Abstract
Diseases of the lung, e. g. chronic obstructive pulmonary disease (COPD), interstitial lung diseases, bronchiectasis or cystic fibrosis, often lead to recurrent severe respiratory infections that cause exacerbations of the underlying disease. These acute or chronic inflammatory processes can result in a progressive destruction of the lung and in an ongoing decline in lung function. Therefore longer inpatient stays for intravenous antibiotic treatment are necessary and the quality of life in these patients is severely limited. A rapid detection of infectious agents in human lungs is often crucial, because the choice of the appropriate therapeutic regime depends at first on the identification of the infecting species. Standard methods for detection and identification are either time consuming, of low sensitivity or expensive. It is known that bacteria, and also mitosporic fungi, produce volatile organic compounds (VOCs) that can be detected in exhaled breath by ion mobility spectrometry (IMS), were a distinct detection of a specific VOC is related to a “peak”. We investigated, whether the detection and characterisation of VOCs by Multi-capillary column coupled to IMS in exhaled breath of patients whose airways are either infected or colonized by Pseudomonas aeruginosa compared to healthy non-smoker controls is capable of identifying those infectious agents. To realize a non invasive identification of pathogens, the exhaled breath of 53 persons (24 patients suffering chronic or infectious on Pseudomonas and 29 healthy controls) was investigated using an ion mobility spectrometer type BioScout. In total 224 different signals were found. Actually, 21 different signals are able to differentiate the two groups, Control and Pseudomonas, with rank sum values less than 0.2. For all 224 signals Box-and-Wisker plots were realized. The peaks with the lowest rank sum values F (0,107) and PS0 (0,112) show rather good separation of both groups. Our preliminary results demonstrate that distinct patterns of a small number of IMS-peaks are sufficient for the identification of these infectious agents. Therefore MCC-IMS seems to be a promising method for the non-invasive identification of patients which are colonized or infected with bacteria such as Pseudomonas aeruginosa.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Actually, there are several analytical detection approaches available for human breath investigations, including gas chromatography–mass spectrometry (GC-MS) [1–7], proton transfer reaction-mass spectrometry (PTR-MS) [8–11], selected ion flow tube-mass spectrometry (SIFT-MS) [12–18] and ion mobility spectrometry (IMS) [2, 19–28] and electronic noses [29–33] and different types of sensors [34–40]. The sampling techniques include direct sampling, partly using sample loops [41–44], Tedlar bags [45–48], SPME [4, 5, 49–51] and different adsorbents [20, 26, 52]. In all cases mentioned a non-invasive and easy method for early diagnosis or therapy monitoring should be developed by identifying disease-specific biomarkers in the breath of patients.
On the other hand, first scientific evidence for microbial VOCs has been presented early in the last century [53, 54]. More recently, the detection of different VOCs and their relations to medical questions was reported, including ion mobility spectrometry [22, 55–71]. Some of the VOCs were related to bacteria taken from headspace of cultures [58, 72, 73].
The analytical technique used in the present paper is the same basic technique used to detect explosives or chemical warfare agents and was developed in a close cooperation of ISAS (Institute for Analytical Sciences, Dortmund) and the Lung Hospital Hemer, to examine hospital patients with lung cancer and airway infections [3, 21–23, 25, 74–78].
The technique, based on ion mobility spectrometry, is able to detect effectively metabolites in human breath down to the pptv or pg/L-range. For investigations of human breath at a comparatively high level of humidity, a Multi-Capillary Column (MCC) for partly pre-separating of the analytes is used in combination with a conventional ion mobility spectrometer (IMS). An IMS coupled to a MCC allows the identification and quantification of volatile metabolites present in human breath, down to the ng/L- and pg/L-range of analytes within less than 500 s and without any pre-concentration. The IMS investigations are based on different drift times of swarms of ions from metabolites formed directly in air at ambient pressure. About 10 mL of breath is necessary to carry out a full analysis.
Patients
All patients were recruited from the Department of Pulmonology, Ruhrlandklinik, University Hospital of Essen, Germany. The diagnosis of Pseudomonas was established according to the actual guidelines. Subjects with any other respiratory disease or any concomitant malignant, heart, renal, liver or collagen disease were excluded. All patients were clinically stable (no evidence of acute exacerbation for at least 4 weeks prior to enrolment). Healthy non-smokers, all employees of the hospital, served as control group. The study was approved by the ethic committee of the University of Essen and all subjects provided an informed consent.
Method
The IMS coupled to a multi-capillary column (MCC/IMS) used was a BioScout (B&S Analytik, Dortmund, Germany), consisting of the MCC/IMS and a SpiroScout (Ganhorn Medizin Electronic, Niederlauer, Germany) as sample inlet unit. The major parameters are summarized elsewhere [2, 3, 21–24, 74, 76, 79, 80]. In this spectrometer a 550 MBq [63]Ni ß-radiation source was applied for the ionization of the carrier gas (air). It is connected to a polar multi-capillary column (MCC, type OV-5, Multichrom Ltd, Novosibirsk, Russia) used as the pre-separation unit. In this MCC, the analytes of exhaled breath were sent through 1.000 parallel capillaries, each with an inner diameter of 40 μm and a film thickness of 200 nm. The total diameter of the separation column was 3 mm. The relevant MCC parameters are listed in Table 1.
All subjects were requested to exhale through a mouth piece connected to a Teflon tube. In each case, end-tidal breath controlled by a flow sensor, was collected in a sample loop of 10 mL in volume. The sample air was collected and transferred to the multi-capillary column for a first chromatographic separation after reaching three times 10 mL above the dead volume. Using the software VOCan 1.7 (B&S Analytik, Dortmund Germany), the dead volume was adjusted and fixed in the present case to 500 mL. The expiration was controlled by a CO2-sensor element integrated in the SpiroScout and recorded for each subject.
A preliminary relation between the peak position and the identity of the analyte was obtained using the database BSIMSDB 1.4 (B&S Analytik, Dortmund, Germany), but parallel measurements using e.g. GC/MSD should be realized with respect of further confirmation.
Statistical evaluation
The peaks were characterized using the software Visual Now 2.5 (B&S Analytik, Dortmund Germany), which is described elsewhere [74, 81–84]. All peaks found were characterized by their position with drift time (corresponding 1/K0-value) and retention time and their concentration represented as the peak height. For all the peaks in both of the groups, Box-and-Wisker plots were realized. The rank sum as provided by Visual Now 2.5 was used to rank the peaks with the maximum difference between both groups. The value of the rank sum is related to the Mann–Whitney–Wilcoxon U value directly (rank sum = norm U = U/n1n2), were n1 and n2 are the numbers of cases in each group.
Results
To realize a non invasive identification of pathogens the exhaled breath of 53 persons (24 patients suffering chronic or infectious on Pseudomonas and 29 healthy controls) was investigated using the ion mobility spectrometer type BioScout. In total 224 signals were found as shown in Fig. 1.
For each of the peak the rank sum was calculated using Visual Now 2.2 to be able to find the signals with the maximum potential to discriminate between both of the groups, see Table 2.
In total, 21 signals seem to be able to differentiate the two groups control and pseudomonas with a rank sum values less than 0.2.
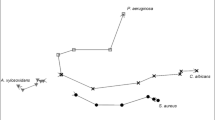
The position of the peaks with the rank sum less than 0.2 is shown in Fig. 2.
Position of the peaks with the maximum potential with respect to discrimination between the group of Pseudomonas and the healthy controls within the IMS-Chromatogram. The cross line related to the single spectrum (below) and the chromatogram (right) shows the position of the peak with the lowest rank sum
Furthermore, for all 224 signals Box-and-Wisker plots were realized. The peaks with the lowest rank sum values F (0,107) and PS0 (0,112) show rather good separation of both groups. A further discrimination between the first two peaks F and P_20, which were rather close to each other, located by 1/K0 and retention time, was not realized in the present study (see Table 2). Therefore, the first and the third peak should be considered more in detail. Surprisingly, in all cases the concentration of the analytes was significantly higher in the control group than in the Pseudomonas group.
The values of sensitivity and specificity, and the positive and negative predictive value were summarized in Table 3 as obtained from visual discrimination. The peak PS0 shows a sensitivity and a negative predictive value of 100%. The sensitivity was higher than the specificity in both cases. But, the peak PS0 was really low and near to the noise and in some cases the measurements were not carried out until 600 s. This becomes visible from null values for four cases as shown in Fig. 3.
In addition, a calculation of the best position of a threshold with respect to differentiation of the two groups was realized for all 224 peaks. The Table 4 compares the results for the positions 1–7 of the rank sum from Table 2.
Surprisingly, the peak with rank sum position 3 delivers the best accuracy (0,88). For Peak PS0 the sensitivity found was 100, the specificity 74, the positive predictive value 82%, the negative predictive value 100%.
Therefore, the signal F should be considered for separation between control and pseudomonas group with the highest accuracy. Generally, the finding needs further confirmation and a higher number of subjects included within the study (Fig. 4).
Summary
To realize a non invasive identification of pathogens, the exhaled breath of 53 persons (24 patients suffering chronic or infectious on Pseudomonas and 29 healthy controls) was investigated using an ion mobility spectrometer type BioScout. In total 224 different signals were found in the exhaled breath. Actually, 21 different signals are able to differentiate the two groups Control and Pseudomonas with rank sum values less than 0.2. The best separation was found by peak F with rank sum 0,107. In this case, the sensitivity found was 89%, the specificity 77%, the positive and negative predictive values were 83% and 86%, respectively. Generally, the finding needs further confirmation and a higher number of subjects included within the study.
References
Mieth M, Schubert JK, Groger T, Sabel B, Kischkel S, Fuchs P, Hein D, Zimmermann R, Miekisch W (2010) Automated needle trap heart-cut GC/MS and needle trap comprehensive two-dimensional GC/TOF-MS for breath gas analysis in the clinical environment. Anal Chem 82:2541–2551. doi:10.1021/ac100061k
Jünger M, Bödeker B, Baumbach JI (2010) Peak assignment in multi-capillary column—ion mobility spectrometry using comparative studies with gas chromatography—mass spectrometry for exhalred breath analysis. Anal Bioanal Chem 396:471–482. doi:10.1007/s00216-009-3168-z
Westhoff M, Litterst P, Freitag L, Urfer W, Bader S, Baumbach JI (2009) Ion mobility spectrometry for the detection of volatile organic compounds in exhaled breath of patients with lung cancer: results of a pilot study. Thorax 64:744–748. doi:10.1136/thx.2008.099465
Buszewski B, Ulanowska A, Ligor T, Denderz N, Amann A (2009) Analysis of exhaled breath from smokers, passive smokers and non-smokers by solid-phase microextraction gas chromatography/mass spectrometry. Biomed Chromatogr 23:551–556
Ligor T, Ligor M, Amann A, Ager C, Bachler M, Dzien A, Buszewski B (2008) The analysis of healthy volunteers’ exhaled breath by the use of solid-phase microextraction and GC-MS. J Breath Res 2:046006/046001–046006/046008
Kushch I et al (2008) Compounds enhanced in a mass spectrometric profile of smokers’ exhaled breath versus non-smokers as determined in a pilot study using PTR-MS. J Breath Res 2:026002/026001–026002/026026
Amann A, Spanel P, Smith D (2007) Breath analysis: the approach towards clinical applications. Mini-Rev Med Chem 7:115–129
Beauchamp J, Kirsch F, Buettner A (2010) Real-time breath gas analysis for pharmacokinetics: monitoring exhaled breath by on-line proton-transfer-reaction mass spectrometry after ingestion of eucalyptol-containing capsules. J Breath Res 4. doi:10.1088/1752-7155/4/2/026006
Herbig J, Mueller M, Schallhart S, Titzmann T, Graus M, Hansel A (2009) On-line breath analysis with PTR-TOF. J Breath Res 3:027004/027001–027004/027010
Warneke C, Kuczynski J, Hansel A (1996) Proton transfer reaction mass spectrometry (PTR-MS) propanol in human breath. Int J Mass Spectrom Ion Proc 154:61–70
Hansel A, Jordan A, Holzinger R (1995) Proton transfer reaction mass spectrometry: on-line trace gas analysis at the ppb level. Int J Mass Spectrom Ion Proc 149:609–619
Smith D, Spanel P, Enderby B, Lenney W, Turner C, Davies SJ (2010) Isoprene levels in the exhaled breath of 200 healthy pupils within the age range 7–18 years studied using SIFT-MS. J Breath Res 4:017101/017101–017101/017107
Seeley MJ, Hu W-P, Scotter JM, Storer MK, Shaw GM (2009) In vitro SIFT-MS validation of a breath fractionating device using a model VOC and ventilation system. J Breath Res 3(1):016001
Spanel P, Smith D (2008) Quantification of trace levels of the potential cancer biomarkers formaldehyde, acetaldehyde and propanol in breath by SIFT-MS. J Breath Res 2:046003/046001–046003/046010
Spanel P, Dryahina K, Smith D (2007) The concentration distributions of some metabolites in the exhaled breath of young adults. J Breath Res 1:1–8
Smith D, Turner C, Spanel P (2007) Volatile metabolites in the exhaled breath of healthy volunteers: their levels and distributions. J Breath Res 1:R1–R12
Turner C, Welch S, Bellingan G, Singer M, Spanel P, Smith D (2005) Analysis of breath using SIFT-MS: a comparison of the breath composition of healthy volunteers and seriously-ill ICU patients. Breath Anal Clin Diagn Ther Monit, [Presentations Conf. “Breath Gas Anal. Med. Diagn.”] FIELD Full Journal Title:Breath Analysis for Clinical Diagnosis and Therapeutic Monitoring, [Based on Presentations at the Conference “Breath Gas Analysis for Medical Diagnostics”], Dornbirn, Austria, Sept. 23–26, 2004, 317–326
Smith D, Diskin AM, Ji Y, Spanel P (2001) Concurrent use of H3O+, NO+, and O2+ precursor ions for the detection and quantification of diverse trace gases in the presence of air and breath by selected ion-flow tube mass spectrometry. Int J Mass Spectrom 209:81–97
Perl T, Bödecker B, Jünger M, Nolte J, Vautz W (2010) Alignment of retention time obtained from multicapillary column gas chromatography used for VOC analysis with ion mobility spectrometry. Anal Bioanal Chem 397:2385–2394. doi:10.1007/s00216-010-3798-1
Vautz W, Nolte J, Fobbe R, Baumbach JI (2009) Breath analysis—performance and potential of ion mobility spectrometry. J Breath Res 3:036004. doi:036010.031088/031752-037155/036003/036003/036004
Bunkowski A, Boedeker B, Bader S, Westhoff M, Litterst P, Baumbach JI (2009) MCC/IMS signals in human breath related to sarcoidosis-results of a feasibility study using an automated peak finding procedure. J Breath Res 3:046001/046001–046001/046010
Westhoff M, Litterst P, Freitag L, Baumbach JI (2007) Ion mobility spectrometry in the diagnosis of Sarcoidosis: results of a feasibility study. J Physiol Pharmacol 58:739–751
Baumbach JI, Westhoff M (2006) Ion mobility spectrometry to detect lung cancer and airway infections. Spectrosc Eur 18:22–27
Baumbach JI (2006) Process analysis using ion mobility spectrometry. Anal Bioanal Chem 384:1059–1070
Ruzsanyi V, Baumbach JI, Sielemann S, Litterst P, Westhoff M, Freitag L (2005) Detection of human metabolites using multi-capillary columns coupled to ion mobility spectrometers. J Chromatogr A 1084:145–151
Basanta M et al (2010) Non-invasive metabolomic analysis of breath using differential mobility spectrometry in patients with chronic obstructive pulmonary disease and healthy smokers. Analyst 135:315–320. doi:10.1039/b916374c
Basanta M, Koimtzis T, Singh D, Wilson I, Thomas CLP (2007) An adaptive breath sampler for use with human subjects with an impaired respiratory function. Analyst (Cambridge, U K) 132:153–163
Basanta M, Koimtzis T, Thomas CLP (2006) Sampling and analysis of exhaled breath on human subjects with thermal desorption gas chromatography—differential mobility spectrometry. Int J Ion Mobility Spectrom 9:45–49
Horvath I, Lazar Z, Gyulai N, Kollai M, Losonczy G (2009) Exhaled biomarkers in lung cancer. Eur Respir J 34:261–275. doi:10.1183/09031936.00142508
Dragonieri S, Annema JT, Schot R, van der Schee MPC, Spanevello A, Carratu P, Resta O, Rabe KF, Sterk PJ (2009) An electronic nose in the discrimination of patients with non-small cell lung cancer and COPD. Lung Cancer 64:166–170. doi:10.1016/j.lungcan.2008.08.008
Cheng ZJ, Warwick G, Yates DH, Thomas PS (2009) An electronic nose in the discrimination of breath from smokers and non-smokers: a model for toxin exposure. J Breath Res 3:036003/036001–036003/036005
Dragonieri S et al (2007) An electronic nose in the discrimination of patients with asthma and controls. J Allergy Clin Immunol 120:856–862. doi:10.1016/j.jaci.2007.05.043
Risby TH, Solga SF (2006) Current status of clinical breath analysis. Appl Phys B Lasers Opt 85:421–426
Silkoff P (2008) History, technical and regulatory aspects of exhaled nitric oxide. J Breath Res 2:037001/037001–037001/037008
Gelperin A, Johnson ATC (2008) Nanotube-based sensor arrays for clinical breath analysis. J Breath Res 2:037015/037011–037015/037016
de Lacy Costello BPJ, Ewen RJ, Ratcliffe NM, Richards M (2008) The characteristics of novel low-cost sensors for volatile biomarker detection. J Breath Res 2:037017/037011–037017/037016
de Lacy Costello BPJ, Ewen RJ, Ratcliffe NM (2008) A sensor system for monitoring the simple gases hydrogen, carbon monoxide, hydrogen sulfide, ammonia and ethanol in exhaled breath. J Breath Res 2:037011/037011–037011/037019
De Lacy Costello B, Ewen R, Ewer AK, Garner CE, Probert CSJ, Ratcliffe NM, Smith S (2008) An analysis of volatiles in the headspace of the faeces of neonates. J Breath Res 2:037023/037021–037023/037028
Mazzone PJ, Hammel J, Dweik R, Na J, Czich C, Laskowski D, Mekhail T (2007) Diagnosis of lung cancer by the analysis of exhaled breath with a colorimetric sensor array. Thorax 62:565–568. doi:10.1136/thx.2006.072892
Toda K, Li J, Dasgupta Purnendu K (2006) Measurement of ammonia in human breath with a liquid-film conductivity sensor. Anal Chem 78:7284–7291
Baumbach JI, Vautz W, Ruzsanyi V, Freitag L (2005) In: Anmann A, Smith D (eds) Breath Analysis for Clinical Diagnosis and Therapeutic Monitoring Breath Anal. Clin. Diagn. Ther. Monit., [Presentations Conf. “Breath Gas Anal. Med. Diagn.”] FIELD Full Journal Title: Breath Analysis for Clinical Diagnosis and Therapeutic Monitoring, [Based on Presentations at the Conference “Breath Gas Analysis for Medical Diagnostics”], Dornbirn, Austria, (Sept. 23–26, 2004) 53–66 (World Scientific)
Borgerding AJ, Wilkerson CW (1996) A comparison of cryofocusing injectors for gas sampling and analysis in fast GC. Anal Chem 68:2874–2878
Lopez-Ferrer D et al (2008) On-line digestion system for protein characterization and proteome analysis. Anal Chem 80:8930–8936. doi:10.1021/ac800927v
Miekisch W, Hengstenberg A, Kischkel S, Beckmann U, Mieth M, Schubert JK (2010) Construction and evaluation of a versatile CO2 controlled breath collection device. IEEE Sens J 10:211–215. doi:10.1109/jsen.2009.2035757
Beauchamp J, Herbig J, Gutmann R, Hansel A (2008) On the use of Tedlar bags for breath-gas sampling and analysis. J Breath Res 2:046001/046001–046001/046019
Lee JH, Hwang SM, Lee DW, Heo GS (2002) Determination of volatile organic compounds (VOCs) using Tedlar bag/solid-phase microextraction/gas chromatography/mass spectrometry (SPME/GC/MS) in ambient and workplace air. Bull Korean Chem Soc 23:488–496
Schulz K, Jensen ML, Balsley BB, Davis K, Birks JW (2004) Tedlar bag sampling technique for vertical profiling of carbon dioxide through the atmospheric boundary layer with high precision and accuracy. Environ Sci Technol 38:3683–3688
Steeghs MML, Cristescu SM, Harren FJM (2007) The suitability of Tedlar bags for breath sampling in medical diagnostic research. Physiol Meas 28:73–84. doi:10.1088/0967-3334/28/1/007
Bajtarevic A et al (2009) Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 9. doi:10.1186/1471-2407-9-348
Deng CH, Zhang J, Yu XF, Zhang W, Zhang XM (2004) Determination of acetone in human breath by gas chromatography-mass spectrometry and solid-phase microextraction with on-fiber derivatization. J Chromatogr B 810:269–275. doi:10.1016/j.jchromb.2004.08.013
Ligor M et al (2009) Determination of volatile organic compounds in exhaled breath of patients with lung cancer using solid phase microextraction and gas chromatography mass spectrometry. Clin Chem Lab Med 47:550–560. doi:10.1515/cclm.2009.133
Benoit MF, Davidson WR, Lovett AM, Nacson S, Ngo A (1983) Breath analysis by atmospheric pressure ionization mass spectrometry. Anal Chem 55:805–807
Zoller HF, Clark WM (1921) The production of volatile fatty acids by bacteria of the dysentry group. J Gen Physiol 3:325–330
Stotzky G, Schenck S (1976) Volatile organic compounds and microorganisms. CRC Crit Rev Microbiol 4:333–382. doi:10.3109/10408417609102303
Shnayderman M et al (2005) Species-specific bacteria identification using differential mobility spectrometry and bioinformatics pattern recognition. Anal Chem 77:5930–5937. doi:10.1021/ac050348i
DeBono R, Jadamec JR, Vinopal RT (2001) Rapid charactization of bacteria by ion mobility spectrometry. Int J Ion Mobil Spectrom 3:83
Schmidt H, Tadjimukhamedov F, Mohrenz I, Smith GB, Eiceman GA (2004) Micro-fabricated differential mobility spectrometry with pyrolysis gas chromatography for chemical characterization of bacteria. Anal Chem 76:5208–5217
Jünger M, Vautz W, Kuhns M, Hofman L, Ulbricht S, Baumbach JI, Quintel M, Perl, T (2011) Ion mobility spectrometry for microbial volatile organic compounds: a new pathogen identification tool by smelling human pathogenic bacteria. Appl Microbiol Biotechn
Vinopal RT, Jadamec JR, deFur P, Demars AL, Jakubielski S, Green C, Anderson CP, Dugas JE, DeBono RF (2002) Fingerprinting bacterial strains using ion mobility spectrometry. Anal Chim Acta 457:83–95
Cheung W, Xu Y, Thomas CLP, Goodacre R (2009) Discrimination of bacteria using pyrolysis-gas chromatography-differential mobility spectrometry (Py-GC-DMS) and chemometrics. Analyst (Cambridge, U K) 134:557–563
Snyder AP, Shoff DB, Eiceman GA, Blyth DA, Parsons JA (1991) Detection of bacteria by ion mobility spectrometry. Anal Chem 63:526–529
Snyder AP, Dworzanski JP, Tripathi A, Maswadeh WM, Wick CH (2004) Correlation of mass spectrometry identified bacterial biomarkers from a fielded pyrolysis-gas chromatography-ion mobility spectrometry biodetector with the microbiological gram stain classification scheme. Anal Chem 76:6492–6499
Prasad S, Pierce KM, Schmidt H, Rao JV, Gueth R, Synovec RE, Smith GB, Eiceman GA (2008) Constituents with independence from growth temperature for bacteria using pyrolysis-gas chromatography/differential mobility spectrometry with analysis of variance and principal component analysis. Analyst (Cambridge, U K) 133:760–767
Harrington PB, Buxton TL, Chen G (2001) Classification of bacteria by thermal methylation hydrolysis ion mobility spectrometry using SIMPLISMA and multidimensional wavelet compression. Int J Ion Mobil Spectrom 4(2):148–151
Ochoa ML, Harrington PB (2004) Characterization and differentiation of bacteria using in situ derivatization ion mobility spectrometry of whole cells and chemometric modeling. Int J Ion Mobil Spectrom 7:C19–C50
Westhoff M, Freitag L, Ruzsanyi V, Baumbach JI (2005) Bacterial differentiation by ion mobility spectrometry: first results of a pilot study. Chest 128:375S–375S
Strachan NJC, Nicholson FJ, Ogden ID (1995) An automated sampling system using ion mobility spectrometry for the rapid detection of bacteria. Anal Chim Acta 313:63–67
Prasad S, Schmidt H, Lampen P, Wang M, Gueth R, Rao JV, Smith GB, Eiceman GA (2006) Analysis of bacterial strains with pyrolysis-gas chromatography/differential mobility spectrometry. Analyst (Cambridge, U K) 131:1216–1225
Prasad S, Pierce KM, Schmidt H, Rao JV, Gueth R, Bader S, Synovec RE, Smith GB, Eiceman GA (2007) Analysis of bacteria by pyrolysis gas chromatography-differential mobility spectrometry and isolation of chemical components with a dependence on growth temperature. Analyst (Cambridge, U K) 132:1031–1039
Phillips M et al (2008) Detection of lung cancer using weighted digital analysis of breath biomarkers. Clin Chim Acta 393:76–84
Chaim W, Karpas Z, Lorber A (2003) New technology for diagnosis of bacterial vaginosis. Eur J Obstet Gynecol Reprod Biol 111:83–87
Scholler C, Molin S, Wilkins K (1997) Volatile metabolites from some gram-negative bacteria. Chemosphere 35:1487–1495
Korpi A, Kasanen J-P, Alarie Y, Kosma V-M, Pasanen A-L (1999) Sensory irritating potency of some Mircobial Volatile Organic Compounds (MVOCs) and a mixture of five MVOCs. Arch Environ Health 54:347–352
Westhoff M, Litterst P, Maddula S, Bödecker B, Rahmann S, Davies AN, Baumbach JI (2010) Differentiation of chronic obstructive pulmonary disease (COPD) including lung cancer from healthy control group by breath analysis using ion mobility spectrometry. Int J Ion Mobil Spectrom 13:131–139
Vautz W, Baumbach JI, Westhoff M, Zuechner K, Carstens ETH, Perl T (2010) Breath sampling control for medical application. Int J Ion Mobil Spectrom 13:41–46
Bunkowski A, Maddula S, Davies AN, Westhoff M, Litterst P, Bödecker B, Baumbach JI (2010) One-year time series of investigations of analytes within human breath using ion mobility spectrometry. Int J Ion Mobil Spectrom 13:141–148. doi:10.1007/s12127-010-0052-7
Baumbach JI, Maddula S, Bödecker B, Westhoff M, Litterst P, Davies AN, Neuziel P (2010) Breath discovery based on ion mobility spectrometry and classification and differentiation models for lung diseases. Biomed Tech 55. doi:10.1515/BMT.2010.537
Baumbach JI, Bödeker B, Westhoff M, Litterst P (2010) Metabolites in human breath during indursulfase therapy of a patient with Hunter disease—first results of time series using MCC/IMS. Biomed Tech 55. doi:10.1515/BMT.2010.677
Bödecker B, Davies AN, Maddula S, Baumbach JI (2010) Biomarker validation—room air variation during human breath investigations. Int J Ion Mobil Spectrom 13:177–184. doi:10.1007/s12127-010-0044-7
Maddula S, Blank L, Schmid A, Baumbach JI (2009) Detection of volatile metabolites of Escherichia coli by multi capillary column coupled ion mobility spectrometry. Anal Bioanal Chem 394:791–800
Bödeker B, Baumbach JI (2009) Analytical description of IMS-signals. Int J Ion Mobil Spectrom 12:103–108. doi:10.1007/s12127-009-0024-y
Bödeker B, Vautz W, Baumbach JI (2008) Peak finding and referencing in MCC/IMS—data. Int J Ion Mobil Spectrom 11:83–88
Bödeker B, Vautz W, Baumbach JI (2008) Peak comparison in MCC/IMS—data—searching for potential biomarkers in human breath data. Int J Ion Mobil Spectrom 11:89–93
Bödeker B, Vautz W, Baumbach JI (2008) Visualisation of MCC/IMS—data. Int J Ion Mobil Spectrom 11:77–82
Acknowledgements
The financial support of the Ministry of Education, Science and Technology (MEST) of the Republic Korea is acknowledged thankfully. Part of the work on this paper has been supported by Deutsche Forschungsgemeinschaft (DFG) within the Collaborative Research Center (Sonderforschungsbereich) SFB 876 “Providing Information by Resource-Constrained Analysis”, project TB1 “Resource-Constrained Analysis of Spectrometry Data”. In addition, the work was supported partly by the German Federal Ministry of Economics and Technology based on a decision of the German Bundestag within the project KF2368102AKO.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rabis, T., Sommerwerck, U., Anhenn, O. et al. Detection of infectious agents in the airways by ion mobility spectrometry of exhaled breath. Int. J. Ion Mobil. Spec. 14, 187–195 (2011). https://doi.org/10.1007/s12127-011-0077-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12127-011-0077-6