Abstract
The physicofunctional and chemical properties of acid-aided protein isolate (AcPi), alkaline-aided protein isolate (AlPi) and soy protein isolate (SPI) prepared from tilapia muscle and defatted soy flour as a function of pH and/or NaCl concentration were investigated. Both acid- and alkali-aided processes lead to significant recoveries (P < 0.05) of proteins with substantial reduction of lipids in AlPi (0.81%) and AcPi (0.96%), the lowest for SPI (0.336%) facilitated by the processing method and sample used. There is greater lipid reduction at alkali pH, effective removal of impurities such as bones and scales, indicated by percentage ash (AcPi, 4.53%; AlPi, 3.75% and SPI, 3.51%). No major difference noted in sodium dodecyl sulphate polyacrylamide gel electrophoresis protein bands (14.4–97.4 kDa) possibly representing partial hydrolysis of myosin. Solubility was the highest at pH 3.0 and 11.0 and the lowest at isoelectric point with foam capacity showing similarity at varying pH. The addition of NaCl improved foam stability, possibly due to the increased solubility and surface activity of the soluble protein. On the whole, AcPi, AlPi and SPI manifested lower solubility and foamability at pH 4.0 and 5.0. AlPi exhibited appreciable levels of solubility, emulsion capacity, oil-holding capacity, viscosity and whiteness, whereas SPI had appreciable water-holding capacity. AcPi, AlPi and SPI have excellent relevance for product development based on their functionality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tilapias (Oreochromis niloticus) have been of major importance in the small-scale commercial or subsistence fish farming practiced worldwide, especially in Africa and Asia. Tilapia is nutritious and forms a healthy portion of a balanced diet that is high in protein (16–25%), low in fat (0.5–3.0%) and substitute well in most sea food recipe. Protein modification is mostly realized by enzymatic, physical and chemical treatment with resultant change in its structure, physicochemical and functional properties (Chobert et al. 1988). Similarly, fish protein isolate (FPI) prepared from different raw material, without retaining the original form of the muscle, is generally not consumed directly and is used as raw material for production of value-added products. In many parts of the world today, fish protein products are gaining increased interest as ingredients in food engineering. Proteins isolates are the basic functional components of various high protein processed food products and thus determine the textural and nutritional properties of the foods. These properties contribute to the quality and sensory attributes of food systems. The successful utilization of fish proteins depends greatly upon the appreciable characteristics that they convey to foods. Therefore, the connections between raw material freshness, processing parameters and providing sufficient amounts and proper balance of the essential amino acids, having an influence on the functional performance of protein products, are worthy of a wide-ranging investigation.
The pH-induced phenomenon of fish proteins during processing is believed to influence useful functionalities such as water-holding capacity (WHC), emulsification and gel-forming ability. This method is thought to be more efficient for complex raw material such as whole fish and rest raw materials. The process has given excellent results for some cold water species as well as temperate and warm water species (Helgi and Ingrid 2009). According to Kristinsson and Hultin (2004), the alkali treatment of cod muscle proteins improved functional properties (emulsification and gelation) of cod myosin and myofibrillar proteins. Functional properties of proteins are the physicochemical properties of those proteins that affect their behaviour in food systems during preparation, processing, storage and consumption and for fish proteins to be useful and successful in food application; they should ideally possess several desirable characteristics (McWatters and Chhinnan 1985; Parks and Carpenter 1987). These properties are fundamental physicochemical characteristics, which affect the behaviour of proteins in food systems during processing, manufacturing, storage and preparation (Pablo et al. 2010; Kinsella 1979).
However, the industrial applications of food proteins are limited because proteins are generally unstable to heating, organic solvents and proteolysis. Nonetheless, attempts have been carried out to modify fish proteins to improve their physical functionality, i.e. gelation, viscosity, emulsification and foaming (Sakamoto et al. 1994). Proteins have unique surface properties due to their large molecular size and their amphiphilic properties. Therefore, if proteins could be converted into stable forms, their applications would be greatly broadened.
Information about the functionality of these protein isolates may be used as the initial step for further investigation to modify and to improve functionality of fish proteins. Therefore, the purpose of this study is to evaluate functionality and physicochemical properties of FPI compared to commercial soy flour protein isolate (SPI) as influenced by acid and alkaline solubilisation technique.
Materials and Methods
Fresh tilapias (O. niloticus), 550–580 g/fish with length range of 22–25 cm/fish, were purchased from a local freshwater fish market in Wuxi, Jiangsu, People’s Republic of China. The fish samples were transported within 24 h on ice to the School of Food Science and Technology laboratory of Jiangnan University, Wuxi, Jiangsu, People’s Republic of China, where the fish muscle was retrieved carefully, separating the bones from the meat, chopped into pieces (0.20 cm) and vacuum packed in polyethylene bags (150–200 g/U), kept frozen at −20 °C until needed for the experiment. The storage time did not exceed 5 days. Commercial soy protein isolate purchased from Sun-Green Biotech Co. Ltd (Nantong, China) is a by-product of soy oil processing industries, is available and is a cheap source of protein. All chemicals used in the experiment were of analytical grade.
Preparation of Fish Protein Isolate (Acid and Alkaline Solubilisation Process)
The acid- and alkaline-aided protein solubilisation process, in the preparation of FPI, was applied according to the method described by Hultin and Kelleher (2000) with trivial modification. The samples were named thereafter as acid-aided protein isolate (AcPi) and alkaline-aided protein isolate (AlPi).
The alkaline-aided solubilisation process was carried out using homogenised minced tilapia fish (Ultra-Turrax T18 homogenizer, Shanghai, China, equipped with a motorized stirrer driven by the rheostat) at a 1:8 (w/v) ratio with cold distilled water for 90 s. The homogenates were adjusted to pH 11.0 with 2 N NaOH, incubated for 30 min before centrifugation at 9,000×g for 25 min at 4 °C. The residues were re-extracted twice more as described above. The alkaline-soluble fraction was collected and adjusted to the isoelectric point (pI) of muscle proteins (pH 4.5–5.0) using 2 N HCl. The precipitate was filtered through a double layer cheese cloth and dewatered by centrifugation at 9,000×g for 25 min at 4 °C. The final pH of the sample was adjusted to pH 7.0 before and or after centrifugation using 2 N NaOH.
The acid-aided process was undertaken as described previously, except that the pH of sample was adjusted to 3.0 with 2 N HCl. Soluble proteins were recovered by isoelectric precipitation at pH 4.5–5.0 and collected as described above. The experiments were conducted in triplicate and the precipitates were adjusted to pH 7.0 by using 2 N NaOH. Samples AcPi and AlPi were lyophilized, stored at −20 ± 2 °C until used.
Proximate Composition
Proximate analyses of AcPi, AlPi and SPI samples were determined (AOAC 2005). Moisture content was determined by the oven-drying method at 105 °C until a constant weight was reached. Ash content was estimated by incinerating the sample in a muffle furnace at 550 °C until whitish appearance. The total crude protein content (N × 6.25) was determined by Kjeldahl assay (AOAC 2005). The extraction and determination of total lipids from the samples were performed using methanol. Triplicate samples were analysed for each sample. The yield was calculated by determining the weight of sample isolates as a percentage of the total wet weight of raw material used:
Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis
The pattern of proteins obtained from different methods was determined by using electrophoresis according to the method of Laemmli (1970). To 3 g of sample was added with 27 ml of 5% sodium dodecyl sulphate (SDS; 85 °C). The mixtures were homogenised at a speed of 11,000 rpm for 1 min using an IKA Labortechnik homogenizer (Selangor, Malaysia). The homogenate was incubated at 85 °C for 60 min, followed by centrifugation at 8,000×g for 5 min at room temperature, using a Mikro 20 centrifuge (Hettich Zentrifugen, Tuttlingen, Germany). The supernatants were mixed at 1:1 (v/v) ratio with the sample buffer (0.5 M Tris–HCl, pH 6.8, containing 4% SDS, 20% glycerol and 10% BME) and boiled for 3 min. The samples (20 μg protein) were loaded into the polyacrylamide gel (10% running and 4% stacking gel) and subjected to electrophoresis at a constant current of 15 mA per gel, using a Mini Protean II unit (Bio-Rad Laboratories, Inc., Richmond, CA, USA). After separation, the proteins were stained with 0.02% (w/v) Coomassie Brilliant Blue R-250 and destained with 50% methanol (v/v) and 7.5% (v/v) acetic acid, followed by 5% methanol (v/v) and 7.5% (v/v) acetic acid. The protein bands were visualized from the gels stained with Coomassie Blue.
Colour Measurement
The colour of AcPi, AlPi and SPI samples were determined at regular intervals using a hand-held Minolta Chromameter (model CR-200b, Minolta Co., Ltd., Japan) and reported as L* (lightness), a* (redness) and b* (yellowness) in triplicate assays whereas values were averaged and whiteness was calculated as (L* − 3b*) referred by Codex Alimentarius (WHO/FAO 2005).
Differential Scanning Calorimetry
Thermal properties of samples were evaluated using differential scanning calorimetry (DSC; Pyris-I-DSC, Perkin-Elmer Corp., Norwalk, CT, and USA). Seventy milligrams of various samples was dissolved in 1 mL of 0.05 M phosphate buffer (pH 7.0) containing 0.1 M NaCl. The protein solutions (45 μL) were transferred and hermetically sealed in a stainless steel pan. The samples were heated by scanning from 25 °C to 135 °C at a rate of 10 °C/min against a reference containing 45 μL buffer without protein in a differential scanning calorimeter (Perkin-Elmer Corp., Norwalk, CT, USA). The denaturation peak temperature and enthalpy were calculated by a thermal analysis data software programme.
Functionality
Three separate experiments for each AcPi, AlPi and SPI samples were conducted, and the results were reported on a protein content basis.
Protein Solubility
Protein solubility was determined following the procedure of Diniz and Martin (1997), with slight modification. Samples were dispersed in distilled water (10 g L−1); the suspensions were adjusted to pH 2.0 up to pH 12.0 with either 0.1 M HCl or 0.1 M NaOH. The solution were shaken (Lab-Line Environ-Shaker; Lab-Line Instrument, Inc., Melrose Park, IL, USA) for 30 min at 25 °C and centrifuged at 4,000×g for 30 min. The protein content of the supernatant was determined by Kjeldahl method, and percent protein solubility was calculated according to the equation:
where
- PS:
-
Amount of protein in supernatant
- PIS:
-
Protein in initial sample
Foaming Capacity/Stability
Foam capacity and stability at different pH levels were estimated following the method of Diniz and Martin (1997), with minor modifications. Thirty millilitres of 30 g L−1 aqueous dispersion was mixed thoroughly using an Ultra-Turrax T25 homogenizer at 9,500 rpm for 3 min at 27 °C in a 250-mL graduated cylinder, and the total volume of the liquid was measured immediately after 30 s. The difference in volume was expressed as the volume of the foam. The foam stability (FS) was determined by measuring the decrease in volume of foam as a function of time up to a period of 60 min.
Oil/Water-Holding Capacity
Following the procedure of Diniz and Martin (1997), with slight modification, 1 g of each sample was stirred in 10 mL of distilled water or soybean oil (Gold Ingots Brand, QS310002012787, Suzhou, China), vortexed for 30 s and then centrifuged at 2,500×g for 25 min. The volume of the supernatant was measured. The water-holding capacity was expressed as the millilitres of water absorbed per gram protein sample. The free oil was decanted and oil-holding capacity was expressed by weight difference.
Emulsifying Capacity
The method of Yatsumatsu et al. (1972) was applied to evaluate the emulsion capacity (EC) of AcPi, AlPi and SPI. Five hundred milligrams of each sample was dissolved in 50 mL of 0.1 M NaCl, and then 50 mL of soybean oil was added into a 250-mL beaker. The homogenizer (Ultra-Turrax T18 homogenizer, Shanghai, China) equipped with a motorized stirrer driven by the rheostat was immersed in the mixture and operated for 3 min at 9,500 rpm to make an emulsion. From the emulsion, three 25-mL aliquots were immediately taken and transferred into three 25-mL graduated cylinders. The emulsions were allowed to stand for 15 min at 25 °C, and then the millilitre of aqueous volume to total volume was calculated:
Bulk Density
The bulk density (BD) of AcPi, AlPi and SPI was determined by adding a known weight of the sample in a graduated cylinder. The cylinder was gently tapped on the lab bench ten times and volume occupied by the sample was determined (grams per millilitre). All experiments were performed in triplicate, and the results are the average of the three values.
Viscosity
The viscosity analysis was carried out after protein solubilisation/precipitation at varying protein concentration according to a modified form of the method described (Yatsumatsu et al. 1972). The homogenates were placed into a 400-mL beaker to control temperature, and viscosity was measured at 25 °C to 27 °C using Bohlin BV88 viscometer (Bohlin Instruments, England), with nr C30 spindle (30 mm in diameter) at 1,000 rpm. The viscosity of the samples was recorded after 30 s of operating instrument, speed setting 6, system switch 6. Measurements were done in triplicate, and the viscosity values (in millipascal seconds) were extrapolated.
Statistical Analysis
The entire experiments were performed in three determinations. Results were subjected to analysis of variance using the SPSS version 16.0 (SPSS, Chicago, IL, USA), and significant differences (P < 0.05) between individual means were identified by the least significant difference procedure. Data were presented as means and standard deviations.
Results and Discussion
Proximate Composition and Protein Recovery
The proximate composition and protein recovery of AcPi, AlPi and SPI is shown in Table 1. AlPi had proximate protein content (92.76%) higher than AcPi protein content (89.95%), following an insignificant difference between SPI (88.66%) and AcPi. The fat content of AcPi (0.96%) was slightly higher (P < 0.05) than AlPi (0.81%) showing almost concurrence in what has been reported by Taskaya et al. (2009), but similarly manifesting lowest result in SPI sample (0.36) probably due to sample used in experiment. The ash content of SPI (3.15%) was the lowest (P < 0.05) to that of AlPi (3.75%) and the AcPi samples (4.53%). Ash content (dry basis) was determined as an indicator of how well the impurities were removed because most of the minerals are located in the impurities. The moisture content was also reported for AcPi (3.9%), AlPi (3.86%) and SPI (4.25%) as shown in Table 1. The protein recovery yield is a critical consideration to initiate the determination of an economically feasible technology. The acid- and alkali-aided processes gave higher protein recoveries (P < 0.05) when compared with conventional lab-scale surimi process (<75%) reported by Kristinsson and Liang (2006). In this experiment, higher yields were obtained from tilapia muscles and similarly exhibiting higher result for soy protein isolate. Results revealed the acid-aided extraction process, significantly (P < 0.05) yielding higher protein recovery for AcPi (84.97%) than the alkali-aided extraction process for AlPi (79.82%) and SPI (67.91%). This is in concurrence with other processing methods (Helgi and Ingrid 2009) and reported fish species, such as mullet (Kristinsson and Liang 2006), Pacific whiting (Choi and Park 2002) and soy protein concentrate/isolate (Bazinet et al. 1997). Other reported recovery for the acid-aided (74%) and alkali-aided process (68%) in herring white muscle, with similarity to the catfish protein recovery, although Kim et al. (1996) reported a 33% recovery from catfish frame mince, which is more or less half that reported in our findings. Protein recovery yields for isoelectric solubilisation technique reported in literature range between 42% and 90% (Kristinsson and Liang 2006). The dissimilarities may be attributed to series of factors ranging from fish species, methodology used to determine recovery yield, water sacroplasmic concentration and centrifugation force during isoelectric solubilisation (Chen and Jaczynski 2007). Hypothetically, the alkali-aided process has shown lower protein denaturation (Table 2) and thus less co-aggregation of proteins compared to the acid-aided process, which could lead to less overall protein recovery for the alkali-aided process (Kristinsson and Hultin 2004).
Proteins Solubility
In Fig. 1, the solubility pattern of AcPi, AlPi and SPI obtained from pH-shift process is illustrated. The solubility of muscle proteins at different pH values using the acid- and alkali-aided processes is important for optimizing protein recoveries. Intended for these processes, low solubility of proteins is needed for high recovery of proteins following precipitation at the isoelectric point, whereas high solubility of muscle proteins is needed for an efficient separation from impurities at acidic or alkali pH values (Kristinsson et al. 2005). Good protein solubility is believed to be a prerequisite for many functional properties, including gelation and emulsification. The solubility curves (Fig. 1) are U-shaped, similar to other fish species such as channel catfish (Kristinsson et al. 2005) and similarly for cereals (Kamara et al. 2009). The AlPi had the highest solubility values followed by AcPi and SPI, respectively, both in acidic and alkaline conditions. All proteins had solubility above 60% at pH 3, the highest being AlPi (81.34%) and the lowest SPI (60.65%). Above pH 7, solubility is above 75% for AlPi and AcPi and lowest for SPI (61.85%) with a difference (P < 0.05). Among protein isolates, the alkaline-aided process provided higher protein solubility than the acid-aided process. The lower solubility was in the pH range of 4.5 to 5.5 and the highest solubilities at pH 2 to 3 and at pH 11 to 12 for both acid- and alkali-aided processes compared with SPI. The sharpest solubility curve occurred between pH 3.5 and 4.0 and between pH 9.5 and 11.0. SPI exhibited similar U-shaped solubility pattern but lowest to AcPi and AlPi on comparative basis (Fig. 1). Low protein solubility in acid- and alkaline-aided processes is probably caused by the denaturation of muscle proteins induced by pH shift, since denaturation designates the response of the native protein to (not only) heat, a variety of other chemical and physical agents, but also significantly to acids and alkali, causing marked changes in the protein structure. Decrease of protein solubility, as a result of protein denaturation, subsequently increased hydrophobic interactions, which caused precipitation of the proteins (Zayas 1997). Protein solubility at various pH values may provide useful pointer of how well protein isolates will perform when incorporated into food systems.
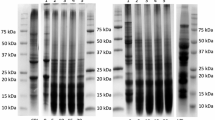
Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis
Figure 2 illustrates electrophoresis (SDS-polyacrylamide gel electrophoresis (PAGE)) of films prepared from AcPi and AlPi to obtain information on the molecular weight and distribution pattern of the protein components. As shown in Fig. 2, AcPi and AlPi revealed polypeptides of a wide range of molecular weights. AcPi and AlPi illustrated slight variation in band patterns. Lower band intensity was revealed in the alkaline-aided process notwithstanding the possibilities of hydrolysis in the acid-aided process resulting to a less intense band. However, the most abundant proteins recovered were polypeptides with estimated molecular weights of 14.4, 20.1, 31.0, 43.0, 66.2 and 97.4 kDa, with the hydrolysates only showing bands below 14.4 kDa (subjectively judged, based on band width and intensity). The results are in conformity with research findings by Kristinsson and Ingadottir (2006) and also compliant results observed for other species, such as pacific whiting and herring (Kristinsson and Ingadottir 2006).
Differential Scanning Calorimetry
The protein denaturation profiles for AcPi and AlPi are given Table 2. According to the result, the DSC thermogram for AcPi, AlPi and SPI showing a single endothermic transition with total enthalpy, ΔH total, values of 0.0066, 0.0206 J/g and 4.18, respectively. And according to the results, the samples have varied denaturation temperatures (P < 0.05) of 53.46 °C, 50.34 °C and 88.41 °C for AcPi, AlPi and SPI, respectively. Industrial applications of food proteins are limited because proteins are generally unstable to heating, organic solvents and proteolysis. The poikilothermic characteristics of fish proteins make them more heat sensitive than mammalian muscle proteins and to that of soy protein with a greater tendency to denature at elevated temperatures (Choi and Park 2002). However, these denaturation temperatures are influenced by heating rate and sample concentration. It could be seen from Table 2 that AlPi demonstrated an early onset of temperature (47.95 °C) compared to AcPi (50.53 °C) and SPI (86.06 °C). Knowledge of the thermal decomposition of the FPI samples can be used to improve their stability and functional properties.
Colour Measurement
Colour characteristics of proteins obtained from tilapia (AcPi and AlPi) compared with SPI, after applying the pH-shift process, is given in Table 2. Whiteness of the protein isolates is marked (P < 0.05) in both acidic- and alkali-aided process for AcPi, AlPi and SPI (L* 86.12, L* − 3b* = 71.51), (L* 82.60, L* − 3b* = 76.24) and (L* 76.83, L* − 3b* = 63.12), respectively. Conversely, AlPi was whiter than protein isolate of AcPi and SPI, and to a practical extent, AlPi manifested less yellowness (b* 3.29) than it is for AcPi (b* 3.70) and SPI (b* 4.57; P < 0.05). Kristinsson and Liang (2006) reported a higher whiteness for both acid- and alkaline-aided isolates of croaker meat, having concurrence with our findings. It is apparent that after the alkali-aided process, a considerable amount of native heme proteins remain soluble at isoelectric point, and on the contrary, only a faded field of oxidized heme proteins seen for the acid-aided process. The manifestation here is that, after the acid-aided process, a substantial fraction of the heme proteins co-precipitated at isoelectric point. Acid and alkali solubilisation technique yields higher L* values and more whiteness that could be attributed to the retention of connective tissue. More yellowness (b*) could be in part due to more retention of lipids for this process and sample type. The slight increase in redness (a*) is likely attributed to the remaining hemoproteins in the recovered protein (Choi and Park 2002). It is therefore likely that the unfolded heme proteins in tilapia muscle co-precipitated with the muscle proteins at pH 5.0, which would explain the higher yellowness and the lower whiteness and L* value. The fish-like and metallic odours may be due to residual fish lipid in the samples. Soy protein isolate made from defatted soy meal by removing most of the fats and carbohydrates has a very neutral flavour because most of the carbohydrates are removed.
Functional Properties of Tilapia Protein Isolate
Water- and Oil-Holding Capacity
The WHC of AcPi, AlPi and SPI range was given (Table 2) as 2.63, 2.51 and 3.01 mL g−1, respectively, with a significant difference (P < 0.05). Interactions of water and oil with proteins are equally very important in the food systems because of their effects on the flavour and texture of foods.
For AlPi and SPI are showing (Table 2) higher (3.38 and 2.81 mL g−1) oil absorption capacity than it is for AcPi (2.50 mL g−1) with significant difference (P < 0.05), that is, in collaboration with other studies (Kristinsson et al. 2005; Diniz and Martin 1997). Furthermore, high oil absorption is essential in the formulation of food systems like sausages, cake, batters, mayonnaise and salad dressings.
Bulk Density
Bulk density is an important parameter that determines the packaging requirement of a product for it measures heaviness of the protein isolate powder. Furthermore, bulk density signifies the behaviour of a product in dry mixes, varying with the fineness of the particles. As shown in Table 2, AcPi, AlPi and SPI had varying bulk densities of 0.30, 0.34 and 0.20 g mL−1, respectively, with a significant difference (P < 0.05). AlPi had the highest bulk density as compared to SPI and AcPi. Present results obtained are compared to reported research values (Kamara et al. 2009) and amidst other findings, solubility, hydrodynamic properties, hydrophobicity and microstructure of proteins play an important role in the bulk density of protein isolates (Kinsella 1979). High bulk density is disadvantageous for the formulation of weaning foods, where low density is required.
Foaming Properties
The effect of pH, NaCl and time (minutes) on FC and FS are given in Figs. 3 and 4a, b (with NaCl). The FC (Fig. 3) was pH dependent and was found to be the lowest at pH 4.0 and 5.0 for AcPi, AlPi and SPI (4.4, 4.5%; 4.71, 5.14% and 3.70, 4.03%), respectively. The lowest FC was attributed to the protein behaviour at around its isoelectric point. Beyond pH 5.0, FC significantly (P < 0.05) improved, especially at pH 9 and 10 for AcPi, AlPi and SPI (102.31, 103.60%; 102.21, 103.61% and 96.86, 95.52%), respectively, and it was likely due to the increased net charges on the protein, which weakened the hydrophobic interactions but increased the flexibility of the protein. This allowed the protein to diffuse more rapidly to the air–water interface to encapsulate air particles and then enhance the foam formation (Wierenga and Gruppen 2010). The profile of FC against pH for the protein isolate was more or less parallel to that of its nitrogen solubility against pH.
The addition of NaCl (1.0 M) at pH 5.0 (Fig. 4b) gradually improved FC of the protein, and a higher percentage increase was observed at this concentration. This may be attributed to the fact that addition of NaCl, at a concentration up to 1.0 M, enhances the protein solubility by weakening the hydrophobic interaction of the protein while high salt concentration had an adverse effect on FC due to the salting effect of NaCl. FS significantly decreased (P < 0.05) with time when the foam stood for an initial period of 15 min (64.63%, 67.22% and 59.75%) to 90 min (31.43%, 31.93% and 25.47%) for AcPi, AlPi and SPI, respectively (Fig. 4a) given AlPi to having superior FS. Addition of salt (1.0 M NaCl) greatly improved FS of the protein, due to increased solubility and surface activity of the soluble protein. Results also revealed that foaming properties were pH dependent and that a decrease in the fat content of the protein isolate and furthermore the isolation conditions influenced foam ability.
Emulsion Capacity
The ability of proteins to form emulsions is important owing to the interactions between proteins and lipids in many food systems. SPI have a significant inferior emulsion capacity than AcPi and AlPi (Table 2). Emulsifying capacities of AcPi, AlPi and SPI ranged from 83.70%, 85.32% and 55.27%, respectively, with a significant difference (P < 0.05). Isolates with high solubility can rapidly diffuse and adsorb at the interface. Higher contents of larger molecular weight peptides or more hydrophobic peptides contribute to emulsion properties (Mutilangi et al. 1996). Results revealed that emulsion capacity was pH dependent, and at alkaline pH, EC improved than at acidic pH. EC of proteins depends upon the hydrophilic–lipophilic balance, which is affected by pH of the proteins. Our data showed that AcPi, AlPi and SPI have relatively good emulsifying properties and that AlPi manifested superior emulsifying capacity. This could be due to the exposure of hydrophobic groups of denatured proteins. Similar observations on the pH dependence of EC have been reported (Sathe et al. 1982).
Viscosity
According to Charles and Peggy (2010), protein content affects viscosity and physical stability to emulsions that is manifested in our findings. Viscosity is believed to play an important role in acid and alkali process since it facilitates the separation of soluble protein from the insoluble. The concentrations, molecular weight’s polydispersity, hydrophobicity and conformation of each protein species affect the viscosity of the solution. All of these factors tend to confound the underlying inverse relationship of protein solubility and viscosity (Kolawole and Bukola 2010). Results presented in Fig. 5 showed that AcPi, AlPi and SPI were able to form viscosity solutions even at high concentrations. AcPi and AlPi dispersions were more viscous at higher concentration than it was for SPI dispersion. The effect of protein concentration on the viscosity showed that sample dispersion in water exhibits pseudoplastic (shear rate thinning) behaviour over a relatively wide range of concentration. This corroborate with results obtained by Kolawole and Bukola (2010).
Conclusion
Tilapia fish protein isolates compared to commercial soy protein isolate as influenced by acid and alkaline solubilisation technique exhibited substantial recovery/amount of protein content. Furthermore, the result of the protein characteristics shows that AlPi had superior. The solubility of the protein isolates was pH dependent with minimum solubility of the protein occurring around the isoelectric precipitation point. For most of these functional parameters, processing methods and source of raw materials served as important variables responsible for the differences in functionality of the samples. The isolates varied in their denaturation temperatures and revealed only slight range of molecular weight polypeptides between AcPi and AlPi. Addition of NaCl greatly improved FS of the protein, due to increased solubility and surface activity of the soluble protein. The effect of protein concentration on apparent viscosity gives additional understanding of muscle proteins and lipids as well as their isoelectric behaviour in food systems which allow the development of this technology at a commercial scale for efficient processing of novel food products.
References
AOAC. (2005). Official methods of analysis (16th ed.). Washington, DC: Association of Official Analytical Chemists.
Bazinet, L., Lamarche, F., Labrecque, R., Toupin, R., Boulet, M., & Ippersiel, D. (1997). Electro-acidification of soybean proteins for the production of isolate. Food Technology, 51, 52–56. 58, 60.
Charles, I. O., & Peggy, M. T. (2010). Gelling properties of tyrosinase-treated dairy proteins. Food and Bioprocess Technology, 3, 554–560.
Chen, Y. C., & Jaczynski, J. (2007). Protein recovery from rainbow trout (Oncorhynchus mykiss) processing by-products via isoelectric solubilization/precipitation and its gelation properties as affected by functional additives. Journal of Agricultural and Food Chemistry, 55(22), 9079–9088.
Chobert, J. M., Bertrand-Harb, C., & Nicolus, M. G. (1988). Solubility and emulsifying properties of caseins and whey proteins modified enzymatically by trypsin. Journal of Agriculture and Food Chemistry, 36, 883–892.
Choi, Y. L., & Park, J. W. (2002). Acid-aided protein recovery from enzyme-rich Pacific whiting. Journal of Food Science, 67(8), 2962–2967.
Diniz, F. M., & Martin, A. M. (1997). Effects of the extent of enzymatic hydrolysis on the functional properties of shark protein hydrolysate. LWT Food Science and Technology, 30, 266–272.
Helgi, N., & Ingrid, U. (2009). The acid and alkaline solubilization process for the isolation of muscle proteins: State of the art. Food and Bioprocess Technology, 2, 1–27.
Hultin, H. O., & Kelleher, S. D. (2000). High efficiency alkaline protein extraction. Patent US6136959.
Kamara, M. T., Zhu, K., Amadou, I., Tarawalie, F., & Zhou, H. (2009). Functionality, in vitro digestibility and physicochemical properties of two varieties of defatted foxtail millet protein concentrates. International Journal of Molecular Sciences, 10, 5224–5238.
Kim, J. M., Liu, C. H., Eun, J. B., Park, J. W., Oshimi, R., Hayashi, K., et al. (1996). Surimi from fillet frames of channel catfish. Journal of Food Science, 61(2), 428–432.
Kinsella, J. E. (1979). Functional properties of soy proteins. Journal of the American Oil Chemists’ Society, 56, 242–249.
Kolawole, O. F., & Bukola, S. O. (2010). Effect of processing methods on physical, chemical, rheological, and sensory properties of okra (Abelmoschus esculentus). Food and Bioprocess Technology, 3, 387–394.
Kristinsson, H. G., & Hultin, H. O. (2004). Changes in trout hemoglobin conformations and solubility after exposure to acid and alkali pH. Journal of Agriculture and Food Chemistry, 52, 3633–3643.
Kristinsson, H. G., & Ingadottir, R. (2006). Recovery and properties of muscle proteins extracted from tilapia (Oreochromis niloticus) light muscle by pH shift processing. Journal of Food Science, 71(3), E132–E141.
Kristinsson, H. G., & Liang, T. (2006). Effect of pH-shift processing and surimi processing on Atlantic croaker (Micropogonias undulates) muscle proteins. Journal of Food Science, 7, 298–306.
Kristinsson, H. G., Theodore, A. E., Demir, N., & Ingadottir, B. (2005). A comparative study between acid- and alkali-aided processing and surimi processing for the recovery of proteins from channel catfish muscle. Journal of Food Science, 70, C298–C306.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage. Nature, 227, 680–685.
McWatters, K. H., & Chhinnan, M. S. (1985). Effect of hydration of cowpea meal on physical and sensory attributes of a traditional West African food. Journal of Food Science, 50(2), 444–446. 453.
Mutilangi, W. A. M., Panyam, D., & Kilara, A. (1996). Functional properties of hydrolysates from proteolysis of heat-denatured whey protein isolate. Journal of Food Science, 61, 270–274. 32.
Pablo, D. R., Gabriela, T. P., María, C. A., & Alberto, E. L. (2010). Optimization of additive combination for improved soy–wheat bread quality. Food and Bioprocess Technology, 3, 395–405.
Parks, L. L., & Carpenter, J. A. (1987). Functionality of six non-meat proteins in meat emulsion systems. Journal of Food Science, 52(2), 271–274.
Sakamoto, H., Kumazawa, Y., & Motoki, M. (1994). Strength of protein gels prepared with microbial transglutaminase as related to reaction conditions. Journal of Food Science, 59, 866–871.
Sathe, S. K., Deshpande, S. S., & Salunkhe, D. K. (1982). Functional properties of lupin seed (Lupinus mutabilis) proteins and protein concentrates. Journal of Food Science, 47, 491–497. 38.
Taskaya, L., Chen, Y. C., & Jaczynski, J. (2009). Functional properties of proteins recovered from whole gutted silver carp (Hypophthalmichthys molitrix) by isoelectric solubilization/precipitation. LWT—Food Science and Technology, 42(6), 1082–1089.
WHO/FAO. (2005). Codex code for frozen surimi. In J. W. Park (Ed.), Park, Surimi and Surimi Sea Food (pp. 869–885). Boca Raton: Taylor and Francis Group.
Wierenga, P. A., & Gruppen, H. (2010). New views on foams from protein solutions. Current Opinion in Colloid and Interface Science, 15(5), 365–373.
Yatsumatsu, K., Sawada, K., & Moritaka, S. (1972). Whipping and emulsifying properties of soybean products. Agriculture and Biological Chemistry, 36, 719–727.
Zayas, J. F. (1997). Functionality of proteins in food. Berlin: Springer.
Acknowledgement
Authors wish to thank the earmarked fund for Modern Agro-industry Technology Research System (NYCYTX-49-22), PCSIRT0627 and 111project-B07029 for providing financial support to carry out this research. We also extend our profound thanks and appreciation to the governments of People’s Republic of China and Sierra Leone.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Foh, M.B.K., Wenshui, X., Amadou, I. et al. Influence of pH Shift on Functional Properties of Protein Isolated of Tilapia (Oreochromis niloticus) Muscles and of Soy Protein Isolate. Food Bioprocess Technol 5, 2192–2200 (2012). https://doi.org/10.1007/s11947-010-0496-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-010-0496-0