Abstract
This study examined the effects of high hydrostatic pressure pretreatment (100–400 MPa) on the properties of heat-induced wheat gluten (WG) gels. The results showed that treatment with pressure higher than 100 MPa significantly increased the strength, water-holding capacity (WHC), non-freezable water (Wnf), and storage modulus (G′) of heat-induced WG gels. When pressure was increased from 100 to 400 MPa, the free SH content of the heat-induced WG gels increased to 23.55 μmol/g protein, and the surface hydrophobicity (H0) increased to 25.36. Fourier transform infrared spectroscopy (FTIR) analysis showed that the β-sheet and random coil content of the WG treated by high-pressure increased with pressure, while the α-helix and β-turn content decreased. Scanning electron microscopy (SEM) showed that the heat-induced WG gels had more uniform and dense three-dimensional networks after high-hydrostatic-pressure pretreatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wheat gluten (WG), an important by-product of wheat starch production, has a protein content of approximately 80% and is one of the major plant protein sources of human food (Irisj et al. 2009). WG is widely used in the structural optimization of foods, such as meat products, cereal products, and cheese analogues, because of its gelation properties and ability to form good viscoelastic networks (Kong et al. 2010). Generally, WG in foods is heated to form heat-induced WG gels during food preparation, which can significantly improve the quality of foods (Santhi et al. 2015). By adding wheat gluten to meat products, the formation of WG gels help to increase the network and burying ability of the meat products, thereby significantly enhancing the viscoelasticity, hardness, and water-holding capacity (WHC) of the meat products (Maningat et al. 1999). Such gel formation includes a heating and a cooling phase. During the heating phase, the compact secondary and tertiary structures of WG are destroyed, and some functional groups are exposed. During the subsequent cooling phase, protein molecules are linked together by intermolecular forces, such as disulfide bonds, hydrophobic interactions, and hydrogen bonds, to form a three-dimensional gel network (Ibanoglu 2005). However, the tight molecular structure and poor solubility of WG are not conducive to its unfolding or to the exposure of its functional groups during the heating phase, which limits improvement of the properties of heat-induced WG gels (Day et al. 2006).
Physical (Byaruhanga et al. 2006) and enzymatic (Dondero et al. 2006) pretreatment are common methods for enhancing the properties of heat-induced protein gels. High-pressure pretreatment is a physical method that can change the tertiary or even secondary structure of proteins, leading to the unfolding of protein molecules and the exposure of groups buried inside them, which allows chemical forces, such as disulfide bonds, hydrophobic interactions, hydrogen bonding, and electrostatic interactions, to affect the functional properties of the protein, such as solubility and gelation properties (He et al. 2014). Studies by Xue et al. and Qiu et al. showed that proper high-pressure treatment can change the structure of muscle protein molecules and enhance interactions between meat protein molecules, thereby increasing the strength and rheology of heat-induced muscle protein gels (Qiu et al. 2013; Xue et al. 2017). These results suggest that high-pressure pretreatment might be used to affect the heating phase of heat-induced WG gel formation and improve properties of the gels.
Therefore, the purpose of this study was to investigate the effects of high pressure on the properties of WG gels. The results of this study may provide a direct reference for improving the gel properties of WG and facilitate the development of new applications for WG in the food processing industry.
Materials and Methods
Materials
WG (> 78% protein, 15.5% carbohydrates, 0.9% fat) was obtained from the Ruifuixang Reagent Company, Anhui, China. Sodium dodecyl sulfate (SDS), 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), tris, glycine, disodium ethylenediaminetetraacetic acid (Na2EDTA), potassium bromide (KBr), 1-anilino-8-naphthalene sulfonate (ANS), Coomassie brilliant blue G-250, and other chemicals were obtained from Solarbio Science and Technology Co., Ltd., Beijing, China. All reagents were analytical grade.
High-Pressure Treatment of WG
This study used a hydrostatic pressure unit equipped with a pressure and temperature controller (Baotou Kefa High Pressure Technology Co., Ltd., Inner Mongolia, China) to process samples. Ten-percent protein dispersions (w/v; 10 g WG/100 mL water) prepared using WG were subjected to high-pressure treatment at 100, 200, 300, and 400 MPa for 10 min. Distilled water was used as a pressure mediator during hydrostatic pressure treatment, and the temperature was maintained 20 °C (Meng and Ma 2001). Prior to pressure treatment, WG dispersions were packaged in polyethylene bags under vacuum. In addition, untreated samples were used as experimental controls.
Preparation of the Heat-Induced WG Gels
Heat-induced WG gels were prepared according to Wang’s method with some modifications (Wang et al. 2017). Sixteen-percent suspensions of WG treated by high pressure were induced at 95 °C for 30 min. After heat treatment, the samples were rapidly cooled in ice water. The prepared heat-induced WG gels were stored at 4 °C.
Determination of Gel Strength
The strength of the heat-induced WG gels was measured using the TA.XT Plus texture analyzer (Texture Technologies, Hamilton, MA, USA), and the maximum force used for puncture was taken as the gel strength according to the method of Hu et al. (2013) with modifications. A section 10 mm in height and 20 mm in diameter was cut from the center of a gel as a sample. Using a probe with a P/0.5 diameter probe attachment, the samples were pierced to a depth of 10 mm at a durable speed of 5.0 mm/s and the maximum force was recorded.
Determination of WHC
The WHC of heat-induced WG gels was measured according to the method of Tang et al. (2011) with modifications. Five-gram gel samples were placed in 10-mL centrifuge tubes and centrifuged at 8000×g for 20 min at 4 °C in a CR22G II centrifuge (Hitachi Ltd., Tokyo, Japan). Water was removed after centrifugation, and the samples were accurately weighed. WHC is defined as follows:
where Wr is the total weigh (g) of the removed water and Wt is the total weight (g) of water.
Differential Scanning Calorimetry
The water distribution in the gel system was determined according to the method of Feng (Chen et al. 2010), with some modification. The DSC-Q200 differential scanning calorimetry system (TA Instruments, New Castle, DE, USA) was used to determine the content of freezable water in the gel system. A small sample (5–10 mg) was encapsulated into an aluminum pan, and an empty pan was used as a control. Thermal scans were taken from − 60 to 50 °C at a rate of 5 °C/min under a 50-mL/min flow of nitrogen gas. The content of freezable water (Wf) was calculated according to the method of Yoshida (Yoshida et al. 1993). Non-freezable water (Wnf) was calculated as follows:
where Wt is the total water content as measured according to Yoshida’s method.
Dynamic Rheological Measurements
According to the modified method of Moreno et al. (2015), the dynamic rheological behavior of WG was determined using the DHR-3 rheometer (TA Instruments). Dynamic rheological evaluations were performed under the following conditions: the temperature was raised from 25 to 95 °C at a rate of 2 °C/min, held for 10 min, and then lowered to 25 °C at a rate of 4 °C/min. At the same time, oscillation frequency was set to 1 Hz and strain to 0.01%, and changes in G′ during the temperature scanning were recorded.
Determination of Free SH Content
The free SH content in the WG gels was measured according to the modified method of Chan et al. (Chan and Wasserman 1993). A 0.1 g sample of WG gel was dissolved in 10 mL phosphate buffer (1 mmol/L EDTA, 1% SDS) and centrifuged at 8000×g for 20 min at 4 °C. Three milliliters of the resulting supernatant and 0.1 mL DTNB solution (4 mg/mL) were added to 3 mL phosphate buffer, incubated at 25 °C for 1 h, and then centrifuged at 8000×g for 30 min. Absorbance of the supernatant was measured at 412 nm using a 722E Visible Spectrophotometer (Shanghai Spectrum Instruments Co., Ltd., Shanghai, China), and phosphate buffer was used as a control. SH content of the WG gels was calculated as follows:
where 73.53 is the molar extinction coefficient value of DTNB, A412 is the absorbance of the sample at 412 nm, D is the dilution factor of the sample, and C is the sample concentration (mg/mL).
Determination of Surface Hydrophobicity (H 0)
Surface hydrophobicity was determined using ANS as a fluorescence probe according to the method described by Zhang et al. (2015). Samples were dissolved in phosphate buffer (pH 7, 1 mg/mL in 0.01 M buffer) and centrifuged at 8000×g for 20 min at 4 °C. The supernatant was diluted to several different concentrations (0.1–0.0005 mg/mL), and 40 μL ANS (8.0 mmol/L) was added to 4 mL of the diluted supernatant. Using an FC Multiskan microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA), the relative fluorescence intensity was measured at an excitation wavelength of 365 nm and emission wavelength of 484 nm. The initial slope of the curve of fluorescence intensity vs. protein concentration was recorded as the H0.
Fourier Transform Infrared Spectroscopy
The secondary structure of WG in the gels was determined using a Nicolet 6700 Fourier transform infrared spectroscopy spectrometer (Thermo Nicolet Co., Madison, WI, USA). A mixture of 1 mg sample and 99 mg KBr was thoroughly ground in an agate mortar and tableted. Full-band scanning (400–4000 cm) was carried out 32 times with the Fourier transform infrared spectroscopy (FTIR). The amide I band (1600–1700 cm) in the spectrum was analyzed using the OMNIC 6.0 (Thermo Fisher Scientific) and Peak Fit v4.12 (Systat Software, Inc., San Jose, CA, USA) software to evaluate secondary structural changes in WG.
Scanning Electron Microscopy
The microscopic network of heat-induced WG gels was observed according to the method described by Qin et al. (2016). Freeze-dried gel samples were mounted onto a scanning electron microscopy (SEM) plate using conductive carbon tabs and coated with gold. The three-dimensional network microstructure of the heat-induced WG gels was then observed and photographed using a JSM-6490LV scanning electron microscope (JEOL Ltd., Tokyo, Japan) at a 20-kV acceleration voltage.
Statistical Analysis
In this study, all experiments were performed in triplicate. Data are presented as means ± standard deviation, and statistical significance levels for the data were analyzed using SPSS 19.0 (IBM, Armonk, NY, USA) and expressed by analysis of variance (ANOVA) (p < 0.05). Figures were generated by the Origin software version 8.0 (OriginLab Corp., Northampton, MA, USA).
Results and Discussion
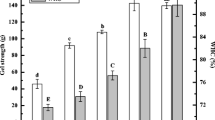
Gel Strength and WHC
Gel strength and WHC are important properties of protein gels and characterize the interactions between protein and water in the gel system. Higher gel strength and WHC contribute to better springiness and chewiness of meat products such as sausages (Colmenero 2000). High-pressure treatment promotes partial unfolding of protein molecules and exposure of hydrophobic groups. This exposure enhances hydrophobic interactions between protein molecules, increasing the strength of the gel network. The changes in gel strength and WHC in heat-induced WG gels are shown in Fig. 1. Gel strength and WHC significantly increased with pressure treatment, reaching their maximums of 93.8 ± 1.93 g and 79.47 ± 0.7%, respectively, at 300 MPa. He et al. (2014) reported that high-pressure treatment significantly enhanced the strength of rapeseed gel, which they speculated to be caused by changes of hydrophobic interactions between protein molecules. In addition, Zhu et al. (2015) reported that a uniform and dense microstructure could better bind the water to the gel network, thus increasing gel strength and WHC. And they found that high-pressure treatment caused proteins from mackerel surimi to form more uniform and compact three-dimensional gel networks. Further, Fig. 1 shows that when the treatment pressure reached 400 MPa, gel strength and WHC decreased. A study of the effects of high pressure on the gel strength and WHC of Nemipterus virgatus surimi gel reported similar results (Ma et al. 2015). This may be due to the excessive pressure resulting in the aggregation and folding of proteins, and some free SH and hydrophobic groups are embedded, which is not conducive to the formation of a more uniform and dense gel network.
Water Distribution Analysis
Differential scanning calorimetry is based on differences in phase transition behavior of different water states and is often used to analyze the migration and distribution of water in polymer systems. In such a system, water might bind to the functional groups of polymers or become encapsulated by the network of the system, both of which significantly affect the texture and WHC of the system (Tananuwong and Reid 2004). Figure 2 shows the changes in water distribution in the gel system. As pressure increased, the Wt and Wnf of the gel system showed an increasing trend, with maximum values of 77.64% and 13.61%, respectively, at 300 MPa. Liu (Liu et al. 2013) studied the effects of high-pressure homogenization on water distribution in tofu. High-pressure treatment significantly increased the Wnf of tofu and enhanced its texture and WHC. Notably, the effects of high pressure on Wt and Wnf were similar to those on the WHC of gel systems in the present study. Cao (Cao et al. 2017) studied the water distribution in tofu prepared using organic acid coagulants and observed that higher Wt and Wnf were related to higher WHC, higher gel strength, and more stable gel structures. This may explain why Wt and Wnf decreased when the pressure reached 400 MPa.
Dynamic Rheological Properties
Rheological measurements were used to characterize the gel formation and gel capabilities of the protein. G′ represents the elasticity of the gel network structure, and the viscoelasticity is usually applied to study the structure of a protein gel during its formation (Yoon et al. 2004). Figure 3 shows the changes in G′ during the cooling phase of heat-induced WG gel formation. During gel formation, gels prepared from high-pressure-treated WG showed higher G′ values as the pressure increased. The values reached a maximum of 5831.70 Pa at 300 MPa, significantly higher than the control group value of 2584.20 Pa. This trend is similar to that in the effect of high pressure on the gel strength and WHC of heat-induced WG gels. Speroni et al. (Speroni and Añón 2013) obtained similar results when preparing cold-set gels of soybean protein with high-pressure pretreatment. This phenomenon is due to the continuous formation of the gel network during the cooling phase, which is related to the formation of new hydrogen bonds and the exposure of free SH groups to generate new disulfide bonds and hydrophobic interactions (Wang et al. 2017). Therefore, the significant increase in the G′ of high-pressure-treated WG can be considered related to the high-pressure treatment exposing more hydrophobic groups and SH, leading to more disulfide bonds, stronger hydrophobic interactions, and more flexible networks during the gel-forming phase.
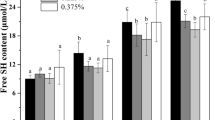
Free SH Content and Surface Hydrophobicity Analysis
Free SH groups are reactive groups on the protein surface, and surface hydrophobicity represents the number of hydrophobic groups on the protein surface. Changes in free SH content and surface hydrophobicity can significantly affect the stability and gelation properties of proteins, and are important indicators for evaluating the conformation of proteins. Figure 4 shows the changes in free SH content and surface hydrophobicity in WG gels. As pressure increased, free SH content shows an upward trend. The free SH content reached its 23.55 ± 0.23-μmol/g maximum at 300 MPa, which was significantly higher than that of the controls (12.41 ± 0.09 μmol/g). Similarly, Zhou et al. (2014) reported that 30–50 min of high-pressure treatment significantly increased the free SH content of golden thread myosin gels. Further, Ko et al. (2003) reported that the free SH content of tilapia myosin gels increased significantly after treatment with pressure greater than 50 MPa.
ANS is an efficient fluorescent probe that can be connected to non-polar moieties of protein molecules, and its fluorescence intensity represents the number of hydrophobic groups on the surfaces of proteins. Alvarez et al. (Pedroa et al. 2008) reported that as treatment pressure increases, more ANS can be attached to the non-polar groups on a molecule, resulting in increased fluorescence intensity. In this study, fluorescence intensity was measured to characterize the changes in surface hydrophobicity of WG gels. Figure 4 shows that as pressure increased, the surface hydrophobicity of WG gradually increased. When the pressure was 300 MPa, the surface hydrophobicity reached its maximum of 25.36 ± 0.21. This indicates that high-pressure treatment promotes partial unfolding of WG, exposing hydrophobic regions and free SH embedded in the interior of the molecules. These changes in free SH content and surface hydrophobicity of WG also support our conclusions related to the effects of high-pressure treatment on gel strength, WHC, and rheological properties.
Secondary Structure Analysis
FTIR is a common analytical method used to study protein conformation and reveals the secondary structure of a protein, including α-helices (1650–1660 cm), β-sheets (1610–1640 and 1670–1690 cm), β-turns (1660–1670 and 1690–1700 cm), and random coils (1640–1650 cm) through a specific vibrational frequency (400–4000 cm). The vibration frequency of the amide I band (1600–1700 cm) depends on hydrogen bonds between C=O and N-H (Wang et al. 2014). Each secondary structure is represented by the percentage of the total amide I band area occupied by the corresponding region. The proportion of each secondary structure in the WG treated by high-pressure was calculated as shown in Table 1. β-Sheet and random coil content increased with pressure. On the other hand, α-helix and β-turn content decreased gradually.
Hydrogen bonding is one of the major forces that maintain the α-helix. In addition, according to Tang and Ma (2009), the strength of the amide I band reflects the degree of stretch of the protein structure, and stretching of the protein structure breaks some hydrogen bonds, decreasing the α-helix content of WG. Hydrogen bonds in the β-sheets of globular proteins can be formed between different peptide chains, between different molecules, and between different peptide segments of the same peptide chain. As the high-pressure treatment upsets the original structure of WG, hydrogen bonds within the molecule also formed, resulting in increased β-sheet content. In addition, a decrease in β-turn content results in increased random coil content.
Microstructure of Heat-Induced WG Gels
SEM determination can be used to evaluate the effect of high-pressure treatment on the three-dimensional network of heat-induced WG gels. The three-dimensional network is an important factor that influences rheological properties, gel strength, and WHC. In general, the uniformity and density of the gel network affect the taste and quality of the gel-based food. Cao et al. prepared a tofu with higher WHC and hardness by making the gel network denser (Cao et al. 2017). Figure 5 shows the changes in microstructure of the heat-induced WG gels. The gel network of control gels exhibits larger and irregular pores, whereas the high-pressure-treated, heat-induced WG gels have smaller pores and uniform and dense three-dimensional networks.
In addition, similar results were observed in studies of the effects of high pressure on the gelation properties of heat-induced chicken breast protein gels (Chen et al. 2014). Our previous studies (Wang et al. 2017) showed that the unfolding and aggregation of the protein molecules are two major steps in the network formation of heat-induced WG gels. During the extension of the molecular structure of WG, exposure of more free SH and hydrophobic groups contributes to the formation of a more dense gel network structure. Therefore, treatment with high pressure leads to more hydrophobic interactions and formation of disulfide bonds and hydrogen bonds, forming a gel network with smaller, uniform, and dense pores. Ma et al. reported that the formation of a more dense gel network may be due to the high-pressure treatment increased the breaking force, rupture activity, and breaking displacement of WG gels (Ma et al. 2015).
Conclusions
This study showed that high-pressure treatment (100–400 MPa) resulted in secondary structural changes in WG due to partial unfolding or folding of its molecular structure. The treatment also increased β-sheet and random coil content and decreased of α-helix and β-turn content, which in turn enhanced the viscoelasticity of WG. At the same time, high-pressure treatment exposed more free SH and hydrophobic groups within WG, significantly enhancing its surface hydrophobicity and resulting in more disulfide bonds during gel formation and enhanced hydrophobic interactions between protein molecules in the gel system. Due to structural changes, the properties of heat-induced WG gels, such as storage modulus (G′), gel strength, water-holding capacity, and non-freezable water content, were significantly enhanced, and a more dense and uniform three-dimensional gel network was formed. The results of this study indicated that the high-pressure treatment-mediated unfolding of protein structure facilitates the formation of WG gels. Therefore, high-pressure treatment might be considered as a method to effectively improve the properties of heat-induced WG gels.
References
Byaruhanga, Y. B., Emmambux, M. N., & Belton, P. S. (2006). Alteration of Kafirin and Kafirin film structure by heating with microwave energy and tannin Complexation. Journal of Agricultural & Food Chemistry, 54(12), 4198–4207.
Cao, F. H., Li, X. J., Luo, S. Z., Mu, D. D., Zhong, X. Y., Jiang, S. T., & Zhao, Y. Y. (2017). Effects of organic acid coagulants on the physical properties of and chemical interactions in tofu. LWT- Food Science and Technology, 85, 58–65.
Chan, K. Y., & Wasserman, B. P. (1993). Direct colorimetric assay of free thiol groups and disulfide bonds in suspensions of solubilized and particulate cereal proteins. Cereal Chemistry, 70(1), 22–26.
Chen, F. L., Wei, Y. M., & Zhang, B. (2010). Characterization of water state and distribution in textured soybean protein using DSC and NMR. Journal of Food Engineering, 100(3), 522–526.
Chen, X., Chen, C. G., Zhou, Y. Z., Li, P. J., Ma, F., Nishiumi, T., & Suzuki, A. (2014). Effects of high pressure processing on the thermal gelling properties of chicken breast myosin containing κ-carrageenan. Food Hydrocolloids, 40(ISSN), 262–272.
Colmenero, F. J. (2000). Relevant factors in strategies for fat reduction in meat products. Trends in Food Science & Technology, 11(2), 56–66.
Day, L., Augustin, M. A., Batey, I. L., & Wrigley, C. W. (2006). Wheat-gluten uses and industry needs. Trends in Food Science & Technology, 17(2), 82–90.
Dondero, M., Figueroa, V., & Morales, X. (2006). Transglutaminase effect on gelation capacity of thermally induced beef protein gels. Food Chemistry, 99(3), 546–554.
He, R., He, H. Y., Chao, D., Ju, X., & Aluko, R. (2014). Effects of high pressure and heat treatments on physicochemical and gelation properties of rapeseed protein isolate. Food & Bioprocess Technology, 7(5), 1344–1353.
Hu, H., Fan, X., Zhou, Z., Xu, X., Fan, G., Wang, L., & Zhu, L. (2013). Acid-induced gelation behavior of soybean protein isolate with high intensity ultrasonic pre-treatments. Ultrasonics Sonochemistry, 20(1), 187–195.
Ibanoglu, E. (2005). Effect of hydrocolloids on the thermal denaturation of proteins. Food Chemistry, 90(4), 621–626.
Irisj, J., Bert, L., & Jana, D. (2009). Endogenous redox agents and enzymes that affect protein network formation during breadmaking -- a review. Journal of Cereal Science, 50(1), 1–10.
Ko, W. C., Jao, C. L., & Hsu, K. C. (2003). Effect of hydrostatic pressure on molecular conformation of Tilapia (Orechromis niloticus) myosin. Journal of Food Science, 68(4), 1192–1195.
Kong, X., Zhou, H., & Hua, Y. (2010). Preparation and antioxidant activity of wheat gluten hydrolysates (WGHs) using ultrafiltration membranes. Journal of the Science of Food & Agriculture, 88(5), 920–926.
Liu, H. H., Chien, J. T., & Kuo, M. I. (2013). Ultra high pressure homogenized soy flour for tofu making. Food Hydrocolloids, 32(2), 278–285.
Ma, X. S., Yi, S. M., Yu, Y. M., Li, J. R., & Chen, J. R. (2015). Changes in gel properties and water properties of Nemipterus virgatus surimi gel induced by high-pressure processing. LWT - Food Science and Technology, 61(2), 377–384.
Maningat, C. C., Demeritt, G. K. J., Chinnaswamy, R., et al. (1999). Properties and applications of texturized wheat gluten. Cereal Foods World, 44(9), 650–655.
Meng, G. T., & Ma, C. Y. (2001). Thermal properties of Phaseolus angularis (red bean) globulin. Food Chemistry, 73(4), 453–460.
Moreno, H. M., Bargiela, V., Tovar, C. A., Cando, D., Borderias, A. J., & Herranz, B. (2015). High pressure applied to frozen flying fish ( Parexocoetus brachyterus ) surimi: Effect on physicochemical and rheological properties of gels. Food Hydrocolloids, 48(4), 127–134.
Pedroa, A., Hosahallis, R., & Ashrafa, I. (2008). High pressure gelation of soy proteins: Effect of concentration, pH and additives. Journal of Food Engineering, 88(3), 331–340.
Qin, X. S., Luo, S. Z., Cai, J., Zhong, X. Y., Jiang, S. T., Zheng, Z., & Zhao, Y. Y. (2016). Effects of microwave pretreatment and transglutaminase crosslinking on the gelation properties of soybean protein isolate and wheat gluten mixtures. Journal of the Science of Food & Agriculture, 96(10), 3559–3566.
Qiu, C., Xia, W., & Jiang, Q. (2013). Effect of high hydrostatic pressure (HHP) on myofibril-bound serine proteinases and myofibrillar protein in silver carp (Hypophthalmichthys molitrix). Food Research International, 52(1), 199–205.
Santhi, D., Kalaikannan, A., & Sureshkumar, S. (2015). Factors influencing meat emulsion properties and product texture: A review. Critical Reviews in Food Technology, 57(10), 2021–2027.
Speroni, F., & Añón, M. C. (2013). Cold-set gelation of high pressure-treated soybean proteins. Food Hydrocolloids, 33(1), 85–91.
Tananuwong, K., & Reid, D. S. (2004). DSC and NMR relaxation studies of starch–water interactions during gelatinization. Carbohydrate Polymers, 58(3), 345–358.
Tang, C. H., & Ma, C. Y. (2009). Effect of high pressure treatment on aggregation and structural properties of soy protein isolate. LWT - Food Science and Technology, 42(2), 606–611.
Tang, C. H., Chen, L., & Foegeding, E. A. (2011). Mechanical and water-holding properties and microstructures of soy protein isolate emulsion gels induced by CaCl2, glucono-δ-lactone (GDL), and transglutaminase: Influence of thermal treatments before and/or after emulsification. Journal of Agricultural & Food Chemistry, 59(8), 4071–4077.
Wang, Z., Li, Y., Jiang, L., Qi, B., & Zhou, L. (2014). Relationship between secondary structure and surface hydrophobicity of soybean protein isolate subjected to heat treatment. Journal of Chemistry, 2014(5), 1–10.
Wang, K. Q., Luo, S. Z., Zhong, X. Y., Cai, J., Jiang, S. T., & Zheng, Z. (2017). Changes in chemical interactions and protein conformation during heat-induced wheat gluten gel formation. Food Chemistry, 214, 393–399.
Xue, S., Yang, H., Wang, H., Tendu, A. A., Bai, Y., Xu, X., & Zhou, G. (2017). High-pressure effects on the molecular aggregation and physicochemical properties of myosin in relation to heat gelation. Food Research International, 99(Pt 1), 413–418.
Yoon, W. B., Gunasekaran, S., & Park, J. W. (2004). Characterization of thermorheological behavior of Alaska Pollock and Pacific whiting surimi. Journal of Food Science, 69(7), 338–343.
Yoshida, H., Hatakeyama, T., & Hatakeyama, H. (1993). Characterization of water in polysaccharide hydrogels by DSC. Journal of Thermal Analysis, 40(2), 483–489.
Zhang, Z., Yang, Y., Tang, X., Chen, Y., & You, Y. (2015). Chemical forces and water holding capacity study of heat-induced myofibrillar protein gel as affected by high pressure. Food Chemistry, 188, 111–118.
Zhou, A., Lin, L., Liang, Y., Benjakul, S., Shi, X., & Liu, X. (2014). Physicochemical properties of natural actomyosin from threadfin bream (Nemipterus spp.) induced by high hydrostatic pressure. Food Chemistry, 156(4), 402–407.
Zhu, Z., Lanier, T. C., & Farkas, B. E. (2015). High pressure effects on heat-induced gelation of threadfin bream (Nemipterus spp.) surimi. Journal of Food Engineering, 146, 23–27.
Funding
This research was funded by The National Key Research and Development Program of China (Grant Nos. 2018YFD0400600 and 2018YFD0400400), the National Natural Science Foundation of China (Grant No. 31701638), and the Key Scientific and Technological Project of Anhui Province of China (Grant Nos. 16030701082 and 17030701014).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, B., Liu, F., Luo, S. et al. Effects of High Hydrostatic Pressure on the Properties of Heat-Induced Wheat Gluten Gels. Food Bioprocess Technol 12, 220–227 (2019). https://doi.org/10.1007/s11947-018-2205-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-018-2205-3