Abstract
Non-invasive methods with potential for diagnosis of lung diseases gain increasing interest. Within the present study the exhaled breath of 132 persons (97 Chronic obstructive pulmonary disease (COPD) patients [35 COPD without lung cancer, 62 COPD with lung cancer] and 35 healthy volunteers) was investigated using an Ion Mobility Spectrometer (IMS) coupled to a Multi-Capillary Column (MCC) without any pre-separation or pre-enrichment. One hundred four different peaks were considered within the IMS-Chromatograms of the 10 mL breath samples of both groups. A principal component analysis (PCA) of these 104 peaks identified a single analyte, that allowed a separation of the healthy persons and the COPD patients (with and without lung cancer). The sensitivity obtained was 60%, the specificity 91%, the positive predictive value 95%. The peak was characterized as cyclohexanone (CAS 108-94-1). Subsequent studies must validate the identity of the peak used for separation of the two groups with a greater population and external standards. Breath gas analysis using ion mobility spectrometry offers a chance of separating healthy persons and COPD patients using a single analyte at a defined concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic obstructive pulmonary disease (COPD) is an inflammatory condition characterized by oxidative stress and the formation of volatile organic compounds (VOCs) secreted via the lungs. Therefore, the analysis of VOCs might be highly relevant in the diagnosis of COPD. A complete description of the volatiles associated with COPD has yet to be developed. Recently, some studies have identified groups of volatiles and other biomarkers in COPD patients that have potentially useful relationships to cell degradation processes. There are several analytical detection approaches available, including gas chromatography-mass spectrometry (GC-MS) [1–7], proton transfer reaction-mass spectrometry (PTR-MS) [8–11], selected ion flow tube-mass spectrometry (SIFT-MS) [12–18], ion mobility spectrometry (IMS) [2, 19–28], and electronic noses [29–33] as well as other types of sensors [34–40].
In the present paper breath gas analysis using ion mobility spectrometry was used to investigate the potential of differentiating healthy persons and COPD patients.
Material and method
The IMS coupled to a multi-capillary column (MCC/IMS) used was a BreathDiscovery (B&S Analytik, Dortmund, Germany). The major parameters are summarized elsewhere [2, 3, 21–24, 41]. In this spectrometer a 555 MBq 63Ni ß-radiation source was applied for the ionisation of the carrier gas (air). It was connected to a polar multi-capillary column (MCC, type OV-5, Multichrom Ltd, Novosibirsk, Russia) used as the pre-separation unit. In this MCC the analytes of exhaled breath were sent through 1,000 parallel capillaries, each with an inner diameter of 40 μm and a film thickness of 200 nm. The total diameter of the separation column was 3 mm. The relevant MCC parameters are listed in Table 1.
In total, 132 persons were included in the study of which 97 had a COPD (35 COPD without Bronchial Carcinoma, 62 COPD with Bronchial Carcinoma) and 35 were healthy volunteers. In each case end-tidal breath, controlled by a flow sensor, was collected in a sample loop of 10 mL volume. The content of the sample loop was then fed into the inlet of a MCC and transported to the IMS after pre-separation. The MCC and the drift tube IMS were held at a constant 30 °C.
The peaks were characterized using the software Visual Now (B&S Analytik, Dortmund Germany), which is described elsewhere [42–45]. All 104 peaks are characterized by their position with drift time (corresponding 1/K0-value) and retention time and their concentration related to the peak height. A principal component analysis (PCA) was realized using the heights of each peak as variable and each peak as sample. Therefore, the scores are related to the individual peak heights and the loadings to the analytes considered as samples. The software Unscrambler (CAMO Software AS, Oslo, Norway) was used for the analysis.
Results
One hundred four peak areas were selected from the IMS chromatogrammes recorded as shown in Fig. 1. They were characterized by their drift and retention time values. Some signals related to benzaldehyde, limonene, 2-ethyl-1-hexanol, nonanal, menthone, menthol and decanal are selected and marked with their names, the other with black surroundings and numbers for clarity.
A principal component analysis was made taking all 104 peak heights in consideration as variable and each peak as sample. Therefore, the scores are related to the individual peak heights and the loadings to the analytes (see Fig. 2 for scores and loadings).
The ammonia peak height dominated PC-1 and in PC-2 it was acetone and 2-pronanol.
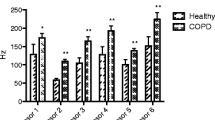
In addition, box-and-whisker plots were realized in addition to show major differences for all of the 104 peaks and the groups selected. The peak with number P7 showed a clear difference between the healthy group and the COPD group, see Fig. 3.
After a further reduction of the analytes by deleting all signals related to the correlation loading less than 50% in the PCA the peaks P33 and again P7 were found to be discriminating peaks (Figs. 4, 5, 6, 7).
Thus, the peaks P7 and P33 were investigated in the IMS-chromatogram in detail. Figure 8 shows the IMS-chromatogram where signal P7 has a high value and another one, where the value is low for comparison. It was found that in case of COPD the peak P7 was rather high. In contrast, for the healthy controls the peak P33 shows low values. Without knowledge of the normal fluctuation of peak P33 the low values are just used for comparison. The increased peak height of P7—related to an additional concentration of the analyte considered seems to be more reliable than missing concentration of P33.
Comparison of the IMS-chromatograms of the signals P7 and P33 (left) and the corresponding single spectra (right) representative for the COPD group (above) and the healthy controls (below)—the single spectra are shown at the peak maximum in the retention time scale and the peak shape within the rectangle used for the automatic peak finding procedure
Therefore, the question must be asked as to, whether a separation between COPD and healthy controls could be realized by only considering peak P7 as positive value with respect to COPD. To answer this question, in all IMS-chromatograms P7 was considered and the individual peak height were re-analysed.
Figure 9 shows all the areas selected. In detail, the peaks and the signal within the district marked black in the center are defined as district with relevance for further automatic peak characterization procedures.
A separation could be realized by definition of a threshold (Fig. 10). In all cases for healthy controls the peak was not detectable or very small in contrast to rather height signals for COPD patients. The corresponding probability density curves show the differences in another way in Fig. 10 (below, left). Within the right part below in Fig. 10 a specific threshold was defined to distinguish between COPD and healthy controls. The signal height of P7 is directly relatable to the concentration of the specific analyte behind.
Using the threshold defined within Fig. 10 a contingency table was obtained, see Table 2. The sensitivity was 60%, the specificity 91% and the positive predictive value 95%. Therefore, obtaining a value of P7 beyond the threshold is a high indicator for COPD. However, the classification below the threshold has no predictive power.
The peak P7 was subsequently identified as relating to cyclohexanone (CAS 108-94-1) using parallel measurements using a GC/MSD but will not be considered in the present paper further. Finally, using the threshold defined in Fig. 10 and the values from Table 2 a value above the threshold shows a positive prediction power of 95% for COPD.
Discussion
The data presented show that MCC/IMS provides a VOC analysis of exhaled breath that allows a discrimination of COPD patients and healthy persons by a single peak (P7) with a high specificity. The underlying analyte could be identified as cyclohexanone.
Basanta et al. [26] reported about using a differential mobility spectrometer and a adaptive sampling technique using Tenax adsorbant traps followed by analysis using GC/DMS after thermodesorption including 26 patients (20 COPD, six healthy smokers). Some signals were found to be characteristic but not named. Electronic noses were used by Fens et al. [46] for profiling of exhaled breath to enable discrimination of COPD and asthma. After 5 min of tidal breathing through an inspiratory VOC filter, an expiratory vital capacity was collected in a Tedlar bag and sampled by electronic nose. Ninety subjects participated in the study (30 patients with COPD, 20 patients with asthma, 20 non-smoking controls and 20 smoking controls). Asthma was separated from COPD with an accuracy of 96%, from non-smoking and from smoking controls by 92.5% each. Dragoniero [30] summarized the possibility of discrimination of patients with non-small cell lung cancer (NSCLC) and COPD using an electronic nose and Tedlar bags including 30 subjects (ten NSCLC, ten COPD and ten healthy persons). Poli et al. [47]. found a combination of 13 VOCs for correct classification of NSCLC, COPD and healthy persons using Tedlar bags, solid-phase micro-extraction and GC/MS for the investigations. Together with conventional diagnostic approaches Poli et al. suggested, the VOC analysis could be used as a complementary test for the early diagnosis of lung cancer. In that case, none of VOCs alone discriminated the study groups and it was not possible to identify one single chemical compound as a specific lung cancer biomarker. However, Poli´s multinomial logistic regression analysis showed that VOC profile can correctly classify about 80% of cases. Corradi et al. [48]. investigated nitrate in exhaled breath condensate of patients with different airway diseases, but no differences were found. This findings correspond with Kharitonov et al. [49]. that exhaled NO is normal in COPD.
In all publications no specific peak or analyte was found to be usable for separation of patients suffering COPD and healthy controls. In contrast to these studies the present study illustrates a discriminating analyte P7 = cyclohexanone. The actual study included COPD patients with and without lung cancer. However, subgroup analysis did not show a difference between both COPD groups using main component analysis, so that lung cancer did not influence the results. Nevertheless, a test which separates patients with airway disease from those without is a first step in the development of screening methods.
Next steps in research have to be performed. The first is the validation of P7 in a larger group of patients. A further one is to find discriminating VOCs for COPD patients with and without lung cancer. As was shown in a former feasibility study [3], lung cancer patients can be separated from healthy persons using MCC/IMS. However, this method is based on a discrimination by a combination of peak regions.
MCC/IMS has the potential to find discriminating VOC’s. Another advantage of this method is the fast examination, as a single measurement only takes about 10 min.
Summary
Within this clinical study the exhaled breath of 132 persons (97 suffering COPD [35 COPD without lung cancer, 62 COPD with lung cancer]) and 35 healthy volunteers) was investigated using an Ion Mobility Spectrometer coupled to a Multi-Capillary Column without any pre-separation or pre-enrichment. In total 104 different peaks were considered within the IMS-chromatograms of the 10 mL breath samples of both groups. Following a principal component analysis (PCA) of the 104 peaks identified it was found that a single analyte was able to separate the two groups of healthy persons and those such suffering from COPD with and without lung cancer. The sensitivity obtained was 60%, the specificity 91%, the positive predictive value 95%. The peak was characterized as cyclohexanone (CAS 108-94-1). Thus, a concentration of cyclohexanone above the threshold could be seen as an indicator for COPD. The identity of the peak used for separation of the two groups must be validated with a greater population and external standards. Breath gas analysis using ion mobility spectrometry offers a chance for separation of healthy persons and COPD patients using a single analyte at a defined concentration. Probably, some other peaks could be included in the consideration in addition to cyclohexanone. Additional studies with a higher population and parallel measurements using GC/MSD will support the identification further.
References
Mieth M, Schubert JK, Groger T, Sabel B, Kischkel S, Fuchs P, Hein D, Zimmermann R, Miekisch W (2010) Automated needle trap heart-cut GC/MS and needle trap comprehensive two-dimensional GC/TOF-MS for breath gas analysis in the clinical environment. Anal Chem 82:2541–2551
Jünger M, Bödeker B, Baumbach JI (2010) Peak assignment in multi-capillary column—ion mobility spectrometry using comparative studies with gas chromatography—mass spectrometry for exhalred breath analysis. Anal Bioanal Chem 396:471–482
Westhoff M, Litterst P, Freitag L, Urfer W, Bader S, Baumbach JI (2009) Ion mobility spectrometry for the detection of volatile organic compounds in exhaled breath of patients with lung cancer: results of a pilot study. Thorax 64:744–748
Buszewski B, Ulanowska A, Ligor T, Denderz N, Amann A (2009) Analysis of exhaled breath from smokers, passive smokers and non-smokers by solid-phase microextraction gas chromatography/mass spectrometry. Biomed Chromatogr 23:551–556
Ligor, T, Ligor M, Amann A, Ager C, Bachler M, Dzien A, Buszewski B (2008) The analysis of healthy volunteers’ exhaled breath by the use of solid-phase microextraction and GC-MS. J Breath Res 2. 046006/046001-046006/046008
Kushch I et al (2008) Compounds enhanced in a mass spectrometric profile of smokers’ exhaled breath versus non-smokers as determined in a pilot study using PTR-MS. J Breath Res 2. 026002/026001-026002/026026
Amann A, Spanel P, Smith D (2007) Breath analysis: the approach towards clinical applications. MiniRev Med Chem 7:115–129
Beauchamp J, Kirsch F, Buettner A (2010) Real-time breath gas analysis for pharmacokinetics: monitoring exhaled breath by on-line proton-transfer-reaction mass spectrometry after ingestion of eucalyptol-containing capsules. J Breath Res 4. No pp. given, doi:10.1088/1752-7155/4/2/026006
Herbig J, Mueller M, Schallhart S, Titzmann T, Graus M, Hansel A (2009) On-line breath analysis with PTR-TOF. J Breath Res 3. 027004/027001-027004/027010
Warneke C, Kuczynski J, Hansel A (1996) Proton transfer reaction mass spectrometry (PTR-MS) propanol in human breath. Int J Mass Spectrom Ion Proc 154:61–70
Hansel A, Jordan A, Holzinger R (1995) Proton transfer reaction mass spectrometry: on-line trace gas analysis at the ppb level. Int J Mass Spectrom Ion Proc 149:609–619
Smith D, Spanel P, Enderby B, Lenney W, Turner C, Davies SJ (2010) Isoprene levels in the exhaled breath of 200 healthy pupils within the age range 7–18 years studied using SIFT-MS. J Breath Res 4. 017101/017101-017101/017107
Seeley MJ, Hu W-P, Scotter JM, Storer MK, Shaw GM (2009) In vitro SIFT-MS validation of a breath fractionating device using a model VOC and ventilation system. J Breath Res 3. Paper No 1, 7 pp
Spanel P, Smith D (2008) Quantification of trace levels of the potential cancer biomarkers formaldehyde, acetaldehyde and propanol in breath by SIFT-MS. J Breath Res 2. 046003/046001-046003/046010
Spanel P, Dryahina K, Smith D (2007) The concentration distributions of some metabolites in the exhaled breath of young adults. J Breath Res 1:1–8
Smith D, Turner C, Spanel P (2007) Volatile metabolites in the exhaled breath of healthy volunteers: their levels and distributions. J Breath Res 1:R1–R12
Turner C, Welch S, Bellingan G, Singer M, Spanel P, Smith D (2005) Analysis of breath using SIFT-MS: a comparison of the breath composition of healthy volunteers and seriously-ill ICU patients. Breath Anal Clin Diagn Ther Monit [Presentations Conf “Breath Gas Anal Med Diagn”] Dornbirn, Austria, Sept. 23–26, 2004, 317–326
Smith D, Diskin AM, Ji Y, Spanel P (2001) Concurrent use of H3O+, NO+, and O2+ precursor ions for the detection and quantification of diverse trace gases in the presence of air and breath by selected ion-flow tube mass spectrometry. Int J Mass Spectrom 209:81–97
Perl T, Bödecker B, Jünger M, Nolte J, Vautz W (2010) Alignment of retention time obtained from multicapillary column gas chromatography used for VOC analysis with ion mobility spectrometry. Anal Bioanal Chem 397:2385–2394. doi:10.1007/s00216-010-3798-1
Vautz W, Nolte J, Fobbe R, Baumbach JI (2009) Breath analysis—performance and potential of ion mobility spectrometry. J Breath Res 3
Bunkowski A, Boedeker B, Bader S, Westhoff M, Litterst P, Baumbach JI (2009) MCC/IMS signals in human breath related to sarcoidosis-results of a feasibility study using an automated peak finding procedure. J Breath Res 3. 046001/046001-046001/046010
Westhoff M, Litterst P, Freitag L, Baumbach JI (2007) Ion mobility spectrometry in the diagnosis of Sarcoidosis: results of a feasibility study. J Physiol Pharmacol 58:739–751
Baumbach JI, Westhoff M (2006) Ion mobility spectrometry to detect lung cancer and airway infections. Spectrosc Eur 18:22–27
Baumbach JI (2006) Process analysis using ion mobility spectrometry. Anal Bioanal Chem 384:1059–1070
Ruzsanyi V, Baumbach JI, Sielemann S, Litterst P, Westhoff M, Freitag L (2005) Detection of human metabolites using multi-capillary columns coupled to ion mobility spectrometers. J Chromatogr A 1084:145–151
Basanta M et al (2010) Non-invasive metabolomic analysis of breath using differential mobility spectrometry in patients with chronic obstructive pulmonary disease and healthy smokers. Analyst 135:315–320. doi:10.1039/b916374c
Basanta M, Koimtzis T, Singh D, Wilson I, Thomas CLP (2007) An adaptive breath sampler for use with human subjects with an impaired respiratory function. Analyst (Cambridge, UK) 132:153–163
Basanta M, Koimtzis T, Thomas CLP (2006) Sampling and analysis of exhaled breath on human subjects with thermal desorption gas chromatography—differential mobility spectrometry. Int J Ion Mobility Spectrom 9:45–49
Horvath I, Lazar Z, Gyulai N, Kollai M, Losonczy G (2009) Exhaled biomarkers in lung cancer. Eur Respir J 34:261–275. doi:10.1183/09031936.00142508
Dragonieri S, Annema JT, Schot R, van der Schee MPC, Spanevello A, Carratu P, Resta O, Rabe KF, Sterk PJ (2009) An electronic nose in the discrimination of patients with non-small cell lung cancer and COPD. Lung Cancer 64:166–170. doi:10.1016/j.lungcan.2008.08.008
Cheng ZJ, Warwick G, Yates DH, Thomas PS (2009) An electronic nose in the discrimination of breath from smokers and non-smokers: a model for toxin exposure. J Breath Res 3. 036003/036001-036003/036005
Dragonieri S et al (2007) An electronic nose in the discrimination of patients with asthma and controls. J Allergy Clin Immunol 120:856–862. doi:10.1016/j.jaci.2007.05.043
Risby TH, Solga SF (2006) Current status of clinical breath analysis. Appl Phys B Lasers Opt 85:421–426
Silkoff, P (2008) History, technical and regulatory aspects of exhaled nitric oxide. J Breath Res 2. 037001/037001-037001/037008
Gelperin A, Johnson ATC (2008) Nanotube-based sensor arrays for clinical breath analysis. J Breath Res 2. 037015/037011-037015/037016
de Lacy Costello BPJ, Ewen RJ, Ratcliffe NM, Richards M (2008) The characteristics of novel low-cost sensors for volatile biomarker detection. J Breath Res 2. 037017/037011-037017/037016
de Lacy Costello BPJ, Ewen RJ, Ratcliffe NM (2008) A sensor system for monitoring the simple gases hydrogen, carbon monoxide, hydrogen sulfide, ammonia and ethanol in exhaled breath. J Breath Res 2. 037011/037011-037011/037019
De Lacy Costello B, Ewen R, Ewer AK, Garner CE, Probert CSJ, Ratcliffe NM, Smith S (2008) An analysis of volatiles in the headspace of the faeces of neonates. J Breath Res 2. 037023/037021-037023/037028
Mazzone PJ, Hammel J, Dweik R, Na J, Czich C, Laskowski D, Mekhail T (2007) Diagnosis of lung cancer by the analysis of exhaled breath with a colorimetric sensor array. Thorax 62:565–568. doi:10.1136/thx.2006.072892
Toda K, Li J, Dasgupta Purnendu K (2006) Measurement of ammonia in human breath with a liquid-film conductivity sensor. Anal Chem 78:7284–7291
Baumbach JI (2009) Ion mobility spectrometry coupled with multi-capillary columns for metabolic profiling of human breath. J Breath Res 3:1–16
Bödeker B, Baumbach JI (2009) Analytical description of IMS-signals. Int J Ion Mobility Spectrom 12:103–108. doi:10.1007/s12127-009-0024-y
Bödeker B, Vautz W, Baumbach JI (2008) Peak finding and referencing in MCC/IMS—data. Int J Ion Mobility Spectrom 11:83–88
Bödeker B, Vautz W, Baumbach JI (2008) Peak comparison in MCC/IMS—data—searching for potential biomarkers in human breath data. Int J Ion Mobility Spectrom 11:89–93
Bödeker B, Vautz W, Baumbach JI (2008) Visualisation of MCC/IMS—data. Int J Ion Mobility Spectrom 11:77–82
Fens N, Zwinderman AH, van der Schee MP, de Nijs SB, Dijkers E, Roldaan AC, Cheung D, Bel EH, Sterk PJ (2009) Exhaled breath profiling enables discrimination of chronic obstructive pulmonary disease and asthma. Am J Respir Crit Care Med 180:1076–1082. doi:10.1164/rccm.200906-0939OC
Poli D, Carbognani P, Corradi M, Goldoni M, Acampa O, Balbi B, Bianchi L, Rusca M, Mutti A (2005) Exhaled volatile organic compounds in patients with non-small cell lung cancer: cross sectional and nested short-term follow-up study. Respir Res 6. No pp. given
Corradi M, Pesci A, Casana R, Alinovi R, Goldoni M, Vittoria Vettori M, Cuomo A (2003) Nitrate in exhaled breath condensate of patients with different airway diseases. Nitric Oxide 8:26–30
Kharitonov SA, Barnes PJ (2002) Biomarkers of some pulmonary diseases in exhaled breath. Biomarkers 7:1–32
Acknowledgements
The continuous support of Mrs. Barbara Obertrifter (Lung clinic Hemer) is acknowledged thankfully. The financial support of the Bundesministerium für Bildung und Forschung and the Ministerium für Innovation, Wissenschaft, Forschung und Technologie des Landes Nordrhein-Westfalen for the work carried out by JIBB at ISAS (Institute for Analytical Sciences, Dortmund, Germany) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Westhoff, M., Litterst, P., Maddula, S. et al. Differentiation of chronic obstructive pulmonary disease (COPD) including lung cancer from healthy control group by breath analysis using ion mobility spectrometry. Int. J. Ion Mobil. Spec. 13, 131–139 (2010). https://doi.org/10.1007/s12127-010-0049-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12127-010-0049-2