning session. Freezing behavior during trace memory retention test (c) and context memory retention test (d) is shown. Each vertical bar represents the mean ± SD. The three study groups of both sham and surgery animals were indicated in “Materials and methods” (n = 8 in each group). *Significant (p < 0.05), ns non-significant (p > 0.05)
For the context retention testing, pairwise comparisons revealed a significant main group effect (p < 0.05, Fig. 3d). Similar to trace memory retention testing, pairwise comparisons indicated that surgical rats froze significantly less than the animals in corresponding sham-surgery (p = 0.82) or surgery (p = 0.76) groups.
Trace fear-conditioning task at 7 days after surgery
During the training session, there were no significant differences in the acquisition of trace fear conditioning between the groups (Fig. 4a, b). In trace memory retention testing, no significant between-group differences were observed (p = 0.68, Fig. 4c). However, context memory retention was significantly impaired in the surgery groups compared with the corresponding sham-surgery groups (Fig. 4d, all p < 0.05), whereas neither Low- nor High-HAL regimen had significant effects within sham-surgery (p = 0.69) or surgery (p = 0.70) groups.
Trace fear-conditioning task in late postoperative period (Experiment-2). Behavioral responses during the training session in sham (a) and surgery (b) animals. Freezing behavior is expressed as a percentage of total time (mean ± SD) across the 8 trials of the conditioning session. Freezing behavior during trace memory retention test (c) and context memory retention test (d) is shown. Each vertical bar represents the mean ± SD. The three study groups of both sham and surgery animals were indicated in “Materials and methods” (n = 8 in each group). *Significant (p < 0.05), ns non-significant (p > 0.05)
Brain levels of pro-inflammatory cytokines
After completion of behavioral testing either 2 (Experiment-1) or 7 (Experiment-2) days after surgery, TNF-α and IL-1β levels in the hippocampus and mPFC were measured. In sham-surgery animals, hippocampal and mPFC levels of TNF-α at 2 (Fig. 5a, b) and 7 (Fig. 5c, d) days after surgery were comparable between groups (all p > 0.05). In contrast, the average TNF-α levels in both brain regions at 2 days after surgery in the surgical group were significantly greater than those in the control groups (all p < 0.05, Fig. 5a, b). However, no significant pharmacological effects of HAL were observed in both hippocampus and mPFC (p > 0.05 in each pairwise comparison). At 7 days after surgery, the hippocampal TNF-α levels in the surgical groups were higher than those in corresponding sham-surgery groups (all p < 0.05, Fig. 5c, d), whereas there were no differences in the TNF-α levels in the mPFC. Neither Low- nor High-HAL regimen showed any significant effects on the TNF-α levels in both regions. Regarding IL-1β levels, the statistical analysis revealed similar main group effects with those observed for TNF-α (Supplementary data 5).
Cytokine concentrations in the brain after surgery. Tumor necrosis factor (TNF)-α levels in the hippocampus (a) and medial prefrontal cortex (mPFC, b) in Experiment-1, as well as TNF-α levels in the hippocampus (c) and mPFC (d) in Experiment-2, are shown. Each vertical bar represents the mean ± SD. The three study groups of both sham and surgery animals were indicated in “Materials and methods” (n = 8 in each group). *Significant (p < 0.05), ns non-significant (p > 0.05)
Brain levels of DA and its metabolite
In the hippocampus of sham-surgery rats at postoperative day 2, HAL did not change the levels of DA (p = 0.67, Fig. 6a), whereas significantly increased the concentrations of DOPAC (+ 197% for Low-HAL, + 443% for High-HAL vs. control, p < 0.05, Fig. 6b) in dose-dependent manner. Along with this, the DA turnover was found to be significantly enhanced by HAL in dose-dependent manner (+ 208% for Low-HAL, + 417% for High-HAL vs. control, p < 0.05, Fig. 6c). Laparotomy also significantly increased the hippocampal levels of DA and DOPAC (both p < 0.05, sham vs. surgery in corresponding control rats), resulting in elevation of the DA turnover (+ 1127%, p < 0.05). However, HAL showed no additional effects on the hippocampal contents of DA, DOPAC, and DA turnover, in surgery group (p = 0.75 for Low-HAL, p = 0.88 for High-HAL vs. control surgery groups). On the other hand, at postoperative day 7, there were no group differences in any of the dopaminergic parameters (Fig. 6d–f). Similar group differences were observed with respect to mPFC (Supplementary data 6).
HPLC analysis of dopamine (DA), its metabolite, and turnover in the hippocampus after surgery. Levels of DA (a), 3,4-dihydroxyphenylacetic acid (DOPAC, b), and DA turnover (DOPAC-to-DA ratio, c) in Experiment-1, as well as levels of DA (d), DOPAC (e), and DA turnover (f) in Experiment-2 are shown. Each vertical bar represents the mean ± SD. The three study groups of both sham and surgery animals were indicated in “Materials and methods” (n = 8 in each group). *Significant (p < 0.05), ns non-significant (p > 0.05)
Correlation analysis between hippocampal neurochemicals and memory retention
The relationships of hippocampal TNF-α and DA turnover levels with freezing rates during trace or context memory retention testing were assessed in control animals of both sham and surgery groups. The results of Pearson’s correlation analyses are summarized in Table 1. Two days after the procedures (Experiment-1), hippocampal levels of TNF-α were inversely correlated with both trace and context memory retentions. Conversely, a significant relationship was not observed between hippocampal levels of DA turnover and both memory performances. At 7 days after the procedures (Experiment-2), the hippocampal TNF-α levels were significantly correlated with context, but not trace, memory retention. However, there was no significant relationship between the hippocampal DA turnover and both memory functions.
Discussion
In the present study, we showed in geriatric rodents that acute neuroinflammation, as well as increased brain DA turnover, was induced secondary to surgery in the early postoperative period. Consistent with our previous study [5], postoperative poor trace and context memory functions were highly correlated with the neuroinflammatory response in both hippocampus and mPFC. Meanwhile, the relationships with DA turnover were less pronounced, suggesting that increased dopamine signaling may not play a major role in the pathogenesis of attentional/cognitive domains of POD. In line with this, our findings indicate that a DA D2 receptor-selective antagonist HAL could not significantly restore the surgery-induced delirious-like cognitive symptoms.
Recent clinical studies show the possibility that neuroinflammation is one of the pathogenesis of schizophrenia [15]. The previous in vitro experiments have also been reported that HAL could inhibit inflammatory signaling and glia-derived pro-inflammatory proteins [16, 17]. These results imply that HAL has an anti-neuroinflammatory activity, which may in turn be involved in its anti-psychotic effects. However, until now, this hypothesis remains to be confirmed under in vivo conditions. One of the obstacles regarding HAL treatment in whole animals is the differences in the pharmacokinetics with humans, e.g., elimination half-life (t1/2) of rodent and human is 3.5 h and 20–24 h, respectively [10]. Therefore, in this study, continuous infusion pumps were applied to extrapolate human- to rodent dose, successfully achieving human equivalent plasma concentrations.
HAL is shown therapeutic activity on psychotic symptoms, schizophrenia and delirium, principally acting as a potent dopaminergic antagonist [6]. In our HPLC analysis, HAL administration did not change DA contents, whereas dose-dependently increased the DOPAC levels and enhanced DA turnover, in non-surgical control animals. Comparably, the previous reports showed that this DA turnover enhancement is a compensatory response to the blockade of DA D2 receptors [18]. Although HAL failed to show the additional effects on surgery-induced elevation of DA turnover, suggesting that no compensatory effects occurred due to an already maximum rate. On the other hand, the HAL-induced compensatory response could be an alternative approach for evaluating the blocking activity of DA antagonists. These findings further confirm that our dosage regimen is pharmacologically active in aged rats. As evidence, HAL treatment dose-dependently produced catalepsy that is predictive of extrapyramidal effects through the blockade of DA receptors in humans. However, despite in vivo therapeutically relevant doses, treatment with HAL failed to treat the surgery-induced acute neuroinflammation.
In late postoperative phase, after the surgery-induced systemic inflammation was resolved, significant neuroinflammation was detected in the hippocampus, but not in mPFC. This may explain the corresponding behavioral consequences in which laparotomy impairs the hippocampus-dependent context memory retention without affecting the mPFC-dependent trace memory retention. Our results further showed that HAL administration during early postoperative period could not prevent the development of these late-phase neurocognitive deficits. Under identical experimental conditions, we previously found that the postoperative chronic neuroinflammation may be associated with age-related pro-inflammatory changes of microglia [19]. The hippocampal specificity may be due to its higher microglia density and aging-related vulnerability. This suggests that the transition from acute to chronic hippocampal neuroinflammation after surgery may represent a pathogenic mechanism producing POD-associated persistent postoperative neurocognitive disorders. Recently, a study demonstrated that in vivo administration of HAL has no significant influence in the microglial population of rat cortex [20]. Consequently, pharmacologically active doses of HAL may lack of in vivo efficacy for microglia-mediated neuroinflammation, and thus no mitigating effects on the long-term cognitive burden of POD.
In addition or association with neuroinflammation, imbalance in various neurotransmitters can contribute to the pathogenesis of delirium [21]. Among them, excess in DA levels within the brain may play a crucial role. Our results also showed that surgery resulted in a moderate increase in DA contents, while a more profound increase of the DA metabolite, DOPAC, levels, i.e., increased DA turnover, during the early postoperative period. Since only extracellular DA is metabolized into DOPAC by monoamine oxidase, the elevated DA turnover could reflect in increased synaptic DA levels [18]. Like a transient nature of POD in human, excessed dopaminergic activity is diminished during the late postoperative phase. However, we could not find significant correlation of this hyperdopaminergic state with any cognitive abnormality. This discrepancy may be due to a wide range of delirious symptoms via several independent mechanisms. Specifically, excessive dopaminergic activity is shown to be associated with symptoms of hyperactive delirium, i.e., restlessness, agitation, and refusal to cooperate with care [21]. Since there is currently no established method for assessing the hyperactive delirious state in rodents, these symptoms have not been detected in our experimental POD model. To better understand these relationships, a comprehensive animal model of POD should be established in the future.
POD is associated with a subsequently increased risk of dementia, contributing to the healthcare burden of elderly patients [3]. Therefore, exploring the mechanism based rather than the symptomatic treatment for POD may be of greater clinical importance. Specifically, evidence consistent with our findings suggests that acute neuroinflammation may be a key pathogenesis of POD [4, 5]. HAL is the most widely used anti-psychotic drug in management of delirium, whereas its rationale is not clear due to limited clinical evidence with conflicting results [1, 2, 7]. Taking into consideration the increased risk of extrapyramidal side effects in elderly, routine use of HAL for preventing or treating POD may not be currently recommended [1, 2]. Our findings further provide preclinical insight that pharmacologically active doses of HAL did not elicit anti-neuroinflammatory effects, and thus may have no impact on the cognitive symptoms of POD. Nevertheless, we cannot rule out the possibility that a different type of surgical model may induce different dopamine responses, which could influence the cognitive outcomes. Furthermore, various critical translational questions remain such as effects of the administration route and timing, new-generation anti-psychotic drugs, as well as the effects on non-cognitive morbidity and mortality. Therefore, future studies are needed to answer these issues before our findings can be translated into clinical practice.
Conclusion
Our data indicate that acute neuroinflammation, but not excess in dopaminergic activity, may contribute to the development of delirious-like cognitive symptoms during the early postoperative period. Postoperative administration of HAL with pharmacologically active doses failed to alleviate the surgery-induced neuroinflammation and related cognitive deficits. Furthermore, HAL treatment could not prevent the hippocampal neuroinflammatory chronification and the consequent long-term cognitive decline. Our findings indicate that perioperative administration with HAL may have no effects on postoperative neuroinflammation and related cognitive impairment.
References
American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;220:136–48.
Aldecoa C, Bettelli G, Bilotta F, Sanders RD, Audisio R, Borozdina A, Cherubini A, Jones C, Kehlet H, MacLullich A, Radtke F, Riese F, Slooter AJ, Veyckemans F, Kramer S, Neuner B, Weiss B, Spies CD. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34:192–214.
Hayden KM, Inouye SK, Cunningham C, Jones RN, Avidan MS, Davis D, Kuchel GA, Tang Y, Khachaturian AS. Reduce the burden of dementia now. Alzheimer Dement. 2018;14:845–7.
Cunningham C. Systemic inflammation and delirium: important co-factors in the progression of dementia. Biochem Soc Trans. 2011;39:945–53.
Kawano T, Yamanaka D, Aoyama B, Tateiwa H, Shigematsu-Locatelli M, Nishigaki A, Iwata H, Locatelli FM, Yokoyama M. Involvement of acute neuroinflammation in postoperative delirium-like cognitive deficits in rats. J Anesth. 2018;32:506–17.
Latronico N. Haloperidol and delirium in the ICU: the finger pointing to the moon. Intensive Care Med. 2018;44:1346.
Shen YZ, Peng K, Zhang J, Meng XW, Ji FH. Effects of haloperidol on delirium in adult patients: a systematic review and meta-analysis. Med Princ Pract. 2018;27:250–9.
Runyan JD, Moore AN, Dash PK. A role for prefrontal cortex in memory storage for trace fear conditioning. J Neurosci. 2004;24:1288–95.
Arias N, Méndez M, Arias JL. The importance of the context in the hippocampus and brain related areas throughout the performance of a fear conditioning task. Hippocampus. 2015;25:1242–9.
Vernon AC, Natesan S, Crum WR, Cooper JD, Modo M, Williams SC, Kapur S. Contrasting effects of haloperidol and lithium on rodent brain structure: a magnetic resonance imaging study with postmortem confirmation. Biol Psychiatry. 2012;71:855–63.
Kawano T, Eguchi S, Iwata H, Yamanaka D, Tateiwa H, Locatelli FM, Yokoyama M. Pregabalin can prevent, but not treat, cognitive dysfunction following abdominal surgery in aged rats. Life Sci. 2016;148:211–9.
Locatelli FM, Kawano T, Iwata H, Aoyama B, Eguchi S, Nishigaki A, Yamanaka D, Tateiwa H, Shigematsu-Locatelli M, Yokoyama M. Resveratrol-loaded nanoemulsion prevents cognitive decline after abdominal surgery in aged rats. J Pharmacol Sci. 2018;137:395–402.
Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, Mapplebeck JC, Wei P, Zhan S, Zhang S, McDougall JJ, King OD, Mogil JS. The rat grimace scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011;7:55.
Coryell W, Miller DD, Perry PJ. Haloperidol plasma levels and dose optimization. Am J Psychiatry. 1998;155:48–53.
Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277–86.
Kowalski J, Blada P, Kucia K, Madej A, Herman ZS. Neuroleptics normalize increased release of interleukin-1 beta and tumor necrosis factor-alpha from monocytes in schizophrenia. Schizophr Res. 2001;50:169–75.
de Souza DF, Wartchow K, Hansen F, Lunardi P, Guerra MC, Nardin P, Gonçalves CA. Interleukin-6-induced S100B secretion is inhibited by haloperidol and risperidone. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:14–22.
Seeman P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse. 1987;1:133–52.
Kawano T, Eguchi S, Iwata H, Tamura T, Kumagai N, Yokoyama M. Impact of preoperative environmental enrichment on prevention of development of cognitive impairment following abdominal surgery in a rat model. Anesthesiology. 2015;123:160–70.
Bloomfield PS, Bonsall D, Wells L, Dormann D, Howes O, De Paola V. The effects of haloperidol on microglial morphology and translocator protein levels: an in vivo study in rats using an automated cell evaluation pipeline. J Psychopharmacol. 2018;32:1264–72.
Ali S, Patel M, Jabeen S, Bailey RK, Patel T, Shahid M, Riley WJ, Arain A. Insight into delirium. Innov Clin Neurosci. 2011;8:25–34.
Acknowledgements
This work was supported by a Grant-in Aid for Scientific Research (C): [Grant number 15K10538 to TK] and a Grant-in Aid for Scientific Research (C): [Grant number 15K10560 to MY] from the Japan Society for the Promotion of Science, Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no financial or scientific conflict of interest regarding the research described in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Nishigaki, A., Kawano, T., Iwata, H. et al. Acute and long-term effects of haloperidol on surgery-induced neuroinflammation and cognitive deficits in aged rats. J Anesth 33, 416–425 (2019). https://doi.org/10.1007/s00540-019-02646-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-019-02646-0