Abstract
Wheat gluten protein (WGP) is a fine-quality plant-based protein resource. However, as its unparalleled reticulation structure, the processing properties of WGP are extremely poor, limiting its application. To overcome these drawbacks, the purpose of this work was to embellish wheat gluten protein by three relatively novel and mainstream chemical modifications. The results showed the pH-shifting treatment altered the apparent morphology of protein, showing a uniform flocculent structure, leading to significant improvements in foaming capacity and emulsification property. After deamidation by acetic acid, the solubility of WGP was greatly improved (60.1%), which was nearly four times that of the control group (15.8%), and its foam stability was also significantly improved. The WGP had the highest thermal stability (deformation temperature up to 148 ℃) after TGase deamidation. These results indicate that the three modification methods enhance the functional characters of WGP in different aspects and expand its application potential.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wheat gluten protein (commonly known as gluten meal, WGP) is a coproduct of wheat starch production process (Dong et al., 2022). The protein content of gluten is as high as 72–85% and contains a variety of complex proteins. Because of its rich natural resources, high nutritional value, food safety, and low price, it is widely used in the food and feed processing industry as a high-quality protein raw material. Nevertheless, as their HMW (high molecular weight) and a mass of hydrophobic amino acids, wheat gluten molecules have a large hydrophobic intramolecular interaction area and a low solubility (Wang & Arntfield, 2016), which limits the application of WGP in food and non-food applications (Qin et al., 2017). In this case, it is difficult for native WGP to have multiple processing functional properties. Hence, it is imperative to adopt appropriate modification ways to enhance and broaden its functional properties to meet the needs of different sectors of industry.
Protein modifications allow efficient use of new protein materials from unconventional sources and customize protein to impart specific and predictable functional properties (Sun et al., 2014). Previous modifications mainly include physical and chemical methods. Physical modifications are favored for their rapidity, greenness, and high safety factor. These methods are a targeted modification of proteins and generally do not involve the primary structure of the protein molecules (Zhang et al., 2020a, b). Relatively speaking, chemical modification has countless merits than other methods, covering low cost, short reaction time, highly visible modification effects, and no need for specialized equipment. Hence, this way has become the mainstream approach for protein modification (Robertson et al., 2014). For purpose of giving a comprehensive realizing of influences of chemical modification on WGP, this work chose three typically chemical approaches to modify WGP and compare their effects on structural and functional characters of WGP.
Firstly, pH-shifting modification is extensively used to modify plant protein due to its simple and novel. Proteins are very sensitive to changes in pH, and pH-shifting treatment of proteins at extreme pH conditions can cause changes in structural and functional properties of proteins. During the pH-shifting modification, the pH of the protein solution is adjusted to a very acidic or basic pH, then strong repulsive forces between the proteins allow the protein molecules to partially unfold. Next, the pH of the solution is adjusted back to neutral, the protein molecules refold, and the protein amphiphilicity changes to form a more flexible structure (Jiang et al., 2017; Kahraman et al. 2022; Wang et al., 2022a, b). This unfolding and refolding process observably alters the structural and functional characters of protein. At present, this method had been applied to vegetable proteins such as soy protein (Lee et al., 2016), pea protein (Jiang et al., 2017), and chickpea protein isolate (Wang et al., 2022a, b). Our latest work demonstrated that the pH-shifting-treated WGP possessed an enhanced emulsifying property and could be utilized in powdered oils (Xiong et al., 2023). This inspired us to further explore the different influence of this method with other chemical modifications.
Secondly, the deamidation is the change of an amide group from glutamine (Gln) and asparagine (Asn) residues to carboxyl groups, including glutamic acid and aspartic acid. Numerous studies had tried to catalyze the deamidation of protein by acid-bases and enzyme through various reaction mechanisms (Yong et al., 2006). One of the most common methods of deamidation is hydrochloric acid treatment. But hydrochloric acid hydrolysis can lead to massive hydrolysis of peptide bonds, producing bitter peptides and reducing the processing properties of the protein (Liao et al., 2010a, b, c). Carboxylic acid has been reported to be a better deamidation option. And it reduces the potential risk of celiac disease in patients and results in little hydrolysis of the protein produced (Qiu et al., 2013). Therefore, in this study, WGP was modified by deamidation with low concentration acetic acid (0.1 M) to observe the changes in the function and property of the protein.
Thirdly, in addition to pH-shifting and acid hydrolysis deamidation, enzymatic treatment is another typical method to modify proteins. Due to its safe, healthy, and environmentally friendly characters, the protein transglutaminase (TGase) can improve the quality of WGP products (Wee & Jeyakumar Henry, 2019). Transglutaminase is an enzyme that forms covalent cross-links between gluten and gliadin; specifically, it catalyzes the reaction between the ammonia (NH2) group of glutamine and lysine to form a covalent ε-(γ-glutamyl) lysine bridge (G-L bond) (Kuraishi et al., 2001), resulting in the formation of a permanent iso-peptide bond between gluten chains (Meerts et al., 2017). TGase has been successfully used in some gluten-free food systems because of their unique ability to facilitate protein cross-linking (Han et al., 2013). Accordingly, the processing properties of WGP would also significantly alter.
Therefore, the purpose of this study is to compare the influences of three representatively chemical ways (pH-shifting treatment, acetate deamidation, and enzymatic treatment) on structural and functional characters of WGP, providing a reference for subsequent WGP modification and applications.
Materials and Methods
Materials
WGP was provided by Henan Danmire Trading Company, China (production date was 11.16, 2021). Transglutaminase was purchased from Aladdin Biochemical Technology Company. All other chemical reagents used in the experiments were chemically pure, including 8-aniline-1-naphthalenesulfonic acid (ANS), phosphate buffer (PBS buffer), potassium bromide, bromophenol blue, trimethylolaminomethane, 2-mercaptoethanol, so-dium dodecyl sulfate, hydrochloric acid, and sodium hydroxide.
pH-Shifting, Acetic Acid, and Enzymatic Treatment
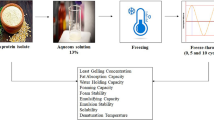
Control: Dissolve 1% WGP (W/V) in distilled water and stir for 1 h. The solution was centrifuged for 10 min (7000 g), and then the supernatant after centrifugation was freeze-dried (FD5-3, China) and stored at -20 °C. This group was the control group without modification treatment. The sample preparation date was December 18, 2021, the same below.
The pH-shifting treatment (pH): the pH-shifting treatment referenced to our previous method with some adjustments (Xiong et al., 2023). The pH of 1% WGP (W/V) suspension was changed to 12 by 1 M NaOH, and then it was placed in an 80 °C water bath for 0.5 h. The suspension was taken out immediately after the water bath and stirred magnetically at 20 °C for 60 min. After stirring was completed, the pH of solution was changed to 7 by l M HCl, then magnetically stirred again for 60 min. Next, this suspension was loaded into centrifuge tubes and centrifuged for 10 min (7000 g). The supernatant was freeze-dried (FD5-3, China) after centrifugation and then stored at − 20 °C.
Acetic acid treatment (AAT): AAT was based on the way of Liao et al. (Liao et al., 2010a, b, c) with a small modification. The 1% WGP (W/V) was mixed with 0.1 M acetic acid to form a suspension, which was stirred for 120 min in an 20 °C water bath and then heated for 10 min at 121℃. After being heated, the suspension was removed and straightway placed in a cold-water bath for 5 min to stop the reaction. Finally, this suspension was loaded into centrifuge tubes and centrifuged for 10 min (7000 g), and it was freeze-dried (FD5-3, China) after centrifugation and then stored at − 20 °C.
Enzymatic treatment (ET): The ET was based on the way of Lei et al. (Lei et al., 2021) with a small modification. The 1% WGP (W/V) was stirred for 30 min, after adding 20 U/g of TGase (transglutaminase), the solution was placed in an 40 °C water bath and shaken for 24 h at 110 r/min. After that, the suspension was cooled in an ice-water bath to inhibit enzyme activity. The suspension was centrifuged for 10 min (7000 g) after the reaction was stopped, and the supernatant was finally freeze-dried (FD5-3, China) and stored at − 20 °C.
Structure of WGP
SDS-PAGE of Soluble WGP
The SDS-PAGE of soluble protein was determined using the method of Jiang et al. (Jiang et al., 2009). In this study, we measured SDS-PAGE under reducing and non-reducing conditions. It was performed using 5% stacking gels (pH 6.8) and 12% separation gels (pH 8.8), and the electrophoretic voltage was always stabilized at 80 V during the assay.
FT-IR
The sample was accurately weighed at 2 mg, added to potassium bromide (1:100), ground to a homogeneous powder using a mortar and pestle, pressed into thin slices, and then scanned by fourier transform infrared spectrometer (Vetex70, Brooke, Germany) for 32 times at full wavelength (400–4,000 cm–1).
Surface Hydrophobicity (H0)
According to the way of Dong et al. (Dong et al., 2022), the H0 of soluble protein was determined. The protein sample of 100 mg was dispersed in 15 mL phosphate buffer (0.01 M, pH 7.0) and centrifuged for 10 min at 5478 g. The 8.0 mM ANS reagent was prepared using the above phosphate-buffered solution. The supernatant was diluted 1.00, 0.75, 0.50, 0.25, and 0.125 times, and then 40 μL ANS was added to the different concentrations of supernatant to form 4 mL of protein mixture. The mixture was stored in the dark for fluorometric determination. The emission wavelength of fluorescence spectrophotometer (LS55, USA) was set to 484 nm, and the excitation wavelength was set to 365 nm, and then the fluorescence intensity of different mass concentrations of protein sample was measured. The slope of the curve was defined as the H0 of samples being tested.
Free Sulfhydryl Content (SH)
According to previous research (Xiong et al., 2023), the free sulfhydryl content was determined. The samples lyophilized were dissolved in Tris-Gly buffer (containing 4 mM NaEDTA, 0.086 M Tris, and 0.09 M glycine) at pH 8.0 to obtain a 0.5% the sample solution. The solution sample was reacted in a 25 ℃ water bath for 30 min and then centrifuged for 15 min (7155 g). Add 0.05 mL DTNB to 5 mL supernatant, and react for 15 min. After the reaction was completed, the absorbance of the solution was measured at 412 nm (UV2550, Shimadzu, Japan). The formula for calculating the free sulfhydryl content was described as follows:
where C (mg/mL) is the concentration of the sample solution; D is the dilution multiple; A412 is the absorbance value at 412 nm; and 1.36 × 104 is the molar extinction coefficient of the DTNB solution.
SEM
At an accelerating voltage of 3.0 kV, the morphology of the modified WGP was observed. The lyophilized sample powder in 2.2 was attached to a circular energized carrier table using black double-sided tape. And under the electron beam, all samples were plated with gold to avoid charge. Finally, images of modified WGP were taken using a scanning electron microscope (SEM, Nano SEM-450, US) at × 10,000 magnification.
Protein Solubility
Protein solubility was expressed by the amount of soluble protein in the supernatant as a percentage of the total protein added to the deionized water (Xiong et al., 2023). The precipitate from the “pH-shifting, acetic acid, and enzymatic treatment” section after 7000 g centrifugation was collected and washed three times with distilled water, and then the washed precipitate was freeze-dried. The total protein mass of WGP was m0, and the protein in the freeze-dried precipitate was m1. The protein solubility was shown in Eq. (2):
Particle Size
The samples were dissolved in 10 mM PBS (pH 7.0) and prepared as a 7% suspension, followed by vortexing with a vortex to mix well. The particle size of the samples was measured using a wet method. This way was using the same concentration of PBS as the aqueous phase and setting the absorbance and refractive index at 0.001 and 1.414 respectively (Day et al., 2009). The particle size was determined using a nano-laser particle sizer (ZEN3600, MALVERN INSTRUMENTS LIMITED, Britain) at room temperature and averaged to obtain a volume-weighted average diameter after every sample was measured 3 times.
Foaming Properties
The foam stability (FS) and foaming capacity (FA) of WGP were measured by the way of Xiong et al. (Xiong et al., 2023). Firstly, the WGP was dissolved in deionized water to form a 2% suspension; then 15 mL solution was vortexed and homogenized for 2 min and immediately placed to a cylinder with scale. The formula for FA and FS was as follows:
where V0 is the volume of the foam at t = 0 min and V30 is the volume of the foam at t = 30 min.
Emulsification Properties
The protein was dissolved in phosphate buffer (0.2 M, pH 8.2) to make a 24 mL 2 mg/mL protein solution, and 8 mL soybean oil was slowly added while stirring. A sample of 50 μL of prepared solution was aspirated from the bottom of the solution at 0 and 10 min and added to 5 mL SDS (0.1%) solution. Then using SDS solution as blank control, the absorbance at 500 nm (A0 and A10) was measured (UV2550, Shimadzu, Japan). The emulsification activity index (EAI) and the emulsion stability index (ESI) were expressed using following equations:
where A0 is the absorbance measured at 0 min; DF is the dilution multiple (DF = 100); φ is the light range (φ = 1 cm); C (g/mL) is the sample mass concentration; θ is the proportion of oil phase in the emulsion (θ = 0.25); and A10 is the absorbance measured at 10 min (Tang et al., 2003).
Apparent Viscosity
According to the way of Jia et al. (Jia et al., 2021), the apparent viscosity of the WGP samples was determined. The protein dispersion of 15 mg/mL was prepared by 10 mM PBS buffer (pH 7.4). First, the prepared protein dispersions were loaded onto parallel plates, then the gap height of the rotational rheometer (DHR-1, USA) was set to 1 mm, and the apparent viscosity of the dispersions was measured by shear scanning (0.01–100 s−1). Apparent viscosity was repeated three times for each set of samples.
Thermal Characterization
The thermal property was measured according to the previous method (Dong et al., 2022), and the differential scanning calorimetry (DSC) measurement was carried out on a differential scanning calorimeter (TA Q2000, TA company, US). The lyophilized sample powder of 3 mg was placed in an aluminum tray and pressed, and the empty tray was used as a control for thermal scanning. The heating rate was set to 10 ℃/min, the nitrogen flow rate was kept at 50 mg/mL, and the temperature scanning range was 20–200℃. The denaturation temperature (Td) was obtained by analyzing the heat flow curve of the sample with the software Universal Analysis 2000.
Statistical Analysis
The measurements were repeated at least three times, and the final results were expressed as the mean ± standard deviation (SD). Analysis of variance (ANOVA) (p < 0.05) was performed using SPSS 26.0 followed by Duncan’s multiple range test.
Results and Discussion
Structure of WGP
SDS-PAGE of Soluble WGP
SDS-PAGE is a familiar method for evaluating changes in the degree of protein cross-linking and protein molecular weight distribution (Lin et al., 2023). In Fig. 1A, the control group was mainly protein of 14 kDa and 40 kDa. These subunit bands were clearly visible in the pH-shifting group, meaning the treatment retained a relatively complete molecular structure. In contrast, the AAT group showed increased intensity of the bands at ~ 34 kDa (LMW-GS + gliadins, low molecular weight-glutenin subunits and gliadins) and 14 kDa (Liu et al., 2021). For the ET group, only the 14 kDa subunit band was shown, with the rest of the bands having reduced intensity. The reduction in molecular weight of the protein after enzymatic digestion suggested that enzymatic deamidation reduced the solubility of WGP in SDS solution. The control and AAT groups showed an increase in band intensity at 43 kDa in reducing conditions (Fig. 1B), which was predominantly a low molecular weight glutenin subunit, rich in β-turn structures (Tatham et al., 1989). This result was the same as that of previous findings that WGP was sulfur-containing protein and that the denaturation process exposed previously unexposed SH groups and reduced existing S–S bonds (Dong et al., 2022).
FT-IR
The amide I region (1700–1600 cm−1) was the particular band related to the secondary structure of WGP in the FT-IR spectrum. Figure 2A and Table 1 show the profiles and contents of the secondary structures of four types (random coil, β-turn, β-sheet, and α-helix) of WGP. In Fig. 2A, the absorption peak around 3600–3300 cm−1 was caused by O–H deformation and N–H stretching vibrations (Guan et al., 2006). The pH group showed broader absorption peak in the 3600–3300 cm−1 regions, showing higher levels of -NH2 and -OH groups. According to previous studies (Lei & Ma, 2021), all treatment groups showed a more pronounced absorption peak at 2364 cm−1 that was due to NH2 stretching vibrations. The absorption peak of the control group was much sharper, resulting that WGP had a high denaturation temperature (Fig. 2B) due to the presence of many intramolecular or intermolecular hydrogen bonds within its molecules, possibly due to stretching vibrations.
In the FT-IR spectrum, the results of the deconvolution of the amide I bands, followed by second order derivative fitting, were used to calculate the proportion of the various secondary structures of WGP after different treatments for the attribution corresponding to each peak as shown in Table 1. Typically, the α-helix structure represents the stronger conformation of the protein interface, and hydrogen bonding is the main force that maintains the α-helix structure (Jarpa-Parra et al., 2015). In Table 1, the control group had the highest α-helix content, meaning that there was more hydrogen bonding within the control protein. As the α-helix decreases, the ordered structure of the protein changed to disordered structures (Liu et al., 2021). Compared to the control group, the three modified groups showed an increase in random coil and a decrease in α-helix, leading to a more disordered structure of the WGP. In addition, the increase in β-sheet led to an increase in random coil content, which was different from the decrease in β-turn content that led to an increase in random coil content in previous studies (Wang et al., 2019). The reason for this may be that all treatments weakened the ordered structure of the hydrogen bonds of the polypeptide chains within the WGP and the protein tended to be more disordered, which was detrimental to the foaming capacity (FA) of the protein, in agreement with previous findings (Wang et al., 2022a, b). The pH group had the lowest β-turn in Table 1, and its emulsion stability was the best (Fig. 5B). The more disordered the protein tends to be, the more favorable its presence at the oil–water interface and the better its emulsification properties, which confirmed 3.3 result that the modified groups had better emulsification than the control group.
Surface Hydrophobicity (H0)
Protein surface hydrophobicity is an index to evaluate the conformational change of protein, which is closely associated with the emulsification, foaming, and gel ability of protein. The pH group observably increased the surface hydrophobicity of WGP from 495 to 1202 compared to the control in Fig. 3A. The reason for this was attributed to the exposure of more hydrophobic groups at alkaline pH, leading to an increase in surface hydrophobicity (Tian et al., 2015). Compared with the acidic condition of AAT group, the alkaline condition was more conducive to the improvement of surface hydrophobicity, which was supported by previous research (Jiang et al., 2014). This was because the unfolding and refolding processes in the acid treatment may not significantly alter the hydrophobic groups in the WGP (Lee et al., 2016), whereas the alkaline treatment (pH 12) was further away from the isoelectric region of the WGP and thus could unfold the WGP molecules more strongly. As a result, the balance between hydrophilic and hydrophobic groups was altered and more hydrophobic parts or non-polar amino acid residues initially buried inside the protein were exposed to the surface of the protein (Jiang et al., 2014). The lower surface hydrophobicity may be related to the lower amount of conformational changes (Abdollahi & Undeland, 2018). In contrast, for the AAT group, there was a significant conformational change (from granular to reticular structure (Fig. 4)), but the surface hydrophobicity was significantly lower, probably due to the lower percentage of hydrophobic groups in their exposed groups. Surprisingly, the surface hydrophobicity of the AT group was as high as 1,356. This may be since the long enzymatic deamidation treatment exposed too many hydrophobic groups of the WGP and the hydrophobic interactions led to the aggregation of the protein, increasing the particle size and surface hydrophobicity of the protein molecules (Fig. 3D).
Free Sulfhydryl Content (SH)
Figure 3B shows the changes in free sulfhydryl content of WGP after different treatments, which can be used to indicate the formation or breakage of disulfide bonds in protein and indirectly the tertiary structure of protein and be important indicators for evaluating the conformation of protein (Wang et al., 2019). Among all the treatments, the pH-shifting group had the highest SH content, and the control and AAT groups had the lowest SH content, followed by the ET group. It has been reported that pH changed in both extremely acidic and alkaline conditions break disulfide bonds and increased the SH (Li et al., 2020). Moreover, thiol groups tend to be more active under alkaline pH conditions, forming sulfhydryl ionic species (S-), which promotes the oxidation of SH (Jiang et al., 2009). The higher SH content of pH-shifting group showed the exposure of internal SH groups, leading to the cleavages of S–S bonds in native protein or protein unfolding. Thus, surface free sulfhydryl content was associated with conformational changing and protein unfolding, showing disruption of disulfide bonds or expose of SH groups (Jiang et al., 2017). And the SH content of the ET was lower than pH-shifting but higher than that in the control group, which was attributed to prolonged enzymatic deamidation induced protein unfolding and degradation, result in exposing more internal sulfhydryl groups (Zhang et al., 2021). The AAT group had the lowest content of free sulfhydryl groups that was consistent with previous findings that the reduction in the number of disulfide bonds after deamidation of WGP by acetic acid led to a decrease in the content of free sulfhydryl groups (Liao et al., 2010a, b, c), resulting in a significant increase in its foam stability (Fig. 5A).
SEM
Figure 4 shows the surface micrographs of the WGP lyophilized powder samples. Compared to the control samples, the microstructure of the modified samples changed observably. It was observed that the protein particles of control were smooth and intact. And the pH-shifting samples had a homogeneous flocculent structure, which resulted in excellent emulsification properties. Due to acetic acid cross-linking, the WGP used in this study resulted in the development of a WGP network (Zhang et al., 2020a, b), forming a three-dimensional lattice structure that wrapped around water molecules and increased the solubility of AAT samples in water. This network was associated with the WGP and interaction of alcoholic through non-covalent bonds and covalent (SS) and the structure of WGP (Zhang et al., 2021). The ET samples were rough lamellar structures rich in small pores due to deamidation (Su et al., 2021), which was consistent with the fact that the protein particle was large (Fig. 3D).
Protein Solubility
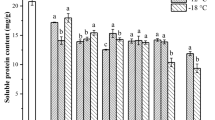
Solubility influences other functional properties of protein, and it is one of the vital functional characters of protein (Zhang et al., 2021). The solubility of WGP was evaluated after different treatments in this work, as shown in Fig. 3C. The acetic acid treatment showed the highest protein solubility of 60.2%, while the control group showed the lowest (15.9%). The solubility of the pH sample was observably higher than that of the ET and control groups, but significantly lower than that of the AAT group. During the pH-shifting, the WGP molecule first unfolded and then folded. As the molecule unfolds, some polar groups were exposed, which increased the elasticity of WGP and enhanced the interaction between the protein and water, finally leading to an increase in solubility (Jiang et al., 2009). As expected, the solubility of the AAT group (60.2%) was nearly four times higher than control (15.9%), as the acetic acid-treated protein exposed more polar groups. A previous study (Jiang et al., 2009) had reported that the increase in ionic strength in the solution led to partial unfolding of the protein, when protein was exposed to extremely basic conditions. Thus, the protein solubility was the highest under acetic acid conditions. For the ET group, the solubility was higher than control but significantly lower than other two treatment groups. The decrease of WGP solubility in this treatment compared to the other treatment groups was mainly due to the significant increase in particle size and surface hydrophobicity of WGP, leading to the formation of larger molecular aggregates in aqueous solution. Protein denaturation led to an increase in solubility, which contrary to the results in previous studies (Foh et al., 2012). This is mainly due to the low solubility of WGP under neutral conditions, which solubility increased with slight changes in the pH of the microenvironment in which WGP was located.
Particle Size
As it can influence protein adsorption at the gas–liquid interface, the particle size of protein has a remarkable influence on protein foaming capacity. The particle size of the three groups of WGP was shown in Fig. 3D. Except for the AAT group, all treatments exhibited soluble WGP aggregates larger than 1000 nm in size. The pH-shifting treatment (1939.7 nm) mildly reduced the average particle size of WGP compared to the control (1982.5 nm). The ET group of WGP had the largest particle size of 5477.7 nm. This treatment was deamidated for 24 h, as previously reported in the literature, which may be related to excessive deamidation causing protein aggregation (Liao et al., 2010a, b, c). And the AAT group had the smallest particle size, which was mainly attributed to acetic acid stabilizing the folded protein structure of the disulfide bond within the WGP molecules (Sun et al., 2019). It also validated that solubility increases at smaller particle sizes. And protein particle size is also related to foaming capacity, which will be explored below.
Foaming Properties
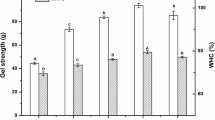
In this work, foam stability (FS) and foaming capacity (FA) were expressed by measuring the volume of the foam. In Fig. 5A, the influence of the different modification ways on FA and FS of WGP was shown. Compared to the control protein sample, the FA and FS of WGP treated by pH-shifting increased from 128 to 138% and from 76 to 80% respectively, which may be related to the increased β-sheet of the protein (Table 1). The β-sheet structure could facilitate the formation and expansion of bubbles, thus improving the FA and FS (Mundi & Aluko, 2012). Also, the WGP partially unfolded at an extreme alkaline environment and then partly folded at a neutral environment, leading to the WGP being in a molten state, followed by a decrease in particle size and an increase in foaming properties and surface hydrophobicity (Jiang et al., 2018). Since proteins readily adsorb at the water–air interface, and the increase in hydrophobic groups promoted the formation of this interface, more stable foams can be formed. For the AAT and ET groups, their FA was significantly lower, but the FS of the protein was significantly higher. Wheat gluten protein treated by acetic acid and enzyme showed an increase in FS, which may be due to their reticulate and lamellar structure (Fig. 4), whose surface structure allowed them to exist longer at the water–air interface. Moreover, physical and chemical properties such as molecular size, free sulfhydryl content, and electrostatic interactions can also influence the interfacial behavior of protein (Han et al., 2019).
Emulsification Properties
The emulsifying activity index (EAI) and emulsifying stability index (ESI) of protein were used to evaluate the absorption capacity of protein at the oil–water interface, that is, the emulsification property of protein. ESI is a measure of emulsion stability over a given time span, and EAI is a measure of how much oil can be emulsified per unit of protein (Boye et al., 2010). Figure 5B shows the EAI and ESI of WGP with different treatments. The pH-shifting and AAT samples had higher EAI and ESI, while the ET group and the control group showed lower EAI and ESI. This could be explained that the AAT and pH-shifting had higher solubility. The EAI of sample after pH-shifting treatment increased from 1.04 to 1.44 m2/g compared with the control, which may be attributed to the disulfide bond breakage and following molecular rearrangement. Another reason for this phenomenon was that the exposure of hydrophobic groups increases the hydrophobicity of WGP during the treatment of pH-shifting, thus enhancing the ability to combine with oil (Liu et al., 2021). The EAI of protein was correlated with their solubility and hydrophobicity, and the lower surface hydrophobicity previously reported may lead to lower EAI (Abdollahi & Undeland, 2018), while the EAI of the low hydrophobicity AAT group (1.51 m2/g) was slightly higher than that of the pH-shifting group (1.44 m2/g), which may be due to acid-induced protein hydrolysis resulting in a dominant role of solubility in EAI. And the reduced size of the protein allowed easier adsorption to the oil–water interface and enhanced emulsification activity. On the contrary, the pH-shifting treatment gave the best emulsion stability with the highest ESI values, which may be related to the homogeneous flocculent structure of its surface (Fig. 4), whose special structure made it more stable at the oil–water interface.
Apparent Viscosity
As a water-insoluble protein, it is difficult to find relative studies on the rheological properties of WGP dispersions. In this work, the viscoelastic interval was determined, and it was found that there was no specific viscoelastic interval for the dispersion. Therefore, only apparent viscosity of samples was explored. Figure 6 shows the flow behavior of the WGP sample dispersion. As shown, the apparent viscosity of all samples decreased with increasing shear rate, exhibiting pseudoplastic behavior (shear thinning) (Jiang et al., 2020). Although the above results showed that the treatment changed the secondary and tertiary structure of the WGP, they did not change the flow properties and remained pseudoplastic. Shear thinning was usually caused by rupture of protein aggregates, alignment of aggregates in the flow direction or disruption of covalent and non-covalent interaction forced, leading to modification of the WGP network structure (Song et al., 2013). This result can be clearly observed in the SEM (Fig. 5). In this case, apparent viscosity of the AAT samples did not decrease steadily with shear rate, but fluctuates somewhat. This was probably due to the fact that there were more the droplets and aggregates were not homogeneous and stable, leading to an increase in viscosity in AAT dispersions (Wang et al., 2022a, b).
Thermal Characterization
The thermal characteristic of the lyophilised sample was analyzed by DSC. And denaturation temperature (Td), the temperature at which the molecular chain of the polymer reaches the point at which polymer chain degradation becomes apparent, is shown in Fig. 2B. Surprisingly, the pH-shifting sample with heat treatment had the lowest Td (110 °C). The ET group had the highest Td at 148 °C, followed by the control and AAT groups. From the SEM results, the surface of pH-shifting protein was the most homogeneous structure, but its small particle flocculent structure made its molecular chains more susceptible to degradation during the warming process. On the contrary, the lamellar structure of the ET sample gave it a high Td and made it less susceptible to degradation during the warming process. Moreover, the excessive exposure of hydrophobic groups during the pH-shifting also suggested that the hydrophobic groups in WGP were not heat resistant and the structure may be altered when the temperature was increased to 110 °C.
Conclusion
In conclusion, we investigated the effects of three representatively chemical modifications on the molecular structure, particle morphology, and functional characters of WGP. The results showed that the pH-shifting treatment changed the apparent morphology of the protein, showing a homogeneous flocculent structure, leading to significant improvements in FA, EAI and ESI. After acetic acid deamidation treatment, the particle size of WGP was reduced and solubility was greatly increased, and the reduction of disulfide bonds led to a decrease in its free sulfhydryl content, which significantly improved the foam stability compared with the other two methods. Furthermore, the WGP treated by TGase has the highest thermal stability.
Supplementary Information
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abdollahi, M., & Undeland, I. (2018). Structural, functional, and sensorial properties of protein isolate produced from salmon, cod, and herring by-products. Food and Bioprocess Technology, 11(9), 1733–1749.
Boye, J. I., Aksay, S., Roufik, S., Ribéreau, S., Mondor, M., Farnworth, E., & Rajamohamed, S. H. (2010). Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Research International, 43(2), 537–546.
Day, L., Xu, M., Lundin, L., & Wooster, T. J. (2009). Interfacial properties of deamidated wheat protein in relation to its ability to stabilise oil-in-water emulsions. Food Hydrocolloids, 23(8), 2158–2167.
Dong, M., Tian, L., Li, J., Jia, J., Dong, Y., Tu, Y., Liu, X., Tan, C., & Duan, X. (2022). Improving physicochemical properties of edible wheat gluten protein films with proteins, polysaccharides and organic acid. LWT, 154, 112868.
Foh, M. B. K., Wenshui, X., Amadou, I., & Jiang, Q. (2012). Influence of pH shift on functional properties of protein isolated of tilapia (Oreochromis niloticus) muscles and of soy protein isolate. Food and Bioprocess Technology, 5(6), 2192–2200.
Guan, J.-J., Qiu, A.-Y., Liu, X.-Y., Hua, Y.-F., & Ma, Y.-H. (2006). Microwave improvement of soy protein isolate–saccharide graft reactions. Food Chemistry, 97(4), 577–585.
Han, A., Romero, H. M., Nishijima, N., Ichimura, T., Handa, A., Xu, C., & Zhang, Y. (2019). Effect of egg white solids on the rheological properties and bread making performance of gluten-free batter. Food Hydrocolloids, 87, 287–296.
Han, L., Cheng, Y., Qiu, S., Tatsumi, E., Shen, Q., Lu, Z., & Li, L. (2013). The effects of vital wheat gluten and transglutaminase on the thermomechanical and dynamic rheological properties of buckwheat dough. Food and Bioprocess Technology, 6(2), 561–569.
Jarpa-Parra, M., Bamdad, F., Tian, Z., Zeng, H., Temelli, F., & Chen, L. (2015). Impact of pH on molecular structure and surface properties of lentil legumin-like protein and its application as foam stabilizer. Colloids and Surfaces B: Biointerfaces, 132, 45–53.
Jia, J., Ji, B., Tian, L., Li, M., Lu, M., Ding, L., Liu, X., & Duan, X. (2021). Mechanism study on enhanced foaming properties of individual albumen proteins by Lactobacillus fermentation. Food Hydrocolloids, 111, 106218.
Jiang, J., Chen, J., & Xiong, Y. L. (2009). Structural and emulsifying properties of soy protein isolate subjected to acid and alkaline pH-shifting processes. Journal of Agricultural and Food Chemistry, 57(16), 7576–7583.
Jiang, J., Wang, Q., & Xiong, Y. L. (2018). A pH shift approach to the improvement of interfacial properties of plant seed proteins. Current Opinion in Food Science, 19, 50–56.
Jiang, J., Zhu, B., Liu, Y., & Xiong, Y. L. (2014). Interfacial structural role of pH-shifting processed pea protein in the oxidative stability of oil/water emulsions. Journal of Agricultural and Food Chemistry, 62(7), 1683–1691.
Jiang, S., Ding, J., Andrade, J., Rababah, T. M., Almajwal, A., Abulmeaty, M. M., & Feng, H. (2017). Modifying the physicochemical properties of pea protein by pH-shifting and ultrasound combined treatments. Ultrasonics Sonochemistry, 38, 835–842.
Jiang, Y., Jia, J., Xiong, D., Xu, X., Yang, Y., Liu, X., & Duan, X. (2020). Effects of short-term fermentation with lactic acid bacteria on egg white: Characterization, rheological and foaming activities. Food Hydrocolloids, 101, 105507.
Kahraman, O., Petersen, G. E., & Fields, C. (2022). Physicochemical and functional modifications of Hemp protein concentrate by the application of ultrasonication and pH shifting treatments. In Foods, vol. 11.
Kuraishi, C., Yamazaki, K., & Susa, Y. (2001). Transglutaminase: Its utilization in the food industry. Food Reviews International, 17(2), 221–246.
Lee, H., Yildiz, G., dos Santos, L. C., Jiang, S., Andrade, J. E., Engeseth, N. J., & Feng, H. (2016). Soy protein nano-aggregates with improved functional properties prepared by sequential pH treatment and ultrasonication. Food Hydrocolloids, 55, 200–209.
Lei, D., & Ma, X. (2021). Effect of enzymatic glycosylation on the structure and properties of wheat gluten protein fibers. Journal of Engineered Fibers and Fabrics, 16, 15589250211000336.
Li, Y., Cheng, Y., Zhang, Z., Wang, Y., Mintah, B. K., Dabbour, M., Jiang, H., He, R., & Ma, H. (2020). Modification of rapeseed protein by ultrasound-assisted pH shift treatment: Ultrasonic mode and frequency screening, changes in protein solubility and structural characteristics. Ultrasonics Sonochemistry, 69, 105240.
Liao, L., Liu, T.-X., Zhao, M.-M., Cui, C., Yuan, B.-E., Tang, S., & Yang, F. (2010a). Functional, nutritional and conformational changes from deamidation of wheat gluten with succinic acid and citric acid. Food Chemistry, 123(1), 123–130.
Liao, L., Qiu, C.-Y., Liu, T.-X., Zhao, M.-M., Ren, J.-Y., & Zhao, H.-F. (2010b). Susceptibility of wheat gluten to enzymatic hydrolysis following deamidation with acetic acid and sensory characteristics of the resultant hydrolysates. Journal of Cereal Science, 52(3), 395–403.
Liao, L., Zhao, M., Ren, J., Zhao, H., Cui, C., & Hu, X. (2010c). Effect of acetic acid deamidation-induced modification on functional and nutritional properties and conformation of wheat gluten. Journal of the Science of Food and Agriculture, 90(3), 409–417.
Lin, Q., Shen, H., Ma, S., Zhang, Q., Yu, X., & Jiang, H. (2023). Morphological distribution and structure transition of gluten induced by various drying technologies and its effects on Chinese dried noodle quality characteristics. Food and Bioprocess Technology.
Liu, Z., Zheng, Z., Zhu, G., Luo, S., Zhang, D., Liu, F., & Shen, Y. (2021). Modification of the structural and functional properties of wheat gluten protein using a planetary ball mill. Food Chemistry, 363, 130251.
Meerts, M., Van Ammel, H., Meeus, Y., Van Engeland, S., Cardinaels, R., Oosterlinck, F., Courtin, C. M., & Moldenaers, P. (2017). Enhancing the rheological performance of wheat flour dough with glucose oxidase, transglutaminase or supplementary gluten. Food and Bioprocess Technology, 10(12), 2188–2198.
Mundi, S., & Aluko, R. E. (2012). Physicochemical and functional properties of kidney bean albumin and globulin protein fractions. Food Research International, 48(1), 299–306.
Qin, X.-S., Chen, S.-S., Li, X.-J., Luo, S.-Z., Zhong, X.-Y., Jiang, S.-T., Zhao, Y.-Y., & Zheng, Z. (2017). Gelation properties of transglutaminase-induced soy protein isolate and wheat gluten mixture with ultrahigh pressure pretreatment. Food and Bioprocess Technology, 10(5), 866–874.
Qiu, C., Sun, W., Zhao, Q., Cui, C., & Zhao, M. (2013). Emulsifying and surface properties of citric acid deamidated wheat gliadin. Journal of Cereal Science, 58(1), 68–75.
Robertson, G. H., Cao, T. K., Gregorski, K. S., Hurkman, W. J., Tanaka, C. K., Chiou, B.-S., Glenn, G. M., & Orts, W. J. (2014). Modification of vital wheat gluten with phosphoric acid to produce high free swelling capacity. Journal of Applied Polymer Science, 131(2).
Song, X., Zhou, C., Fu, F., Chen, Z., & Wu, Q. (2013). Effect of high-pressure homogenization on particle size and film properties of soy protein isolate. Industrial Crops and Products, 43, 538–544.
Su, G., Zheng, X., Zou, J., Waterhouse, G. I. N., & Sun-Waterhouse, D. (2021). Insight into the advantages of premixing yeast-wheat gluten and combining ultrasound and transglutaminase pretreatments in producing umami enzymatic protein hydrolysates. Food Chemistry, 342, 128317.
Sun, L.-C., Lin, Y.-C., Liu, W.-F., Qiu, X.-J., Cao, K.-Y., Liu, G.-M., & Cao, M.-J. (2019). Effect of pH shifting on conformation and gelation properties of myosin from skeletal muscle of blue round scads (Decapterus maruadsi). Food Hydrocolloids, 93, 137–145.
Sun-Waterhouse, D., Zhao, M., & Waterhouse, G. I. N. (2014). Protein modification during ingredient preparation and food processing: Approaches to improve food processability and nutrition. Food and Bioprocess Technology, 7(7), 1853–1893.
Tang, S., Hettiarachchy, N. S., Horax, R., & Eswaranandam, S. (2003). Physicochemical properties and functionality of rice bran protein hydrolyzate prepared from heat-stabilized defatted rice bran with the aid of enzymes. Journal of Food Science, 68(1), 152–157.
Tatham, A. S., Drake, A. F., & Shewry, P. R. (1989). Conformational studies of a synthetic peptide corresponding to the repeat motif of C hordein. Biochemical Journal, 259(2), 471–476.
Tian, J., Wang, Y., Zhu, Z., Zeng, Q., & Xin, M. (2015). Recovery of tilapia (Oreochromis niloticus) protein isolate by high-intensity ultrasound-aided alkaline isoelectric solubilization/precipitation process. Food and Bioprocess Technology, 8(4), 758–769.
Wang, B., Liu, F., Luo, S., Li, P., Mu, D., Zhao, Y., Zhong, X., Jiang, S., & Zheng, Z. (2019). Effects of high hydrostatic pressure on the properties of heat-induced wheat gluten gels. Food and Bioprocess Technology, 12(2), 220–227.
Wang, K., & Arntfield, S. D. (2016). Modification of interactions between selected volatile flavour compounds and salt-extracted pea protein isolates using chemical and enzymatic approaches. Food Hydrocolloids, 61, 567–577.
Wang, Y., Wang, S., Li, R., Wang, Y., Xiang, Q., Li, K., & Bai, Y. (2022a). Effects of combined treatment with ultrasound and pH shifting on foaming properties of chickpea protein isolate. Food Hydrocolloids, 124, 107351.
Wang, Y., Zhao, J., Zhang, S., Zhao, X., Liu, Y., Jiang, J., & Xiong, Y. L. (2022b). Structural and rheological properties of mung bean protein emulsion as a liquid egg substitute: The effect of pH shifting and calcium. Food Hydrocolloids, 126, 107485.
Wee, M. S., & Jeyakumar Henry, C. (2019). Effects of transglutaminase on the protein network and in vitro starch digestibility of Asian wheat noodles. Foods, 8(12), 607.
Xiong, D., Xu, Q., Tian, L., Bai, J., Yang, L., Jia, J., Liu, X., Yang, X., & Duan, X. (2023). Mechanism of improving solubility and emulsifying properties of wheat gluten protein by pH cycling treatment and its application in powder oils. Food Hydrocolloids, 135, 108132.
Yong, Y. H., Yamaguchi, S., & Matsumura, Y. (2006). Effects of enzymatic deamidation by protein-glutaminase on structure and functional properties of wheat gluten. Journal of Agricultural and Food Chemistry, 54(16), 6034–6040.
Zhang, C., Yang, Y.-H., Zhao, X.-D., Zhang, L., Li, Q., Wu, C., Ding, X., & Qian, J.-Y. (2021). Assessment of impact of pulsed electric field on functional, rheological and structural properties of vital wheat gluten. LWT, 147, 111536.
Zhang, H., Chen, G., Liu, M., Mei, X., Yu, Q., & Kan, J. (2020a). Effects of multi-frequency ultrasound on physicochemical properties, structural characteristics of gluten protein and the quality of noodle. Ultrasonics Sonochemistry, 67, 105135.
Zhang, Z., Li, Y., Lee, M. C., Ravanfar, R., Padilla-Zakour, O. I., & Abbaspourrad, A. (2020b). The impact of high-pressure processing on the structure and sensory properties of egg white-whey protein mixture at acidic conditions. Food and Bioprocess Technology, 13(2), 379–389.
Acknowledgements
The authors would like to thank the instrument shared platform of the College of Food Science & Engineering of NWAFU, for the assistance in the nanometer particle size and zeta potentiometer, dynamic shear rheometer, Fourier transform infrared spectrometer, and differential scanning calorimeter.
Funding
This work was supported by the Key Research & Development Program of Shaanxi Province (No. 2022NY-010) and National Natural Science Foundation of China (No. 32172205).
Author information
Authors and Affiliations
Contributions
Mengxue Dong, Yusha Sun, and Dandan Xiong wrote the main manuscript text, and Qi Song and Jie Jia prepared Figs. 1–6. Xuebo Liu, Long Sheng, and Xiang Duan mainly revised the manuscript files and Figs. 1–6. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, M., Sun, Y., Xiong, D. et al. Comparison of the Effects of pH-Shifting, Acetic Acid Modification, and TGase Treatment on the Physicochemical and Functional Properties of Wheat Gluten Protein. Food Bioprocess Technol 17, 245–256 (2024). https://doi.org/10.1007/s11947-023-03130-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03130-0