Abstract
Purpose
Cardiac images using I-123 metaiodobenzylguanidine (MIBG) are widely used to evaluate cardiac sympathetic denervation in Parkinson’s disease (PD). The aim of this study was to evaluate the utility of segmental analysis on cardiac MIBG SPECT in PD patients.
Materials and Methods
In total, 36 patients with PD (n = 26) or essential tremor (ET, n = 10) who underwent MIBG cardiac SPECT were enrolled. The heart-to-mediastinum (H/M) ratios of MIBG uptake were acquired on planar images. For the segmental analysis of SPECT images, we evaluated the summed defect score (SDS) using a 17-segment model. The diagnostic abilities of H/M ratios and segmental parameters on MIBG SPECT were assessed by ROC curve analysis.
Results
The H/M ratios were significantly lower in PD than in ET patients (p < 0.05). On segmental analysis, SDS was significantly higher in PD patients than in the ET group (7.04 ± 4.09 vs. 2.90 ± 2.80; p = 0.006). The defect score of the anteroseptal region showed a significant difference between the groups (p = 0.002). The ROC analysis suggested only SDS (AUC = 0.785, p = 0.0003) and defect scores in the anteroseptal (AUC = 0.800, p < 0.0001) and inferior (AUC = 0.667, p = 0.013) regions showed significant diagnostic ability to differentiate PD from ET.
Conclusions
Segmental parameters from cardiac MIBG SPECT images can provide additional information to differentiate PD from ET patients. Beyond H/M ratios from planar images, we recommend an MIBG SPECT study to evaluate sympathetic denervation in PD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative diseases. It is characterized by several cardinal symptoms, such as resting tremor, rigidity, bradykinesia, and postural instability. Differential diagnosis between PD and other parkinsonisms using clinical criteria is often difficult because neurological symptoms overlap in these disorders. In this context, cardiac sympathetic innervation imaging using I-123 metaiodobenzylguanidine (MIBG) has emerged as a valuable tool for diagnosing PD [1].
MIBG is a norepinephrine analog that is taken up and stored in sympathetic nerve endings and myocytes. Early uptake of myocardial MIBG may indicate presynaptic sympathetic integrity, and delayed uptake may reflect the functional status or tone of the sympathetic nervous system [2, 3].
Cardiac MIBG scintigraphy has been investigated in several neurodegenerative disorders associated with parkinsonism. Previous reports revealed that cardiac MIBG is significantly reduced in patients with PD, but cardiac MIBG uptake was within the normal range in patients with essential tremor, drug-induced parkinsonism, vascular parkinsonism, and other ‘Parkinson plus’ syndromes [2, 4–6]. Furthermore, MIBG scintigraphy was reported to be an effective tool for distinguishing early PD without significant autonomic symptoms [7, 8].
Most previous studies using MIBG scintigraphy were based on the heart-to-mediastinum (H/M) ratio derived from early and delayed planar images [4–8]. However, further evaluation of segmental analysis of cardiac sympathetic denervation could be available using single photon emission computed tomography (SPECT) images. To our knowledge, there have been few previous studies reporting the usefulness of segmental analysis of MIBG cardiac SPECT. The aim of this study was to evaluate the usefulness of myocardial segmental analysis on MIBG SPECT in PD patients.
Materials and Methods
Patients
This study included 36 patients [17 females, 19 males; mean age ± standard deviation (SD), 60.5 ± 12.2 years; range, 26–83 years] with clinically diagnosed PD (n = 26) or essential tremor (ET, n = 10), who underwent MIBG cardiac SPECT between June 2013 and June 2014.
PD was diagnosed according to the UK Parkinson’s Disease Society Brain Bank Clinical Diagnosis Criteria [9], and the diagnostic criteria of the Tremor Investigation Group [10] were used for ET patients. The mean tremor score was calculated in PD patients as the mean of the following nine items of the Unified Parkinson’s Disease Rating Scale (UPDRS): right and left arm tremor, as determined by history, tremor at rest of either the face, lips, or chin, all four limbs, and action or postural tremor in both arms, as determined by an investigator’s examination.
The patients had no history of diabetes mellitus or ischemic heart disease that could affect the autonomic nervous system. No patient had received any substance known or expected to interfere with MIBG uptake.
The clinical design of this retrospective study was approved by the Institutional Review Board (IRB) of Ajou University (MED-MDB-14-318), with waiver of the need for informed consent.
Cardiac MIBG Image Acquisition
The I-123-labeled MIBG (111 MBq) was injected intravenously into each subject. Planar images of the chest region were acquired twice, at 15 min (early) and 3 h (delayed), after injection using a dual-head gamma-camera system with a low-energy, high-resolution collimator (Symbia E, Siemens Medical Solutions, IL, USA).
Also, in the delayed phase, SPECT data were acquired with the step-and-shoot mode, in which the two detector heads made an alternate motion in a 180° range. Energy discrimination was provided by a 15 % window centered on the 140-keV photopeak. The data were stored in a 64 × 64 matrix, and the images were reconstructed using an iterative algorithm.
Image Analysis
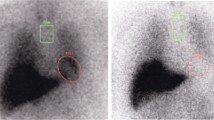
To evaluate the heart-to-mediastinum (H/M) ratio, rectangular regions of interest (ROIs) of 5 × 5 pixels were drawn manually on the left ventricle and upper mediastinum of the anterior planar image (Fig. 1a). The H/M count ratios of MIBG uptake were calculated at 15 min (early H/M) and at 180 min (delayed H/M), defined as the average counts/pixel in the myocardium divided by that of the upper mediastinum.
Regions of interest (ROIs) used for image analyses. a The rectangular ROIs, located at the upper mediastinum (ROI 1) and heart (ROI 2), were used to evaluate the heart-to-mediastinum ratio in the anterior planar image. b The circular ROI was carefully positioned over the heart on SPECT images to avoid overlap with adjacent liver and lung uptake for segmental analyses
For the segmental analysis, a circular ROI was placed carefully over the heart on SPECT images to avoid overlap with adjacent liver and lung uptake (Fig. 1b). Cardiac uptake of MIBG was assessed semiquantitatively with 17-segment automatic quantification of the summed defect score (SDS), reflecting the extent and severity of cardiac sympathetic dysfunction using the Quantitative Perfusion SPECT (QPS) software. We also analyzed the 17 segments in three categories—anteroseptal, lateral, and inferior wall—according to vessel territory.
Statistical Analyses
Student’s t-test and the χ 2 test were used to compare the characteristics and results of cardiac MIBG findings between the PD and ET patients. The diagnostic ability of the H/M ratios and segmental analytic values on MIBG SPECT was assessed by ROC curve analysis. The area under the ROC curve (AUC) was compared with 0.5, and the ‘best’ cutoff value was determined as that with the highest Youden Index. Additionally, the McNemar test was performed to compare the diagnostic performance of two modalities using the H/M ratio from planar images and SDS from SPECT. The MedCalc software (version 14.8.1; MedCalc Software bvba, Ostend, Belgium) was used in all statistical analyses. P values < 0.05 were considered to indicate statistical significance.
Results
Patient Characteristics
The clinical characteristics of the patients are shown in Table 1. There was no significant difference in age or gender between the PD and ET groups. The duration of disease was significantly longer in PD than ET patients (3.2 ± 2.1 vs. 0.6 ± 0.3 years; p < 0.001).
Comparison of Cardiac MIBG Findings Between PD and ET Patients
The early and delayed H/M ratios from planar images were significantly lower in patients with PD than in those with ET (early H/M ratio, p = 0.020; delayed H/M ratio, p = 0.008; Table 2). On segmental analysis, SDS from SPECT images was significantly higher in PD patients than in the ET group (7.04 ± 4.09 vs. 2.90 ± 2.80, p = 0.006). In terms of the cardiac territory, the defect score of the anteroseptal wall showed significant differences between the groups (p = 0.002), but not the lateral or inferior walls (Table 2, Fig. 2).
Diagnostic Ability of Cardiac MIBG Parameters
Table 3 and Fig. 3 show the results of the ROC curve analysis as an indicator of the diagnostic ability of each cardiac MIBG parameter. The AUC value of SDS was 0.785, significantly greater than 0.5 (p < 0.001). When an SDS of 4 was used as the cutoff value for positivity, the sensitivity was 73.1 % and the specificity was 80.0 %. Also, the defect scores of the anteroseptal and inferior walls had AUC values greater than 0.5, showing significant diagnostic utility for differentiating PD from ET. However, the AUC values of early and delayed H/M ratios were not significantly different from 0.5. The direct comparison of diagnostic performance of the two modalities using the H/M ratio from planar images and SDS with a cutoff of 4 from SPECT did not show a significant difference (early H/M ratio vs. SDS, p = 0.227; delayed H/M ratio vs. SDS, p = 0.146).
Discussion
Cardiac MIBG scintigraphy has been suggested as a useful diagnostic test to differentiate PD from other neurodegenerative disorders associated with Parkinson. Imaging of the cardiac sympathetic system can be used to quantify the functional severity of damaged neural activity and to identify PD patients with risk stratification [11]. To date, H/M ratios from planar images have typically been used to evaluate cardiac MIBG studies, and the investigation of SPECT images has been limited. To our knowledge, this is the first report of use of the segmental analysis of cardiac MIBG SPECT images in patients with PD to differentiate them from ET patients.
According to our study, PD patients showed significantly lower uptake of cardiac MIBG than ET patients on SPECT images, and the anteroseptal wall region was particularly affected in PD patients on a segmental analysis. Most of the segmental analysis study using MIBG scintigraphy was performed in patients with heart failure, and only a few studies were carried out in PD patients [12]. Only one previous study has reported a regional pattern of cardiac MIBG uptake in PD patients: Courbon et al. [7]. They reported that PD patients demonstrated a prominent regional reduction in MIBG uptake in the apex region compared with multiple systemic atrophy (MSA) patients. Their study was somewhat different from ours. We assessed regional MIBG uptake in three regions (anteroseptal, lateral, inferior); thus, their ‘apex’ was generally included within the anteroseptal region in our study. These findings suggest that cardiac sympathetic denervation might affect mainly the anteroseptal region, including the apex, in PD patients.
In our study, the defect score in the inferior wall also had significant diagnostic ability for PD patients on an ROC curve analysis. However, caution is needed before accepting this result. Previous studies reported that cardiac MIBG uptake was reduced in the inferior wall in old normal subjects and tended to decrease in the whole myocardium with age, especially above 60 years [13, 14]. Also, inferior wall uptake shows a wide range of variation because of an artifact in SPECT reconstruction caused by high MIBG uptake in the nearby liver [7]. This suggests that the inferior wall signals are readily changeable because of physiological conditions and artifacts. Based on the negative results from other studies, it is difficult to conclude that cardiac MIBG uptake patterns in the inferior wall strongly predict PD patients from ET patients.
Our study showed that the H/M ratios from planar images in PD patients were significantly lower than in ET patients, consistent with a previous report [4]. However, the early and delayed H/M ratios did not show significant diagnostic efficacy for PD from ROC curve analyses, because some values were below the reference line (Fig. 3). A previous study by Treglia et al. reported that H/M ratios had significant diagnostic ability for PD with high sensitivity, specificity and AUC value [1]. The discrepancy of results between our and previous studies might arise from the diversity of clinical and methodological parameters between different studies and patient-related factors among different patient populations. Our results suggest that parameters from SPECT images had additional information to differentiate PD from ET patients. Beyond H/M ratios from planar images, the segmental assessment of cardiac MIBG SPECT images could be a useful method for diagnosing PD. Indeed, a previous study emphasized the analysis of cardiac MIBG SPECT images, because regional MIBG uptake alterations might precede global uptake reduction, as assessed by H/M ratios, in PD patients [7].
This study had several limitations. First, the numbers of patients were relatively small, and further studies in a larger cohort are essential to confirm the diagnostic ability of cardiac MIBG SPECT parameters in PD. Second, concurrent myocardial perfusion imaging was not performed in our patients. Although our study excluded patients who had a history of ischemic heart disease, in elderly patients there is the possibility of hidden coronary artery disease without related symptoms. Further studies including myocardial perfusion imaging are needed to validate our results. Finally, there is no standardized method of drawing an ROI on a heart SPECT image, so it is difficult to place the ROI exactly, minimizing any effects of lung and liver MIBG uptake. This process can be both time-consuming and operator-dependent. A well-trained researcher may be needed to obtain accurate results from SPECT images.
Conclusions
We showed that the segmental parameters in cardiac MIBG images can provide additional information to differentiate PD from ET patients. Thus, in addition to H/M ratios from planar images, we recommend using MIBG SPECT studies to evaluate sympathetic denervation in PD.
References
Treglia G, Cason E, Stefanelli A, Cocciolillo F, Di Giuda D, Fagioli G, et al. MIBG scintigraphy in differential diagnosis of parkinsonism: a meta-analysis. Clin Auton Res Off J Clin Auton Res Soc. 2012;22(1):43–55. doi:10.1007/s10286-011-0135-5.
Kashihara K, Yamamoto M. Myocardial 123I-MIBG scintigraphy in patients with PSP, CBD and MSA. J Neurol. 2006;253(3):iii35–40.
Taki J, Yoshita M, Yamada M, Tonami N. Significance of123I-MIBG scintigraphy as a pathophysiological indicator in the assessment of Parkinson’s disease and related disorders: it can be a specific marker for Lewy body disease. Ann Nucl Med. 2004;18(6):453–61.
Lee PH, Kim JW, Bang OY, Joo IS, Yoon SN, Huh K. Cardiac 123I-MIBG scintigraphy in patients with essential tremor. Mov Disord. 2006;21(8):1235–8.
Lee PH, Kim JS, Shin DH, Yoon S-N, Huh K. Cardiac 123I-MIBG scintigraphy in patients with drug induced parkinsonism. J Neurol Neurosurg Psychiatry. 2006;77(3):372–4.
Kim JS, Lee PH, Lee KS, Park JW, Kim YI, Chung YA, et al. Cardiac [123I] metaiodobenzylguanidine scintigraphy for vascular parkinsonism. Mov Disord. 2006;21(11):1990–4.
Courbon F, Brefel-Courbon C, Thalamas C, Alibelli MJ, Berry I, Montastruc JL, et al. Cardiac MIBG scintigraphy is a sensitive tool for detecting cardiac sympathetic denervation in Parkinson’s disease. Mov Disord. 2003;18(8):890–7.
Orimo S, Suzuki M, Inaba A, Mizusawa H. 123 I-MIBG myocardial scintigraphy for differentiating Parkinson’s disease from other neurodegenerative parkinsonism: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2012;18(5):494–500.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–4.
Findley LJ. Classification of tremors. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 1996;13(2):122–32.
Lucio CG, Vincenzo C, Antonio R, Oscar T, Antonio R, Luigi M. Neurological applications for myocardial MIBG scintigraphy. Nucl Med Rev Cent East Eur. 2013;16(1):35–41. doi:10.5603/NMR.2013.0007.
Carrió I, Cowie MR, Yamazaki J, Udelson J, Camici PG. Cardiac sympathetic imaging with mIBG in heart failure. J Am Coll Cardiol Img. 2010;3(1):92–100.
Somsen GA, Verberne HJ, Fleury E, Righetti A. Normal values and within-subject variability of cardiac I-123 MIBG scintigraphy in healthy individuals: implications for clinical studies. J Nucl Cardiol. 2004;11(2):126–33.
Estorch M, Carrió I, Berná L, López-Pousa J, Torres G. Myocardial iodine-labeled metaiodobenzylguanidine 123 uptake relates to age. J Nucl Cardiol. 1995;2(2):126–32.
Conflict of Interest
Soo Hyun Kwon, Joon-Kee Yoon, Jung Han Yoon, Su Jin Lee, Kyung Sook Jo, Dong Hyun Lee, and Young-Sil An declare that they have no conflict of interest.
Ethical Statement
This study was approved by the Institutional Review Board (IRB) of Ajou University (MED-MDB-14-318), with waiver of the need for informed consent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kwon, S.H., Yoon, JK., Yoon, J.H. et al. The Utility of Segmental Analysis in Cardiac I-123 MIBG SPECT in Parkinson’s Disease. Nucl Med Mol Imaging 49, 298–302 (2015). https://doi.org/10.1007/s13139-015-0354-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-015-0354-0