Abstract
The enzymes glucose oxidase and transglutaminase are frequently used to improve the breadmaking performance of wheat flours, as they have the ability to considerably alter the viscoelastic nature of the gluten network. To evaluate a flour’s breadmaking performance, rheological tests offer an attractive framework. In this study, the rheological impact of adding glucose oxidase or transglutaminase to wheat flour dough is investigated by means of linear oscillatory shear tests, creep-recovery shear tests and startup extensional tests. The former tests reveal that the enzymes render the dough stiffer and enhance its elastic character, until saturation is reached. In the breadmaking process, the use of excessive amounts of enzyme is known to be counterproductive. The strain-hardening index clearly reveals this overcross-linking effect. Besides enzymes, the gluten network can also be reinforced by adding supplementary gluten, which was indeed found to enhance the extent of strain-hardening.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The breadmaking performance of wheat flours is mostly determined by the gluten network, and may differ significantly from one wheat flour type to another. In addition to gluten quantity, gluten quality was also found to be an important factor. Lack of high molecular weight glutenin proteins will result in a loss of dough cohesiveness and elasticity, whereas a surplus of these long protein chains will likely thwart a proper expansion of the gas cells. In both cases, only very small bread volumes with poor crumb characteristics will be obtained (Veraverbeke and Delcour 2002). The gluten quality can be adjusted by the use of enzymes, resulting in an improved loaf volume, crumb structure, shelf-life and/or flavour. The enzymes glucose oxidase (GO) and transglutaminase (TG) are known to have applications in baked goods (Gerrard et al. 2001 and Caballero et al. 2005, amongst others). The GO and TG enzymes both have the ability to create covalent cross-links between the gluten polypeptide chains, but the two enzymes differ with regard to the reaction mechanism and the nature of the established cross-links.
The GO enzyme is able to alter the structure and functional properties of the gluten network by creating additional covalent disulphide cross-links through the intermediary action of H2O2 (Poulsen and Høstrup 1998; Decamps et al. 2013). In the presence of O2, GO catalyses the oxidation of β-D-glucose into gluconic acid and H2O2 (Bankar et al. 2009). The produced H2O2 will oxidise the available thiol groups on the gluten proteins to create disulphide cross-links. Disulphide cross-links are naturally present in dough, and form an important means to set up the gluten network, besides hydrogen bonds, hydrophobic interactions, etc. (Kontogiorgos 2011). The disulphide cross-links are generally considered to be non-permanent, as the cross-links can be rearranged through the SH-SS interchange mechanism (Bloksma 1975). The glutenin fraction appears to be affected to a greater extent by the action of GO than the gliadin fraction (Rasiah et al. 2005; Bonet et al. 2006), possibly because of the latter’s compact structure (Bonet et al. 2006).
GO is an oxygen-consuming enzyme. O2 is naturally present in wheat flour, and an additional amount of O2 is incorporated in the dough during mixing. However, several other enzymes, such as lipoxygenase and polyphenol oxidase, compete with GO for the limited amount of O2 present in bread dough (Decamps et al. 2012a). Consequently, O2 is probably the limiting substrate for GO. For that reason, the activity of GO is restricted to the mixing stage (Dunnewind et al. 2002; Rasiah et al. 2005). Additional complications arise as not all of the generated H2O2 is used for the formation of disulphide cross-links. For instance, Tilley et al. (2001) mention that H2O2 is also able to cross-link tyrosine residues in gluten. Even so, Hanft and Koehler (2005) and Decamps et al. (2013) found the formation of dityrosine cross-links to be very limited in GO-supplemented dough and model systems, respectively. Additionally, in vitro experiments have indicated that H2O2 promotes the cross-linking of arabinoxylans by means of ferulic acid bridges (Decamps et al. 2013). At present, the question remains to what extent cross-linking of arabinoxylans effectively occurs in dough. There is furthermore no consensus yet on the exact nature of the formed network (see for instance Labat et al. 2001 vs. Piber and Koehler 2005).
The main action mechanism of the TG enzyme is the catalysis of the acyl-transfer reaction between the ε-amino groups of peptide-bound lysine residues and the γ-carboxyamide group of peptide-bound glutamine residues, resulting in permanent iso-peptide bonds between the gluten chains (Nonaka et al. 1989). Besides the ε-amino group of lysine residues, other primary amines can equally serve as substrate for TG; when insufficient primary amines are present, also hydrolysis of glutamine to glutamic acid may take place. However, according to Larré et al. (2000) and Ohtsuka et al. (2001), these side reactions are probably only of minor importance.
Protein extraction experiments have indicated that TG has the ability to substantially alter the molecular weight distribution of the gluten proteins present in wheat flour. With increasing TG concentration, a dramatic shift in the amount of gliadins and glutenins towards the highest molecular weights occurs, resulting in the creation of large, insoluble protein aggregates (Larré et al. 2000; Gerrard et al. 2001; Bauer et al. 2003a; Rosell et al. 2003; Autio et al. 2005; Bagagli et al. 2014). As glutamine is abundantly present in all gluten fractions, the reactivity of each gluten fraction is most likely determined by its lysine content. Lysine residues can mainly be found in the high molecular weight glutenins (HMW-GS), which are indeed affected the most by TG (Larré et al. 2000; Bauer et al. 2003a; Autio et al. 2005). The bread-improving effect of TG is typically attributed to the increase in the amount of high molecular weight gluten proteins. Contrary to GO, TG does not require O2 to create covalent cross-links, and consequently the enzymatic reaction can proceed for very long times (up to 18 h, according to Larré et al. 2000). The degree of cross-linking induced by TG is therefore dependent on both the concentration level and the reaction time, which makes TG a very flexible bread-improving agent (Basman et al. 2002).
The rheological properties of dough are known to relate to the final product quality (Dobraszczyk and Morgenstern 2003). In a previous publication (Meerts et al. 2017), we noted that only non-linear rheological tests allow to obtain a good indication of flour quality, because in the linear region, the starch granules may mask the differences between doughs containing different gluten systems. The objective of this study is to assess the potential of the GO and TG enzymes as quality-improving agents, by means of non-linear rheological tests (i.e. uniaxial extension and non-linear creep-recovery tests in shear). The extensional tests are performed by means of an extensional viscosity fixture (EVF), which enables us to apply a pure extensional flow field, in contrast to the more commonly used Kieffer extensibility rig. In addition, the impact of adding the GO and TG enzymes to a weak wheat flour (thereby aiming at improving its gluten quality) is compared to the beneficial effect of increasing the gluten quantity of the flour, in order to establish which parameter is the most important with regard to the flour’s baking performance.
Materials and Methods
Materials
In this study, two wheat flour types (Bilux and Bison) were used. Both flours were obtained from Dossche Mills (Deinze, Belgium). The moisture content of the flours was determined to be 13.4 ± 0.07 and 12.7 ± 0.05 wt% for Bilux and Bison, respectively, according to AACC method 44-19.01 (AACC International 2000). The protein content (N × 5.7) of the flours was measured with an automated Dumas protein analysis system (EAS, VarioMax N/CN, Elt, Gouda, The Netherlands) following an adaptation of the AOAC method 990.03 (AOAC International 1995). Compared to the Bison flour, the Bilux flour had a significantly higher protein content (15.1 ± 0.2 vs. 12.4 ± 1.0 wt% on a dry matter basis) and a far superior Farinograph mixing stability (10.8 ± 0.8 vs. 1.0 ± 0.2 min). Hence, the Bilux flour could be classified as a strong flour, and the Bison flour as a weak flour. It is reasonable to assume that approx. 80% (for a weak flour) to 85% (for a strong flour) of the proteins present in wheat flour are gluten proteins. Consequently, the gluten content of the strong Bilux flour amounts to ca. 13 wt% (dm), whereas the weak Bison flour contains ca. 10 wt% (dm) gluten. To assess the impact of the gluten quantity on the flour performance, additional gluten were added to the weak Bison flour to obtain gluten contents of ca. 11.5 wt% and 13 wt% (dm), respectively. The commercial gluten proteins used to upgrade the Bison flour were purchased from Tereos Syral (Aalst, Belgium), had a protein content of 77.8 ± 0.13 wt% (dm), and a water content of 6.5 ± 0.09 wt%. GO produced by Aspergillus niger was obtained from Sigma-Aldrich (Bornem, Belgium). The enzymatic activity of GO was determined with the 2-2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assay as described in Decamps et al. (2012a), and turned out to be 5900 U/g. An equivalent of 1 U GO enzyme is able to catalyse the oxidation of 1 μmol of glucose per min. The microbial ACTIVA\(^{{\circledR }}\)WM TG was kindly provided by Ajinomoto Foods Europe S.A.S. (Mesnil-Saint-Nicaise, France). The enzymatic activity unit for TG is defined as the amount of enzyme resulting in the formation of 1 μmol of hydroxamic acid per min. The enzymatic activity was determined by the manufacturer with the hydroxamate procedure originally developed by Folk and Cole (1966), and was reported to be 100 U/g.
Dough Preparation
The optimal baking absorption and mixing time were determined with a Farinograph (Brabender, Duisburg, Germany) and a Mixograph (National Manufacturing, Lincoln, NE, USA) in accordance with AACC methods 54-40.02 and 54-21.02, respectively (AACC International 2000). The dough samples consisted of 10 g flour (on 14% moisture base), 1.5 wt% sodium chloride, 6 wt% sucrose, 1 ml sodium phosphate buffer (pH 6.5) and 4.80 ml/4.40 ml/4.40 ml/4.45 ml water for standard Bilux, standard Bison, Bison (+ 1.5 wt% gluten) and Bison (+ 3 wt% gluten) flour, respectively (AACC method 10-10.03). Suitable amounts of GO or TG were added to the dough by dissolving them in water. All ingredients were mixed in a 10 g pin bowl mixer (National Manufacturing). The standard Bilux and Bison flours were mixed for 3 min 30 s to reach their optimal consistency, whereas the Bison (+ 1.5 wt% gluten) and Bison (+ 3 wt% gluten) flours had to be mixed for 4 min. After mixing, the dough samples were shaped with a pasta machine to obtain a cylindrical shape with a height of ca. 4 mm and a diameter larger than 40 mm. The dough samples were allowed to rest for 30 min prior to loading in the rheometer.
The pH of the dough was determined with a pH probe (HI 9126, Hanna Instruments, Temse, Belgium), which was placed directly in the sample. The addition of the sodium phosphate buffer resulted in a small but significant increase in the dough pH (from 5.76 ± 0.02 to 5.87 ± 0.01), which coincides with the optimal range of pH values (5.5–6.0) for both enzymes (Decamps et al. 2012a; Kieliszek and Misiewicz 2014).
Rheological Methods
Oscillatory Tests
Small amplitude oscillatory shear (SAOS) tests were performed at 25 °C on a stress-controlled MCR501 rheometer (Anton Paar, Graz, Austria) with a 40-mm parallel plate geometry in direct strain mode (Läuger et al. 2002). To prevent dehydration of the dough samples, a solvent trap combined with wet cotton wool was used. Slip effects were eliminated by coating the top and bottom plates with sandpaper. After loading in the rheometer, the dough sample was allowed to rest for an additional 30 min to allow the remaining stresses to relax. Subsequently, frequency sweeps were obtained. All dynamic measurements were performed at least in triplicate on separately prepared batches of dough (with the average values being shown). Good reproducibility was obtained with relative standard deviations less than 10%. To determine whether the observed differences between dough samples prepared with different enzyme concentrations were statistically significant, we made use of the one-tailed unpaired Welch’s t test (p < 0.05).
Creep-Recovery Tests
Non-linear creep-recovery experiments in shear deformation mode were performed with the same setup as that used for the dynamic measurements. The creep-recovery data were also obtained after a resting period of 30 min following the loading of the sample in the rheometer. The shear stress was applied for 30 min, after which the dough sample was allowed to recover for 60 min. The applied stress (σ = 500 Pa) is known to result in non-linear creep behaviour (Meerts et al. 2017). The compliance J(t) [Pa−1] was determined as the ratio of the observed strain γ(t) to the applied shear stress σ. Creep-recovery measurements were performed at least in duplicate and the relative standard deviation was less than 17% for all cases.
Extensional Tests
Dough behaviour in extension was studied at ambient temperature by means of the extensional viscosity fixture (EVF) mounted on a strain-controlled ARES-G2 rheometer (TA Instruments, New Castle, DE, USA). The EVF setup consists of two drums to which the dough strand can be attached. Extension at a constant rate is obtained as one drum remains stationary and the other moves in a circular orbit around it whilst also rotating around its own axis. As a measure for deformation, we use the Hencky strain ε(t) [-]:
L 0 stands for the initial length of the dough strand, whereas L(t) is the actual length during extension. The transient extensional viscosity ηe+(ε) [Pa s] is defined as:
In this expression, σ 11(ε) corresponds to the longitudinal stress [Pa] registered by the transducer of the rheometer. The extension rate \(\dot {\varepsilon }\) was kept constant at 0.1 s−1. The maximum achievable strain with the EVF setup was limited to 2.9; hence, it was not possible to determine the extensibility of the dough, as the samples could not be stretched until their point of failure. In extension, both Bilux and Bison dough show substantial strain-hardening, the extent of which can be quantified by means of the strain-hardening index (SHI) (see also Meerts et al. 2017):
In this expression, ηe+(ε max ) is the actual value of the transient extensional viscosity at the maximum strain (2.9), and ηe0+(ε max ) is the value of the linear extensional viscosity extrapolated to the maximum strain. The extensional viscosity curves are the average of 10–25 measurements on 2–4 separately prepared batches. As these measurements are not all independent, the pooled standard deviation s p has been used to determine the data variability (McNaught and Wilkinson 1997):
In this expression, s 1 and s 2 represent the standard deviations for the measurements of batch 1 and 2, respectively. The number of measurements in each batch are denoted by n 1 and n 2, whereas the total number of batches is given by N. In the expression above N = 2, but the expression can easily be adjusted in case N > 2. The relative standard deviation varied between 7 and 22%. For additional information on the rheological setups and methodologies the reader is referred to Meerts et al. (2017).
Results and Discussion
Impact of GO and TG on Dough Behaviour
Oscillatory Tests
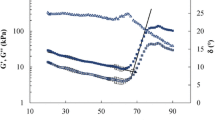
Even though the breadmaking potential of dough is known to be related mostly to its non-linear rheological properties, it is still worthwhile to perform small-amplitude oscillatory shear (SAOS) as well, as these tests may yield additional information on the kinetics and the concentration-dependent stiffening effect of both GO and TG. Figure 1a gives the storage modulus G ′ as a function of angular frequency ω for the untreated Bilux dough and for Bilux dough supplemented with GO or TG. The oscillatory data were obtained exactly 1 h after mixing of the dough. Upon addition of either GO or TG, the storage modulus G ′ increases substantially. The loss modulus G ″ increases as well, albeit to a lesser extent, as evidenced by the slight decrease in the phase angle δ. The addition of GO results in a quasi-parallel upward shift in the G ′ and G ″ curves, whereas TG appears to also affect the frequency dependency of the dynamic moduli (as shown for G ′ in Fig. 1a). The effect of GO and TG on the dynamic moduli has already been documented in literature, both for dough (see Vemulapalli et al. 1998 and Dunnewind et al. 2002 for GO, and Caballero et al. 2005 for TG) and for pure gluten (Hilhorst et al. 1999 for GO, and Larré et al. 2000 for TG). A decrease in the frequency dependency of the dynamic moduli was also observed for pure gluten supplemented with TG (Larré et al. 2000). The change in the frequency dependency of the dynamic moduli is not a time effect (for a discussion of the reaction time of TG, see below), but most likely stems from the particularly high sensitivity of the HMW-GS fraction towards TG. Indeed, since the HMW-GS fraction is mainly associated with the slowest relaxation mechanisms, the high sensitivity of the HMW-GS fraction towards TG is likely to result in an increase in the dynamic moduli predominantly at the lowest frequencies. Rasiah et al. (2005) and Bonet et al. (2006) reported that GO also prefers to interact with the glutenin rather than the gliadin fraction, yet this preference is apparently not sufficiently pronounced to be detected in linear oscillatory shear tests.
a Storage modulus G ′ and phase angle δ for Bilux dough plotted versus angular frequency ω in the linear region (strain amplitude γ 0 = 0.06%). The three samples correspond to standard dough, dough supplemented with 1.8 U GO/g flour, and dough supplemented with 5 U TG/g flour. b Dynamic moduli G ′ and G ″ at ω = 1 rad/s for the standard Bilux dough and Bilux dough supplemented with different concentrations of GO (0.03, 0.6, 1.8 and 3.6 U/g flour) and TG (0.5, 2, 5 and 10 U/g flour). The dynamic moduli were determined exactly 1 h after mixing. Error bars indicate the standard deviation; G ′ values indicated with different letters are significantly different (p < 0.05)
Another difference between GO and TG is the reaction time. Whereas the action of GO is mostly restricted to the mixing stage because of O2 limitations, the TG reaction may continue for several hours. As did Caballero et al. (2007a), we found the dynamic moduli of TG-supplemented dough to increase substantially over the course of three hours, without reaching steady-state. According to Larré et al. (2000) the TG reaction might require up to 18 h to complete.
In Fig. 1b, the dynamic moduli are given for Bilux dough supplemented with different concentrations of GO and TG. The optimal enzyme levels as recommended by the suppliers are 0.3 U/g flour and 0.5 U/g flour for GO and TG, respectively (Caballero et al. 2007a). To allow for a proper comparison of TG with GO, the data were collected after a fixed resting period (i.e., 1 h after mixing). Figure 1b shows that G ′ and G ″ increase significantly with increasing enzyme concentration, but at sufficiently high concentrations saturation occurs for both enzymes. For GO, the saturation effect most likely results from the limited availability of O2. Indeed, addition of extra glucose did not have any effect on the dynamic moduli (results not shown), the extensional behaviour (Dunnewind et al. 2002) nor on the extent of gluten cross-linking (Rasiah et al. 2005). For TG, it is more difficult to assign a specific cause to the observed saturation. Larré et al. (2000) suggested three possible explanations: (a) all available lysine residues in the gluten network have reacted; (b) the gluten network has become so dense that the diffusion of the enzyme is severely hampered, and consequently, the enzyme cannot locate any free lysine residues in its vicinity; (c) the gluten network has become so dense that the creation of additional cross-links does not have any further effect on its viscoelastic response. However, a more in-depth study of the mechanisms underlying the saturation effect of TG is not within the scope of the present work.
Extensional Tests
The linear oscillatory measurements discussed above point out that both GO and TG have a strong impact on the dough behaviour. However, previous work has indicated that linear rheological tests are not the best for assessing the breadmaking performance of a given flour (Amemiya and Menjivar 1992; Dunnewind et al. 2002; Schiedt et al. 2013; Meerts et al. 2017). The flour quality is known to be inherently linked to the gluten network, which cannot be probed adequately by linear rheological tests as the starch granules play a pivotal role in the linear response of dough, and may partially mask the contributions of the gluten network. As a result, only non-linear rheological tests can be trusted to expose differences in flour quality. Non-linear extensional tests in particular constitute a very promising quality assessment tool, as in the actual breadmaking process dough is also subjected to large extensional deformations (Kokelaar et al. 1996).
The impact of GO on the extensional behaviour of dough is shown in Fig. 2a. At low GO concentrations, only the final part of the extensional viscosity curve, the so-called region of strain-hardening, is affected (as indicated by arrow (I) in Fig. 2a). The strain-hardening effect is very sensitive to the presence of long, branched chains. Since the reaction time of GO is limited, low concentrations of this enzyme will result in the cross-linking of only a small number of gluten chains. Yet, as GO appears to interact preferentially with the glutenins rather than the gliadins (Bonet et al. 2006), low GO concentrations already have a significant effect on the dough response at large strains. Conversely, at higher GO concentrations, the enzyme will cross-link numerous small and large gluten molecules alike, and consequently the entire extensional viscosity curve is shifted upwards, but mainly at smaller strains (arrow (II) in Fig. 2a).
a Extensional behaviour of standard strong Bilux dough and strong Bilux dough supplemented with 0.03 U GO/g flour and 0.90 U GO/g flour. b The strain-hardening index of strong Bilux dough as a function of GO and TG concentration. All measurements were performed 30 min after mixing. Error bars indicate the standard deviation
The two-step strengthening mechanism of GO is readily reflected in the value of the strain-hardening index, as shown in Fig. 2b. At low GO concentrations, the strain-hardening index tends to increase because of the dough’s increased resistance to flow at larger strains, whilst the viscosity curve at small strains remains relatively unaffected. Low GO concentrations have indeed been reported to be beneficial for the bread volume (Poulsen and Høstrup 1998; Vemulapalli et al. 1998; Bonet et al. 2006; Dagdelen and Gocmen 2007), and this improvement is clearly reflected in the strain-hardening index as its value increases significantly (from 17.0 ± 1.6 to 23.3 ± 4.5). By contrast, the use of excessive amounts of GO (> 0.015–0.100 U/g flour, depending on the flour characteristics) often proves counterproductive, as the gluten network becomes too stiff to allow a proper leavening of the dough (Bonet et al. 2006; Steffolani et al. 2010; Decamps et al. 2012b). This overcross-linking of the gluten network is again clearly revealed in the strain-hardening index, as its value tends to go down at high GO concentrations (> 0.030 U/g flour for the Bilux dough), eventually reaching a more or less constant value (which is significantly below the SHI value of the standard Bilux dough) as the saturation point is approached. The attenuation of the strain-hardening phenomenon at high GO concentrations is a direct consequence of the pronounced increase of the extensional viscosity at low strains (Fig. 2a). In the end, this increased resistance of the gluten network towards extension will result in undesirably small bread volumes. The enzyme dosage recommended by the supplier (0.3 U/g flour) is thus rather on the high side for the Bilux dough system, at least when evaluated under the mild conditions associated with a lab environment (see also Bueno et al. 2016). Besides bread volume, GO has also been reported to have a positive influence on the crumb texture (Vemulapalli et al. 1998; Rasiah et al. 2005; Bonet et al. 2006).
Compared to GO, the addition of TG has similar effects on the extensional behaviour of dough. Low concentrations of TG predominantly result in an increased flow resistance of the dough at large strains, whereas higher concentrations produce a viscosity increase over the whole strain region (results not shown). Consequently, the concentration dependency of the strain-hardening index is fairly similar for both enzymes (Fig. 2b). The SHI again exhibits a local maximum (which differs significantly from the SHI value of standard Bilux dough). Yet, in the case of TG, this local maximum is somewhat less pronounced, as also at higher TG concentrations the final part of the extensional viscosity curve continues to be affected significantly by the enzyme (until a saturation point is reached). This observation is in accordance with the linear oscillatory measurements, which indicated that TG has a more pronounced preference for the HMW-GS fraction than GO (cf. supra). Likewise, Kieffer rig experiments (Basman et al. 2002; Autio et al. 2005; Bagagli et al. 2014) and Alveograph studies (Caballero et al. 2007b) also signalled a continuous increase in the dough’s maximum resistance to extension with increasing TG concentration.
The evolution of the strain-hardening index with TG concentration again follows closely the trend in breadmaking performance established in other studies (Basman et al. 2002; Bauer et al. 2003b; Autio et al. 2005; Huang et al. 2008). Small amounts of TG generally have a positive effect on the bread volume, whereas the use of larger amounts (> 0.3–1 U/g flour, depending on the flour characteristics) entails the risk of overcross-linking the dough. Other beneficial effects of TG are a significant decrease in dough stickiness, and therefore improved machinability (Bauer et al. 2003b); improved bread crumb strength (Caballero et al. 2007a); and a finer and more uniform crumb structure (Basman et al. 2002; Caballero et al. 2007a). Considering the latter, van Vliet (2008) showed that strain-hardening indeed stimulates an equal growth of expanding gas cells, and tends to prevent coalescence between adjacent gas cells.
Creep-Recovery Tests
Our extensional rheological tests highlight the potential of the strain-hardening index as a quality indicator. The SHI is not only able to capture the beneficial effect of adding small amounts of GO/TG to dough, but also provides an indication of the onset of overcross-linking, which tends to occur when excessive amounts of GO/TG are used. The extensional tests thus allow us to determine, in an approximate way, the “optimal” concentration levels of GO and TG that will improve the breadmaking performance of a given flour to the maximal extent. Besides extensional tests, non-linear creep-recovery tests have also been proposed for assessing the flour quality. Important parameters that can be derived from the creep-recovery curves are the maximum creep compliance \(J_{c}^{max}\) (i.e. the compliance at the end of the creep phase) and the total recovery compliance \(J_{r}^{max}\) (i.e. a measure for the amount of deformation that can be recovered following the removal of the stress). Van Bockstaele et al. (2008) found the total recovery compliance \(J_{r}^{max}\) to correlate well with the breadmaking performance, as it provides a measure of the dough elasticity, which in turn can be traced back to the gluten network. In a previous study, we verified this correlation for the untreated Bilux and Bison dough systems (Meerts et al. 2017).
Figure 3 illustrates the impact of GO on the creep-recovery behaviour of Bilux dough. The addition of GO substantially lowers its flowability, and leads to a decrease in the recoverable and non-recoverable strain. At sufficiently high GO concentrations, the creep-recovery curves superimpose, indicating that saturation has been reached. The saturation concentration turns out to be similar for the linear oscillatory and the non-linear creep-recovery tests. The parameters characterising the creep-recovery behaviour of Bilux dough supplemented with GO are summarised in Table 1 for two GO concentrations. The low concentration corresponds to the “optimal” GO concentration as obtained from the extensional tests, whereas the high concentration is situated close to the saturation point. With increasing GO concentration, the maximum creep compliance \(J_{c}^{max}\) and the total recovery compliance \(J_{r}^{max}\) both exhibit a steady decline. Contrary to the strain-hardening index, the total recovery compliance \(J_{r}^{max}\) does not show a local maximum with respect to the GO concentration, and neither does the %-recovery. Consequently, the creep-recovery experiments cannot be used to determine the onset of overcross-linking. It is evident, however, that at the highest GO concentrations the gluten network has become too dense to allow for a proper leavening of the dough, indicating that overcross-linking has indeed occurred. At the same time, it is surprising to note that even at the highest GO concentrations, the dough still retains its ability to flow. This observation suggests that the SS cross-links created by GO in the gluten network are not permanent. Instead, a SH/SS interchange reaction mechanism has been suggested to allow for relative movement within the gluten matrix without compromising its stability (Bloksma 1975). It is equally possible that the observed stiffening effect of GO is only partially the result of an increased number of SS cross-links. Kontogiorgos (2011), amongst others, suggests that the continuity of the gluten network also depends strongly on non-covalent interactions (such as hydrogen bonds, hydrophobic interactions, etc.). It does not seem unthinkable, therefore, that conformational changes induced by the creation of additional SS cross-links may lead to improved, non-covalent interaction possibilities.
The effect of TG on the creep-recovery behaviour of Bilux dough resembles that of GO. The maximum creep compliance \(J_{c}^{max}\) and the total recovery compliance \(J_{r}^{max}\) both decrease upon addition of TG, yet the relative extent to which the dough is able to recover after removal of the load increases significantly (from 12.5 to 60.0%, see again Table 1), even more so than with the addition of GO. In other words, TG increases the dough’s viscosity as well as elasticity, but the latter is affected the most since TG has a particularly high affinity for the longer gluten chains. The impact of TG on the dough behaviour observed in creep-recovery tests is thus fully in line with the observations from oscillatory tests (see above). Other studies on the creep-recovery behaviour of TG-supplemented dough have yielded comparable results (Bauer et al. 2003b; Šimurina et al. 2014).
Upgrading Weak Flour Dough: Gluten Quality versus Gluten Quantity
Extensional Tests
In the baking industry, GO and TG are applied to improve the gluten quality, and hence the characteristics of the baked product. Another way of improving the baking performance of a wheat flour would be to increase the gluten content of that flour by adding supplementary gluten. With our rheological toolbox, we will now compare the potential of these different routes to improve the rheological response of a weak flour dough.
To assess the potential of the GO and TG enzymes as quality-improving agents, we tested multiple enzyme concentrations in combination with the weak Bison dough, and we compared the rheological performance of the upgraded Bison dough to that of the untreated strong Bilux dough, for shear as well as extensional deformations. As with Bilux dough, we observed that the strain-hardening index of GO- and TG-supplemented Bison dough exhibits a local maximum as a function of enzyme concentration (results not shown). Moreover, for Bison dough, the “optimal” enzyme concentrations turned out to be very similar to those for Bilux dough. These “optimal” enzyme concentrations were subsequently used to improve the rheological performance of Bison dough, as shown in Fig. 4a, which compares the extensional behaviour of weak Bison dough, supplemented with 0.03 U/g flour GO or 0.5 U/g flour TG, to that of strong Bilux dough. The small amounts of GO and TG turn out to affect the extensional viscosity of weak Bison dough predominantly at larger strains, as was also the case with strong Bilux dough (see Fig. 2a). It is furthermore clear that the stiffening effect of GO and TG with regard to the weak Bison dough is fairly significant, as the use of these enzymes allows to emulate the strong Bilux dough. The value of the extensional viscosity at the maximum strain, ηe+(ε max ), remains somewhat lower for the improved Bison dough as compared to the strong Bilux dough (see Fig. 4a). Yet, the SHI reaches practically the same values (differences are not statistically significant) for the improved Bison dough as for the strong Bilux dough: the use of GO or TG results in an increase of the SHI from 13.1 (for untreated Bison dough) to 16.7 and 17.1 for GO-treated and TG-treated Bison dough, respectively, whereas the untreated strong Bilux dough has a SHI value of 17.0. Adjustment of the gluten quality by the use of enzymes thus constitutes a valuable means to enhance the rheological performance of a weak flour dough. Furthermore, the two enzymes GO and TG seem to improve the wheat flour dough to the same extent.
a Extensional behaviour of standard weak Bison dough and Bison dough supplemented with “optimal” amounts of GO (0.03 U/g flour) and TG (0.50 U/g flour) or b with 1.5 wt% and 3.0 wt% supplementary gluten. The response of the strong Bilux dough is added for comparison in (a) and (b). All measurements were performed 30 min after mixing. SHI values are given as mean ± standard deviation
Besides the gluten quality, it is also possible to adjust the gluten quantity of a flour, by adding commercial gluten to the dough system. To assess the impact of changes in gluten quantity on the rheological performance of wheat flour dough, commercial gluten were added to the weak Bison flour in two different concentrations, in order to bridge the gap in gluten content between the standard Bilux and Bison flours (see also Materials and Methods section). In Fig. 4b, the performance of the standard Bison dough (ca. 10 wt% gluten) and the standard Bilux dough (ca. 13 wt% gluten) in extension is compared to that of the upgraded Bison dough samples with 11.5 and 13 wt% gluten. The addition of commercial gluten to the weak Bison dough turns out to have a clear impact on its rheological behaviour. The commercial gluten proteins are readily integrated in the native gluten network, as evidenced by the upward shift in the extensional viscosity curves with increasing gluten content. In contrast to the GO and TG enzymes, the addition of supplementary gluten has an effect on the extensional viscosity curves of dough at both small and large strains, probably because the supplementary gluten proteins have a wide distribution in molecular weight. The increase in extensional viscosity is most pronounced at higher strains, which implies that the addition of supplementary gluten has a positive effect on the SHI; the SHI value increases from 13.1 (for untreated Bison dough) to 17.8 and 17.2 for Bison (+ 1.5 wt% gluten) and Bison (+ 3 wt% gluten), respectively. By adding gluten to the weak Bison flour, it is thus possible to obtain SHI values similar to that of the (untreated) strong Bilux flour. Yet, as with the use of enzymes, adjustment of the protein content also entails the risk of over-reinforcing the gluten network: as the gluten content of the Bison flour is further increased from 11.5 to 13 wt%, the SHI value appears to decrease slightly (but the decrease is not statistically significant).
Creep-Recovery Tests
Non-linear creep-recovery tests can also be used to evaluate the changes in rheological performance of weak Bison dough supplemented with either enzymes or extra gluten. Fig. 5a shows the creep-recovery behaviour of Bison dough supplemented with GO or TG. The characteristics of the creep-recovery curves are summarised in Table 2. In creep-recovery, the effect of TG on Bison dough is much more apparent than the effect of GO, even though their impact on the extensional behaviour of weak Bison dough was fairly similar (see Fig. 4a). For weak Bison dough, we thus observe the same trends as for strong Bilux dough (cf. Table 1). As with the Bilux dough, both \(J_{c}^{max}\) and \(J_{r}^{max}\) exhibit a steady decline, whereas the relative amount of recoverable strain increases. The impact of an increase in the gluten content on the creep-recovery behaviour of weak Bison dough is shown in Fig. 5b. The addition of commercial gluten results in a substantial reinforcement of the native gluten network already present in Bison dough, to such an extent that the weak Bison dough supplemented with 3 wt% gluten attains a much higher shear viscosity (Fig. 5b), and judging from the %-recovery value in Table 2, also a slightly greater elasticity than the untreated strong Bilux dough.
a Non-linear creep-recovery curves for standard weak Bison dough and Bison dough supplemented with “optimal” amounts of GO (0.03 U/g flour) and TG (0.50 U/g flour) or b with 1.5 wt% and 3.0 wt% supplementary gluten. The creep-recovery response of the strong Bilux dough is added for comparison in (a) and (b). All measurements were performed one hour after mixing
Conclusions
The enzymes glucose oxidase and transglutaminase significantly affect the viscoelastic behaviour of wheat flour dough. Even though their assumed reaction mechanisms are different, the outcome is rather similar, as they both render the dough more stiff and elastic. The enzymes can be used to improve the strain-hardening behaviour of dough, and their effect can be quantified by means of a strain-hardening index (SHI). The SHI value of the weak flour dough used in this study can be brought to the same level as that of the strong flour dough by the action of GO or TG. The use of excessive amounts of enzyme has already proven to have severe ramifications for the breadmaking performance, and turns out to have an adverse effect on the SHI value as well. The SHI thus appears to correlate well with the breadmaking performance. In addition to gluten quality, a flour’s breadmaking performance can also be improved by increasing the gluten quantity. Supplementary gluten were found to become readily incorporated in the existing gluten network, thereby increasing the SHI value of the weak flour dough to equal that of the strong flour dough. Consequently, from a rheological point of view it appears that the degree of strain-hardening of a wheat flour dough can be improved to an equivalent extent by adjusting either the gluten quality or the gluten quantity. However, additional research on other wheat flour types is required before these conclusions can be generalised. Moreover, future breadmaking trials will have to decide whether these two bread-improving strategies are truly equivalent or not.
References
AACC International. (2000). Approved methods of analysis, 11th edition. St. Paul (MN): AACC International.
Amemiya, J.I., & Menjivar, J.A. (1992). Comparison of small and large deformation measurements to characterize the rheology of wheat flour doughs. Journal of Food Engineering, 16(1-2), 91–108.
AOAC International. (1995). Official methods of analysis of AOAC international, 16th edition. Washington: AOAC International.
Autio, K., Kruus, K., Knaapila, A., Gerber, N., Flander, L., & Buchert, J. (2005). Kinetics of transglutaminase-induced cross-linking of wheat proteins in dough. Journal of Agricultural and Food Chemistry, 53 (4), 1039–1045.
Bagagli, M.P., Jazaeri, S., Bock, J.E., Seetharaman, K., & Sato, H.H. (2014). Effect of transglutaminase, citrate buffer, and temperature on a soft wheat flour dough system. Cereal Chemistry, 91(5), 460–465.
Bankar, S.B., Bule, M.V., Singhal, R.S., & Ananthanarayan, L. (2009). Glucose oxidase - an overview. Biotechnology Advances, 27(4), 489–501.
Basman, A., Köksel, H., & Ng, P.K.W. (2002). Effects of increasing levels of transglutaminase on the rheological properties and bread quality characteristics of two wheat flours. European Food Research and Technology, 215(5), 419–424.
Bauer, N., Koehler, P., Wieser, H., & Schieberle, P. (2003a). Studies on effects of microbial transglutaminase on gluten proteins of wheat. I. Biochemical analysis. Cereal Chemistry, 80(6), 781–786.
Bauer, N., Koehler, P., Wieser, H., & Schieberle, P. (2003b). Studies on effects of microbial transglutaminase on gluten proteins of wheat. II. Rheological properties. Cereal Chemistry, 80(6), 787–790.
Bloksma, A.H. (1975). Thiol and disulfide groups in dough rheology. Cereal Chemistry, 52(3s), 170r–183r.
Bonet, A., Rosell, C.M., Caballero, P.A., Gómez, M., Pérez-Munuera, I., & Lluch, M.A. (2006). Glucose oxidase effect on dough rheology and bread quality: a study from macroscopic to molecular level. Food Chemistry, 99(2), 408–415.
Bueno, M.M., Thys, R.C.S., & Rodrigues, R.C. (2016). Microbial enzymes as substitutes of chemical additives in baking wheat flour? Part I: individual effects of nine enzymes on flour dough rheology. Food and Bioprocess Technology, 9(12), 2012–2023.
Caballero, P.A., Bonet, A., Rosell, C.M., & Gómez, M. (2005). Effect of microbial transglutaminase on the rheological and thermal properties of insect damaged wheat flour. Journal of Cereal Science, 42(1), 93–100.
Caballero, P.A., Gómez, M., & Rosell, C.M. (2007a). Improvement of dough rheology, bread quality and bread shelf-life by enzymes combination. Journal of Food Engineering, 81(1), 42–53.
Caballero, P.A., Gómez, M., & Rosell, C.M. (2007b). Bread quality and dough rheology of enzyme-supplemented wheat flour. European Food Research and Technology, 224(5), 525–534.
Dagdelen, A.F., & Gocmen, D. (2007). Effects of glucose oxidase, hemicellulase and ascorbic acid on dough and bread quality. Journal of Food Quality, 30(6), 1009–1022.
Decamps, K., Joye, I.J., Haltrich, D., Nicolas, J., Courtin, C.M., & Delcour, J.A. (2012a). Biochemical characteristics of Trametes multicolor pyranose oxidase and Aspergillus Niger glucose oxidase and implications for their functionality in wheat flour dough. Food Chemistry, 131(4), 1485–1492.
Decamps, K., Joye, I.J., Courtin, C.M., & Delcour, J.A. (2012b). Glucose and pyranose oxidase improve bread dough stability. Journal of Cereal Science, 55(3), 380–384.
Decamps, K., Joye, I.J., Rakotozafy, L., Nicolas, J., Courtin, C.M., & Delcour, J.A. (2013). The bread dough stability improving effect of pyranose oxidase from Trametes multicolor and glucose oxidase from Aspergillus niger: unraveling the molecular mechanism. Journal of Agricultural and Food Chemistry, 61(32), 7848– 7854.
Dobraszczyk, B., & Morgenstern, M.P. (2003). Rheology and the breadmaking process. Journal of Cereal Science, 38(3), 229– 245.
Dunnewind, B., van Vliet, T., & Orsel, R. (2002). Effect of oxidative enzymes on bulk rheological properties of wheat flour doughs. Journal of Cereal Science, 36(3), 357–366.
Folk, J.E., & Cole, P.W. (1966). Transglutaminase: mechanistic features of the active site as determined by kinetic and inhibitor studies. Biochimica et Biophysica Acta, 122(2), 244–264.
Gerrard, J.A., Fayle, S.E., Brown, P.A., Sutton, K.H., Simmons, L., & Rasiah, I. (2001). Effects of microbial transglutaminase on the wheat proteins of bread and croissant dough. Journal of Food Science, 66(6), 782–786.
Hanft, F., & Koehler, P. (2005). Quantitation of dityrosine in wheat flour and dough by liquid chromatography-tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 53(7), 2418–2423.
Hilhorst, R., Dunnewind, B., Orsel, R., Stegeman, P., van Vliet, T., Gruppen, H., & Schols, H.A. (1999). Baking performance, rheology, and chemical composition of wheat dough and gluten affected by xylanase and oxidative enzymes. Journal of Food Science, 64(5), 808–813.
Huang, W.N., Yuan, Y.L., Kim, Y.S., & Chung, O.K. (2008). Effects of transglutaminase on rheology, microstructure, and baking properties of frozen dough. Cereal Chemistry, 85(3), 301–306.
Kieliszek, M., & Misiewicz, A. (2014). Microbial transglutaminase and its application in the food industry. A review. Folia Microbiology, 59(3), 241–250.
Kokelaar, J.J., van Vliet, T., & Prins, A. (1996). Strain hardening properties and extensibility of flour and gluten doughs in relation to breadmaking performance. Journal of Cereal Science, 24(3), 199–214.
Kontogiorgos, V. (2011). Microstructure of hydrated gluten network. Food Research International, 44(9), 2582–2586.
Labat, E., Morel, M.H., & Rouau, X. (2001). Effect of laccase and manganese peroxidase on wheat gluten and pentosans during mixing. Food Hydrocolloids, 15(1), 47–52.
Larré, C., Denery-Papini, S., Popineau, Y., Deshayes, G., Desserme, C., & Lefebvre, J. (2000). Biochemical analysis and rheological properties of gluten modified by transglutaminase. Cereal Chemistry, 77(2), 121–127.
Läuger, J., Wollny, K., & Huck, S. (2002). Direct strain oscillation: a new oscillatory method enabling measurements at very small shear stresses and strains. Rheologica Acta, 41(4), 356–361.
McNaught, A.D., & Wilkinson, A. (1997). Compendium of Chemical Terminology. Oxford: Blackwell Scientific Publications.
Meerts, M., Cardinaels, R., Oosterlinck, F., Courtin, C.M., & Moldenaers, P. (2017). The interplay between the main flour constituents in the rheological behaviour of wheat flour dough. Food and Bioprocess Technology, 10(2), 249–265.
Nonaka, M., Tanaka, H., Okiyama, A., Motoki, M., Ando, H., Umeda, K., & Matsuura, A. (1989). Polymerization of several proteins by Ca+2-independent transglutaminase derived from microorganisms. Agricultural and Biological Chemistry, 53(10), 2619–2623.
Ohtsuka, T., Umezawa, Y., Nio, N., & Kubota, K. (2001). Comparison of deamidation activity of transglutaminases. Journal of Food Science, 66(1), 25–29.
Piber, M., & Koehler, P. (2005). Identification of dehydro-ferulic acid-tyrosine in rye and wheat: evidence for a covalent cross-link between arabinoxylans and proteins. Journal of Agricultural and Food Chemistry, 53(13), 5276–5284.
Poulsen, C., & Høstrup, P.B. (1998). Purification and characterization of a hexose oxidase with excellent strengthening effects in bread. Cereal Chemistry, 75(1), 51–57.
Rasiah, I.A., Sutton, K.H., Low, F.L., Lin, H.-M., & Gerrard, J.A. (2005). Crosslinking of wheat dough proteins by glucose oxidase and the resulting effects on bread and croissants. Food Chemistry, 89(3), 325–332.
Rosell, C.M., Wang, J., Aja, S., Bean, S., & Lookhart, G. (2003). Wheat flour proteins as affected by transglutaminase and glucose oxidase. Cereal Chemistry, 80(1), 52–55.
Schiedt, B., Baumann, A., Conde-Petit, B., & Vilgis, T.A. (2013). Short- and long-range interactions governing the viscoelastic properties during wheat dough and model dough development. Journal of Texture Studies, 44(4), 317–332.
Šimurina, O.D., Filipčev, B.V., Dapčević Hadnađev, T.R., Ikonić, B.B., & Bodroža Solarov, M.I. (2014). Modification of the rheological properties of substandard quality wheat dough with different doses of selected enzymes. Food and Feed Research, 41(2), 93–102.
Steffolani, M.E., Ribotta, P.D., Pérez, G.T., & León, A.E. (2010). Effect of glucose oxidase, transglutaminase, and pentosanase on wheat proteins: relationship with dough properties and bread-making quality. Journal of Cereal Science, 51(3), 366–373.
Tilley, K.A., Benjamin, R.E., Bagorogoza, K.E., Okot-Kotber, B.M., Prakash, O., & Kwen, H. (2001). Tyrosine cross-links: molecular basis of gluten structure and function. Journal of Agricultural and Food Chemistry, 49(5), 2627–2632.
Van Bockstaele, F., De Leyn, I., Eeckhout, M., & Dewettinck, K. (2008). Rheological properties of wheat flour dough and the relationship with bread volume. I. Creep-recovery measurements. Cereal Chemistry, 85(6), 753–761.
van Vliet, T. (2008). Strain hardening as an indicator of bread-making performance: a review with discussion. Journal of Cereal Science, 48(1), 1–9.
Vemulapalli, V., Miller, K.A., & Hoseney, R.C. (1998). Glucose oxidase in breadmaking systems. Cereal Chemistry, 75(4), 439–442.
Veraverbeke, W.S., & Delcour, J.A. (2002). Wheat protein composition and properties of wheat glutenin in relation to breadmaking functionality. Critical Reviews in Food Science and Nutrition, 42(3), 179–208.
Acknowledgements
MM is indebted to the Research Foundation - Flanders (FWO) for a doctoral fellowship at KU Leuven. The authors would also like to express their gratitude to the Research Fund KU Leuven (IDO/12/011) for financial support. Nore Struyf and Mohammad Naser Rezaei are also gratefully acknowledged for determining the flour characteristics (protein content, moisture content, optimal mixing time and water absorption). Finally, we would like to thank Ajinomoto Foods Europe S.A.S. for providing us with a free sample of TG (ACTIVA\(^{{\circledR }}\)WM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meerts, M., Van Ammel, H., Meeus, Y. et al. Enhancing the Rheological Performance of Wheat Flour Dough with Glucose Oxidase, Transglutaminase or Supplementary Gluten. Food Bioprocess Technol 10, 2188–2198 (2017). https://doi.org/10.1007/s11947-017-1986-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1986-0