Abstract
Parkinson’s disease is now widely recognized to be a multisystem disorder affecting the brain and peripheral autonomic nerves. Extensive pathology is present in both the sympathetic and parasympathetic nervous system and the intrinsic gastrointestinal plexuses in patients. Autonomic pathology and symptoms such as constipation can predate the clinical diagnosis by years or decades. Imaging studies have contributed greatly to our understanding of Parkinson’s disease but focused primarily on imaging cerebral pathology. However, given the importance of understanding the nature, chronology, and functional consequences of peripheral pathology, there has been renewed interest in imaging peripheral organs in Parkinson’s disease. Suitable imaging tools can be divided into two types: radiotracer studies that directly estimate loss of sympathetic or parasympathetic nerve terminals, and imaging modalities to quantitate dysphagia, gastric emptying, esophageal and intestinal transit times, and anorectal dyssynergia. In this review, we summarize current knowledge about peripheral imaging in Parkinson’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Functional imaging studies have contributed greatly to our understanding of Parkinson’s disease (PD) in recent decades, but mainly focused on imaging cerebral pathology [1, 2]. However, PD is now in many ways considered a systemic disorder with profound involvement of the peripheral and enteric nervous systems [3]. Pathological α-synuclein aggregates are present in the sympathetic and parasympathetic nervous system and throughout the gastrointestinal canal in all PD patients [4••, 5]. These pathological features can be detected in the pre-clinical disease phase [6, 7], and autonomic symptoms of the gastrointestinal and genitourinary tracts may also predate clinical diagnosis [8–10]. The progressive aggregation of α-synuclein seems to be initiated in terminals of hyperbranching, non-myelinated neurons and spread via retrograde axonal transport, explaining why autonomic nerves are so prone to formation of Lewy pathology [11•, 12].
Functional imaging may play an important part in elucidating the importance and chronological sequence of peripheral pathology in PD. The imaging modalities can be divided into two types: (1) radiotracer studies with the ability to directly image the loss of cellular or molecular structures, such as sympathetic or parasympathetic nerve terminals, and (2) imaging methods that measure the functional consequences of these pathologies, such as radiotracer and radiological studies of dysphagia, gastric emptying, esophageal and intestinal transit times, and anorectal dysfunction. The present review will summarize principles and main findings of these diverse methods for imaging peripheral pathology in PD.
Imaging the Sympathetic Nervous System
Pathological α-synuclein inclusions are present in the sympathetic ganglia and intermediolateral column of the medulla in the vast majority of examined PD patients [4••, 13]. Orimo and colleagues showed that accumulation of α-synuclein aggregates in distal, cardiac sympathetic axons precedes that of the neuronal cell bodies [14]. A recent, large post-mortem series reported that the density of α-synuclein inclusions in the ganglia is greater than that found in the intermediolateral column [13]. Taken together, these finds are compatible with a chronological, peripheral-to-central spreading pattern of α-synuclein inclusions in the sympathetic nervous system.
The post-ganglionic, noradrenergic nerve terminals of the sympathetic nervous system can be imaged with 123I-meta-iodobenzylguanidin (MIBG), which is an analogue of the adrenergic blocking ligand guanethidine. Its uptake and storage is similar to norepinephrine, and MIBG can therefore be used to assess in vivo the integrity of sympathetic nerve terminals. Function of these terminals can also be imaged with the PET ligand 18F-fluorodopamine, which is enzymatically converted to 18F-fluoronorepinephrine by dopamine-β-hydroxylase, and stored in noradrenergic vesicles [15].
Cardiac Imaging
The majority of sympathetic imaging studies in PD have investigated myocardial denervation. This literature has been extensively reviewed in several recent publications [16–18], so only main findings and principles will be summarized here.
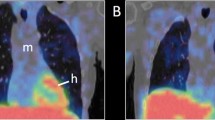
MIBG is administered intravenously and its myocardial uptake detected by gamma camera recordings at 15 min (early phase) and 3–4 h post-injection (late phase). Most studies acquire simple planar images of the thoracic region and a striking loss of cardiac signal is visually apparent in the majority of PD patients (Fig. 1). In addition to simple visual interpretation, the cardiac uptake is semi-quantitatively evaluated by calculating the heart-to-mediastinum (H/M) signal ratio. The purpose of early and late imaging is to evaluate tracer delivery and then storage. Whereas most healthy subjects display increasing H/M ratios from the early to late image time points, the opposite is true for PD and dementia with Lewy bodies (DLB) patients [19]. The more rapid tracer wash-out is believed to be caused by a lack of storage vesicles in diseased sympathetic terminals, which facilitates enhanced tracer clearance [20]. Recently, several MIBG studies have demonstrated that 3D tomographical imaging has superior diagnostic accuracy to planar imaging, as it allows detailed cardiac segmental analysis [21, 22].
Cardiac MIBG signal is decreased in 80–90 % of patients with clinical probable PD [23–26], though only ∼50 % of Hoehn and Yahr 1 cases, whereas patients with atypical parkinsonian disorders, such as PSP, CBD, and multiple system atrophy (MSA) most often show normal or near-normal cardiac signal [19, 23]. Two meta-analyses demonstrated pooled sensitivity and specificity of ∼85 % for separating PD from other parkinsonian disorders [27, 28]. Cardiac MIBG uptake is also decreased in a majority of rapid-eye-movement sleep behavior disorder (RBD) patients [29], many of whom go on to develop an alpha-synucleinopathy [30•]. One study has reported that RBD patients display a reduced cardiac MIBG signal comparable to that seen in moderate-to-late stage PD patients, and exceeding the signal loss seen in newly diagnosed PD patients without RBD [31]. Similar to PD, cardiac MIBG signal is decreased in ∼90 % of patients with DLB [18]. A recent study reported that the combination of MIBG and dopamine transporter SPECT imaging yielded sensitivity and specificity of 95 and 91 % for differentiating DLB from patients with Alzheimer’s disease, where cardiac sympathetic innervation and nigrostriatal dopamine innervation is preserved [32].
Some studies have reported an inverse correlation between cardiac MIBG uptake and Hoehn and Yahr stage and with the UPDRS motor score, but other studies have failed to detect such a correlation—recently reviewed by Orimo et al. [16]. The reduction of cardiac MIBG uptake may also be more pronounced in patients with the akinetic-rigid phenotype than tremor-predominant patients [33]. Orthostatic hypotension is a common symptom in treated PD [34], but its presence correlates only poorly with cardiac MIBG uptake, often being absent in those early cases who show reduced sympathetic cardiac innervation [35–37].
Imaging Non-cardiac Regions
The thyroid receives rich sympathetic innervation [38] and is visible on a normal MIBG planar image (Fig. 1). A marked reduction in the thyroid uptake of 18F-fluorodopamine and 123I-MIBG has been reported in PD patients compared to controls [39, 40] (Fig. 1c). Decreased 18F-fluorodopamine uptake has also been reported in the renal cortex of PD patients [39, 41]. Involvement of the kidneys in PD has received very little attention, but the renal cortex receives extensive sympathetic innervation [42], so it seems probable that autonomic denervation could also affect this organ.
Several recent studies have demonstrated the presence of pathological α-synuclein inclusions in the submandibular salivary glands of PD patients [4••, 43], and these glands are also innervated by the sympathetic nervous system [44]. However, the submandibular and parotid glands did not exhibit decreased 18F-fluorodopamine uptake in moderate-stage PD patients [39, 41], suggesting that sympathetic denervation of the salivary glands is insignificant. Alternatively, 18F-fluorodopamine may not be a specific marker of the sympathetic innervation of the salivary glands.
In summary, established PD and nearly all DLB patients display decreased uptake of MIBG and fluorodopamine in the heart, as do a majority of RBD cases; however, only 50 % of new PD cases have been reported to show loss of sympathetic innervation. These observations underscore the point that RBD constitutes a unique phenotype of PD. Some studies report a correlation of loss of cardiac MIBG uptake and motor severity, but levels of cardiac sympathetic denervation on imaging correlate poorly with cardiac autonomic symptoms. Decreased signal is also seen in the thyroid and renal cortex of PD cases but the clinical significance of this is unclear.
Imaging the Parasympathetic Nervous System
The dorsal motor nucleus of the vagus (DMV) may be the initial target structure in the brain stem of PD patients [45]. At post-mortem, the DMV exhibits a ∼50 % neuron loss [46, 47] and α-synuclein inclusions are found in the vagus nerve of most PD patients [4••]. Approximately 90 % of α-synuclein pathology in the gastrointestinal tract is in the form of neurites rather than inclusion bodies in the soma [48–50]. The distribution of pathological gastrointestinal α-synuclein inclusions displays a rostro-caudal gradient [4••, 5], which is similar to the density of vagal innervation [51]. These findings suggest that α-synuclein pathology may preferentially be located in vagal efferents rather than intrinsic enteric neurons. This view is further supported by reports of only minimal or no loss of enteric neurons in PD [52, 53]. It has been suggested that a neurotrophic pathogen may initiate or exacerbate the formation of α-synuclein inclusions in vagal terminals of the gut with secondary spreading in a prion-like fashion to the DMV [54]. This hypothesis has been reinforced by the observation that total truncal vagotomy in ulcer patients reduced their risk of PD by ∼50 % after decades of follow-up [55].
The ability to image parasympathetic dysfunction is an unmet medical need, but very little research has been done to develop functional imaging of this branch of the autonomic nervous system. One reason may be that parasympathetic, cholinergic neurons possess no distinctive molecular target, which would allow highly specific imaging. In histology, measurements of acetylcholinesterase (AChE) activity have been used for decades to assess the integrity of the parasympathetic and enteric nervous system [56, 57], and cardiac cholinergic innervation [58]. Since AChE is not exclusively produced by cholinergic neurons, immunohistochemical staining of a more specific target, the vesicular acetylcholine transporter (VAChT), has largely replaced AChE staining in histology. However, VAChT is also not a specific marker of parasympathetic neurons. For instance, the majority of the enteric neurons are cholinergic and express VAChT [59].
In addition to the lack of distinct molecular targets, successful parasympathetic imaging poses a unique problem, at least if the gastrointestinal innervation is to be visualized. Many otherwise suitable imaging tracers accumulate rapidly in the liver, and radioactive metabolites are soon excreted into the duodenum via the biliary system, rendering valid measurements of the small-intestine wall activity difficult or impossible. As such, a radiotracer for the gastrointestinal system requires slow biliary excretion kinetics or complete excretion via renal filtration.
The PET ligand 5-11C-methoxy-donepezil binds non-competitively and reversibly to AChE, and has been successfully used to visualize the AChE density in brains of patients with PD or Alzheimer’s disease [60, 61]. 11C-donepezil also exhibits specific, displaceable binding with nano-molar affinity to a range of peripheral tissues, including the stomach, intestine, and pancreas [62]. Importantly, no biliary excretion of radiometabolites is seen during a 60-min PET scan [63•]. An early study showed that sub-diaphragmatic vagotomy in guinea pigs induced a 50 % decrease of AChE activity in the upper gastrointestinal tract [57], suggesting that a PET ligand with affinity for AChE could be a valid marker of parasympathetic denervation.
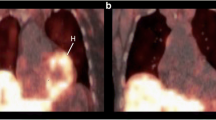
We recently investigated the suitability of 11C-donepezil to image parasympathetic denervation in 12 early-to-moderate stage PD patients and 12 healthy controls [63•]. The PD patients displayed a highly significant 35 % decrease in small-intestine signal, which was visually apparent in most individual patients (Fig. 2). Decreased signal in the pancreas was also seen in the majority of patients. No correlations were seen between the PET signal and disease duration, gastric emptying time, or severity of constipation.
11C-donepezil PET images. a Whole body PET scan of a healthy, male subject. b Summed PET images (55–60 min post-injection) of the upper abdominal region in a healthy control (top) and a PD patient (bottom). Note the visually apparent decrease in the small-intestine signal. l liver, p pancreas, s small intestine
The cardiac 11C-donepezil signal was significantly decreased by 9 % in the PD group, a much smaller reduction than seen with MIBG. It was recently demonstrated that the cardiomyocytes synthesize and secrete acetylcholine in a paracrine fashion and express VAChT [64]. It seems highly likely that the break-down enzyme AChE would also be synthesized by myocytes, although this was not measured by the authors. Nevertheless, this raises the possibility that the decreased cardiac 11C-donepezil signal in PD patients may reflect considerable parasympathetic denervation, the detection of which is obscured by concomitant intrinsic signal arising from the cardiomyocytes.
More 11C-donepezil studies are required to confirm that these findings are caused by parasympathetic denervation in PD patients. Studies should be conducted in groups of de novo PD patients, and prodromal RBD patients, given that parasympathetic dysfunction is thought to be among the first non-motor manifestations in prodromal PD.
A VAChT imaging ligand should theoretically be a better marker of parasympathetic innervation. A detailed biodistribution study of the VAChT ligand, 18F-fluoroethoxybenzovesamicol (FEOBV), was recently published by Petrou and colleagues [65]. However, radioactive FEOBV metabolites are rapidly excreted via the biliary system in 10–20 min in rodents and pigs (Borghammer, unpublished observation). Data on biliary excretion kinetics of FEOBV in humans have not been published, so it remains possible that FEOBV could successfully image the density of VAChTs in the gut, given that humans often exhibit slow liver metabolism compared to rodents. Even if it cannot, FEOBV may be able to estimate parasympathetic innervation in other organs, including the salivary glands, thyroid, heart, pancreas, and pelvic organs, none of which would be confounded by the presence of radiometabolites in the gastrointestinal lumen.
Gastrointestinal Functional Imaging
A full description of gastrointestinal symptoms in PD has recently been covered in detail [66, 67], and will be only briefly summarized in this review. The main focus here will be on imaging methods suitable for studying functional changes of the gastrointestinal tract in PD.
Oropharyngeal Imaging
A recent meta-analysis reported a pooled prevalence of subjective dysphagia of 35 % in PD patients, but prevalence estimates in individual studies ranged from 16 to 55 % [68•]. Aspiration is also a common problem in PD [69]. Dysphagia typically develops in later PD stages, but occasionally, it can be the presenting feature [70].
The pathophysiological substrate of dysphagia is not known, but has often been attributed to bradykinesia and rigidity secondary to basal ganglia dysfunction. However, hypometabolism in the supplementary motor area and the anterior cingulate cortex was found to correlate with dysphagia in PD [71]. Esophageal motility is primarily determined by neural pattern generators in the medulla oblongata. The upper esophagus receives vagal innervation from the nucleus ambiguus, and the lower esophagus receives its innervation from the DMV [72]. Lewy body pathology is present in both of these nuclei, although the DMV is much more severely affected [45, 73].
Swallowing function is most commonly evaluated by barium videofluoroscopy, where subjects swallow a bolus of thin barium suspension during a continuous x-ray [74]. Swallowing function for solids can be determined using barium-coated bread. Oral and pharyngeal transit times and oropharyngeal swallow efficiency are typically calculated. Residues in the vallecula and pyriform sinus and aspiration of liquid or solids can also be visualized.
Leopold and Kagel conducted a very detailed videofluoroscopic study of 72 PD patients, and found epiglottic dysmotility in 56 %, pharyngeal constrictor dysfunction in 42 %, esophageal dysmotility in 91 %, and gastro-esophageal reflux in 56 % of the patients [75]. Another study found disturbances of the oral and pharyngeal phase in 75 % of PD patients compared to only 8 % of controls. Seven of the patients improved their performance after levodopa [76]. A more recent study compared swallowing function in controls to PD patients with and without dyskinesias and surprisingly found no difference in oral and pharyngeal transit time among the groups. The efficacy of swallowing was decreased in the non-dyskinetic group, but not in the dyskinetic group perhaps explained by the greater levodopa intake in this group [77]. In addition, silent aspiration in PD patients was a frequent finding in these videofluoroscopy studies.
Dysphagia can also be investigated using scintigraphic methodology [78]. In short, the test subject swallows a bolus of radiolabeled water or semi-solid food, the passage of which is recorded by a gamma camera. Time-activity curves can then be determined in the oropharyngeal and esophageal subregions and stomach. Using combined scintigraphy and EMG measurements, Potulska et al. reported abnormal findings in all of 18 PD patients, although only 13 patients had subjective dysphagia. Importantly, prolonged esophageal transit time was the most prominent feature in the PD group [79].
In summary, subjective dysphagia is a relative common non-motor symptom in PD patients, but often shows poor correlation to objective measures. Moreover, imaging studies and other modalities demonstrate that the vast majority of patients have objectively quantifiable dysfunction in the oropharyngeal and esophageal motility [68•]. Interestingly, objective esophageal dysfunction may be even more prevalent than oropharyngeal dysfunction, which could be explained by the observation that the esophageal mucosa consistently displays the most severe α-synuclein pathology in the entire gastrointestinal tract [4••, 5].
Gastric Imaging
The rhythmic contractions of the stomach wall are generated by interstitial pacemaker cells of Cajal [80], but considerable autonomic modulatory input from the CNS also controls volume, contraction strength, and acid secretion [81, 82].
Gastroparesis is a common manifestation in PD and presents with symptoms of nausea, vomiting, early satiety, and bloating [83, 84]. Nausea and vomiting is present in 15 % of PD patients [85] and abdominal fullness in up to 50 % [86]. The underlying pathology of these symptoms is unclear, but α-synuclein pathology in the DMV and vagal efferents and intrinsic pathology in the enteric neurons of the stomach wall [4••, 87] is believed to interfere with gastric motility.
Solid meal gastric emptying scintigraphy is considered the reference standard for evaluation of gastric emptying time (GET) [88]. A radioactive standard meal is ingested and followed by serial scintigraphic images until 90 % emptying is reached. The presence of prolonged GET in PD is often cited in the literature, but relatively few studies have employed solid meal scintigraphy to measure GET in PD patients. An early study saw no difference in GET between PD patients and elderly controls, but both groups differed from young controls [89]. Another study found that PD patients with fluctuating symptoms showed delayed GET compared to non-fluctuating patients, and both patient groups had delayed GET compared to controls [90]. Krygowska-Wajs et al. detected delayed GET in patients with familial PD, but only a non-significant trend towards prolonged GET in idiopathic PD [91]. In contrast, a recent study of 12 early-to-moderate PD patients in the off-state found that GET was significantly faster in the patients [63•]. No significant difference in GET was detected between treated and untreated PD patients [92]. Thus, although many PD patients certainly have delayed gastric emptying, the findings in the literature is somewhat mixed. It is also not clear to what degree the presence of prolonged GET correlates with subjective symptoms of gastroparesis or medication state. Most of the cited studies reported a wide range in the GET data, often with substantial overlap with control values. Indeed, in our recent series, we saw two PD patients with extremely rapid GET (T 1/2 < 30 min) suggestive of gastric dumping [63•]. Similar rapid emptying in some PD patients was also reported by other authors [91, 92].
Gastric emptying can also be estimated with the 13C-sodium breath test. In short, a meal containing 13C-sodium octanoate or acetate is ingested, subsequently absorbed in the jejunum, and metabolized in the liver to 13CO2. The concentration of 13CO2 expired from the lungs is measured and can be converted to an estimate of GET [93]. Both solid and liquid meal breath tests have been utilized to examine GET in PD patients [93–99]. Most of these studies reported significantly increased GET in the PD group compared to controls with both meal types. However, the breath test is dependent not only on gastric emptying but also successful small-intestine absorption, which is known to be deranged in PD [100]. Thus, the somewhat more consistently prolonged GET in breath test studies could be explained by a dual pathology of delayed gastric emptying and small-intestine dysfunction.
Small-Intestine Imaging
The small intestine has received little attention in PD research, and very few imaging studies have been conducted. Recently, Dutkiewicz and colleagues assessed the small-bowel transit time using serial SPECT scans in 10 PD patients with no gastrointestinal complaints and 10 control subjects after ingestion of a capsule containing the gamma emitting isotope 99mTc [101]. In all healthy controls, the capsule had left the small intestine within 4 h, whereas the capsule was still present in the small intestine in seven PD patients at 4 h and in one patient at 24 h. This study suggests that the oro-cecal transit time, and probably the small-intestine transit time, is prolonged in PD patients. This interpretation is supported by another study that evaluated colonic transit time in six PD patients using radio-opaque markers and CT scans [102]. Five PD patients had prolonged colonic transit time, and two patients also retained radio-opaque markers in the small intestine 24 h after ingestion of the final capsule suggesting prolonged small intestinal transit time.
Colonic Imaging
Constipation is among the most frequent non-motor symptoms in PD with prevalence estimates ranging from 30 to 70 % [103–105]. Thus, subjectively reported constipation shows considerable variation in PD, which is probably related to variable definitions of constipation applied in the studies. Constipation is more frequent in PD patients with RBD compared to those without RBD [106], and it may be the first non-motor symptom in prodromal PD, appearing >10 years prior to diagnosis in a sizeable fraction of PD patients [8, 9].
Several studies reported Lewy pathology in the nerve terminals of the submucosal and myenteric plexus of the colon [50, 53]. The vagus innervates the upper two thirds of the colon, and consistently exhibits Lewy pathology in the axons and the DMV. The distal part of the colon receives its parasympathetic innervation from preganglionic neurons situated in the sacral part of the intermediolateral cell column. These neurons are involved in controlling colonic motility and also cause contraction of the striated detrusor bladder muscles during micturition [107, 108]. The sacral parasympathetic neurons display consistent α-synuclein pathology and probably cell loss in PD patients and incidental Lewy body cases, but it remains unclear to what degree these pathologies are responsible for the colonic dysmotility [109•, 110].
The most widely used method for measuring colonic transit is the radio-opaque marker (ROM) technique. A defined number of radio-opaque plastic markers containing barium sulfate enclosed within a gelatin capsule are ingested for a defined number of days prior to radiological imaging. A planar x-ray image of the abdomen is performed 24 h after ingestion of the last capsule, and the segmental and total colonic transit time (CTT) can be calculated based on the number of retained markers [111].
Using ROM methodology, several studies reported increased CTT in PD patients compared to healthy controls [112–115]. An early study reported that many de novo PD patients did not have increased CTT [116]. However, the authors applied a very strict definition of increased CTT in the study. Had they used the most commonly and best validated cutoff score for number of retained ROM [111], a different conclusion emerges from their data, namely that 80 % of de novo PD patients exhibit prolonged CTT. Ashraf et al. found no correlation between number of bowel movements per week and CTT or subjectively reported constipation in PD patients, suggesting that objectively measured colonic pathology correlates poorly with subjective symptoms [117]. Tateno and colleagues found no difference in CTT in PD patients on and off levodopa treatment, suggesting that colonic function is affected mainly by disease involvement [118]. Interestingly, one study found no significant difference in CTT between PD and MSA patients, although their sample size was relatively small [119].
It has been proposed that an even distribution of ROM throughout the colon is indicative of slow-transit time constipation, whereas a predominance of ROM in the descending segments signifies outlet obstruction constipation [120]. This distinction is important in the context of PD, since many patients suffer from dyssynergic defecation characterized by inability of the pelvic muscles to relax during defecation [115, 121]. This observation is further supported by the consistent high prevalence of subjectively reported straining during defecation in PD patients, which tends to be a more prevalent symptom than decreased number of defecations [103, 122]. Three CTT studies investigated segmental distribution of ROM in the colon of PD patients, and generally reported particularly prolonged rectosigmoid CTT [112, 115, 118]. This suggests that constipation in PD is often of the “outlet obstruction” type.
We recently measured colonic volume in 24 early-to-moderate stage PD patients and 15 controls on abdominal CT scans, and found significantly larger volumes of transverse and descending colonic segments in the patients [102]. Volume measurements of the colon may provide a novel method to study gastrointestinal pathology in PD. Finally, two very early case studies reported severe megacolon in relatively late stage patients [123, 124]. Massive dilation and, in several cases, obstruction of the colon was seen subsequent to a barium meal or enema. The dilation was often more distinct in the rectosigmoid segment. Taken together, these results show that the colon volume is significantly increased in the more distal segments in PD patients and confirm that constipation may often be of the outlet obstruction type.
Rectoanal Imaging
Propulsive reflexes of the distal colorectum and defecation are controlled by central defecation centers in the lumbosacral spinal cord [125, 126]. As covered above, the sacral parasympathetic nuclei, including Onuf’s nucleus, show consistent Lewy body pathology in PD patients at post-mortem, which may contribute to dyssynergic defecation.
Rectoanal dysfunction is very common in PD and contributes to the high prevalence of constipation. Several studies reported that straining for defecation is present in up to 83 % of PD patients [103, 122, 127]. Edwards et al. showed that 67 % PD patients at early-to-moderate disease stage exhibit defecatory problems such as straining and feeling of incomplete emptying. Only 30 % of the same patients had less than three bowel movements per week as a marker of constipation [128]. Excessive straining and a feeling of partial or incomplete emptying are known to be indicators of outlet obstruction constipation as opposed to slow-transit constipation [120].
Rectoanal function can be evaluated using defecography. In short, a barium contrast medium is instilled into the rectum and subsequently monitored by x-ray images at rest, contraction, straining, and defecation to measure emptying rate, relaxation of pelvic floor muscles, and pathological features such as rectal prolapse [114, 120]. Defecography is evaluated along with physiological measures, including anorectal manometry, which record rectal and anal pressure during rest and contraction, and electromyography (EMG), exploring the contraction function of the puborectalis muscles [120].
Several studies investigated rectoanal function in PD patients. An early study of six patients in the off-state reported paradoxical contraction of the puborectalis and external anal sphincter muscles during straining and incomplete rectal emptying, which was improved after apomorphine administration [121]. Other studies showed that PD patients had non-significantly increased rectal volume at first sensation, decreased rectal contraction, and significantly increased post-defecation residual volume [115, 129]. Wang et al. found a significant correlation between difference in anorectal angle at rest and straining and total CTT, demonstrating that prolonged CTT is in part determined by dyssynergy in the pelvic floor musculature [112]. Botulinum toxin injection in the puborectalis muscle significantly decreased manometric anorectal tone and improved the anorectal angle during straining [130].
Conclusions
Imaging methods provide attractive non-invasive tools to study the involvement of the peripheral nervous system in PD. Most PD patients show sympathetic denervation of the myocardium and probably also the thyroid and renal cortex. The PET ligand 11C-donepezil may provide a novel method of visualizing the marked parasympathetic denervation known to occur in most patients. A range of functional techniques clearly demonstrate dysfunction in all parts of the gastrointestinal tract, which, importantly, are often more frequent than the subjective symptoms experienced by the patients. This is important since the presence of subjective non-motor symptoms is increasingly being used to categorize different phenotypes of PD patients and to identify prodromal PD patients in the population [131]. Thus, objective measures of peripheral pathology in PD may have utility not only for expanding our understanding of this disorder but also for guiding treatment of the troublesome non-motor symptoms and for facilitating prodromal diagnosis.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Weingarten CP, Sundman MH, Hickey P, Chen NK. Neuroimaging of Parkinson’s disease: expanding views. Neurosci Biobehav Rev. 2015;59:16–52. doi:10.1016/j.neubiorev.2015.09.007.
Brooks DJ, Pavese N. Imaging biomarkers in Parkinson’s disease. Prog Neurobiol. 2011;95:614–28. doi:10.1016/j.pneurobio.2011.08.009.
Jellinger KA. The pathomechanisms underlying Parkinson’s disease. Expert Rev Neurother. 2014;14:199–215. doi:10.1586/14737175.2014.877842.
Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi:10.1007/s00401-010-0664-3. The study demonstrated widespread alpha-synuclein pathology throughout the gastrointestinal canal in most PD patients, and furthermore that a distinct cranio-caudal gradient is present in the severity of pathological involvement.
Gelpi E, Navarro-Otano J, Tolosa E, Gaig C, Compta Y, Rey MJ, et al. Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov Disord. 2014;29:1010–8. doi:10.1002/mds.25776.
Hilton D, Stephens M, Kirk L, Edwards P, Potter R, Zajicek J, et al. Accumulation of alpha-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2014;127:235–41. doi:10.1007/s00401-013-1214-6.
Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord. 2012;27:716–9. doi:10.1002/mds.25020.
Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57:456–62.
Adams-Carr KL, Bestwick JP, Shribman S, Lees A, Schrag A, Noyce AJ. Constipation preceding Parkinson’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2015. doi:10.1136/jnnp-2015-311680
Noyce AJ, Lees AJ, Schrag AE. The prediagnostic phase of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2016. doi:10.1136/jnnp-2015-311890
Uchihara T, Giasson BI. Propagation of alpha-synuclein pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol. 2016;131:49–73. doi:10.1007/s00401-015-1485-1. This is a thoughtful and critical review of whether or not PD is characterized by predictable spreading of misfolded protein along specific anatomic pathways.
Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna). 2003;110:517–36. doi:10.1007/s00702-002-0808-2.
Sumikura H, Takao M, Hatsuta H, Ito S, Nakano Y, Uchino A, et al. Distribution of alpha-synuclein in the spinal cord and dorsal root ganglia in an autopsy cohort of elderly persons. Acta Neuropathol Commun. 2015;3:57. doi:10.1186/s40478-015-0236-9.
Orimo S, Uchihara T, Nakamura A, Mori F, Kakita A, Wakabayashi K, et al. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain. 2008;131:642–50. doi:10.1093/brain/awm302.
Goldstein DS, Chang PC, Eisenhofer G, Miletich R, Finn R, Bacher J, et al. Positron emission tomographic imaging of cardiac sympathetic innervation and function. Circulation. 1990;81:1606–21.
Orimo S, Yogo M, Nakamura T, Suzuki M, Watanabe H. Brain imaging in Aging Special Issue of Ageing Research Reviews I-meta-iodobenzylguanidine (MIBG) cardiac scintigraphy in alpha-synucleinopathies. Ageing research reviews. 2016. doi:10.1016/j.arr.2016.01.001
Sakakibara R, Tateno F, Kishi M, Tsuyusaki Y, Terada H, Inaoka T. MIBG myocardial scintigraphy in pre-motor Parkinson’s disease: a review. Parkinsonism Relat Disord. 2014;20:267–73. doi:10.1016/j.parkreldis.2013.11.001.
Chung EJ, Kim SJ. (123)I-metaiodobenzylguanidine myocardial scintigraphy in Lewy body-related disorders: a literature review. J Mov Disord. 2015;8:55–66. doi:10.14802/jmd.15015.
Kashihara K, Ohno M, Kawada S, Okumura Y. Reduced cardiac uptake and enhanced washout of 123I-MIBG in pure autonomic failure occurs conjointly with Parkinson’s disease and dementia with Lewy bodies. J Nucl Med. 2006;47:1099–101.
Goldstein DS, Holmes C, Kopin IJ, Sharabi Y. Intra-neuronal vesicular uptake of catecholamines is decreased in patients with Lewy body diseases. J Clin Invest. 2011;121:3320–30. doi:10.1172/JCI45803.
Kwon SH, Yoon JK, Yoon JH, Lee SJ, Jo KS, Lee DH, et al. The utility of segmental analysis in cardiac I-123 MIBG SPECT in Parkinson’s disease. Nucl Med Mol Imaging. 2015;49:298–302. doi:10.1007/s13139-015-0354-0.
Oh JK, Choi EK, Song IU, Kim JS, Chung YA. Comparison of I-123 MIBG planar imaging and SPECT for the detection of decreased heart uptake in Parkinson disease. J Neural Transm (Vienna). 2015;122:1421–7. doi:10.1007/s00702-015-1409-1.
Orimo S, Ozawa E, Nakade S, Sugimoto T, Mizusawa H. (123)I-metaiodobenzylguanidine myocardial scintigraphy in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;67:189–94.
Yoshita M, Hayashi M, Hirai S. Decreased myocardial accumulation of 123I-meta-iodobenzyl guanidine in Parkinson’s disease. Nucl Med Commun. 1998;19:137–42.
Slaets S, Van Acker F, Versijpt J, Hauth L, Goeman J, Martin JJ, et al. Diagnostic value of MIBG cardiac scintigraphy for differential dementia diagnosis. Int J Geriatr Psychiatry. 2015;30:864–9. doi:10.1002/gps.4229.
Tateno F, Sakakibara R, Kishi M, Ogawa E, Terada H, Ogata T, et al. Sensitivity and specificity of metaiodobenzylguanidine (MIBG) myocardial accumulation in the diagnosis of Lewy body diseases in a movement disorder clinic. Parkinsonism Relat Disord. 2011;17:395–7. doi:10.1016/j.parkreldis.2011.02.001.
Treglia G, Cason E, Stefanelli A, Cocciolillo F, Di Giuda D, Fagioli G, et al. MIBG scintigraphy in differential diagnosis of Parkinsonism: a meta-analysis. Clin Auton Res. 2012;22:43–55. doi:10.1007/s10286-011-0135-5.
Orimo S, Suzuki M, Inaba A, Mizusawa H. 123I-MIBG myocardial scintigraphy for differentiating Parkinson’s disease from other neurodegenerative parkinsonism: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2012;18:494–500. doi:10.1016/j.parkreldis.2012.01.009.
Miyamoto T, Miyamoto M, Inoue Y, Usui Y, Suzuki K, Hirata K. Reduced cardiac 123I-MIBG scintigraphy in idiopathic REM sleep behavior disorder. Neurology. 2006;67:2236–8. doi:10.1212/01.wnl.0000249313.25627.2e.
Iranzo A, Tolosa E, Gelpi E, Molinuevo JL, Valldeoriola F, Serradell M, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12:443–53. doi:10.1016/S1474-4422(13)70056-5. A landmark study describing conversion rate of patients with RBD.
Kashihara K, Imamura T, Shinya T. Cardiac 123I-MIBG uptake is reduced more markedly in patients with REM sleep behavior disorder than in those with early stage Parkinson’s disease. Parkinsonism Relat Disord. 2010;16:252–5. doi:10.1016/j.parkreldis.2009.12.010.
Shimizu S, Hirao K, Kanetaka H, Namioka N, Hatanaka H, Hirose D, et al. Utility of the combination of DAT SPECT and MIBG myocardial scintigraphy in differentiating dementia with Lewy bodies from Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2016;43:184–92. doi:10.1007/s00259-015-3146-y.
Saiki S, Hirose G, Sakai K, Kataoka S, Hori A, Saiki M, et al. Cardiac 123I-MIBG scintigraphy can assess the disease severity and phenotype of PD. J Neurol Sci. 2004;220:105–11. doi:10.1016/j.jns.2004.02.018.
Fereshtehnejad SM, Lokk J. Orthostatic hypotension in patients with Parkinson’s disease and atypical parkinsonism. Parkinsons Dis. 2014;2014:475854. doi:10.1155/2014/475854.
Braune S, Reinhardt M, Schnitzer R, Riedel A, Lucking CH. Cardiac uptake of [123I]MIBG separates Parkinson’s disease from multiple system atrophy. Neurology. 1999;53:1020–5.
Reinhardt MJ, Jungling FD, Krause TM, Braune S. Scintigraphic differentiation between two forms of primary dysautonomia early after onset of autonomic dysfunction: value of cardiac and pulmonary iodine-123 MIBG uptake. Eur J Nucl Med. 2000;27:595–600.
Berganzo K, Tijero B, Somme JH, Llorens V, Sanchez-Manso JC, Low D, et al. SCOPA-AUT scale in different parkinsonisms and its correlation with (123) I-MIBG cardiac scintigraphy. Parkinsonism Relat Disord. 2012;18:45–8. doi:10.1016/j.parkreldis.2011.08.018.
Melander A, Ericson LE, Sundler F, Ingbar SH. Sympathetic innervation of the mouse thyroid and its significance in thyroid hormone secretion. Endocrinology. 1974;94:959–66. doi:10.1210/endo-94-4-959.
Goldstein DS, Holmes CS, Dendi R, Bruce SR, Li ST. Orthostatic hypotension from sympathetic denervation in Parkinson’s disease. Neurology. 2002;58:1247–55.
Matsui H, Udaka F, Oda M, Tamura A, Kubori T, Nishinaka K, et al. Metaiodobenzylguanidine (MIBG) uptake in Parkinson’s disease also decreases at thyroid. Ann Nucl Med. 2005;19:225–9.
Tipre DN, Goldstein DS. Cardiac and extracardiac sympathetic denervation in Parkinson’s disease with orthostatic hypotension and in pure autonomic failure. J Nucl Med. 2005;46:1775–81.
DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197.
Adler CH, Dugger BN, Hinni ML, Lott DG, Driver-Dunckley E, Hidalgo J, et al. Submandibular gland needle biopsy for the diagnosis of Parkinson disease. Neurology. 2014. doi:10.1212/WNL.0000000000000204
Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Autonomic neuroscience : basic & clinical. 2007;133:3–18. doi:10.1016/j.autneu.2006.10.006.
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211.
Gai WP, Blumbergs PC, Geffen LB, Blessing WW. Age-related loss of dorsal vagal neurons in Parkinson’s disease. Neurology. 1992;42:2106–11.
Eadie MJ. The pathology of certain medullary nuclei in parkinsonism. Brain. 1963;86:781–92.
Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi:10.1016/j.neulet.2005.11.012.
Greene JG. Causes and consequences of degeneration of the dorsal motor nucleus of the vagus nerve in Parkinson’s disease. Antioxid Redox Signal. 2014;21:649–67. doi:10.1089/ars.2014.5859.
Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol. 1988;76:217–21.
Hopkins DA, Bieger D, devente J, Steinbusch WM. Vagal efferent projections: viscerotopy, neurochemistry and effects of vagotomy. Prog Brain Res. 1996;107:79–96.
Annerino DM, Arshad S, Taylor GM, Adler CH, Beach TG, Greene JG. Parkinson’s disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol. 2012;124:665–80. doi:10.1007/s00401-012-1040-2.
Lebouvier T, Neunlist M, Bruley des Varannes S, Coron E, Drouard A, N'Guyen JM, et al. Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS One. 2010;5:e12728. doi:10.1371/journal.pone.0012728.
Hawkes CH, Shephard BC, Daniel SE. Olfactory dysfunction in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1997;62:436–46.
Svensson E, Horvath-Puho E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78:522–9. doi:10.1002/ana.24448.
Giacobini E. Cholinesterases and cholinesterase inhibitors. London: Martin Dunitz Ltd; 2000.
Schmid W, van der Zypen E, Keller H. Die Wirkung einer subtotalen Vagotomie auf den Plexus myentericus (Auerbach) verschiedener Darmabschnitte. Acta Anat (Basel). 1979;104:36–51.
Pauza DH, Saburkina I, Rysevaite K, Inokaitis H, Jokubauskas M, Jalife J, et al. Neuroanatomy of the murine cardiac conduction system: a combined stereomicroscopic and fluorescence immunohistochemical study. Auton Neurosci. 2013;176:32–47. doi:10.1016/j.autneu.2013.01.006.
Anlauf M, Schafer MK, Eiden L, Weihe E. Chemical coding of the human gastrointestinal nervous system: cholinergic, VIPergic, and catecholaminergic phenotypes. J Comp Neurol. 2003;459:90–111. doi:10.1002/cne.10599.
Okamura N, Funaki Y, Tashiro M, Kato M, Ishikawa Y, Maruyama M, et al. In vivo visualization of donepezil binding in the brain of patients with Alzheimer’s disease. Br J Clin Pharmacol. 2008;65:472–9. doi:10.1111/j.1365-2125.2007.03063.x.
Hiraoka K, Okamura N, Funaki Y, Hayashi A, Tashiro M, Hisanaga K, et al. Cholinergic deficit and response to donepezil therapy in Parkinson’s disease with dementia. Eur Neurol. 2012;68:137–43. doi:10.1159/000338774.
Gjerloff T, Jakobsen S, Nahimi A, Munk OL, Bender D, Alstrup AK, et al. In vivo imaging of human acetylcholinesterase density in peripheral organs using 11c-donepezil: dosimetry, biodistribution, and kinetic analyses. J Nucl Med. 2014;55:1818–24. doi:10.2967/jnumed.114.143859.
Gjerloff T, Fedorova T, Knudsen K, Munk OL, Nahimi A, Jacobsen S, et al. Imaging acetylcholinesterase density in peripheral organs in Parkinson’s disease with 11C-donepezil PET. Brain. 2015;138:653–63. doi:10.1093/brain/awu369. This study demonstrated that 11C-donepezil PET may be the first successful method for imaging parasympathetic denervation in peripheral organs of PD patients.
Roy A, Fields WC, Rocha-Resende C C, Resende RR, Guatimosim S, Prado VF, et al. Cardiomyocyte-secreted acetylcholine is required for maintenance of homeostasis in the heart. Faseb J. 2013. doi:10.1096/fj.13-238279.
Petrou M, Frey KA, Kilbourn MR, Scott PJ, Raffel DM, Bohnen NI, et al. In vivo imaging of human cholinergic nerve terminals with (−)-5-(18)F-fluoroethoxybenzovesamicol: biodistribution, dosimetry, and tracer kinetic analyses. J Nucl Med. 2014;55:396–404. doi:10.2967/jnumed.113.124792.
Fasano A, Visanji NP, Liu LW, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015;14:625–39. doi:10.1016/S1474-4422(15)00007-1.
Cersosimo MG, Benarroch EE. Pathological correlates of gastrointestinal dysfunction in Parkinson’s disease. Neurobiol Dis. 2012;46:559–64. doi:10.1016/j.nbd.2011.10.014.
Kalf JG, de Swart BJ, Bloem BR, Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2012;18:311–5. doi:10.1016/j.parkreldis.2011.11.006.
Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Parkinsonism Relat Disord. 2011;17:10–5. doi:10.1016/j.parkreldis.2010.08.003.
Noyce AJ, Silveira-Moriyama L, Gilpin P, Ling H, Howard R, Lees AJ. Severe dysphagia as a presentation of Parkinson’s disease. Mov Disord. 2012;27:457–8. doi:10.1002/mds.24006.
Kikuchi A, Baba T, Hasegawa T, Kobayashi M, Sugeno N, Konno M, et al. Hypometabolism in the supplementary and anterior cingulate cortices is related to dysphagia in Parkinson’s disease: a cross-sectional and 3-year longitudinal cohort study. BMJ open. 2013;3. doi:10.1136/bmjopen-2012-002249
Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–69.
Kingsbury AE, Bandopadhyay R, Silveira-Moriyama L, Ayling H, Kallis C, Sterlacci W, et al. Brain stem pathology in Parkinson’s disease: an evaluation of the Braak staging model. Mov Disord. 2010;25:2508–15. doi:10.1002/mds.23305.
Logemann J. Measurements of swallow from videofluoroscopic studies. 2 ed: Texas Pro-ed.; 1993.
Leopold NA, Kagel MC. Pharyngo-esophageal dysphagia in Parkinson’s disease. Dysphagia. 1997;12:11–8. discussion 9–20.
Bushmann M, Dobmeyer SM, Leeker L, Perlmutter JS. Swallowing abnormalities and their response to treatment in Parkinson’s disease. Neurology. 1989;39:1309–14.
Monte FS, da Silva-Junior FP, Braga-Neto P, Souza MA N e, de Bruin VM. Swallowing abnormalities and dyskinesia in Parkinson’s disease. Mov Disord. 2005;20:457–62. doi:10.1002/mds.20342.
Klein H, Wald A. Esophageal transit scintigraphy. New York: New Raven Press; 1988.
Potulska A, Friedman A, Krolicki L, Spychala A. Swallowing disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2003;9:349–53.
Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–43. doi:10.1146/annurev.physiol.68.040504.094718.
Schubert ML, Peura DA. Control of gastric acid secretion in health and disease. Gastroenterology. 2008;134:1842–60. doi:10.1053/j.gastro.2008.05.021.
Hasler WL. In: Yamada T, editor. Gastroenterology. Philadelphia: Lippincott Williams & Wilkins; 2003. p. 195–219.
Marrinan S, Emmanuel AV, Burn DJ. Delayed gastric emptying in Parkinson’s disease. Mov Disord. 2014;29:23–32. doi:10.1002/mds.25708.
Heetun ZS, Quigley EM. Gastroparesis and Parkinson’s disease: a systematic review. Parkinsonism Relat Disord. 2012;18:433–40. doi:10.1016/j.parkreldis.2011.12.004.
Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, MacPhee G, et al. Prevalence of nonmotor symptoms in Parkinson’s disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord. 2007;22:1623–9. doi:10.1002/mds.21586.
Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, van Hilten JJ. Patient-reported autonomic symptoms in Parkinson disease. Neurology. 2007;69:333–41. doi:10.1212/01.wnl.0000266593.50534.e8.
Pouclet H, Lebouvier T, Coron E, Neunlist M, Derkinderen P. Lewy pathology in gastric and duodenal biopsies in Parkinson’s disease. Mov Disord. 2012;27:708. doi:10.1002/mds.24993.
Donohoe KJ, Maurer AH, Ziessman HA, Urbain JL, Royal HD, Martin-Comin J, et al. Procedure guideline for adult solid-meal gastric-emptying study 3.0. J Nucl Med Technol. 2009;37:196–200. doi:10.2967/jnmt.109.067843.
Evans MA, Broe GA, Triggs EJ, Cheung M, Creasey H, Paull PD. Gastric emptying rate and the systemic availability of levodopa in the elderly parkinsonian patient. Neurology. 1981;31:1288–94.
Djaldetti R, Baron J, Ziv I, Melamed E. Gastric emptying in Parkinson’s disease: patients with and without response fluctuations. Neurology. 1996;46:1051–4.
Krygowska-Wajs A, Cheshire Jr WP, Wszolek ZK, Hubalewska-Dydejczyk A, Jasinska-Myga B, Farrer MJ, et al. Evaluation of gastric emptying in familial and sporadic Parkinson disease. Parkinsonism Relat Disord. 2009;15:692–6. doi:10.1016/j.parkreldis.2009.04.003.
Hardoff R, Sula M, Tamir A, Soil A, Front A, Badarna S, et al. Gastric emptying time and gastric motility in patients with Parkinson’s disease. Mov Disord. 2001;16:1041–7. doi:10.1002/mds.1203.
Tanaka Y, Kato T, Nishida H, Yamada M, Koumura A, Sakurai T, et al. Is there a delayed gastric emptying of patients with early-stage, untreated Parkinson’s disease? An analysis using the 13C-acetate breath test. J Neurol. 2011;258:421–6. doi:10.1007/s00415-010-5769-z.
Goetze O, Wieczorek J, Mueller T, Przuntek H, Schmidt WE, Woitalla D. Impaired gastric emptying of a solid test meal in patients with Parkinson’s disease using 13C-sodium octanoate breath test. Neurosci Lett. 2005;375:170–3. doi:10.1016/j.neulet.2004.11.007.
Epprecht L, Schreglmann SR, Goetze O, Woitalla D, Baumann CR, Waldvogel D. Unchanged gastric emptying and visceral perception in early Parkinson’s disease after a high caloric test meal. J Neurol. 2015;262:1946–53. doi:10.1007/s00415-015-7799-z.
Goetze O, Nikodem AB, Wiezcorek J, Banasch M, Przuntek H, Mueller T, et al. Predictors of gastric emptying in Parkinson’s disease. Neurogastroenterol Motil. 2006;18:369–75. doi:10.1111/j.1365-2982.2006.00780.x.
Unger MM, Moller JC, Mankel K, Schmittinger K, Eggert KM, Stamelou M, et al. Patients with idiopathic rapid-eye-movement sleep behavior disorder show normal gastric motility assessed by the 13C-octanoate breath test. Mov Disord. 2011;26:2559–63. doi:10.1002/mds.23933.
Arai E, Arai M, Uchiyama T, Higuchi Y, Aoyagi K, Yamanaka Y, et al. Subthalamic deep brain stimulation can improve gastric emptying in Parkinson’s disease. Brain. 2012;135:1478–85. doi:10.1093/brain/aws086.
Doi H, Sakakibara R, Sato M, Masaka T, Kishi M, Tateno A, et al. Plasma levodopa peak delay and impaired gastric emptying in Parkinson’s disease. J Neurol Sci. 2012;319:86–8. doi:10.1016/j.jns.2012.05.010.
Davies KN, King D, Billington D, Barrett JA. Intestinal permeability and orocaecal transit time in elderly patients with Parkinson’s disease. Postgrad Med J. 1996;72:164–7.
Dutkiewicz J, Szlufik S, Nieciecki M, Charzynska I, Krolicki L, Smektala P, et al. Small intestine dysfunction in Parkinson’s disease. J Neural Transm (Vienna). 2015;122:1659–61. doi:10.1007/s00702-015-1442-0.
Knudsen K, Fedorova T, Borghammer P. Colonic volume and gastrointestinal symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2016;22:e29ee75. P 1.067.
Damian A, Adler CH, Hentz JG, Shill HA, Caviness JN, Sabbagh MN, et al. Autonomic function, as self-reported on the SCOPA-autonomic questionnaire, is normal in essential tremor but not in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:1089–93. doi:10.1016/j.parkreldis.2012.06.008.
Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord. 2009;24:1641–9. doi:10.1002/mds.22643.
Chaudhuri KR, Odin P. The challenge of non-motor symptoms in Parkinson’s disease. 2010;184:325–41. doi:10.1016/s0079-6123(10)84017-8
Nihei Y, Takahashi K, Koto A, Mihara B, Morita Y, Isozumi K, et al. REM sleep behavior disorder in Japanese patients with Parkinson’s disease: a multicenter study using the REM sleep behavior disorder screening questionnaire. J Neurol. 2012;259:1606–12. doi:10.1007/s00415-011-6386-1.
Holstege G. Central nervous system control of ejaculation. World J Urol. 2005;23:109–14. doi:10.1007/s00345-004-0484-y.
Winge K, Skau AM, Stimpel H, Nielsen KK, Werdelin L. Prevalence of bladder dysfunction in Parkinsons disease. Neurourol Urodyn. 2006;25:116–22. doi:10.1002/nau.20193.
Del Tredici K, Braak H. Spinal cord lesions in sporadic Parkinson’s disease. Acta Neuropathol. 2012;124:643–64. doi:10.1007/s00401-012-1028-y. This study demonstrated consistent, severe pathology in sacral parasympathetic nuclei of patients with manifest PD.
VanderHorst VG, Samardzic T, Saper CB, Anderson MP, Nag S, Schneider JA, et al. alpha-Synuclein pathology accumulates in sacral spinal visceral sensory pathways. Ann Neurol. 2015;78:142–9. doi:10.1002/ana.24430.
Abrahamsson H, Antov S, Bosaeus I. Gastrointestinal and colonic segmental transit time evaluated by a single abdominal x-ray in healthy subjects and constipated patients. Scand J Gastroenterol Suppl. 1988;152:72–80.
Wang CP, Sung WH, Wang CC, Tsai PY. Early recognition of pelvic floor dyssynergia and colorectal assessment in Parkinson’s disease associated with bowel dysfunction. Colorectal Dis. 2013;15:e130–7. doi:10.1111/codi.12105.
Jost WH, Schimrigk K. The effect of cisapride on delayed colonic transit time in patients with idiopathic Parkinson’s disease. Wien Klin Wochenschr. 1994;106:673–6.
Edwards LL, Quigley EM, Harned RK, Hofman R, Pfeiffer RF. Characterization of swallowing and defecation in Parkinson’s disease. Am J Gastroenterol. 1994;89:15–25.
Sakakibara R, Odaka T, Uchiyama T, Asahina M, Yamaguchi K, Yamaguchi T, et al. Colonic transit time and rectoanal videomanometry in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2003;74:268–72.
Jost WH, Schimrigk K. Constipation in Parkinson’s disease. Klin Wochenschr. 1991;69:906–9.
Ashraf W, Pfeiffer RF, Park F, Lof J, Quigley EM. Constipation in Parkinson’s disease: objective assessment and response to psyllium. Mov Disord. 1997;12:946–51. doi:10.1002/mds.870120617.
Tateno F, Sakakibara R, Yokoi Y, Kishi M, Ogawa E, Uchiyama T, et al. Levodopa ameliorated anorectal constipation in de novo Parkinson’s disease: the QL-GAT study. Parkinsonism Relat Disord. 2011;17:662–6. doi:10.1016/j.parkreldis.2011.06.002.
Liu Z, Sakakibara R, Odaka T, Uchiyama T, Uchiyama T, Yamamoto T, et al. Mosapride citrate, a novel 5-HT4 agonist and partial 5-HT3 antagonist, ameliorates constipation in parkinsonian patients. Mov Disord. 2005;20:680–6. doi:10.1002/mds.20387.
Steele SR, Mellgren A. Constipation and obstructed defecation. Clin Colon Rectal Surg. 2007;20:110–7. doi:10.1055/s-2007-977489.
Mathers SE, Kempster PA, Swash M, Lees AJ. Constipation and paradoxical puborectalis contraction in anismus and Parkinson’s disease: a dystonic phenomenon? J Neurol Neurosurg Psychiatry. 1988;51:1503–7.
Visser M, Marinus J, Stiggelbout AM, Van Hilten JJ. Assessment of autonomic dysfunction in Parkinson’s disease: the SCOPA-AUT. Mov Disord. 2004;19:1306–12. doi:10.1002/mds.20153.
Lewitan A, Nathanson L, Slade Jr WR. Megacolon and dilatation of the small bowel in parkinsonism. Gastroenterology. 1951;17:367–74.
Caplan LH, Jacobson HG, Rubinstein BM, Rotman MZ. Megacolon and volvulus in Parkinson’s disease. Radiology. 1965;85:73–9. doi:10.1148/85.1.73.
de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981;3:135–60.
Lynch AC, Frizelle FA. Colorectal motility and defecation after spinal cord injury in humans. Prog Brain Res. 2006;152:335–43. doi:10.1016/S0079-6123(05)52022-3.
Rodriguez-Blazquez C, Forjaz MJ, Frades-Payo B, de Pedro-Cuesta J, Martinez-Martin P, Longitudinal Parkinson’s Disease Patient Study ELdPcEdPG. Independent validation of the scales for outcomes in Parkinson’s disease-autonomic (SCOPA-AUT). Eur J Neurol. 2010;17:194–201. doi:10.1111/j.1468-1331.2009.02788.x.
Edwards LL, Pfeiffer RF, Quigley EM, Hofman R, Balluff M. Gastrointestinal symptoms in Parkinson’s disease. Mov Disord. 1991;6:151–6. doi:10.1002/mds.870060211.
Chiu CM, Wang CP, Sung WH, Huang SF, Chiang SC, Tsai PY. Functional magnetic stimulation in constipation associated with Parkinson’s disease. J Rehabil Med. 2009;41:1085–9. doi:10.2340/16501977-0456.
Cadeddu F, Bentivoglio AR, Brandara F, Marniga G, Brisinda G, Maria G. Outlet type constipation in Parkinson’s disease: results of botulinum toxin treatment. Aliment Pharmacol Ther. 2005;22:997–1003. doi:10.1111/j.1365-2036.2005.02669.x.
Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30:1600–11. doi:10.1002/mds.26431.
Acknowledgments
The study was financially supported by a grant from the Lundbeck Foundation. The authors would like to thank Dr. Stanley Fahn for taking the time to review this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Per Borghammer and Karoline Knudsen declare that they have no conflict of interest.
David J. Brooks has received consultancy fees from GE Healthcare.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neuroimaging
Rights and permissions
About this article
Cite this article
Borghammer, P., Knudsen, K. & Brooks, D.J. Imaging Systemic Dysfunction in Parkinson’s Disease. Curr Neurol Neurosci Rep 16, 51 (2016). https://doi.org/10.1007/s11910-016-0655-4
Published:
DOI: https://doi.org/10.1007/s11910-016-0655-4