Abstract
The interpretation of exhaled breath analysis needs to address to the influence of exogenous factors, especially to a transfer of confounding analytes by the test persons. A test person who was exposed to a disinfectant had exhaled breath analysis by MCC/IMS (Bioscout®) after different time intervals. Additionally, a new sampling method with inhalation of synthetic air before breath analysis was tested. After exposure to the disinfectant, 3-Pentanone monomer, 3-Pentanone dimer, Hexanal, 3-Pentanone trimer, 2-Propanamine, 1-Propanol, Benzene, Nonanal showed significantly higher intensities, in exhaled breath and air of the examination room, compared to the corresponding baseline measurements. Only one ingredient of the disinfectant (1-Propanol) was identical to the 8 analytes. Prolonging the time intervals between exposure and breath analysis showed a decrease of their intensities. However, the half-time of the decrease was different. The inhalation of synthetic air –more than consequently airing the examination room with fresh air –reduced the exogenous and also relevant endogenous analytes, leading to a reduction and even changing polarity of the alveolar gradient. The interpretation of exhaled breath needs further knowledge about the former residence of the proband and the likelihood and relevance of the inhalation of local, site-specific and confounding exogenous analytes by him. Their inhalation facilitates a transfer to the examination room and a detection of high concentrations in room air and exhaled breath, but also the exhalation of new analytes. This may lead to a misinterpretation of these analytes as endogenous resp. disease-specific ones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is increasing attention to breath analysis in clinical application. Gas chromatography / mass spectrometry [1,2,3,4,5,6] and e-Nose [7,9,10,10] represent the most known methods. Most recently, another method, multi-capillary column coupled to ion mobility spectrometry (MCC/IMS) [11,13,14,15,16,17,17] is becoming useful, especially with respect to bed-site applications.
IMS is based on the ionisation of gaseous metabolites which are separated by short impulses (about 10–100 ms) in drift tubes with lengths of only a few centimetres at ambient pressure [18, 19]. The very small electric current (nA to pA) generated at a Faraday plate forms the spectrum of the running time of the ions. The combination of IMS with gas chromatographic columns guarantees the pre-separation of gaseous metabolites before entering the drift tube. Thus, MCC/IMS enables the detection and separation of all VOCs in exhaled breath and their visualisation in a three-dimensional IMS chromatogram. Although IMS does not identify unknown compounds in a gas, its main advantages are its ability to detect very low concentrations of compounds (ng/l to pg/l, ppmv to pptv range) without any pre-concentration and the short time for analysis, with spectrums taken in less than 50 ms and complete breath analysis in less than 500 s.
All analytical methods which are applied in clinical setting need to be standardized and validated for their clinicals usefulness. An important issue is the consideration of exogenous factors that might influence the interpretation of exhaled breath. So exhaled breath analysis cannot be regarded without knowing the composition of inhaled air, especially room air. This was shown in time series of exhaled breath by calculating alveolar gradients [20] and by times series of room air [21, 22]. Room air, as well as exhaled breath, can change during the day and longer time spaces. Regarding analytes only in exhaled breath may lead to different results than calculating alveolar gradients respectively considering analytes and their peak intensities in inhaled and exhaled air. Different influential factors have to be taken into consideration: the patient himself, the technician, the frequency of airing the examination room and patients who stayed in the room before.
The high sensitivity of methods, which are applied in exhaled breath analysis, especially of ion mobility spectrometry, also entails a high risk to detect analytes which do not derive from endogenous production but have to be regarded as exogenous VOCs and thus confounding pollutants. Accordingly, it is not clear sometimes, if a VOC originates from room air or is an endogenous one. This is especially relevant when comparing healthy and sick persons as well as patients of different locations. Then a differentiation of these groups might take place not by disease-specific analytes but based on local specificities.
Therefore, the local conditions need to be known and the sampling procedures to be standardized, as has been highlighted by the actual ERS/ATS recommendation [23] for breath analysis.
We wondered which influence exogenous pollutants may have on the analytes which are detected in exhaled breath by MCC/IMS. Therefore, the present study intended to clarify if substances that are inhaled in an environment, which is different from the examination room, influence the exhaled breath analysis and if so, how long they can be detected in exhaled breath. Additionally, it was tested, which measurements can reduce or eliminate such interfering influences and which effect they have on the relation of analytes in exhaled breath to room air. The resulting question is whether an additional sampling and analysis of room air is necessary before or after the sampling procedure, so that it can be included in the interpretation.
Material and methods
For room air and exhaled breath measurements an IMS coupled to a multi-capillary column (MCC/IMS) [Bio-Scout® - B&S Analytik GmbH, Dortmund, Germany] was used. The major parameters of the MCC/IMS are described elsewhere [3, 13, 14, 16, 24,26,27,28,29,29]. In the spectrometer, a 550 MBq 63Ni ß-radiation source was applied for the ionization of the drift gas. As drift gas Synthetic air (Air Liquide, Krefeld, Germany) was used as drift gas, which has a purity of 99,999% and no further peaks, besides the RIP. The IMS was connected to a polar multi-capillary column (MCC, type OV-5, Multichrom Ltd., Novosibirsk, Russia), which was used as a pre-separation unit and in which the analytes of a 10 ml sample of room air resp. exhaled breath were sent through 1000 parallel capillaries, each with an inner diameter of 40 mm and a film thickness of 200 nm. The total diameter of the pre-separation column was 3 mm. The relevant MCC parameters are listed in Table 1.
All measurements were realized within the same room, using the same instrumentation and measurement procedure. The MCC and the drift tube IMS were held constant at 40 °C. The peaks were characterized using the Software Visual Now (B&S Analytik GmbH, Dortmund Germany), which is described elsewhere [26,28,29,29]. All analytes are characterized by their position with drift time (corresponding 1/K0-value) and retention time; their concentration is related to the peak height.
For a total period of 5 min the test person sat in a separate room at a table on which a cloth was deposited that had been impregnated with a disinfectant (Sterillium® - BODE Chemistry), with a composition of active ingredients in 100 g: Propan-2-ol 45.0 g, propan-1-ol 30.0 g, mecetroniumetilsulfate 0.2 g and other ingredients as Glycerol 85%, tetradecan-1-ol, fragrances, patent blute V 85% and purified water.
The succeeding test was composed of 4 settings: 1. Direct way to exhaled breath analysis by MCC/IMS (Bioscout® - Fa. B&S-Analytik GmbH, Dortmund, Germany). 2. 5 min-walk to exhaled breath analysis. 3. Direct way to the examination room, but before breath sampling inhalation of synthetic air for 5 min out of a reservoir bag and exhalation by a so-called „non-rebreathing “N.R.B.-valve (Hans Rudolph Inc.) (Fig. 1). 4. repetition of procedure 3 with 10 min inhalation of synthetic air out of the reservoir bag.
The reservoir bag (A) (Fig. 1) was connected to a mouthpiece of the Spiro-Scout-device® (Fa. Ganshorn, Germany). With a pressure of 2 bar the reservoir bag was filled with synthetic air (B). The exhaled breath, which was contaminated with the analytes of the disinfectant, was directly exhaled to the room by the “non-rebrathing”-NRB-valve (C, orange arrow), thus avoiding an exhalation into the reservoir bag. Before starting the measurements the reservoir bag was continuously flushed with synthetic air in order to avoid contaminations and a detection of components of the bag itself. After the test person had inhaled synthetic air for 5 resp. 10 min, the mouth piece was connected to the Spiroscout-unit of the Bioscout® and the exhaled breath measurements were carried out. During the tests, the room air was aired continuously. In order to exclude a retainment of analytes of the disinfectant in the sampling tube, leading to a signal carryover, the IMS-device was flushed by a FN-manoever (programm mode FN, i.e. taking humid air through the system) between the different tests.
The room-air-measurements after having aired the room (Fig. 2) and the exhaled breath measurement of the healthy and fasting test person (Fig. 3) before having inhaled the disinfectant were chosen as reference measurements and compared with the exhaled breath measurements after the inhalation of the disinfectant.
The peak identification was realized using the software package MIMA (version 1.1, provided by the Max Planck Institute for Informatics, Saarbrücken, Germany, see http://mima.mpi-inf.mpg.de/index.htm) and the database 20160426_SubstanzDbNIST_122 (B & S Analytik GmbH, Dortmund, Germany).
The MCC/IMS studies were approved by the Ethic committee and the participant gave his written informed consent for participation in the study.
Results
The room air measurements and the exhaled breath measurement before the inhalation of the disinfectant did not show any abnormalities regarding a contamination with unusual VOCs and represented a good comparison to the 4 stages of the test.
The peak pattern of the initial breath analysis (test 1) after inhalation of the room air, which was contaminated by the analytes of the disinfectant showed considerable changes in the chromatogram with the appearance of new peaks (Fig. 4).
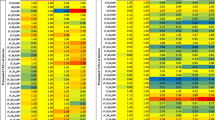
8 conspicuous peaks in test 1 (P0 – P7), which could be identified as 3-Pentanone monomer, 3-Pentanone dimer, Hexanal, 3-Pentanone trimer, 2-Propanamine, 1-Propanol, Benzene and Nonanal (Table 2), were marked in the heatmap, in order to analyze changes in their intensity, depending on the different measurements to reduce their concentrations in exhaled breath resp. their intensity in the chromatogram.
Measurement 2 – with a 5 min rest in a different room before exhaled breath analysis – shows a marked reduction of the peak intensities in the heatmap (Fig. 5). The reduction of the intensities of peak P0 and P1 (3-Pentanone monomer, 3-Pentanone dimer) is significant. Furthermore, the peaks resp. peak regions, which are marked with red rectangle in Figs. 4, 5 and 6., show a reduction of their retention time from 110 s to 45 s (yellow rectangle in Fig. 5).
The continuous inhalation of synthetic air in measurement 3 resulted in a further reduction of the VOC-concentration resp. the peak intensity (Fig. 6).
The retention time, which had been reduced to 45 s in test 2 (Fig. 5) was now reduced to 30 s (green rectangle in Fig. 6). Further peaks, which are marked with black arrows in Fig. 6. had almost completely disappeared compared to test 1 and 2 and the initial chromatogram of the room air.
The synopsis of peak P0 – P7 (3-Pentanone monomer, 3-Pentanone dimer, Hexanal, 3-Pentanone trimer, 2-Propanamine, 1-Propanol, Benzene, Nonanal) shows the influence of the different measures on the peak intensities (Fig. 7).
P0, P6 and P7 (3-Pentanone monomer, Benzene, Nonanal) can be detected from the beginning, i.e. also in the room air. The trend of these 3 peaks is presented in a time series (Fig. 8), which also considers the initial exhaled breath analysis of the test person.
The chosen peaks have a lower intensity in the exhaled breath of the test person than in the room air. The intensities of peak P0 and P6 (3-Pentanone monomer, Benzene) increase in test 1, directly after having inhaled the disinfectant, while peak 7 (Nonanal) shows a decrease of its intensity.
Already in the heatmap of test 2 a relevant reduction can be observed, after 5 min delay and rest in another room, before having taken the breath sample (see Fig. 5). After inhalation of synthetic air (test 3), the intensity of peak P6 and P7 (Benzene, Nonanal) has decreased further and reached the one of the baseline measurement of the test person.
Peak 2 (Hexanal) has a higher intensity in the exhaled breath of the test person and reveals a further increase after the inhalation of the disinfectant resp. its analytes. A 5 min delay of the breath analysis does not really affect the intensity. Even the inhalation of synthetic air only reduces the intensity by 40% from 0.125 V to 0.075 V, without reaching the baseline intensity (Fig. 9).
By prolonging the inhalation of synthetic air over 10 min in test 4 no further influence on the peak intensities can be achieved (Fig. 10).
Discussion
The results show that even a short stay in a contaminated room with interfering VOCs can cause a relevant burden of inhaled breath, leading to a detection of high intensities of confounding analytes in exhaled breath. Except 1-Propanol, the detected VOCs were different to the ingredients of the disinfectant, which suggests either a fast metabolization of the inhaled analytes or a rapid induction of a production of different VOCs in the airways.
Additionally, the results confirm the assumption that VOCs, after having been incorporated and metabolized during even a short stay in a surrounding other than the examination room, are delivered to the room air of the examination room by exhalation, so that it may influence following breath analysis. If a breath analysis is scheduled directly after having entered the examination room, without sufficient time for a reduction of the VOC concentration in exhaled breath or a release to the room air resulting in an equilibrium, this condition can influence the amount and polarity of the alveolar gradient. This implies an important clinical relevance, especially if patients of different locations – wards, hospitals or even countries - are tested, for example of wards with mainly tumor or COPD-patients.
The results reveal the risk that not the disease-specific analytes are detected and classified as significant and discriminating ones, but site-specific room analytes, which are inhaled from the patient or tested person and then transferred to the examination room.
Furthermore, there is a time dependent reduction of the concentration of single analytes, as could be shown by a decrease in peak intensities with a delay of the start of breath sampling by staying longer outside the contaminated room. The longer the time lag to measurement, respectively the way to it, the lower the peak intensity in exhaled breath and the consecutive contamination of the examination room.
However, the times series also highlight a different behaviour of the single analytes. Whereas peak P0, 6 and 7 (3-Pentanone monomer, Benzene, Nonanal) even experience a relevant reduction only after a delay of 5 min, peak P2 (Hexanal) is not influenced by this. Also the inhalation of synthetic air is without significant effect. This means that contaminating analytes have different time constants and half times regarding their elimination by exhalation. Additionally, the chosen analytes in our study have different peak intensities in exhaled breath and room air before the proband was exposed to the disinfectant, resulting in alveolar gradients with different polarities. After the exposition with high intensities in exhaled breath, the alveolar gradients would all be positive, if the baseline room air intensity is used for calculation. Therefore, if an unperceived contamination of inhaled breath has taken place and breath analysis is done without a sufficient equilibrium with the examination room and without additional sampling and analysis of the room air, analytes might falsely be regarded as endogenous instead of realizing them as exogenous ones. Without knowing these facts and without measurement of room air analytes after a sufficient time of equilibrium, the interpretation of exhaled breath resp. its chromatograms would lead to false conclusions.
The performance of exhaled breath analysis after a preceded inhalation of synthetic air seems to be another alternative in order to reduce exogenous and contaminating VOCs in exhaled breath. However, it has to be discussed if such a procedure – much more than consequently airing the examination room with fresh air – does not only reduce the exogenous but also relevant endogenous analytes, leading to a reduction and even changing polarity of the alveolar gradient. Such effects have been described by inhalation of synthetic air [30]. These results also confirm recent observations [22], that also room airing not only leads to an expected reduction of peak intensities in exhaled breath, but may also result in a reversion of the relation of peak intensities between room air and exhaled breath and a negative alveolar gradient. With regard to the selection of analytes for disease classification, a negligence of this circumstance would result in false interpretations.
Time series of exhaled breath analysis [20,22,22] showed that the composition of inhaled air, mainly room air, as well as the intensity of single analytes in exhaled breath, may change in the course of a day or longer time periods, including changing polarities of the alveolar gradient. Moreover, when using short sampling intervals, circadiane rhythms of peak intensities can be detected [22]. Circadiane variations can also be found in synthetic air [21]. Without knowing the composition and intensity of analytes, breath analysis using synthetic air for inhalation not only implies the risk of a wash-out-phenomenon of the originary exhaled breath analytes but also of misinterpretation.
Such influences, especially when breath sampling is performed at different daytimes, have to be taken into account. Therefore, the additional analysis of inhaled air, mainly room air, but also - if used - of synthetic air, is advisable. Thereby specific local factors of influences can be reduced, especially if crosstown tests are scheduled.
Principally, these results, including the inhalation of synthetic air, prompt the question of optimal composition of inhaled air respectively standardization of sampling procedures for breath analysis.
Comparable to the observations in the present study, smoking related analytes are released or re-released into the room air by a contamination of clothes. The mechanisms in “third hand smoke” [31, 32] might be transferred to other volatile compounds.
The knowledge of influential and confounding factors is relevant for the implementation of breath analysis especially in pulmonary diseases, as was shown for patients who still smoke. Data about the behaviour of smoking related analytes in exhaled breath are comparable to the present study. Gordon et al. [33], when testing smokers and non-smokers, showed that the concentration of analytes in exhaled breath, in this case benzene, is not only influenced by the time lag between the inhalation of cigarette smoke and breath analysis, but also by the stay in a room which is contaminated with cigarette smoke. The VOC concentration in room air increased with the number of cigarettes smoked and non-smokers - after having inhaled contaminated room air - had higher VOC-concentrations in exhaled breath than was measured in room air [33]. Thus non-smokers would incorrectly be classified as putative smokers.
Data from Capone et al. [34] confirmed these observations by showing a distinct time dependency of the concentrations resp. intensity of exogenous analytes in exhaled breath. So the influence of smoking on exhaled VOCs was distinctly time dependent. By applying a 2-class model it was possible to proof with high sensitivity and specificity if a person was a smoker with abstinence from smoking of at least 1 h or a smoker without having smoked overnight (“blanked smoker”). Blanked smokers behaved as never-smokers. This confirms former results that smoking does not influence breath analysis, provided an appropriate time lag between last smoking and breath analysis is adhered to [16]. On the other hand non-sufficient time lags – either after inhalation of tobacco smoke or other volatile analytes – can influence breathograms or analyte compositions of exhaled breath negatively. As we found an increase of the benzene peak after the exposure to the disinfectant, the relevance of benzene even as a marker for smoking has to be challenged.
Gaida et al. [35] pointed out that using VOCs in the diagnosis and management of diseases as COPD also locational differences and features have to be considered, even if exogenous influences by cleaning agents have been excluded. This confirms the relevance of an information about the local analytes and their influence on room air and exhaled breath.
Several consequences for the standardization of breath sampling have to be addressed. This is a consequent airing of the examination room in order to avoid an accumulation of contaminating exogenous analytes [22] and to induce a rapid decline of analytes which have been transferred and released to the examination room by a proband during a preceded measurement. Additionally, after having left a ward or another location with possible exposure to confounding exogenous analytes, a longer time lag - usefully not in the examination room - before starting the breath analysis is advisable. Furthermore, a method- and device-specific standardization should be sought for every application of breath analysis, as methodological and device-depending pecularities can influence the detection and the concentration of analytes [33, 36,38,38].
Considering the results not only of this study but also of the aforementioned ones [22, 30, 33, 34], the most reliable and exact procedure for exclusion of exogenous factors of influence would be an immediate sequential and thus nearly simultaneous analysis of inhaled air – i.e. room air - and exhaled air. This should be combined with a further standardization of room airing and waiting times of the testing person in the examination room in order to achieve an equilibrium between exhaled air and inhaled room air, especially if a transfer of exogenous and confounding analytes has to be supposed.
The study has certain limitations as it included only one testing person. It did not calculate alveolar gradients, which would have allowed a more distinct statement to the differences of the intensities of the analytes in room and exhaled air, as well as a wash-out phenomenon by inhaling synthetic air.
In order to close this gap, a procedure is developed to realize a “real-time” subtraction-breathogram.
Conclusion
The interpretation of exhaled breath needs further knowledge about the former residence of the patient resp. proband and the likelihood and relevance of the inhalation of local and confounding exogenous analytes by him. The inhalation of confounding, site-specific exogenous analytes facilitates their transfer to the examination room and a detection of high concentrations in exhaled breath, but also the exhalation of new analytes. This may lead to a misinterpretation of these analytes as endogenous ones. A comparison of persons or patient groups with different diseases as well as studies at different sites implicates a risk of false classification by exogenous and site-specific, but not disease-specific analytes. Not only the analyte composition and concentration in exhaled breath but also in room air can be influenced by a transfer of exogenous analytes. The half-time of the decrease of analyte concentrations can be different and has to be taken into consideration. A method-specific standardisation of the sampling procedure is necessary, optimally with a simultaneous registration of inhaled air and exhaled breath.
References
Amann A, Spanel P, Smith D (2007) Breath analysis: the approach towards clinical applications. Mini Rev Med Chem 7(2):115–129
Buszewski B, Ulanowska A, Ligor T, Denderz N, Amann A (2009) Analysis of exhaled breath from smokers, passive smokers and non-smokers by solid-phase microextraction gas chromatography/mass spectrometry. Biomed Chromatogr 23(5):551–556
Jünger M, Bödeker B, Baumbach JI (2010) Peak assignment in multi-capillary column - ion mobility spectrometry using comparative studies with gas chromatography - mass spectrometry for exhaled breath analysis. Anal Bioanal Chem 396(1):471–482
Kushch I, Schwarz K, Schwentner L, Baumann B, Dzien A, Schmid A, Unterkofler K, Gastl G, Spaněl P, Smith D, Amann A (2008) Compounds enhanced in a mass spectrometric profile of smokers’ exhaled breath versus nonsmokers as determined in a pilot study using PTR-MS. J Breath Res 2(2):026002
Ligor T, Ligor M, Amann A, Ager C, Bachler M, Dzien A, Buszewski B (2008) The analysis of healthy volunteers’ exhaled breath by the use of solid-phase microextraction and GC-MS. J Breath Res 2(4):046006
Mieth M, Schubert JK, Groger T, Sabel B, Kischkel S, Fuchs P, Hein D, Zimmermann R, Miekisch W (2010) Automated needle trap heart-cut GC/MS and needle trap comprehensive two-dimensional GC/TOF-MS for breath gas analysis in the clinical environment. Anal Chem 82:2541–255122
Cheng ZJ, Warwick G, Yates DH, Thomas PS (2009) An electronic nose in the discrimination of breath from smokers and non-smokers: a model for toxin exposure. J Breath Res 3(3):036003
Dragonieri S, Annema JT, Schot R, van der Schee MPC, Spanevello A, Carratu P, Resta O, Rabe KF, Sterk PJ (2009) An electronic nose in the discrimination of patients with non-small cell lung cancer and COPD. Lung Cancer 64(2):166–170
Horvath I, Lazar Z, Gyulai N, Kollai M, Losonczy G (2009) Exhaled biomarkers in lung cancer. Eur Respir J 34(1):261–275
Dragonieri S, Schot R, Mertens BJA, le Cessie S, Gauw SA, Spanevello A, Resta O, Willard NP, Vink TJ, Rabe KF, Bel EH, Sterk PJ (2007) An electronic nose in the discrimination of patients with asthma and controls. J Allergy Clin Immunol 120(4):856–862
Baumbach JI, Vautz W, Ruzsanyi V, Freitag L (2005) Metabolites in human breath: ion mobility spectrometers as diagnostic tools for lung diseases. In: Ammann A, Smith D (eds) Breath analysis for clinical diagnosis and therapeutic monitoring. World Scientific Publishing Co. Ptd. Ltd, Singapore, pp 53–66
Ruzsanyi V, Baumbach JI, Sielemann S, Litterst P, Westhoff M, Freitag L (2005) Detection of human metabolites using multi-capillary columns coupled to ion mobility spectrometers. J Chromatogr A 1084(1-2):145–151
Westhoff M, Litterst P, Freitag L, Baumbach JI (2007) Ion mobility spectrometry in the diagnosis of sarcoidosis: results of a feasibility study. J Physiol Pharmacol 58:739–751
Bunkowski A, Bödeker S, Bader S, Westhoff M, Litterst P, Baumbach JI (2009) MCC/IMS signals in human breath related to sarcoidosis—results of a feasibility study using an automated peak finding procedure. J Breath Res 3(4):046001
Vautz W, Nolte J, Fobbe R, Baumbach JI (2009) Breath analysis—performance and potential of ion mobility spectrometry. J Breath Res 3(3):036004
Westhoff M, Litterst P, Freitag L, Urfer W, Bader S, Baumbach JI (2009) Ion mobility spectrometry for the detection of volatile organic compounds in exhaled breath of patients with lung cancer: results of a pilot study. Thorax 64(9):744–748
Perl T, Bödecker B, Jünger M, Nolte J, Vautz W (2010) Alignment of retention time obtained from multicapillary column gas chromatography used for VOC analysis with ion mobility spectrometry. Anal Bioanal Chem 397(6):2385–2394
Baumbach JI, Eiceman GA (1999) Ion mobility spectrometry: arriving on site and moving beyond a low profile. Appl Spectrosc 53(9):338 A–355 A
Eiceman GA, Karpas Z (1994) Ion mobility spectrometry. CRC Press, Boca Raton, Ann Arbor, London, Tokyo, pp 1–228
Bunkowski A, Maddula S, Davies AN, Westhoff M, Litterst P, Bödecker B, Baumbach JI (2010) One-year time series of investigations of analytes within human breath using ion mobility spectrometry. Int J Ion Mobil Spectrom 13(3-4):141–148
Bödeker B, Davies AN, Maddula S, Baumbach JI (2010) Biomarker validation—room air variation during human breath investigations. Int J Ion Mobil Spectrom 13(3-4):177–184
Westhoff M, Rickermann M, Franieck E, Littterst P, Baumbach JI (2019) Time series of indoor analytes and influence of exogenous factors on interpretation of breath analysis using ion mobility spectrometry (MCC/IMS). Int J Ion Mobil Spectrom 22 (1):39–49
Horváth I, Barnes PJ, Loukides S, Sterk PJ, Högman M, Olin AC, Amann A, Antus B, Baraldi E, Bikov A, Boots AW, Bos LD, Brinkman P, Bucca C, Carpagnano GE, Corradi M, Cristescu S, de Jongste JC, Dinh-Xuan AT, Dompeling E, Fens N, Fowler S, Hohlfeld JM, Holz O, Jöbsis Q, Van De Kant K, Knobel HH, Kostikas K, Lehtimäki L, Lundberg J, Montuschi P, Van Muylem A, Pennazza G, Reinhold P, Ricciardolo FLM, Rosias P, Santonico M, van der Schee MP, van Schooten FJ, Spanevello A, Tonia T, Vink TJ (2017) A European Respiratory Society technical standard: exhaled biomarkers in lung disease. Eur Respir J 49(4):1600965
Baumbach JI (2006) Process analysis using ion mobility spectrometry. Anal Bioanal Chem 384(1):059–1070
Baumbach JI (2009) Ion mobility spectrometry coupled with multi-capillary columns for metabolic profiling of human breath. J Breath Res 3:1–16
Bödeker B, Baumbach JI (2009) Analytical description of IMS-signals. Int J Ion Mobil Spectrom 12(3):103–108
Bödeker B, Vautz W, Baumbach JI (2008a) Peak finding and referencing in MCC/IMS - data. Int J Ion Mobil Spectrom 11(1-4):83–88
Bödeker B, Vautz W, Baumbach JI (2008b) Peak comparison in MCC/IMS – data – searching for potential biomarkers in human breath data. Int J Ion Mobil Spectrom 11(1-4):89–93
Bödeker B, Vautz W, Baumbach JI (2008c) Visualisation of MCC/IMS – data. Int J Ion Mobil Spectrom 11(1-4):77–82
Maurer F, Wolf A, Fink T, Rittershofer B, Heim N, Volk T, Baumbach JI, Kreuer S (2014) Wash-out of ambient air contaminations for breath measurements. J Breath Res 8(2):027107
Collins DB, Wang C, Abbatt JPD (2018) Selective uptake of third-hand tobacco smoke components to inorganic and organic aerosol particles. Environ Sci Technol 52(22):13195–13201
Díez-Izquierdo A, Cassanello-Peñarroya P, Lidón-Moyano C, Matilla-Santander N, Balaguer A, Martínez-Sánchez JM (2018) Update on thirdhand smoke: a comprehensive systematic review. Environ Res 167:341–371
Gordon SM, Wallace LA, Brinkman MC, Callahan PJ, Kenny DV (2002) Volatile organic compounds as breath biomarkers for active and passive smoking. Environ Health Perspect 110(7):689–698
Capone S, Tufariello M, Forleo A, Longo V, Giampetruzzi L, Radogna AV, Casino F, Siciliano P (2018) Chromatographic analysis of VOC patterns in exhaled breath from smokers and nonsmokers. Biomed Chromatogr 32:e4132
Gaida A, Holz O, Nell C, Schuchardt S, Lavae-Mokhtari B, Kruse L, Boas U, Langejuergen J, Allers M, Zimmermann S, Vogelmeier C, Koczulla AR, Hohlfeld JM (2016 Apr 15) A dual center study to compare breath volatile organic compounds from smokers and non-smokers with and without COPD. J Breath Res. 10(2):026006
Bajtarevic A, Ager C, Pienz M, Klieber M, Schwarz K, Ligor M, Ligor T, Filipiak W, Denz H, Fiegl M (2009) Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 9(1):348
Pleil JD, Lindstrom AB (1995) Collection of a single alveolar exhaled breath for volatile organic compounds analysis. Am J Ind Med 27:109–112
Allers M, Langejuergen J, Gaida A, Holz O, Schuchardt S, Hohlfeld JM, Zimmermann S (2016) Measurement of exhaled volatile organic compounds from patients with chronic obstructive pulmonary disease (COPD) using closed gas loop GC-IMS and GC-APCI-MS. J Breath Res 10(2):026004
Acknowledgements
The authors thank Mrs. B. Obertrifter, Lung Clinic Hemer, for her valuable technical assistance in MCC/IMS studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JIBB declared to be an employee of a company producing spectrometers used. There are no other conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Westhoff, M., Rickermann, M., Litterst, P. et al. Exogenous factors of influence on exhaled breath analysis by ion-mobility spectrometry (MCC/IMS). Int. J. Ion Mobil. Spec. 22, 59–69 (2019). https://doi.org/10.1007/s12127-019-00247-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12127-019-00247-x