Opinion statement
Medulloblastoma (MB) is the most common pediatric brain malignancy, with a 5-year overall survival (OS) rate of around 65%. The conventional MB treatment, comprising surgical resection followed by irradiation and adjuvant chemotherapy, often leads to impairment in normal body functions and poor quality of life, especially with the increased risk of recurrence and subsequent development of secondary malignancies. The development and progression of MB are facilitated by a variety of immune-evading mechanisms such as the secretion of immunosuppressive molecules, activation of immunosuppressive cells, inhibition of immune checkpoint molecules, impairment of adhesive molecules, downregulation of the major histocompatibility complex (MHC) molecules, protection against apoptosis, and activation of immunosuppressive pathways. Understanding the tumor-immune relationship in MB is crucial for effective development of immune-based therapeutic strategies. In this comprehensive review, we discuss the immunological aspect of the brain, focusing on the current knowledge tackling the mechanisms of MB immune suppression and evasion. We also highlight several key immunotherapeutic approaches developed to date for the treatment of MB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction to medulloblastoma
Definition and epidemiology
Brain and other central nervous system (CNS) tumors are the most common form of solid tumors in infants and children [1] and are the leading cause of cancer-related deaths in childhood [2]. Medulloblastoma (MB) is the most common malignant childhood brain tumor, accounting for around 20% of all pediatric CNS tumors [3], and 63% of childhood embryonal tumors [1]. MB is a heterogeneous group of embryonal tumors that affect children more frequently than adults, with an incidence of 0.55 per 100,000 population in children aged 0-4 years and 0.16 per 100,000 population in adolescents aged 15–19 years [4]. Most MBs are known to arise from the cerebellar vermis in children; however, the cerebellar hemispheres are more common sites of origin among adults [5]. Males are more frequently diagnosed with MB than females in the children populations, with no racial or ethnic predisposition [6]. The 5-year overall survival (OS) rate of MB patients is 64.7%, although the prognosis varies greatly among the different tumor subgroups [4].
Classification of MB
In its most recent update of tumor classification, the WHO recognized four main histologic types of MB, namely classic MB, large cell/anaplastic MB (LC/A-MB), desmoplastic/nodular MB (D/N-MB), and MB with extensive nodularity (MBEN) [7], which are differentiated according to cellular and histological criteria [8, 9]. Nevertheless, this update requires the integration of histological classification with molecular classification in MB diagnosis [7].
Four main molecular subtypes of MB were identified based on genotyping results: WNT-activated, sonic hedgehog (SHH)-activated and TP53-mutant, SHH-activated and TP53-wildtype, and non-WNT/non-SHH MB [7]. Wnt-activated subtype comprises about 10% of all MB cases, and is characterized by mutations rendering the Wnt pathway constitutively active [3, 10]. Almost all WNT-activated MB cases exhibit classic histology [11], and patients have the most favorable prognosis, with nearly 90% surviving more than 5 years [12, 13]. SHH-activated MB is the most common subtype in infants and adults. It exhibits a favorable prognosis (77% 10-year OS rate in infants) that worsens with the increase in age [11]. This subtype is associated with mutations in genes critical for the SHH pathway [14]. This subtype is further subdivided according to the presence of mutations in TP53 gene, which has profound prognostic implications [7]. TP53-mutant subtype is associated with a 5-year OS rate of 41% as compared to 81% in TP53-wildtype subtype [15]. Non-WNT/non-SHH subtype comprises Group 3 and Group 4 MB, which affect males twice as often as females, and have no identified alterations in specific signaling pathways. Group 3 has the highest metastasis frequency and the worst prognosis among all subtypes, with a 5-year OS rate of 45% and 58% in infants and children, respectively [11]. On the other hand, Group 4 MB represents the most common subtype overall among the different age groups, with an intermediate prognosis (50% 5-year OS rate in infants and children) [11].
Treatment strategies in MB
The current standard of care for MB includes surgical resection followed by craniospinal irradiation and adjuvant chemotherapy. However, although 5-year OS is approximately 80% for patients older than 3 years with no metastasis and with gross total tumor resection, it declines to around 60% in children with metastatic disease and/or having subtotal tumor resection [16,17,18,19]. Moreover, treatment options in MB have been shown to be neurotoxic especially on infants, with studies demonstrating a decrease in intellectual functions among this patient group [20, 21], besides deficits in processing speed, broad attention, and working memory [22, 23] and increased risk of postoperative cerebellar mutism syndrome [24]. Therefore, there is a dire need for improving the conventional therapy and introducing safer and more effective alternatives. Recently, immunotherapy has gained much attention as an attractive approach to target cancer cells while sparing the adjacent non-tumor tissues. Clinical evidence has shown benefits of immunotherapy in many cancers including melanoma and leukemias among others [25,26,27]. Nevertheless, to develop safe and effective immunotherapies for MB, we need to better understand the normal and tumor immunology of the brain.
Brain-immune interaction
Aspects of immunity in the brain
The brain was originally described as an immune-privileged site, due to the restriction of immune cell entry by the blood-brain barrier (BBB), the absence of conventional lymphatic vessels, the inability of microglia to present antigens to T cells, and the low expression of major histocompatibility complex (MHC) molecules [28]. Nevertheless, it is now accepted that the brain shows persistent immune surveillance. Trafficking of immune cells across the BBB has been observed to be low and tightly controlled under normal physiological conditions, which increases when neuroinflammatory reactions develop [29, 30]. The cerebral-spinal fluid (CSF), which drains in the cervical lymph node, is also considered as a pathway for trafficking immune cells and macromolecules, providing a continuous and highly regulated communication between the brain and the immune system [31,32,33,34]. Moreover, functional lymphatic vessels have been recently discovered to line the dural sinuses, assisting in the drainage of macromolecules and enabling immune cells to enter the cervical lymph nodes [35,36,37].
Another unique feature of the brain-immune microenvironment is the unique population of immune cells. The brain immune cell population is composed of microglia (80% of brain immune cells), dendritic cells (DCs), macrophages, T cells, B cells, and natural killer (NK) cells [38]. Microglia are considered as the first line of response to pathological insults by which they show morphological [39] and molecular changes [40], exhibit phagocytic ability, express MHC molecules (although at low levels), release proinflammatory cytokines, and activate other immune cells [41,42,43]. On the other hand, DCs, which are the antigen-presenting cells (APCs) of the brain, have the ability to capture, process, and present foreign antigens, and also express different molecules such as CD11c, CD205, and MHC class II molecules. In the cervical lymph nodes, they encounter naïve lymphocytes and induce T cell activation and recruitment into the brain [44,45,46]. Recent studies have shown that B cells as well migrate to the infected brain area and aid in functional recovery after a focal stroke [47, 48].
Immunity in brain tumors
Brain tumors have evolved multiple mechanisms to escape and inhibit the antitumor activity of immune cells [49]. Brain tumor cells secrete a number of chemokines, cytokines, interleukins, and growth factors that stimulate the infiltration of immune cells such as microglia, macrophages, myeloid-derived suppressor cells (MDSCs), and T cells [50,51,52,53,54] into the tumor niche. Nevertheless, this microenvironment reprograms attacking immune cells by releasing cytokines and interleukins such as interleukin (IL)-10, tissue growth factor (TGF)-β, prostaglandin E2 (PGE2), and vascular endothelial growth factor (VEGF) that promote anti-inflammatory responses [55,56,57]. Furthermore, microglia acquires an M2-like phenotype by the influence of IL-4, IL-10, and IL-13 released by glioblastoma (GB) cells. In turn, microglia promotes glioma growth and survival by secreting growth factors such as TGF-β [58], VEGF, and members of fibroblast growth factor (FGF) family [59]. Besides, NK cells are functionally suppressed by the inflammatory molecules secreted by glioma cells such as cyclooxygenase (COX2), PGE2 [60], and TGF-β [61]. Furthermore, glioma stem cells have been shown to secrete cytokines that are known to reprogram tumor-associated macrophages (TAM) to become immunosuppressive [62].
Lymphocyte apoptosis is another immune-escape mechanism. GB cells were shown to contribute to lymphocyte apoptosis via interaction of CD70 expressed on glioma cells with CD27 expressed on T- and B-lymphocytes [63]. T cell apoptosis is also mediated by Fas-Fas ligand (FasL) interaction, which directly induces T cell apoptosis [64]. Another study revealed that FasL-positive microglia also mediate apoptosis of Fas-positive T cells [65].
Furthermore, intracranial tumors frequently display low numbers of tumor-infiltrating lymphocytes (TILs). Instead, naïve T cells are sequestered in large numbers in the bone marrow [66]. TILs that successfully infiltrate are exposed to further suppression [67]. This “cold tumor” phenotype is usually associated with immune checkpoint molecules [68, 69], such as programmed cell death-ligand 1 (PD-L1). Binding of PD-L1 with PD-1 on lymphocytes mediates T cell inactivation. Studies showed that the expression of PD-L1 on glioma cells and PD-1 on T cells is correlated with tumor progression into high-grade glioma [70]. Microglia also express PD-L1 and impede T cell activation [71].
Mechanisms of immunosuppression and resistance in MB
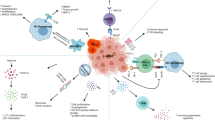
Tumor microenvironment (TME) is a specialized niche in which dynamic interactions between malignant cells and host cells occur [72, 73]. These interactions are mediated by junctions, receptors, secretions, and signals of different cell types encased in a three-dimensional (3D) extracellular matrix (ECM), which promotes tumor progression and invasion [74]. MB exhibits a cold immune microenvironment which is largely attributed to the low levels of pro-inflammatory cytokines and T cell infiltration [75]. Besides MB cells, the main players in this malignant tumor are the innate and adaptive immune cells along with stromal cells including fibroblasts and vascular endothelial cells [76]. Immune cells within the MB TME include immunosuppressive cells such as TILs [77], TAMs [73, 75, 78], MDSCs [79], regulatory T (Treg) cells [80], tumor-associated astrocytes (TAA) [81], as well as NK cells [76] (Fig. 1). To enhance the aggressive impact of MB TME, MB cells secrete immunosuppressive molecules including cytokines such as TGF-β [82], ILs [83], chemokines [84], PGE2 [85], VEGF [86], immune checkpoint inhibitors [87], and gangliosides [88], to evade the immune recognition and promote tumor progression [89] (Fig. 2).
MB microenvironment and immunotherapeutic strategies. MB exhibits an immunosuppressive microenvironment that involves the extracellular matrix including fibroblast and collagen as well as the immunosuppressive cells such as NK cells (NKs), tumor infiltrating lymphocytes (TILs), tumor-associated macrophages (TAMs), regulatory T cells (Treg), and tumor-associated astrocytes (TAA). MB is targeted via several immunotherapy approaches including the chimeric antigen receptor (CAR) T cell therapy, oncolytic virotherapy, immune checkpoint inhibitors, and cytokine inhibitor/administration.
Schematic representation of major immunosuppressive mechanisms of MB. Key mechanisms of MB immunoresistance are briefly described in the figure, including immunosuppressive cells, secretion of immunosuppressive molecules, impairment of adhesive molecules, inhibition of immune checkpoints, downregulation of HLA molecule, protection against apoptosis, and activation of immunosuppressive pathways. These mechanisms are also involved in chemoresistance and radioresistance.
Immunosuppressive cells in MB microenvironment
Tumor-infiltrating lymphocytes (TILs)
In MB, T-lymphocytes can only migrate to TME by the influence of a plethora of secretions. Once they migrate to TME, T-lymphocytes known as tumor infiltrating lymphocytes (TILs) play a critical role in promoting the survival and the progression of tumor cells as well as sustaining the tumor niche [77]. Notably, MB is characterized by a higher neutrophil-to-lymphocyte count ratio which reflects a poor prognosis in patients and is considered a feature of immunosuppression [76]. Several studies showed that MB induces the systemic immune suppression in the patients due to significant reduction in the absolute lymphocyte count and elevation in the neutrophil-to-lymphocyte count ratio in different MB subgroups [90•, 91].
Tumor-associated macrophages (TAMs)
Macrophages in TME exhibit the anti-inflammatory M2 phenotype [92]. Recent studies showed that monocyte-derived TAM2 are immunosuppressive cells responsible for suppressing the anti-tumor immunity within the TME and mediating poor clinical prognosis [92, 93]. In this context, TAMs are considered as a major player in the MB microenvironment, where they modulate the immune response through the secretion of cytokines, chemokines, and growth factors [75] to promote tumor progression, survival, invasion, metastasis, angiogenesis, and inflammation [94], as well as resistance to chemotherapy [92].
Myeloid-derived suppressor cells (MDSCs)
MDSCs are immature myeloid cells that act as a potent suppressor of anti-tumor immunity within the TME [79]. Under pathologic conditions, MDSCs promote tumor growth and invasion [95] through the suppression of T cells within MB TME in response to secreted IL-6 cytokines [79]. A recent study showed that the activation of CD200R on MDSCs by its ligand (CD200) leads to the negative regulation of immune response and promotes tumorigenesis [96].
Regulatory T (Treg) cells
Treg cells are a subgroup of CD4+ T-lymphocytes which serve as potent inhibitors of anti-tumor immunity through the suppression of T cell activation and progression by the production of nitrogen-oxygen species (NOS) under the control of MDSCs [97], downregulation of IL-2, and induction of the cytokine secretion by T-helper type 2 (Th2) cells. Several studies revealed the role of STAT3 in myeloid cells to maintain the Treg populations in MB [79].
Tumor-associated astrocytes (TAAs)
In the normal brain, astrocytes are responsible for maintaining homeostasis, however, in the pathological state, they adapt the “reactive state” through a specialized transition called “astrogliosis” to impact the immune surveillance and promote inflammation [98, 99]. In MB, TAAs enhance MB cell proliferation and growth through the expression and secretion of the mitogen SHH in the TME in response to nestin expression [100]. Moreover, TAA-derived C-C ligand 2 (CCL2) chemokine has been shown to trigger the stemness in MB stem cells (MBSCs) through the activation of the Notch signaling pathways, in addition to inducing necroptosis (programmed necrosis) [101].
Secreted immunosuppressive molecules
Cytokines
Transforming growth factor (TGF-β)
TGF-β is a pleiotropic cytokine [102] secreted by MB cells. It is considered as a potent immune suppressive factor that helps evade the cytotoxicity of the T cell populations [82] and mediates the negative effect on NK cells [103] either directly through the disruption of IFN-γ secretion, or indirectly through the inhibition of IL-12 production by DCs [104]. Previous studies showed that the expression of TGF-β1 and TGF-β2 promotes autoregulation in MB [105].
Interleukins (ILs)
In the early stage of tumorigenesis, the elimination of cancer cells is mediated by the activation of pro-inflammatory and Th1-associated factors including IL-1, IL-12, and IL-15. However, in the advanced stages of tumor progression, the prolonged inflammatory state in the TME promotes the activation of the suppressive response of Th2-associated factors such as IL-4, IL-5, IL-8, IL-10, and IL-13 [83].
IL-6 is a paracrine growth factor that triggers development, proliferation [106], inflammation, and immune regulation of MB cells [106, 107]. In MB cells, IL-6 activates the oncogenic JAK/STAT pathway [107,108,109], leading to MB cell viability, proliferation, glycolysis, clonogenicity [107], as well as the chemoresistance in Group 3 of MB cell lines [108]. In addition, Th17 cells produce a repertoire of interleukins that promote tumor growth and impair immune surveillance. Accumulated evidence revealed the high levels of Th17 and IL-17 both in the patients’ peripheral blood and among MB-infiltrating T cells [109, 110]. Besides, IL-8 has been attributed to the survival, growth, and immunosuppressive and pro-angiogenic properties of MB cells [111].
Chemokines
Chemokines are chemoattractant cytokines that control the migration of immune cells via the interaction with their cell surface receptors [84]. C-C motif ligand 2 (CCL2), is one of the chemokines that is highly expressed in MB and considered as a pro-inflammatory mediator that has the potency to promote the spreading and proliferation of the tumor cells and is sufficient to drive the leptomeningeal dissemination through the interaction with its C-C chemokine receptor type 2 (CCR2) [112, 113]. To add, the overexpression and activation of the chemokine receptor CXCR4 may be essential for the maximal growth of the SHH-driven MB [114].
Prostaglandin E2 (PGE2)
Prostaglandin E2 (PGE2) is bioactive lipid compound [115] that plays a role in many human cancers including MB [85, 116]. It mediates cellular proliferation, angiogenesis, inhibition of apoptosis, invasion, and suppression of immune responses via the interaction with four distinct G-protein coupled receptors (EP1, EP2, EP3, and EP4). Notably, MB cells express high levels of PGE2 and its receptors in a way that promotes their proliferation [85].
Vascular endothelial growth factor (VEGF)
VEGF is a pro-angiogenic growth factor that serves as one of the most potent mitogens in CNS malignancies [86, 117] Among the immunosuppressive factors released by malignant cells, VEGF-A plays an essential role in the induction of immunosuppressive microenvironment through a cascade of events including the impedence of DCs maturation and differentiation, aggregation of MDSC, and induction of Treg cells proliferation [118].In MB, VEGF-A mediates the invasion and metastasis through the CSF and reflects a poor prognostic status [119, 120]. Besides, the soluble and receptor isotypes of VEGF are found in most MB patients [121].
Gangliosides
Gangliosides are sialic acid-containing glycosphingolipids [122] and one of the tumor cell surface molecules. They contribute to the immunosuppression in MB cell lines through the inhibition of the leukocytes’ response, enhancement of platelet activation, angiogenesis, and tumor cell invasion. MB cell lines are characterized by a rapid shedding rate of gangliosides in CSF [123], which serves as a prognostic factor in MB patients [88].
HLA class I defect
HLA class I molecules play a pivotal role in anti-neoplastic immune response [124] by either presenting a range of self and foreign antigens to cytotoxic T-lymphocytes (CTL), or serving as ligands for killer immunoglobulin-like receptors (KIRs) on NK cells [125]. Nevertheless, MB tumor downregulates the HLA class I molecules to evade T cell-mediated immunity and NK cell recognition [89]. This deregulation and structural alteration in the MHC class I antigen presenting machinery results in facilitating MB expansion, aggression, and metastasis. Furthermore, several studies reported the co-expression of the negative prognostic markers such as c-MYC and HLA class I molecules in MB [126]. They have shown the interaction of β2 microglobulin (β2m) that is produced and secreted into the extracellular matrix of MB tissues with the MHC class I molecules on adjacent cells leading to the enhancement of the migratory potential and invasiveness of MB cell lines [126, 127].
Impairment of adhesive molecules
Extracellular matrix (ECM) proteins
The key proteins in the ECM include fibronectin, collagen, tenascin, and laminin, which bind to integrins [128]. Integrins are multifunctional cell surface heterodimers, expressed in tumor cells and are responsible for promoting tumor growth, stemness and metastasis. [129]. In vivo and in vitro studies reported the interaction of cell surface integrins with ECM tenascin mediating the adhesion, survival and proliferation of MB cells [128]. Another ECM protein, known as type I collagen, plays a role in MB angiogenesis and vasculature [130].
Cadherins
Cadherins are Ca2+-dependent hemophilic cell-cell adhesion molecules [131] that maintain homeostasis [132] through the interaction with β-catenin. In this sense, the dysfunctional expression of these classical cadherins seems to be related to cell differentiation, proliferation, tumor invasion, and metastasis. Moreover, mutations in β-catenin have been associated with MB development. Accumulated evidence has shown the immunoreactivity of most MBs and high grade-gliomas for N-cadherin and β-catenin, which are responsible for dissemination, proliferation, and poor prognosis [131].
Immune checkpoint molecules
B7-H3 is an inhibitory immune checkpoint ligand [87], which plays a critical role in promoting MB angiogenesis, progression, tumor cell signaling and chemoresistance [133]. Notably, B7-H3 is highly expressed in MB tumor tissues compared to other immune checkpoints, like the PD-1/PD-L1 and the cytotoxic T-lymphocyte antigen (CTLA)-4 [87]. Besides, the persistent expression of PD-1/PD-L1 immune checkpoint in MB shows a worse prognosis in patients [89].
Protection against apoptosis
Apoptosis is induced in response to different anti-neoplastic agents [134, 135•, 136,137,138,139]. Notably, MB mediates the resistance to apoptosis [140] through the expression of intracellular apoptotic inhibitor (IAP) and the caspase inhibitors to escape from death receptors-induced apoptosis and granzymes-mediated killing pathways [89, 141]. Besides, MB cells undergo a high mitotic activity under the cIAP1 expression [142]. In addition, MB has the potency to express the anti-apoptotic genes including Bcl-2 and c-FLIP, resulting in the resistance to TNF-related apoptosis-inducing ligand (TRAIL) [141]. Another anti-apoptotic molecule, known as microRNA 21 (miR-21), is widely upregulated in all MB subgroups [143]. Moreover, glioma transcription factor (Gli1) binds directly to the Bcl-2 promoter, resulting in the regulation of the MB cell survival [144].
Novel immunosuppressive pathways in MB
Several studies have demonstrated the role of tumor’s intrinsic signaling pathways in mediating the resistance to the immune aggression including the activity of STAT [145], PI3K/Akt/mTOR [146], MAPK [147], and ERK pathways [148]. In MB, PI3K/Akt/mTOR pathway is activated to trigger cell development, proliferation, migration, angiogenesis, and chemoresistance [146,147,148,149,150,151,152,153]. Furthermore, STAT3 is a master cell survival protein that plays an important role in anti-apoptosis, angiogenesis, and cell evasion of the immune cells in many brain tumors, but its elevated level is noticed mainly in MB for its maintenance and development [145]. Recent studies reported the implication of cyclin-dependent kinase (CDK) inhibitor, known as P27, in MB cell migration [154].
Subgroup-specific immune microenvironment in MB
Understanding the immune microenvironment of MB subgroups is essential in order to develop strategies for immunotherapies in pediatric populations. These subgroups have distinct immune profiles that exploit several mechanisms to facilitate immunosuppression and evasion [155]. Recent studies showed a significant difference in immune and stromal cell populations that reside in the MB subgroups-related microenvironment except for NK cells and B cells. First, SHH-derived MB is characterized by more macrophages, DCs, T cells and myeloid cells compared to Group 3 MB, which reflects a better prognosis [76, 89, 155]. On the other hand, SHH-derived MB expresses high levels of inflammation-related genes including TAM-related genes such as CD163 and colony-stimulating factor-1 receptor (CSF1R) and the inhibition of this receptor can arrest the protumor effects in the TME [94].
Moreover, SHH- and Wnt-derived MBs exhibit a monocytic/macrophage and Treg supported pro-tumorigenic microenvironment, while most Group 3 and Group 4 MBs belong to a pattern of tumors that suppresses the infiltration of lymphocytes by immunosuppressive cytokines and checkpoint pathways. Such differentiation of immunological characteristics among the different MB subtypes supports the development of subtype-specific immunotherapeutic strategies [76].
Immune biomarkers in MB
To identify the heterogeneous phenotypes of distinct MB subtypes, several studies highlight the characterization of novel cell surface markers expressed by these tumor cells. Based on the cancer stem cell (CSC) theory, some tumors including MB contain a subpopulation of cells that exhibit stem cell-like properties, responsible for tumor initiation and maintenance [134, 135•, 156,157,158,159••]. CSCs in brain tumors express neural precursor cell surface markers such as CD133 (prominin1) which is responsible for the resistance against apoptosis, drugs, and ionized radiations, and for enhancing the self-renewal capacity [156, 160, 161]. Another cell surface marker, CD271, is a member of the tumor necrosis factor receptor family and is commonly referred to as p75 or p75NTR. It plays a critical role in neurodevelopment including growth cone elongation, axon guidance, cell survival, and cell death depending on neurotrophin ligands as NGF, BDNF, NT-3, and NT-4 [156, 160]. Furthermore, MB exhibits an inverse relationship among the cells that express both cell surface markers (CD133 and CD271). Higher self-renewing cells capacity exhibits an increased expression of CD271 and a decreased expression of CD133 to maintain tumorigenesis, while the opposite expression pattern of these cell surface markers exhibits a highly motile displayed cell [160]. To add, matrix metalloproteinase (MMP) triggers the MB invasion. MB tissues express MMP-9 and membrane type-1 (MT1)-MMP, which is likely responsible for invasion, radio-resistance, and triggering the hypoxic status [161]. Moreover, tumor propagating cell (TPC) expresses another marker, known as CD15, which was found on progenitors and stem cells in the embryonic and adult CNS, plays an essential role in the maintenance and growth of human MB [162]. Interestingly, recent studies highlight a critical immune marker that mediates the immune response in both normal brain and brain tumors, known as MHC class I. In addition, aggressive types of MB are associated with a negative prognostic marker which is c-myc gene. As a result, MHC class I is expressed in aggressive MB with poor prognosis, which in turn induces ERK1/2 phosphorylation and mediates MB metastasis [126]. Besides, all MB cells express a potent CD146 marker, which is responsible for the growing tumor rate and metastasis in the pediatric population [163].
Immunotherapy in medulloblastoma
MB patients usually benefit from different therapeutic paradigms of maximal safe resection, radiation, and adjuvant chemotherapy [18, 164] that can lead to long-term limitations in behavioral, social, cognitive, and physical functions [165, 166]. In the last few decades, there has been a significant progress in the field of immunotherapy that aimed to improve the host immune response against tumor cells [167, 168]. In MB, various experimental approaches in immunotherapy have highlighted the increasing potential for MB treatment (Fig. 1).
Adoptive cellular therapy/cellular immunotherapy
Adaptive cellular therapy involves the direct isolation and modification of host immune cells to enhance their cancer-fighting capabilities, followed by their subsequent infusion back into the host for direct targeting of cancer cells [169]. This approach can be deployed in different ways: NK cell therapy, chimeric antigen receptor (CAR) T cell therapy, engineered T cell receptor (TCR) therapy, and TIL therapy.
NK cells therapy
NK cells can effectively target MB cells mainly through activation of different NK cell receptors (NKG2D, DNAM-1, NKp30, and NKp46) upon the binding of specific MB cell ligands [163]. Blocking of NKG2D receptors on the NK cells and specific ligands expressed on HTB-186 MB cell line, major histocompatibility complex class I-related chain A (MICA) and UL16 binding protein (ULBP-2), have increased the resistance to NK cells lysis in vitro [170], indicating the impact of appropriate stimulation of NK cells for potential targeting of MB cells. However, blocking of HLA class I on MB cells and stimulation of NK cells with IL-15 increased the cytotoxicity against MB cells in vitro [170]. Accordingly, stimulation of NK cells via IL-15 could be a valuable strategy for MB treatment, as has already been suggested for other pediatric solid tumors [171]. Modification of cord blood NK cells to express a dominant negative TGF-β receptor II (DNRII) by retroviral transduction proved an effective therapeutic intervention for brain tumors through evading TGF-β [172]. An in vitro study reported that MB cells were rendered susceptible to NK cells expressing DNRII receptors and also suggested that DNRII can restore the function of NK cells by neutralization of TGF-β [103]. Another study showed that the intracranial injection of human NK cells resulted in a remarkable regression of orthotropic human MB xenografts in NOD/SCID mice, suggesting that NK cells alone were able to significantly delay MB growth [173]. One clinical study has shown the safety of autologous NK cell therapy in patients with recurrent malignant gliomas [174]. A phase I clinical trial conducted on children with recurrent MB and ependymoma showed no dose-limiting toxicities of the intraventricular infusion of autologous ex vivo expanded NK cells, with one out of nine patients showing a stable disease for one month at the end of study follow-up [175]. Another phase I clinical trial has focused on the side effects and the accurate dose of NK cell in pediatric patients with recurrent/ refractory brain tumors including MB, but the results have not been released yet (ClinicalTrial.gov; Phase I clinical trial; NCT02271711) (Table 1).
Chimeric antigen receptor (CAR) T cell therapy
CAR T cell therapy represents a new era of personalized cellular cancer therapy. It is based on genetically engineering patient’s own T cells to express a particular receptor that specifically binds a tumor antigen [176]. CAR T cell treatment holds great promise in the treatment of brain malignancies [177, 178]. It has also been translated clinically and showed an effective therapeutic potential in GB patients [179]. Since HER2 is overexpressed in MB, it can be targeted by CAR T cell therapy. HER2 CAR T cells were able to proliferate and secrete IFN-γ and IL-2 in a HER2-dependent manner. They recognized and attacked MB cell lines and autologous primary MB cells ex vivo and reduced the growth of MB in the orthotopic xenogenic SCID mouse model [180]. Nellan et al. showed that HER2-BBz-CAR T cells therapy effectively cleared MB that was implanted in the posterior fossa of NSG mice via both regional and intravenous delivery in a xenograft mouse model without causing any considerable treatment-related toxicity [181]. Similar results were obtained for NKG2D-specific CAR T cells (KD-025) where KD-025 eliminated MB xenograft in NSG mice without any noticeable safety issues [182]. Another study showed a remarkable antitumor activity B7-H3 CAR T cells in vivo where they mediated MB regression in NSG mice model [183]. Given these data, advances in CAR T cell therapy allow this approach to be translated clinically into patients with MB. Two phase I clinical trials, (ClinicalTrial.gov; Phase I clinical trial; NCT03500991) and (ClinicalTrial.gov; Phase I clinical trial; NCT03638167), are currently evaluating the feasibility, safety, and tolerability of CAR T cell therapy in CNS tumors including MB (Table 1).
Engineered T cell receptor (TCR) therapy
Like CAR T cell therapy, engineered TCR therapy involves targeting tumors via new T cell surface receptors. However, in engineered TCR therapy, the artificial receptor relies on the TCR-peptide/MHC to mark cancer cells with recognizable antigens [184]. MB cells are characterized by a high expression level of several immunogenic antigens that belong to an expanding family of immunogenic cancer testicular antigens (CTAs) [185,186,187]. PRAME, an immunogenic CTA, is frequently expressed in various cancers including MB with a limited expression in normal tissues [186, 188]. Orlando et al. targeted MB by using genetically modified T cells with PRAME-specific TCR (SLL TCR T cells) [189]. Engineered SLL TCR T cells have effectively destroyed MB HLA-A*02+ Daoy cells. Besides, orthotopic MB NSG mice showed a dynamic tumor regression after injection of SLL TCR T cells [189]. These data suggest that engineered TCR therapy could be an effective strategy for targeting MB and should be tested in early-stage clinical trials for MB patients.
TIL therapy
TIL therapy depends on harvesting naturally occurring lymphocytes that have already infiltrated into the tumor site, followed by their activation, expansion, and subsequent infusion back into patients. This approach was initially established by Eberlein et al. in which the injection of lymphocytes expanded in IL-2 has mediated the cure of mice with syngeneic lymphoma [190]. Regarding MB, tumor-reactive T cells were generated and adoptively transferred into MB-bearing mice. These TIL clones have retained their antitumor activity against MB and exhibited a considerable in vivo clonal expansion and persistence within the peripheral blood [191].
Immune checkpoint inhibitors
Immune checkpoint inhibitors have been approved for the use in various types of cancers due to their potential in producing durable tumor regression [136, 137, 192, 193]. These drugs are predominantly monoclonal antibodies that are developed to block immune checkpoints on the surface of immune cells, preventing the interaction with the respective checkpoint ligands, reducing the suppression of T cells, and thereby restoring the function of immune cells [138]. Several studies have discussed the activity of checkpoint inhibitors on MB. Pham et al. demonstrated that the molecular subtypes of murine MB possess distinct immunologic profiles that differentially respond to immune checkpoint inhibitors in mice bearing intracranial MB tumors [155]. Murine group 3 MB contained a higher percentage of PD-1+ CD8+ cells than the SHH MB group, whereas no appreciable difference was detected for the CTLA-4+ CD4+ T cells between the two MB groups. This further suggested that group 3 MB has a more pronounced response to PD-1 blockade [155]. However, another study has demonstrated a complete absence of PD-L1 expression in pediatric MB, and correspondingly PD1/PD-L1 blockers might be inappropriate for MB treatment [89]. While Vermeulen et al. has recognized limited PD-L1 level in MB [89], Martin et al. showed higher PD-L1 expression level in SHH compared to group 3 and 4 MB [139]. The uncommon PD-L1 expression and rare PD-1+ T cell tumor infiltration inferred that MB may not be a suitable candidate for immune checkpoint inhibitors [194]. Several clinical trials, (ClinicalTrials.gov; Phase II clinical trial; NCT03173950), (ClinicalTrials.gov; Phase I clinical trial; NCT02359565), (ClinicalTrials.gov; Phase II clinical trial; NCT03130959), (ClinicalTrials.gov; Phase I clinical trial; NCT00089245), (ClinicalTrials.gov; Phase I clinical trial; NCT02502708) and (ClinicalTrials.gov; Phase I clinical trial; NCT04049669) are investigating checkpoint inhibitor drugs in MB patients (Table 1). However, many of those results have not been released yet.
Vaccination
The expression of tumor-specific antigens holds immunotherapeutic potential, as they have been successfully targeted by vaccination for cancer patients [195, 196]. A series of tumor-specific antigens could render MB amenable to vaccine therapy [197]. Monocyte-derived DCs were generated to be used as a source of tumor-associated antigens. DC-based vaccination showed a more favorable response in high-grade glioma and atypical teratoid-rhabdoid tumors than that in MB patients [198]. Nair et al. have investigated the potential to generate DCs to evaluate ex vivo-generated DCs-RNA based vaccination approach in MB patients [199]. This study is currently in phase I and II clinical trial (ClinicalTrials.gov; Phase I and II clinical trial; NCT01326104). Another autologous DC vaccination based on targeting peptides derived from NY-ESO-1, MAGE-A1, or MAGE-A3, was administrated to MB patients (ClinicalTrials.gov; Phase I and II clinical trial; NCT02332889). However, the study was terminated due to a serious adverse event and disease progression. Furthermore, a Phase I clinical trial was organized for patients with malignant brain tumors including MB using vaccination with brain tumor stem cells-loaded DC. Nevertheless, this trial was terminated without any posted results (ClinicalTrials.gov; Phase I clinical trial; NCT01171469) (Table 1). The use of MB vaccines seems, at least at present time, to be limited and not considered as a therapy of choice mainly for MB patients.
Oncolytic virotherapy
Oncolytic viruses (OVs) are biotherapeutics that have been genetically engineered to selectively infect and kill tumor cells, gaining attention for the treatment of several types of cancers [200,201,202]. An in vitro study has reported the ability of rhinovirus, a recombinant form of poliovirus, to inhibit the proliferation and subsequently kill MB cells [203]. The oncolytic virotherapy with myxoma virus was also studied in MB where it prolonged the survival of tumor-bearing mice. The combinational therapy with rapamycin further enhanced the oncolytic potential of the myxoma virus and reduced tumor metastasis [204]. Another study has reported that the intracerebellar MB xenograft was eliminated after a single intravenous injection of picornavirus SVV-001, resulting in a significant increase in survival [205]. Studebaker et al. evaluated the oncolytic therapeutic potential of herpes virus eRp450 using orthotopic xenograft group 3 and 4 MB, in which eRp450 therapy displayed a significantly prolonged survival following a single intratumoral injection. It also revealed the enhanced efficacy of rRp450 upon the combination with chemotherapeutic drug cyclophosphamide [152]. This is in-line with another study supporting the role of engineered herpes simplex virus therapy for infecting and killing pediatric MB cells [153]. These results underline the potent preclinical antitumor activity of oncolytic virotherapy against MB. To date, various oncolytic viruses are currently under clinical evaluation, (ClinicalTrials.gov; Phase I clinical trial; NCT02962167), (ClinicalTrials.gov; Phase I clinical trial; NCT03615404), (ClinicalTrials.gov; Phase I clinical trial; NCT03299309), (ClinicalTrials.gov; Phase I clinical trial; NCT03043391), (ClinicalTrials.gov; Phase I clinical trial; NCT02444546) and (ClinicalTrials.gov; Phase I clinical trial; NCT03911388) (Table 1).
Cytokine inhibitors/administration
Targeting cytokines and their receptors have been investigated in many studies due to their critical role in several aspects of cancer biology. An in vitro study demonstrated that the blocking of pro-inflammatory IL-6 signaling by the FDA-approved drug bazedoxifene inhibited cell proliferation and colony formation, and decreased cell viability and glycolysis in Daoy and UW288 MB cell lines [107]. Another study reported that targeting IL-13 receptors by IL-13 cytotoxin induced cytotoxicity in UW228 MB cell lines [206]. Additionally, CXCR4 inhibitors (AMD3100 and AMD3465) have shown significant antitumor effects by inhibiting MB cell line growth and migration, inducing apoptosis in MB tumors, and reducing MB allograft growth in mice [114, 207, 208].
In contrast to the therapeutic role of cytokine inhibition, contradictory results have been obtained upon chemokines and ILs administration. Zhou et al. reported a considerable reduction in MB growth in IL-17-injected mice suggesting its antitumor properties [109]. Regarding chemokines, treatment with chemokine CXCL3 elicited a complete disappearance of MB lesion, and further forced the migration and differentiation of pre-neonatal precursor cells (pGCPs) in Ptch1+/- /Tis21-/- mice [209]. These results were consistent with other findings that confirmed the role of CXCL3 in reducing lesion size and promoting pGCPs migration [210].
Conclusions
Medulloblastoma is one of the most devastating pediatric tumors in which long-term survival is achievable nonetheless with significant morbidity from current therapeutic strategies. There has been a great advance in the knowledge of MB immunology over the last few decades. Substantial research work focused on the immunosuppressive mechanisms of MB has been performed providing an initial understanding of how MB can improve its own immunosuppressive mechanisms and evade the immune responses. Standard chemotherapy and radiotherapy are both profoundly immunosuppressive regimens. On the other hand, numerous studies concentrated on the promising potential of various immunotherapeutic approaches to target MB. However, intrinsic immunosuppressive properties, antigenic heterogeneity, physical barriers, the most suitable immunotherapeutic strategy, the dose for each case, and the route of drug delivery are some of the challenges for successful immunotherapy. Novel immunotherapeutic strategies including combination therapy should be developed to overcome the aforementioned obstacles and to achieve the most favorable results.
Data Availability
Not applicable.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro-Oncology. 2018;20(suppl_4):iv1–iv86. https://doi.org/10.1093/neuonc/noy131.
Pollack IF. Brain tumors in children. N Engl J Med. 1994;331(22):1500–7. https://doi.org/10.1056/nejm199412013312207.
Kaur K, Kakkar A, Kumar A, Mallick S, Julka PK, Gupta D, et al. Integrating Molecular Subclassification of Medulloblastomas into Routine Clinical Practice: A Simplified Approach. Brain Pathol. 2016;26(3):334–43. https://doi.org/10.1111/bpa.12293.
Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009-2013. Neuro-Oncology. 2016;18(suppl_5):v1–v75. https://doi.org/10.1093/neuonc/now207.
Millard NE, De Braganca KC. Medulloblastoma. J Child Neurol. 2016;31(12):1341–53. https://doi.org/10.1177/0883073815600866.
Smoll NR, Drummond KJ. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci. 2012;19(11):1541–4. https://doi.org/10.1016/j.jocn.2012.04.009.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–20. https://doi.org/10.1007/s00401-016-1545-1.
Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61(3):215–25; discussion 226-219. https://doi.org/10.1093/jnen/61.3.215.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. https://doi.org/10.1007/s00401-007-0243-4.
Northcott PA, Buchhalter I, Morrissy AS, Hovestadt V, Weischenfeldt J, Ehrenberger T, et al. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017;547(7663):311–7. https://doi.org/10.1038/nature22973.
Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123(4):473–84. https://doi.org/10.1007/s00401-012-0958-8.
Ellison DW, Dalton J, Kocak M, Nicholson SL, Fraga C, Neale G, et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011;121(3):381–96. https://doi.org/10.1007/s00401-011-0800-8.
Ellison DW, Onilude OE, Lindsey JC, Lusher ME, Weston CL, Taylor RE, et al. beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom Children's Cancer Study Group Brain Tumour Committee. J Clin Oncol. 2005;23(31):7951–7. https://doi.org/10.1200/JCO.2005.01.5479.
Kool M, Jones DT, Jager N, Northcott PA, Pugh TJ, Hovestadt V, et al. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell. 2014;25(3):393–405. https://doi.org/10.1016/j.ccr.2014.02.004.
Zhukova N, Ramaswamy V, Remke M, Pfaff E, Shih DJ, Martin DC, et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol. 2013;31(23):2927–35. https://doi.org/10.1200/jco.2012.48.5052.
Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. The Lancet Oncology. 2006;7(10):813–20. https://doi.org/10.1016/s1470-2045(06)70867-1.
Lannering B, Rutkowski S, Doz F, Pizer B, Gustafsson G, Navajas A, et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol. 2012;30(26):3187–93. https://doi.org/10.1200/jco.2011.39.8719.
Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–8. https://doi.org/10.1200/jco.2006.06.4980.
von Bueren AO, Kortmann RD, von Hoff K, Friedrich C, Mynarek M, Müller K, et al. Treatment of Children and Adolescents With Metastatic Medulloblastoma and Prognostic Relevance of Clinical and Biologic Parameters. J Clin Oncol. 2016;34(34):4151–60. https://doi.org/10.1200/jco.2016.67.2428.
Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children's Cancer Group study. J Clin Oncol. 2001;19(15):3470–6. https://doi.org/10.1200/jco.2001.19.15.3470.
Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in Neurocognitive Functioning After Treatment With Cranial Radiation in Childhood. J Clin Oncol. 2004;22(4):706–13. https://doi.org/10.1200/jco.2004.05.186.
Knight SJ, Conklin HM, Palmer SL, Schreiber JE, Armstrong CL, Wallace D, et al. Working memory abilities among children treated for medulloblastoma: parent report and child performance. J Pediatr Psychol. 2014;39(5):501–11. https://doi.org/10.1093/jpepsy/jsu009.
Palmer SL, Armstrong C, Onar-Thomas A, Wu S, Wallace D, Bonner MJ, et al. Processing speed, attention, and working memory after treatment for medulloblastoma: an international, prospective, and longitudinal study. J Clin Oncol. 2013;31(28):3494–500. https://doi.org/10.1200/JCO.2012.47.4775.
Pols S, van Veelen MLC, Aarsen FK, Gonzalez Candel A, Catsman-Berrevoets CE. Risk factors for development of postoperative cerebellar mutism syndrome in children after medulloblastoma surgery. J Neurosurg Pediatr. 2017;20(1):35–41. https://doi.org/10.3171/2017.2.PEDS16605.
Cuevas LM, Daud AI. Immunotherapy for melanoma. Seminars in cutaneous medicine and surgery. 2018;37(2):127–31. https://doi.org/10.12788/j.sder.2018.028.
Bhojwani D, Sposto R, Shah NN, Rodriguez V, Yuan C, Stetler-Stevenson M, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Leukemia. 2019;33(4):884–92. https://doi.org/10.1038/s41375-018-0265-z.
Pollard JA, Loken M, Gerbing RB, Raimondi SC, Hirsch BA, Aplenc R, et al. CD33 Expression and Its Association With Gemtuzumab Ozogamicin Response: Results From the Randomized Phase III Children's Oncology Group Trial AAML0531. J Clin Oncol. 2016;34(7):747–55. https://doi.org/10.1200/JCO.2015.62.6846.
Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. https://doi.org/10.1111/j.1600-065X.2006.00441.x.
Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28(2):254–60. https://doi.org/10.1002/jnr.490280213.
Lawrence JM, Morris RJ, Wilson DJ, Raisman G. Mechanisms of allograft rejection in the rat brain. Neuroscience. 1990;37(2):431–62. https://doi.org/10.1016/0306-4522(90)90413-X.
Bradbury MW, Cserr HF, Westrop RJ. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am J Phys. 1981;240(4):F329–36. https://doi.org/10.1152/ajprenal.1981.240.4.F329.
Szentistványi I, Patlak CS, Ellis RA, Cserr HF. Drainage of interstitial fluid from different regions of rat brain. Am J Phys. 1984;246(6 Pt 2):F835–44. https://doi.org/10.1152/ajprenal.1984.246.6.F835.
Goldmann J, Kwidzinski E, Brandt C, Mahlo J, Richter D, Bechmann I. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J Leukoc Biol. 2006;80(4):797–801. https://doi.org/10.1189/jlb.0306176.
Kaminski M, Bechmann I, Kiwit J, Glumm J. Migration of monocytes after intracerebral injection. Cell Adhes Migr. 2012;6(3):164–7. https://doi.org/10.4161/cam.20281.
Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–9. https://doi.org/10.1084/jem.20142290.
Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. 2018;21(10):1380–91. https://doi.org/10.1038/s41593-018-0227-9.
Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature. 2018;560(7717):185–91. https://doi.org/10.1038/s41586-018-0368-8.
Korin B, Ben-Shaanan TL, Schiller M, Dubovik T, Azulay-Debby H, Boshnak NT, et al. High-dimensional, single-cell characterization of the brain's immune compartment. Nat Neurosci. 2017;20(9):1300–9. https://doi.org/10.1038/nn.4610.
Fernández-Arjona MDM, Grondona JM, Granados-Durán P, Fernández-Llebrez P, López-Ávalos MD. Microglia Morphological Categorization in a Rat Model of Neuroinflammation by Hierarchical Cluster and Principal Components Analysis. Front Cell Neurosci. 2017;11:235. https://doi.org/10.3389/fncel.2017.00235.
Kaminska B, Mota M, Pizzi M. Signal transduction and epigenetic mechanisms in the control of microglia activation during neuroinflammation. Biochim Biophys Acta. 2016;1862(3):339–51. https://doi.org/10.1016/j.bbadis.2015.10.026.
Forrester JV, McMenamin PG, Dando SJ. CNS infection and immune privilege. Nat Rev Neurosci. 2018;19(11):655–71. https://doi.org/10.1038/s41583-018-0070-8.
Ulvestad E, Williams K, Bjerkvig R, Tiekotter K, Antel J, Matre R. Human microglial cells have phenotypic and functional characteristics in common with both macrophages and dendritic antigen-presenting cells. J Leukoc Biol. 1994;56(6):732–40. https://doi.org/10.1002/jlb.56.6.732.
Haley MJ, Brough D, Quintin J, Allan SM. Microglial Priming as Trained Immunity in the Brain. Neuroscience. 2019;405:47–54. https://doi.org/10.1016/j.neuroscience.2017.12.039.
Karman J, Ling C, Sandor M, Fabry Z. Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol. 2004;173(4):2353–61. https://doi.org/10.4049/jimmunol.173.4.2353.
Steinbach K, Vincenti I, Kreutzfeldt M, Page N, Muschaweckh A, Wagner I, et al. Brain-resident memory T cells represent an autonomous cytotoxic barrier to viral infection. J Exp Med. 2016;213(8):1571–87. https://doi.org/10.1084/jem.20151916.
Nishihara H, Soldati S, Mossu A, Rosito M, Rudolph H, Muller WA, et al. Human CD4+ T cell subsets differ in their abilities to cross endothelial and epithelial brain barriers in vitro. Fluids and Barriers of the CNS. 2020;17(1):3. https://doi.org/10.1186/s12987-019-0165-2.
Ortega SB, Torres VO, Latchney SE, Whoolery CW, Noorbhai IZ, Poinsatte K, et al. B cells migrate into remote brain areas and support neurogenesis and functional recovery after focal stroke in mice. Proc Natl Acad Sci U S A. 2020;117(9):4983–93. https://doi.org/10.1073/pnas.1913292117.
Engler-Chiurazzi EB, Monaghan KL, Wan ECK, Ren X. Role of B cells and the aging brain in stroke recovery and treatment. Geroscience. 2020;42(5):1199–216. https://doi.org/10.1007/s11357-020-00242-9.
Perng P, Lim M. Immunosuppressive Mechanisms of Malignant Gliomas: Parallels at Non-CNS Sites. Front Oncol. 2015;5:153. https://doi.org/10.3389/fonc.2015.00153.
Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R, et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin Cancer Res. 2011;17(13):4296–308. https://doi.org/10.1158/1078-0432.Ccr-10-2557.
Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–302. https://doi.org/10.1158/0008-5472.Can-05-3773.
Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008;57(1):123–31. https://doi.org/10.1007/s00262-007-0336-x.
Geranmayeh F, Scheithauer BW, Spitzer C, Meyer FB, Svensson-Engwall AC, Graeber MB. Microglia in gemistocytic astrocytomas. Neurosurgery. 2007;60(1):159–66; discussion 166. https://doi.org/10.1227/01.Neu.0000249192.30786.67.
Hattermann K, Sebens S, Helm O, Schmitt AD, Mentlein R, Mehdorn HM, et al. Chemokine expression profile of freshly isolated human glioblastoma-associated macrophages/microglia. Oncol Rep. 2014;32(1):270–6. https://doi.org/10.3892/or.2014.3214.
Fontana A, Bodmer S, Frei K, Malipiero U, Siepl C. Expression of TGF-beta 2 in human glioblastoma: a role in resistance to immune rejection? CIBA Found Symp. 1991;157:232–8; discussion 238-241. https://doi.org/10.1002/9780470514061.ch15.
Huettner C, Paulus W, Roggendorf W. Messenger RNA expression of the immunosuppressive cytokine IL-10 in human gliomas. Am J Pathol. 1995;146(2):317–22.
Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, et al. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther. 2010;9(1):67–78. https://doi.org/10.1158/1535-7163.Mct-09-0734.
Matias D, Balça-Silva J, da Graça GC, Wanjiru CM, Macharia LW, Nascimento CP, et al. Microglia/Astrocytes-Glioblastoma Crosstalk: Crucial Molecular Mechanisms and Microenvironmental Factors. Front Cell Neurosci. 2018;12:235. https://doi.org/10.3389/fncel.2018.00235.
Nishie A, Ono M, Shono T, Fukushi J, Otsubo M, Onoue H, et al. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res. 1999;5(5):1107–13.
Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, et al. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell. 2018;172(5):1022–1037.e1014. https://doi.org/10.1016/j.cell.2018.01.004.
Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT. TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro-Oncology. 2010;12(1):7–13. https://doi.org/10.1093/neuonc/nop009.
Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro-Oncology. 2010;12(11):1113–25. https://doi.org/10.1093/neuonc/noq082.
Wischhusen J, Jung G, Radovanovic I, Beier C, Steinbach JP, Rimner A, et al. Identification of CD70-mediated apoptosis of immune effector cells as a novel immune escape pathway of human glioblastoma. Cancer Res. 2002;62(9):2592–9.
Didenko VV, Ngo HN, Minchew C, Baskin DS. Apoptosis of T lymphocytes invading glioblastomas multiforme: a possible tumor defense mechanism. J Neurosurg. 2002;96(3):580–4. https://doi.org/10.3171/jns.2002.96.3.0580.
Badie B, Schartner J, Prabakaran S, Paul J, Vorpahl J. Expression of Fas ligand by microglia: possible role in glioma immune evasion. J Neuroimmunol. 2001;120(1-2):19–24. https://doi.org/10.1016/s0165-5728(01)00361-7.
Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Farber SH, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24(9):1459–68. https://doi.org/10.1038/s41591-018-0135-2.
Woroniecka K, Chongsathidkiet P, Rhodin K, Kemeny H, Dechant C, Farber SH, et al. T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in Glioblastoma. Clin Cancer Res. 2018;24(17):4175–86. https://doi.org/10.1158/1078-0432.Ccr-17-1846.
Dyck L, Mills KHG. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol. 2017;47(5):765–79. https://doi.org/10.1002/eji.201646875.
Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020;20(1):12–25. https://doi.org/10.1038/s41568-019-0224-7.
Wei B, Wang L, Zhao X, Du C, Guo Y, Sun Z. The upregulation of programmed death 1 on peripheral blood T cells of glioma is correlated with disease progression. Tumour Biol. 2014;35(4):2923–9. https://doi.org/10.1007/s13277-013-1376-9.
Magnus T, Schreiner B, Korn T, Jack C, Guo H, Antel J, et al. Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS. J Neurosci. 2005;25(10):2537–46. https://doi.org/10.1523/jneurosci.4794-04.2005.
Staneva R, Burla F, Koenderink GH, Descroix S, Vignjevic DM, Attieh Y, et al. A new biomimetic assay reveals the temporal role of matrix stiffening in cancer cell invasion. Mol Biol Cell. 2018;29(25):2979–88. https://doi.org/10.1091/mbc.E18-01-0068.
Maximov V, Chen Z, Wei Y, Robinson MH, Herting CJ, Shanmugam NS, et al. Tumour-associated macrophages exhibit anti-tumoural properties in Sonic Hedgehog medulloblastoma. Nat Commun. 2019;10(1):2410. https://doi.org/10.1038/s41467-019-10458-9.
Franchi M, Piperigkou Z, Karamanos KA, Franchi L, Masola V. Extracellular Matrix-Mediated Breast Cancer Cells Morphological Alterations, Invasiveness, and Microvesicles/Exosomes Release. Cells. 2020;9(9). https://doi.org/10.3390/cells9092031.
Diao S, Gu C, Zhang H, Yu C. Immune cell infiltration and cytokine secretion analysis reveal a non-inflammatory microenvironment of medulloblastoma. Oncol Lett. 2020;20(6):397. https://doi.org/10.3892/ol.2020.12260.
Bockmayr M, Mohme M, Klauschen F, Winkler B, Budczies J, Rutkowski S, et al. Subgroup-specific immune and stromal microenvironment in medulloblastoma. Oncoimmunology. 2018;7(9):e1462430. https://doi.org/10.1080/2162402x.2018.1462430.
Salsman VS, Chow KK, Shaffer DR, Kadikoy H, Li XN, Gerken C, et al. Crosstalk between medulloblastoma cells and endothelium triggers a strong chemotactic signal recruiting T lymphocytes to the tumor microenvironment. PLoS One. 2011;6(5):e20267. https://doi.org/10.1371/journal.pone.0020267.
Bouterfa H, Darlapp AR, Klein E, Pietsch T, Roosen K, Tonn JC. Expression of different extracellular matrix components in human brain tumor and melanoma cells in respect to variant culture conditions. J Neuro-Oncol. 1999;44(1):23–33. https://doi.org/10.1023/a:1006331416283.
Abad C, Nobuta H, Li J, Kasai A, Yong WH, Waschek JA. Targeted STAT3 disruption in myeloid cells alters immunosuppressor cell abundance in a murine model of spontaneous medulloblastoma. J Leukoc Biol. 2014;95(2):357–67. https://doi.org/10.1189/jlb.1012531.
Gururangan S, Reap E, Schmittling R, Kocak M, Reynolds R, Grant G, et al. Regulatory T cell subsets in patients with medulloblastoma at diagnosis and during standard irradiation and chemotherapy (PBTC N-11). Cancer Immunol Immunother. 2017;66(12):1589–95. https://doi.org/10.1007/s00262-017-2051-6.
Shevelkin AV, Terrillion CE, Hasegawa Y, Mychko OA, Jouroukhin Y, Sawa A, et al. Astrocyte DISC1 contributes to cognitive function in a brain region-dependent manner. Hum Mol Genet. 2020;29(17):2936–50. https://doi.org/10.1093/hmg/ddaa180.
Gate D, Danielpour M, Rodriguez J Jr, Kim GB, Levy R, Bannykh S, et al. T-cell TGF-β signaling abrogation restricts medulloblastoma progression. Proc Natl Acad Sci U S A. 2014;111(33):E3458–66. https://doi.org/10.1073/pnas.1412489111.
Sandén E, Enríquez Pérez J, Visse E, Kool M, Carén H, Siesjö P, et al. Preoperative systemic levels of VEGFA, IL-7, IL-17A, and TNF-β delineate two distinct groups of children with brain tumors. Pediatr Blood Cancer. 2016;63(12):2112–22. https://doi.org/10.1002/pbc.26158.
Kofuku Y, Yoshiura C, Ueda T, Terasawa H, Hirai T, Tominaga S, et al. Structural basis of the interaction between chemokine stromal cell-derived factor-1/CXCL12 and its G-protein-coupled receptor CXCR4. J Biol Chem. 2009;284(50):35240–50. https://doi.org/10.1074/jbc.M109.024851.
Baryawno N, Sveinbjörnsson B, Eksborg S, Orrego A, Segerström L, Oqvist CO, et al. Tumor-growth-promoting cyclooxygenase-2 prostaglandin E2 pathway provides medulloblastoma therapeutic targets. Neuro-Oncology. 2008;10(5):661–74. https://doi.org/10.1215/15228517-2008-035.
Slongo ML, Molena B, Brunati AM, Frasson M, Gardiman M, Carli M, et al. Functional VEGF and VEGF receptors are expressed in human medulloblastomas. Neuro-Oncology. 2007;9(4):384–92. https://doi.org/10.1215/15228517-2007-032.
Purvis IJ, Avilala J, Guda MR, Venkataraman S, Vibhakar R, Tsung AJ, et al. Role of MYC-miR-29-B7-H3 in Medulloblastoma Growth and Angiogenesis. J Clin Med. 2019;8(8). https://doi.org/10.3390/jcm8081158.
Chang F, Li R, Noon K, Gage D, Ladisch S. Human medulloblastoma gangliosides. Glycobiology. 1997;7(4):523–30. https://doi.org/10.1093/glycob/7.4.523.
Vermeulen JF, Van Hecke W, Adriaansen EJM, Jansen MK, Bouma RG, Villacorta Hidalgo J, et al. Prognostic relevance of tumor-infiltrating lymphocytes and immune checkpoints in pediatric medulloblastoma. Oncoimmunology. 2018;7(3):e1398877. https://doi.org/10.1080/2162402x.2017.1398877.
• Patel S, Wang S, Snuderl M, Karajannis MA. Pre-treatment lymphopenia and indication of tumor-induced systemic immunosuppression in medulloblastoma. J Neuro-Oncol. 2018;136(3):541–4. https://doi.org/10.1007/s11060-017-2678-3 Reason: Retrospective analysis of pretreatment neutrophil and lymphocyte counts in pediatric patients with medulloblastoma compared to a control group of children with posterior fossa pilocytic astrocytoma, suggesting the presence of tumor-induced systemic immune suppression in medulloblastoma patients already present at the time of diagnosis, with potential implications for the development of immune therapies in this population.
Sharma R, Katiyar V, Gurjar H, Sharma M, Goda R, Vora Z. Is medulloblastoma associated with systemic immunomodulation? - A comparative analysis of preoperative inflammatory markers. Surg Neurol Int. 2020;11:86. https://doi.org/10.25259/sni_336_2019.
Petty AJ, Li A, Wang X, Dai R, Heyman B, Hsu D, et al. Hedgehog signaling promotes tumor-associated macrophage polarization to suppress intratumoral CD8+ T cell recruitment. J Clin Invest. 2019;129(12):5151–62. https://doi.org/10.1172/jci128644.
Dang MT, Gonzalez M, Gaonkar KS, Rathi KS, Young P, Arif S, et al. Single-cell transcriptomic profile reveals macrophage heterogeneity in medulloblastoma and their treatment-dependent recruitment. bioRxiv. 2020:2020.2002.2012.945642. https://doi.org/10.1101/2020.02.12.945642.
Margol AS, Robison NJ, Gnanachandran J, Hung LT, Kennedy RJ, Vali M, et al. Tumor-associated macrophages in SHH subgroup of medulloblastomas. Clin Cancer Res. 2015;21(6):1457–65. https://doi.org/10.1158/1078-0432.Ccr-14-1144.
Kumar V, Cheng P, Condamine T, Mony S, Languino LR, McCaffrey JC, et al. CD45 Phosphatase Inhibits STAT3 Transcription Factor Activity in Myeloid Cells and Promotes Tumor-Associated Macrophage Differentiation. Immunity. 2016;44(2):303–15. https://doi.org/10.1016/j.immuni.2016.01.014.
Moertel CL, Xia J, LaRue R, Waldron NN, Andersen BM, Prins RM, et al. CD200 in CNS tumor-induced immunosuppression: the role for CD200 pathway blockade in targeted immunotherapy. J Immunother Cancer. 2014;2(1):46. https://doi.org/10.1186/s40425-014-0046-9.
Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68(13):5439–49. https://doi.org/10.1158/0008-5472.Can-07-6621.
Henrik Heiland D, Ravi VM, Behringer SP, Frenking JH, Wurm J, Joseph K, et al. Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat Commun. 2019;10(1):2541. https://doi.org/10.1038/s41467-019-10493-6.
Gronseth E, Gupta A, Koceja C, Kumar S, Kutty RG, Rarick K, et al. Astrocytes influence medulloblastoma phenotypes and CD133 surface expression. PLoS One. 2020;15(7):e0235852. https://doi.org/10.1371/journal.pone.0235852.
Liu Y, Yuelling LW, Wang Y, Du F, Gordon RE, O'Brien JA, et al. Astrocytes Promote Medulloblastoma Progression through Hedgehog Secretion. Cancer Res. 2017;77(23):6692–703. https://doi.org/10.1158/0008-5472.Can-17-1463.
Liu H, Sun Y, O'Brien JA, Franco-Barraza J, Qi X, Yuan H, et al. Necroptotic astrocytes contribute to maintaining stemness of disseminated medulloblastoma through CCL2 secretion. Neuro-Oncology. 2020;22(5):625–38. https://doi.org/10.1093/neuonc/noz214.
Vaez F, Farazmand A, Shaaheen S, Mostafaei S, Jamshidi A, Vojdanian M, et al. Upregulation of transforming growth factor-B1 gene in ankylosing spondylitis patients. Rheumatology Research. 2017;2(3):103–7. https://doi.org/10.22631/rr.2017.69997.1026.
Powell AB, Yadavilli S, Saunders D, Van Pelt S, Chorvinsky E, Burga RA, et al. Medulloblastoma rendered susceptible to NK-cell attack by TGFβ neutralization. J Transl Med. 2019;17(1):321. https://doi.org/10.1186/s12967-019-2055-4.
Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol. 2005;6(6):600–7. https://doi.org/10.1038/ni1197.
Jennings MT, Kaariainen IT, Gold L, Maciunas RJ, Commers PA. TGF beta 1 and TGF beta 2 are potential growth regulators for medulloblastomas, primitive neuroectodermal tumors, and ependymomas: evidence in support of an autocrine hypothesis. Hum Pathol. 1994;25(5):464–75. https://doi.org/10.1016/0046-8177(94)90118-x.
Jia L, Hong L, Marie-France H, de Tribolet N. Promotion ofin vitro growth of human medulloblastoma cells by exogeneous IL-6. Chin J Cancer Res. 1996;8(2):85–90. https://doi.org/10.1007/BF02675043.
Chen X, Wei J, Li C, Pierson CR, Finlay JL, Lin J. Blocking interleukin-6 signaling inhibits cell viability/proliferation, glycolysis, and colony forming activity of human medulloblastoma cells. Int J Oncol. 2018;52(2):571–8. https://doi.org/10.3892/ijo.2017.4211.
Sreenivasan L, Wang H, Yap SQ, Leclair P, Tam A, Lim CJ. Autocrine IL-6/STAT3 signaling aids development of acquired drug resistance in Group 3 medulloblastoma. Cell Death Dis. 2020;11(12):1035. https://doi.org/10.1038/s41419-020-03241-y.
Zhou P, Zhang Q, Zhao Y, Sha H, Cao X, Wang Y. IL-17 promoted the inhibition of medulloblastoma in mice by splenocyte injection. Eur J Med Res. 2015;20(1):98. https://doi.org/10.1186/s40001-015-0191-8.
Zhou P, Sha H, Zhu J. The role of T-helper 17 (Th17) cells in patients with medulloblastoma. J Int Med Res. 2010;38(2):611–9. https://doi.org/10.1177/147323001003800223.
Sandén E, Dyberg C, Krona C, Gallo-Oller G, Olsen TK, Enríquez Pérez J, et al. Establishment and characterization of an orthotopic patient-derived Group 3 medulloblastoma model for preclinical drug evaluation. Sci Rep. 2017;7:46366. https://doi.org/10.1038/srep46366.
Low SYY, Bte Syed Sulaiman N, Tan EEK, Ng LP, Kuick CH, Chang KTE, et al. Cerebrospinal fluid cytokines in metastatic group 3 and 4 medulloblastoma. BMC Cancer. 2020;20(1):554. https://doi.org/10.1186/s12885-020-07048-0.
Garzia L, Kijima N, Morrissy AS, De Antonellis P, Guerreiro-Stucklin A, Holgado BL, et al. A Hematogenous Route for Medulloblastoma Leptomeningeal Metastases. Cell. 2018;172(5):1050–1062.e1014. https://doi.org/10.1016/j.cell.2018.01.038.
Sengupta R, Dubuc A, Ward S, Yang L, Northcott P, Woerner BM, et al. CXCR4 activation defines a new subgroup of Sonic hedgehog-driven medulloblastoma. Cancer Res. 2012;72(1):122–32. https://doi.org/10.1158/0008-5472.Can-11-1701.
Kock A, Larsson K, Bergqvist F, Eissler N, Elfman LHM, Raouf J, et al. Inhibition of Microsomal Prostaglandin E Synthase-1 in Cancer-Associated Fibroblasts Suppresses Neuroblastoma Tumor Growth. EBioMedicine. 2018;32:84–92. https://doi.org/10.1016/j.ebiom.2018.05.008.
Kökoğlu E, Tüter Y, Sandikçi KS, Yazici Z, Ulakoğlu EZ, Sönmez H, et al. Prostaglandin E2 levels in human brain tumor tissues and arachidonic acid levels in the plasma membrane of human brain tumors. Cancer Lett. 1998;132(1-2):17–21. https://doi.org/10.1016/s0304-3835(98)00127-x.
Grun D, Adhikary G, Eckert RL. VEGF-A acts via neuropilin-1 to enhance epidermal cancer stem cell survival and formation of aggressive and highly vascularized tumors. Oncogene. 2016;35(33):4379–87. https://doi.org/10.1038/onc.2015.507.
Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212(2):139–48. https://doi.org/10.1084/jem.20140559.
Huang Y, Chen X, Dikov MM, Novitskiy SV, Mosse CA, Yang L, et al. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood. 2007;110(2):624–31. https://doi.org/10.1182/blood-2007-01-065714.
Gao Y, Li P, Liu Z, Diao X, Song C. Expression levels of vascular endothelial cell growth factor and microRNA-210 are increased in medulloblastoma and metastatic medulloblastoma. Exp Ther Med. 2015;10(6):2138–44. https://doi.org/10.3892/etm.2015.2810.
Hervey-Jumper SL, Garton HJ, Lau D, Altshuler D, Quint DJ, Robertson PL, et al. Differences in vascular endothelial growth factor receptor expression and correlation with the degree of enhancement in medulloblastoma. J Neurosurg Pediatr. 2014;14(2):121–8. https://doi.org/10.3171/2014.4.Peds13244.
Hirschberg K, Zisling R, van Echten-Deckert G, Futerman AH. Ganglioside synthesis during the development of neuronal polarity. Major changes occur during axonogenesis and axon elongation, but not during dendrite growth or synaptogenesis. J Biol Chem. 1996;271(25):14876–82. https://doi.org/10.1074/jbc.271.25.14876.
Chang F, Li R, Ladisch S. Shedding of gangliosides by human medulloblastoma cells. Exp Cell Res. 1997;234(2):341–6. https://doi.org/10.1006/excr.1997.3619.
Fonsatti E, Nicolay HJ, Sigalotti L, Calabrò L, Pezzani L, Colizzi F, et al. Functional up-regulation of human leukocyte antigen class I antigens expression by 5-aza-2'-deoxycytidine in cutaneous melanoma: immunotherapeutic implications. Clin Cancer Res. 2007;13(11):3333–8. https://doi.org/10.1158/1078-0432.Ccr-06-3091.
Tang J, Malhotra R, Song W, Brill I, Hu L, Farmer PK, et al. Human leukocyte antigens and HIV type 1 viral load in early and chronic infection: predominance of evolving relationships. PLoS One. 2010;5(3):e9629. https://doi.org/10.1371/journal.pone.0009629.
Smith C, Santi M, Rajan B, Rushing EJ, Choi MR, Rood BR, et al. A novel role of HLA class I in the pathology of medulloblastoma. J Transl Med. 2009;7:59. https://doi.org/10.1186/1479-5876-7-59.
Smith C, Santi M, Rushing EJ, Cornelison R, MacDonald TJ, Vukmanovic S. Characterization of signaling function and expression of HLA class I molecules in medulloblastoma. J Neuro-Oncol. 2011;103(2):197–206. https://doi.org/10.1007/s11060-010-0378-3.
Fiorilli P, Partridge D, Staniszewska I, Wang JY, Grabacka M, So K, et al. Integrins mediate adhesion of medulloblastoma cells to tenascin and activate pathways associated with survival and proliferation. Lab Investig. 2008;88(11):1143–56. https://doi.org/10.1038/labinvest.2008.89.
Vannini A, Leoni V, Barboni C, Sanapo M, Zaghini A, Malatesta P, et al. αvβ3-integrin regulates PD-L1 expression and is involved in cancer immune evasion. Proc Natl Acad Sci U S A. 2019;116(40):20141–50. https://doi.org/10.1073/pnas.1901931116.
Liang Y, Diehn M, Bollen AW, Israel MA, Gupta N. Type I collagen is overexpressed in medulloblastoma as a component of tumor microenvironment. J Neuro-Oncol. 2008;86(2):133–41. https://doi.org/10.1007/s11060-007-9457-5.
Utsuki S, Oka H, Sato Y, Tsutiya B, Kondo K, Tanizaki Y, et al. E, N-cadherins and beta-catenin expression in medulloblastoma and atypical teratoid/rhabdoid tumor. Neurol Med Chir (Tokyo). 2004;44(8):402–6; discussion 407. https://doi.org/10.2176/nmc.44.402.
Sasaki M, Akiyama-Oda Y, Oda H. Evolutionary origin of type IV classical cadherins in arthropods. BMC Evol Biol. 2017;17(1):142. https://doi.org/10.1186/s12862-017-0991-2.
Purvis IJ, Velpula KK, Guda MR, Nguyen D, Tsung AJ, Asuthkar S. B7-H3 in Medulloblastoma-Derived Exosomes; A Novel Tumorigenic Role. Int J Mol Sci. 2020;21(19). https://doi.org/10.3390/ijms21197050.
Bahmad HF, Chalhoub RM, Harati H, Bou-Gharios J, Assi S, Ballout F, et al. Tideglusib attenuates growth of neuroblastoma cancer stem/progenitor cells in vitro and in vivo by specifically targeting GSK-3β. Pharmacol Rep. 2021;73(1):211–26. https://doi.org/10.1007/s43440-020-00162-7.
• Bahmad HF, Poppiti RJ. Medulloblastoma cancer stem cells: molecular signatures and therapeutic targets. J Clin Pathol. 2020;73(5):243–9. https://doi.org/10.1136/jclinpath-2019-206246 Reason: Comprehensive review providing a synopsis of the novel therapeutic approaches that specifically target medulloblastoma cancer stem cells to attain enhanced anti-tumorous effects and overcome therapy resistance.
Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30(17):2046–54. https://doi.org/10.1200/jco.2011.38.4032.
Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13(6):1810–5. https://doi.org/10.1158/1078-0432.Ccr-06-2318.
May KF Jr, Roychowdhury S, Bhatt D, Kocak E, Bai XF, Liu JQ, et al. Anti-human CTLA-4 monoclonal antibody promotes T-cell expansion and immunity in a hu-PBL-SCID model: a new method for preclinical screening of costimulatory monoclonal antibodies. Blood. 2005;105(3):1114–20. https://doi.org/10.1182/blood-2004-07-2561.
Martin AM, Nirschl CJ, Polanczyk MJ, Bell WR, Nirschl TR, Harris-Bookman S, et al. PD-L1 expression in medulloblastoma: an evaluation by subgroup. Oncotarget. 2018;9(27):19177–91. https://doi.org/10.18632/oncotarget.24951.
Shinwari Z, Al-Hindi H, Al-Shail E, Khafaga Y, Al-Kofide A, El-Kum N, et al. Response of medulloblastoma cells to vincristine and lomustine: role of TRKC, CTNNB1 and STK15. Anticancer Res. 2011;31(5):1721–33.
Chen SM, Li YY, Tu CH, Salazar N, Tseng YY, Huang SF, et al. Blockade of Inhibitors of Apoptosis Proteins in Combination with Conventional Chemotherapy Leads to Synergistic Antitumor Activity in Medulloblastoma and Cancer Stem-Like Cells. PLoS One. 2016;11(8):e0161299. https://doi.org/10.1371/journal.pone.0161299.
Chen SM, Lin TK, Tseng YY, Tu CH, Lui TN, Huang SF, et al. Targeting inhibitors of apoptosis proteins suppresses medulloblastoma cell proliferation via G2/M phase arrest and attenuated neddylation of p21. Cancer Med. 2018;7(8):3988–4003. https://doi.org/10.1002/cam4.1658.
Keating J, Tsoli M, Hallahan AR, Ingram WJ, Haber M, Ziegler DS. Targeting the inhibitor of apoptosis proteins as a novel therapeutic strategy in medulloblastoma. Mol Cancer Ther. 2012;11(12):2654–63. https://doi.org/10.1158/1535-7163.Mct-12-0352.
Bar EE, Chaudhry A, Farah MH, Eberhart CG. Hedgehog signaling promotes medulloblastoma survival via Bc/II. Am J Pathol. 2007;170(1):347–55. https://doi.org/10.2353/ajpath.2007.060066.
Yang F, Jove V, Xin H, Hedvat M, Van Meter TE, Yu H. Sunitinib induces apoptosis and growth arrest of medulloblastoma tumor cells by inhibiting STAT3 and AKT signaling pathways. Mol Cancer Res. 2010;8(1):35–45. https://doi.org/10.1158/1541-7786.Mcr-09-0220.
Salm F, Dimitrova V, von Bueren AO, Ćwiek P, Rehrauer H, Djonov V, et al. The Phosphoinositide 3-Kinase p110α Isoform Regulates Leukemia Inhibitory Factor Receptor Expression via c-Myc and miR-125b to Promote Cell Proliferation in Medulloblastoma. PLoS One. 2015;10(4):e0123958. https://doi.org/10.1371/journal.pone.0123958.
Eckerdt F, Beauchamp E, Bell J, Iqbal A, Su B, Fukunaga R, et al. Regulatory effects of a Mnk2-eIF4E feedback loop during mTORC1 targeting of human medulloblastoma cells. Oncotarget. 2014;5(18):8442–51. https://doi.org/10.18632/oncotarget.2319.
Pócza T, Sebestyén A, Turányi E, Krenács T, Márk A, Sticz TB, et al. mTOR pathway as a potential target in a subset of human medulloblastoma. Pathol Oncol Res. 2014;20(4):893–900. https://doi.org/10.1007/s12253-014-9771-0.
Folgiero V, Miele E, Carai A, Ferretti E, Alfano V, Po A, et al. IDO1 involvement in mTOR pathway: a molecular mechanism of resistance to mTOR targeting in medulloblastoma. Oncotarget. 2016;7(33):52900–11. https://doi.org/10.18632/oncotarget.9284.
Zhao P, Hall J, Durston M, Voydanoff A, VanSickle E, Kelly S, et al. BKM120 induces apoptosis and inhibits tumor growth in medulloblastoma. PLoS One. 2017;12(6):e0179948. https://doi.org/10.1371/journal.pone.0179948.
Chaturvedi NK, Kling MJ, Coulter DW, McGuire TR, Ray S, Kesherwani V, et al. Improved therapy for medulloblastoma: targeting hedgehog and PI3K-mTOR signaling pathways in combination with chemotherapy. Oncotarget. 2018;9(24):16619–33. https://doi.org/10.18632/oncotarget.24618.
Studebaker AW, Hutzen BJ, Pierson CR, Haworth KB, Cripe TP, Jackson EM, et al. Oncolytic Herpes Virus rRp450 Shows Efficacy in Orthotopic Xenograft Group 3/4 Medulloblastomas and Atypical Teratoid/Rhabdoid Tumors. Mol Ther Oncolytics. 2017;6:22–30. https://doi.org/10.1016/j.omto.2017.05.005.
Friedman GK, Moore BP, Nan L, Kelly VM, Etminan T, Langford CP, et al. Pediatric medulloblastoma xenografts including molecular subgroup 3 and CD133+ and CD15+ cells are sensitive to killing by oncolytic herpes simplex viruses. Neuro-Oncology. 2016;18(2):227–35. https://doi.org/10.1093/neuonc/nov123.
Mohan AL, Friedman MD, Ormond DR, Tobias M, Murali R, Jhanwar-Uniyal M. PI3K/mTOR signaling pathways in medulloblastoma. Anticancer Res. 2012;32(8):3141–6.
Pham CD, Flores C, Yang C, Pinheiro EM, Yearley JH, Sayour EJ, et al. Differential Immune Microenvironments and Response to Immune Checkpoint Blockade among Molecular Subtypes of Murine Medulloblastoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(3):582–95. https://doi.org/10.1158/1078-0432.CCR-15-0713.
Liang L, Aiken C, McClelland R, Morrison LC, Tatari N, Remke M, et al. Characterization of novel biomarkers in selecting for subtype specific medulloblastoma phenotypes. Oncotarget. 2015;6(36):38881–900. https://doi.org/10.18632/oncotarget.6195.
Bahmad HF, Chamaa F, Assi S, Chalhoub RM, Abou-Antoun T, Abou-Kheir W. Cancer Stem Cells in Neuroblastoma: Expanding the Therapeutic Frontier. Front Mol Neurosci. 2019;12:131. https://doi.org/10.3389/fnmol.2019.00131.
Bou-Gharios J, Assi S, Bahmad HF, Kharroubi H, Araji T, Chalhoub RM, et al. The potential use of tideglusib as an adjuvant radio-therapeutic treatment for glioblastoma multiforme cancer stem-like cells. Pharmacol Rep. 2021;73(1):227–39. https://doi.org/10.1007/s43440-020-00180-5.
•• Hammoud H, Saker Z, Harati H, Fares Y, Bahmad HF, Nabha S. Drug Repurposing in Medulloblastoma: Challenges and Recommendations. Curr Treat Options in Oncol. 2020;22(1):6. https://doi.org/10.1007/s11864-020-00805-0 Reason: Comprehensive review providing a synopsis of the drug repurposing approaches that have been used in medulloblastoma and how this selective compilation of approaches could move drug repurposing to the next level.
Morrison LC, McClelland R, Aiken C, Bridges M, Liang L, Wang X, et al. Deconstruction of medulloblastoma cellular heterogeneity reveals differences between the most highly invasive and self-renewing phenotypes. Neoplasia. 2013;15(4):384–98. https://doi.org/10.1593/neo.13148.
Annabi B, Rojas-Sutterlin S, Laflamme C, Lachambre MP, Rolland Y, Sartelet H, et al. Tumor environment dictates medulloblastoma cancer stem cell expression and invasive phenotype. Mol Cancer Res. 2008;6(6):907–16. https://doi.org/10.1158/1541-7786.Mcr-07-2184.
Read TA, Fogarty MP, Markant SL, McLendon RE, Wei Z, Ellison DW, et al. Identification of CD15 as a marker for tumor-propagating cells in a mouse model of medulloblastoma. Cancer Cell. 2009;15(2):135–47. https://doi.org/10.1016/j.ccr.2008.12.016.
Castriconi R, Dondero A, Negri F, Bellora F, Nozza P, Carnemolla B, et al. Both CD133+ and CD133- medulloblastoma cell lines express ligands for triggering NK receptors and are susceptible to NK-mediated cytotoxicity. Eur J Immunol. 2007;37(11):3190–6. https://doi.org/10.1002/eji.200737546.
Mazza C, Pasqualin A, Da Pian R, Donati E. Treatment of medulloblastoma in children: long-term results following surgery, radiotherapy and chemotherapy. Acta Neurochir. 1981;57(3-4):163–75. https://doi.org/10.1007/bf01664835.
Ness KK, Gurney JG, Zeltzer LK, Leisenring W, Mulrooney DA, Nathan PC, et al. The impact of limitations in physical, executive, and emotional function on health-related quality of life among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Arch Phys Med Rehabil. 2008;89(1):128–36. https://doi.org/10.1016/j.apmr.2007.08.123.
Schultz KA, Ness KK, Whitton J, Recklitis C, Zebrack B, Robison LL, et al. Behavioral and social outcomes in adolescent survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2007;25(24):3649–56. https://doi.org/10.1200/jco.2006.09.2486.
Cai J, Liu P, Huang H, Li Y, Ma S, Zhou H, et al. Combination of anti-PD-1 antibody with P-GEMOX as a potentially effective immunochemotherapy for advanced natural killer/T cell lymphoma. Signal Transduct Target Ther. 2020;5(1):289. https://doi.org/10.1038/s41392-020-00331-3.
Tanaka F, Hashimoto W, Okamura H, Robbins PD, Lotze MT, Tahara H. Rapid generation of potent and tumor-specific cytotoxic T lymphocytes by interleukin 18 using dendritic cells and natural killer cells. Cancer Res. 2000;60(17):4838–44.
Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26(4):332–42. https://doi.org/10.1097/00002371-200307000-00005.
Fernández L, Portugal R, Valentín J, Martín R, Maxwell H, González-Vicent M, et al. In vitro Natural Killer Cell Immunotherapy for Medulloblastoma. Front Oncol. 2013;3:94. https://doi.org/10.3389/fonc.2013.00094.
Cho D, Shook DR, Shimasaki N, Chang Y-H, Fujisaki H, Campana D. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(15):3901–9. https://doi.org/10.1158/1078-0432.CCR-10-0735.
Yvon ES, Burga R, Powell A, Cruz CR, Fernandes R, Barese C, et al. Cord blood natural killer cells expressing a dominant negative TGF-β receptor: Implications for adoptive immunotherapy for glioblastoma. Cytotherapy. 2017;19(3):408–18. https://doi.org/10.1016/j.jcyt.2016.12.005.
Liu D, Song L, Brawley VS, Robison N, Wei J, Gao X, et al. Medulloblastoma expresses CD1d and can be targeted for immunotherapy with NKT cells. Clin Immunol. 2013;149(1):55–64. https://doi.org/10.1016/j.clim.2013.06.005.
Ishikawa E, Tsuboi K, Saijo K, Harada H, Takano S, Nose T, et al. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004;24(3b):1861–71.
Khatua S, Cooper LJN, Sandberg DI, Ketonen L, Johnson JM, Rytting ME, et al. Phase I study of intraventricular infusions of autologous ex vivo expanded NK cells in children with recurrent medulloblastoma and ependymoma. Neuro-Oncology. 2020;22(8):1214–25. https://doi.org/10.1093/neuonc/noaa047.
Kiani J, Naderi M, Torabi-Rahvar M, Ranjbar A, Aghayan HR, Janzamin E, et al. Generation of CD19-Targeted Chimeric Antigen Receptor T Cells. Arch Iran Med. 2019;22(1):7–10.
Brown CE, Aguilar B, Starr R, Yang X, Chang W-C, Weng L, et al. Optimization of IL13Rα2-Targeted Chimeric Antigen Receptor T Cells for Improved Anti-tumor Efficacy against Glioblastoma. Mol Ther. 2018;26(1):31–44. https://doi.org/10.1016/j.ymthe.2017.10.002.
Kong S, Sengupta S, Tyler B, Bais AJ, Ma Q, Doucette S, et al. Suppression of Human Glioma Xenografts with Second-Generation IL13R-Specific Chimeric Antigen Receptor–Modified T Cells. Clin Cancer Res. 2012;18(21):5949. https://doi.org/10.1158/1078-0432.CCR-12-0319.
Brown CE, Badie B, Barish ME, Weng L, Ostberg JR, Chang W-C, et al. Bioactivity and Safety of IL13Rα2-Redirected Chimeric Antigen Receptor CD8<sup>+</sup> T Cells in Patients with Recurrent Glioblastoma. Clin Cancer Res. 2015;21(18):4062. https://doi.org/10.1158/1078-0432.CCR-15-0428.
Ahmed N, Ratnayake M, Savoldo B, Perlaky L, Dotti G, Wels WS, et al. Regression of Experimental Medulloblastoma following Transfer of HER2-Specific T Cells. Cancer Res. 2007;67(12):5957. https://doi.org/10.1158/0008-5472.CAN-06-4309.
Nellan A, Rota C, Majzner R, Lester-McCully CM, Griesinger AM, Mulcahy Levy JM, et al. Durable regression of Medulloblastoma after regional and intravenous delivery of anti-HER2 chimeric antigen receptor T cells. J Immunother Cancer. 2018;6(1):30. https://doi.org/10.1186/s40425-018-0340-z.
H-j D, Sun B, Yang D, Xu H, Zhu J, Wei J, et al. Eradication of medulloblastoma by NKG2D-specific CAR T-cells. J Clin Oncol. 2020;38(15_suppl):2522. https://doi.org/10.1200/JCO.2020.38.15_suppl.2522.
Majzner RG, Theruvath JL, Nellan A, Heitzeneder S, Cui Y, Mount CW, et al. CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin Cancer Res. 2019;25(8):2560. https://doi.org/10.1158/1078-0432.CCR-18-0432.
Anderson KG, Voillet V, Bates BM, Chiu EY, Burnett MG, Garcia NM, et al. Engineered Adoptive T-cell Therapy Prolongs Survival in a Preclinical Model of Advanced-Stage Ovarian Cancer. Cancer Immunol Res. 2019;7(9):1412–25. https://doi.org/10.1158/2326-6066.CIR-19-0258.
Bodey B, Bodey V, Siegel SE. Expression in childhood primary brain tumors of NY-ESO-1, a cancer/testis antigen: an immunohistochemical study. Vivo. 2008;22(1):83–7.
Boon K, Edwards JB, Siu IM, Olschner D, Eberhart CG, Marra MA, et al. Comparison of medulloblastoma and normal neural transcriptomes identifies a restricted set of activated genes. Oncogene. 2003;22(48):7687–94. https://doi.org/10.1038/sj.onc.1207043.
Oba-Shinjo SM, Caballero OL, Jungbluth AA, Rosemberg S, Old LJ, Simpson AJG, et al. Cancer-testis (CT) antigen expression in medulloblastoma. Cancer Immun. 2008;8:7–7.
Vulcani-Freitas TM, Saba-Silva N, Cappellano A, Cavalheiro S, Toledo SR. PRAME gene expression profile in medulloblastoma. Arq Neuropsiquiatr. 2011;69(1):9–12. https://doi.org/10.1590/s0004-282x2011000100003.
Orlando D, Miele E, De Angelis B, Guercio M, Boffa I, Sinibaldi M, et al. Adoptive Immunotherapy Using PRAME-Specific T Cells in Medulloblastoma. Cancer Res. 2018;78(12):3337–49. https://doi.org/10.1158/0008-5472.Can-17-3140.
Eberlein TJ, Rosenstein M, Rosenberg SA. Regression of a disseminated syngeneic solid tumor by systemic transfer of lymphoid cells expanded in interleukin 2. J Exp Med. 1982;156(2):385–97. https://doi.org/10.1084/jem.156.2.385.
Flores C, Wildes T, Dean BD, Moore G, Drake J, Abraham R, et al. Massive clonal expansion of medulloblastoma-specific T cells during adoptive cellular therapy. Sci Adv. 2019;5(11):eaav9879. https://doi.org/10.1126/sciadv.aav9879.
Ansell SM, Hurvitz SA, Koenig PA, LaPlant BR, Kabat BF, Fernando D, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15(20):6446–53. https://doi.org/10.1158/1078-0432.Ccr-09-1339.
Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joseph-Pietras D, et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med. 2014;6(254):254ra128. https://doi.org/10.1126/scitranslmed.3008918.
Hashimoto Y, Penas-Prado M, Zhou S, Wei J, Khatua S, Hodges TR, et al. Rethinking medulloblastoma from a targeted therapeutics perspective. J Neuro-Oncol. 2018;139(3):713–20. https://doi.org/10.1007/s11060-018-2917-2.
Peethambaram PP, Melisko ME, Rinn KJ, Alberts SR, Provost NM, Jones LA, et al. A phase I trial of immunotherapy with lapuleucel-T (APC8024) in patients with refractory metastatic tumors that express HER-2/neu. Clin Cancer Res. 2009;15(18):5937–44. https://doi.org/10.1158/1078-0432.Ccr-08-3282.
Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Herndon JE 2nd, Lally-Goss D, et al. An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther. 2009;8(10):2773–9. https://doi.org/10.1158/1535-7163.Mct-09-0124.
Behrends U, Schneider I, Rössler S, Frauenknecht H, Golbeck A, Lechner B, et al. Novel tumor antigens identified by autologous antibody screening of childhood medulloblastoma cDNA libraries. Int J Cancer. 2003;106(2):244–51. https://doi.org/10.1002/ijc.11208.
Ardon H, De Vleeschouwer S, Van Calenbergh F, Claes L, Kramm CM, Rutkowski S, et al. Adjuvant dendritic cell-based tumour vaccination for children with malignant brain tumours. Pediatr Blood Cancer. 2010;54(4):519–25. https://doi.org/10.1002/pbc.22319.
Nair SK, Driscoll T, Boczkowski D, Schmittling R, Reynolds R, Johnson LA, et al. Ex vivo generation of dendritic cells from cryopreserved, post-induction chemotherapy, mobilized leukapheresis from pediatric patients with medulloblastoma. J Neuro-Oncol. 2015;125(1):65–74. https://doi.org/10.1007/s11060-015-1890-2.
Kellish P, Shabashvili D, Rahman MM, Nawab A, Guijarro MV, Zhang M, et al. Oncolytic virotherapy for small-cell lung cancer induces immune infiltration and prolongs survival. J Clin Invest. 2019;129(6):2279–92. https://doi.org/10.1172/jci121323.
Liu TC, Hwang T, Park BH, Bell J, Kirn DH. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol Ther. 2008;16(9):1637–42. https://doi.org/10.1038/mt.2008.143.
Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252(5007):854–6. https://doi.org/10.1126/science.1851332.
Thompson EM, Brown M, Dobrikova E, Ramaswamy V, Taylor MD, McLendon R, et al. Poliovirus Receptor (CD155) Expression in Pediatric Brain Tumors Mediates Oncolysis of Medulloblastoma and Pleomorphic Xanthoastrocytoma. J Neuropathol Exp Neurol. 2018;77(8):696–702. https://doi.org/10.1093/jnen/nly045.
Lun XQ, Zhou H, Alain T, Sun B, Wang L, Barrett JW, et al. Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res. 2007;67(18):8818–27. https://doi.org/10.1158/0008-5472.Can-07-1214.
Yu L, Baxter PA, Zhao X, Liu Z, Wadhwa L, Zhang Y, et al. A single intravenous injection of oncolytic picornavirus SVV-001 eliminates medulloblastomas in primary tumor-based orthotopic xenograft mouse models. Neuro-Oncology. 2011;13(1):14–27. https://doi.org/10.1093/neuonc/noq148.
Joshi BH, Leland P, Puri RK. Identification and characterization of interleukin-13 receptor in human medulloblastoma and targeting these receptors with interleukin-13-pseudomonas exotoxin fusion protein. Croat Med J. 2003;44(4):455–62.
Ward SA, Warrington NM, Taylor S, Kfoury N, Luo J, Rubin JB. Reprogramming Medulloblastoma-Propagating Cells by a Combined Antagonism of Sonic Hedgehog and CXCR4. Cancer Res. 2017;77(6):1416–26. https://doi.org/10.1158/0008-5472.CAN-16-0847.
Yang L, Jackson E, Woerner BM, Perry A, Piwnica-Worms D, Rubin JB. Blocking CXCR4-Mediated Cyclic AMP Suppression Inhibits Brain Tumor Growth <em>In vivo</em>. Cancer Res. 2007;67(2):651. https://doi.org/10.1158/0008-5472.CAN-06-2762.
Ceccarelli M, Micheli L, Tirone F. Suppression of Medulloblastoma Lesions by Forced Migration of Preneoplastic Precursor Cells with Intracerebellar Administration of the Chemokine Cxcl3. Front Pharmacol. 2016;7:484. https://doi.org/10.3389/fphar.2016.00484.
Farioli-Vecchioli S, Cinà I, Ceccarelli M, Micheli L, Leonardi L, Ciotti MT, et al. Tis21 knock-out enhances the frequency of medulloblastoma in Patched1 heterozygous mice by inhibiting the Cxcl3-dependent migration of cerebellar neurons. J Neurosci. 2012;32(44):15547–64. https://doi.org/10.1523/JNEUROSCI.0412-12.2012.
Acknowledgements
We would like to thank all members of the Neuroscience Research Center, Faculty of Medicine, Lebanese University (Beirut, Lebanon) and the Arkadi M. Rywlin M.D. Department of Pathology and Laboratory Medicine, Mount Sinai Medical Center (Miami Beach, FL, USA) for their help on this work. This work was not funded.
Code Availability
Not applicable.
Author information
Authors and Affiliations
Contributions
H.F.Bahmad and S.Nabha conceived the concept and idea of the present
review. H.F.Bahmad and S.Nabha worked on the study design strategy and selected the topics to be discussed. Z.Audi, Z.Saker, and M.Rizk did literature searches and screened titles and abstracts for relevance, abstracted the data from the eligible full text articles, analyzed and interpreted the data, and drafted the manuscript. H.Harati and Y.Fares critically revised the manuscript. H.F.Bahmad and S.Nabha critically revised the manuscript with input from the entire team. All authors have read and approved the final draft.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
Zahraa F. Audi declares no conflict of interest. Zahraa Saker declares no conflict of interest. Mahdi Rizk declares no conflict of interest. Hayat Harati declares no conflict of interest. Youssef Fares declares no conflict of interest. Hisham F. Bahmad declares no conflict of interest. Sanaa M. Nabha declares no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuro-oncology
Rights and permissions
About this article
Cite this article
Audi, Z.F., Saker, Z., Rizk, M. et al. Immunosuppression in Medulloblastoma: Insights into Cancer Immunity and Immunotherapy. Curr. Treat. Options in Oncol. 22, 83 (2021). https://doi.org/10.1007/s11864-021-00874-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s11864-021-00874-9