Opinion Statement
The treatment of malignant gliomas has undergone a significant intensification during the past decade, and the interdisciplinary treatment team has learned that all treatment opportunities, including surgery and radiotherapy (RT), also have a central role in recurrent gliomas. Throughout the decades, re-irradiation (re-RT) has achieved a prominent place in the treatment of recurrent gliomas. A solid body of evidence supports the safety and efficacy of re-RT, especially when modern techniques are used, and justifies the early use of this regimen, especially in the case when macroscopic disease is present. Additionally, a second adjuvant re-RT to the resection cavity is currently being investigated by several investigators and seems to offer promising results. Although advanced RT technologies, such as stereotactic radiosurgery (SRS), fractionated stereotactic radiotherapy (FSRT), intensity-modulated radiotherapy (IMRT), and image-guided radiotherapy (IGRT) have become available in many centers, re-RT should continue to be kept in experienced hands so that they can select the optimal regimen, the ideal treatment volume, and the appropriate techniques from their tool-boxes. Concomitant or adjuvant use of systemic treatment options should also strongly be taken into consideration, especially because temozolomide (TMZ), cyclohexyl-nitroso-urea (CCNU), and bevacizumab have shown a good safety profile; they should be considered, if available. Nonetheless, the selection of patients for re-RT remains crucial. Single factors, such as patient age or the progression-free interval (PFI), fall too short. Therefore, powerful prognostic scores have been generated and validated, and these scores should be used for patient selection and counseling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The primary treatment of malignant gliomas has undergone a profound evolution during the past 20 years. The introduction of fluorescence-guided surgery and sophisticated imaging techniques before, during, and after surgery improved the efficacy and safety of the treatment of patients with glioma [1,2,3]. Adjuvant RT to the resection cavity has become a standard of care [4], and RT is frequently combined with concomitant or adjuvant systemic agents, most frequently with temozolomide (TMZ) or multi-agent regimens such as PCV (procarbazine, CCNU, and vincristine) [5,6,7,8]. The introduction of these multimodal regimens resulted in the prolongation of overall survival (OS) times, which currently range between 9.3 and 14.6 months in glioblastoma (GBM) and between 40 months and more than 7 years in anaplastic gliomas [5,6,7,8]. Additionally, the progression-free survival (PFS) is now within a range of 5.3 months in elderly patients with non-hypermethylated O-6-methylguanine-DNA methyltransferase (MGMT) promotor GBM and 42.8 months in patients with anaplastic astrocytoma (AA) who are being treated with RT and up to 12 cycles of TMZ [5, 7].
The length of the PFS has implications for the options that the neuro-\oncologist can offer their patients in the case of recurrent disease. In many centers, a second surgery is offered to patients who have recurrent gliomas, especially when the patients are in good physical health and present with low-volume recurrences in non-eloquent brain regions (Fig. 1) [9]. When the extent of resection reaches an almost gross total resection (GTR), then a second surgery results in an improved survival time [10, 11]. There is also an advantage of performing debulking surgery in cases of repeated surgeries for later occurring recurrences [12], and debulking surgery itself is a positive prognostic factor in the context of further salvage therapies, including re-RT [13,14,15].
When a second surgery is not deemed to be feasible or when residual disease in situ cannot be extracted during a second surgery, then re-RT is frequently considered. An important prerequisite for re-RT is an adequate normal tissue tolerance for RT, and the limitations of this tolerance are the major limitation of re-RT. Usually, doses around 60 Gy in 1.8–2.0 Gy per fraction are applied to a volume of approximately 2 cm around the resection cavity as well as tumor remnants in primary RT. This dose is assumed to be safe, yet radiation necrosis can occur in some patients. A risk of approximately 5% for this complication is extrapolated for a total dose of 72 Gy [16]. Because most recurrences occur in the direct vicinity of the initial tumor site [17,18,19], the available dose for re-RT seems to be limited. This necessitates a concept of recovery of radiation tolerance. Preclinical models estimate a recovery of approximately 50% after 1 to 2 years [20], and the application of these models seems to hold in clinical practice [21, 22]. Hence, re-RT is now deemed to be feasible, especially when the interval from first RT is longer than 6 months. Nonetheless, the remaining tolerance is assumed to be smaller in re-RT than in primary RT, which forces compromises to be made in the final dose and target volume. Therefore, one option to enhance the efficacy of re-RT is to add systemic agents, either concomitant or adjuvant to re-RT.
This narrative review of recently published literature discusses current applications of re-RT in recurrent malignant glioma, also termed recurrent high-grade gliomas (rHGG), with a focus on recurrent glioblastoma (rGBM). We will focus on issues and tools for appropriate patient selection, target volume concepts, and evidence for re-RT in general.
Literature search
This review is based on a selective literature search on Medline/Pubmed for articles published within the past 5 years (01/2014 to 12/2018). Only original articles that reported on more than 20 adult patients treated with external beam radiotherapy (EBRT) as re-RT for rHGG including rGBM were eligible. For PubMed, the search terms “glioblastoma,” “high-grade glioma,” “recurrence,” and “re-irradiation” were used. We also included articles from a thorough manual search. In order to unify the reporting and to enhance comparability of the data, the median OS is reported for all articles in this review. In some articles, the median OS was not described within the text; therefore, the mOS was measured from the published Kaplan Meier survival tools using a semi-automatic tool (Digitzeit, www.digitzeit.de). A summary of 15 selected publications from 2014 to 2018 is presented in Table 1.
Patient selection
Re-RT is generally considered appropriate for patients who have a good to fair Karnofsky performance status (KPS ≥ 50–60%) and who present with recurrences not earlier than 6 months after first-line radiotherapy with a recurrent glioma not larger than 6 cm [41, 42]. Patients who have a good performance status and a limited volume of recurrent disease profit most from re-RT. Several scores have been generated to estimate the survival times of patients who have recurrent gliomas after re-irradiation, and do thereby allow valid patient counseling and shared decision-making. The Combs score was one of the first scores to be validated. It included the initial histology, age of the patients, and the time between RT and re-RT as factors for a prognostic score [43, 44]. In a recently updated version, additional factors including the KPS, the tumor volume, and the extent of a second surgery were added to the score [35•].
Another scoring system, the re-irradiation risk score (RRRS), was generated and validated based on a multi-institutional database of the German cancer consortium Deutsches Konsortium für Translationale Krebsforschung, (DKTK); it is based on the age, KPS, and WHO grade (GBM vs. non-GBM) [37]. Importantly and in contrast to the Combs-score, the RRRS uses the age as a continuous variable, which avoids a sharp gradient from which a “high risk” is postulated [35•, 37, 43]. Hence, elderly patients with rGBM may fall into an intermediate-risk group and have a good chance of benefiting from re-RT. This general assumption was recently shown in a small cohort from our institution [45]. However, effectively used scoring systems should remain simple and easy to use, which certainly holds true for the initially developed score that improved over time by our group [22, 41, 42].
We believe that risk scores can be valid tools to estimate the post-progression survival of glioma patients and can be very helpful for the counseling of our patients. However, overestimated conclusions leading to a hasty denial of re-RT based solely on a score or even a single factor should be avoided, and shared decision-making together with the patient remains crucial. Taken together, an effective score will serve as a “digital biomarker” in glioma care (Kessel K et al., Phys Med Submitted).
In addition to selecting the right patients for re-RT, it is also necessary to choose the right technique for the specific patients. The discussion of all available techniques is beyond the scope of this article; however, a basic understanding of differences between the techniques is necessary to identify potential selection biases that are introduced by the choice of a technique. High-precision techniques such as stereotactic radiosurgery (SRS) using linear accelerators or the Gamma Knife (GK) are usually applied to small recurrences (< 2 cm) because these approaches can deliver very high doses to the tumor volume while the surrounding tissue is spared due to sharp dose gradients. SRS is usually delivered in one fraction [14, 28, 29, 34]. Hypofractionated stereotactic radiotherapy (HFSRT) uses the same advantage as SRS, but it is applicable to larger volumes (2–4 cm), as the healthy tissue can recover partially between the single doses of a treatment course (the so-called fractionation effect) [25,26,27, 31, 34, 36]. Both SRS and HFSRT usually have only very small margins to treat infiltrative disease because larger margins increase the risk of radiation necrosis. Fractionated RT, delivered either as 3D-conformal or intensity-modulated RT (IMRT), also takes advantage of the fractionation effect. This technique is appropriate for a variety of treatment volumes (up to more than 5 cm) and usually includes a margin to account for infiltrative disease [23, 24, 35•, 44, 46,47,48, 49••]. The advantages and disadvantages of the different techniques are well summarized in articles from Combs et al. and Nieder et al. [41, 50].
Efficacy
Up until now, there have only been few trials that have randomized patients to re-RT or a non-re-RT regimen, and there has not been a trial that has directly compared re-RT to best supportive care (BSC). We summarized the 15 most important articles from the past 5 years in Table 1.
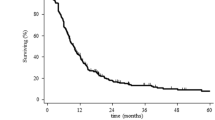
Prospective data concerning the efficacy of salvage strategies were derived from RTOG 0525 in a secondary analysis in 2018 [51•]. Shi and colleagues compared the OS of 637 patients who were previously treated in RTOG 0525 and who experienced progression during follow-up. Only patients who survived more than one half of a month after progression were included in the analysis, and the median time to progression before salvage treatment or BSC ranged between 7.5 and 9.7 months. The median OS for re-RT with chemotherapy was 12.2 and 8.2 months for re-RT only. BSC resulted in a median OS of 4.8 months, and a systemic treatment without re-RT reached a median survival of 10.5 months. There was no difference in the efficacy of chemotherapy-only to re-RT. However, only 13.8% of patients received re-RT with or without chemotherapy, which limits the power of this analysis. Nonetheless, the report generated evidence for the efficacy of either of these salvage regimens and concluded that prospective trials are warranted [51•].
The same question was also analyzed from van Linde et al. in a retrospective, multicenter analysis of 299 patients. The authors compared different treatment strategies upon progression, namely re-RT with or without chemotherapy (ChT), ChT alone, and BSC. The authors showed a significant difference in the OS of patients managed with BSC (median OS 3 months) compared with patients who were managed with chemotherapy (ChT), Re-RT, or Re-OP followed by ChT (median OS 7.3, 9.1, and 11.5 months). Unfortunately, only relatively few patients (21 from 299) underwent re-RT, and the cohort was underpowered to discriminate among the active treatment strategies. However, while the difference among the treatment regimens remained unclear from this data set, the authors pointed out the general efficacy of re-RT over BSC. As in all retrospective analyses of re-RT that compare to BSC, this trial suffers from a risk of selection bias, as patients with large recurrences who had a poor performance status and who were taking higher doses of steroids were more likely to receive BSC [32].
Another important article comparing re-RT to a non-re-RT regimen was published only recently by Chun and colleagues. The authors compared a cohort of patients who underwent a second surgery for either rGBM or rHGG, with those who either received adjuvant re-RT to the resection cavity or no additional treatment. The re-RT was performed as a fractionated radiotherapy and prescribed to the resection cavity and a safety margin of 5 mm. The OS reached borderline significance with a median OS of 12.7 vs. 28.1 months (without vs. with re-RT, p 0.066), and the PFS was significantly improved by re-RT (3.5 vs. 9 months, p 0.025). This article is of special interest to the field for two reasons. First, the trial has a relatively low risk of bias, as all patients underwent surgery and thus were likely able to undergo re-RT. Furthermore, the Re-RT generated a favorable outcome despite the fact that the Re-RT-cohort was more heavily pretreated (33.3% with ChT in the Re-OP-group vs. 66.7% in the re-RT-group, p 0.002), which would generally be estimated to be a negative prognostic factor. Secondly, the article argues in favor of a new concept in neuro-oncology, which is using re-RT as an adjuvant modality after surgery, even after GTR [40]**. This concept was substantiated by analysis of the pattern of recurrence and is currently tested in the prospective GlioCave-trial [52, 53].
The addition of ChT to re-RT may enhance the efficacy of re-RT [28]. Kim and colleagues analyzed 144 rGBM patients who were treated with either re-RT using the Gamma Knife (GK), TMZ, or a combination of GK and TMZ. A fourth group received a second surgery. The target volumes were, as in many cases with GK radiosurgery, relatively small. The authors found a significant improvement of the OS induced by a combination of SRS with TMZ compared with SRS alone or TMZ alone (15.5 vs. 9.2 vs. 5.6 months, p 0.009 and 0.005) [14]. Greenspoon investigated the safety and efficacy of hypofractionated re-RT with concomitant TMZ in 2014. The median OS was 9.3 months in this prospective study, and four patients (12.9%) experienced grade 3 or 4 toxicities [26]. This relatively high incidence of grades 3 and 4 toxicities was potentially attributed to the fractionation scheme. Combs reported on 25 patients treated with standard-fractionated re-RT and concomitant TMZ without severe toxicities and with a median OS of 9 months [54].
In addition to these normo- or hypofractionated regimens, there are two articles that used rather unconventional low-dose or low dose-rate fractionation schemes. Magnuson et al. reported on re-RT in patients with rGBM who progressed after Bevacizumab (Bev). The group re-irradiated relatively large recurrences (2.0–8.1 cm diameter) and further included a 2-cm margin about the GTV. The total dose was 54 Gy in 27 fractions, but each fraction was subdivided in 10 pulses with 0.2 Gy per pulse. The regimen included Bev-treatment, which may have contributed to the low toxicity rate reported in the trial; hence, the regimen was well tolerated with no grades 3–4 toxicities. However, 40% of patients required an increase in steroid consumption. The PFS was 3.3 months, and the OS 6.9 months [23].
A second alternative fractionation regimen, combined with either TMZ or with cisplatin/fotemustine, was presented by Balducci et al. in 2014. RT was delivered in as low as 0.3 to 0.4 Gy per fraction, given twice daily for 5 to 6 days and repeated once after 28 to 42 days. As anticipated by the low dose, the regimen was very well tolerated, with no RT-induced grades 3–4 toxicities, even though 3 cm around the GTV were included into the PTV, and patients were eligible as early as 3 months after first-line RT. The dosing regimen is based on the idea that gliomas may have a hypersensitivity to low-dose RT, but this regimen requires an evaluation in larger cohorts and with randomization to a non-RT arm [24].
A non-re-RT regimen that used the experimental drug APG-101 was compared to re-RT by Wick et al. in 2014. The trial compared re-RT with 36 Gy in 18 fractions with or without APG-101 in adult patients who had rGBM and measurable disease. However, the trial was not designed to be powered for a comparison of the two arms regarding OS and PFS, and the re-RT arm was only included to calibrate for the primary endpoint, which was the PFS rate at 6 months. The 6 months PFS rate in the re-RT arm was only 3.8% compared with 20.7% in the re-RT/APG-101 arm; however, there was an equal median OS of 11.5 months in both arms [55]. This trial used the outdated MacDonald criteria for response assessment. Notably, bevacizumab can reduce the amount of contrast enhancement in gliomas without affecting the real extent of the disease. This phenomenon is termed pseudo-regression. In contrast, radiation can induce early changes in the vascular permeability, which lead to contrast enhancements as early as a few weeks after re-RT, a phenomenon termed pseudo-progression. Noteworthy, the MacDonald criteria only focus on the extent of the contrast enhancement, which may lead to an overestimation of the effect of a drug and an underestimation of the effect of re-RT [56].
Recently, Kazmi et al. published the first meta-analysis of reports about re-irradiation in rGBM. The analysis included 2095 treated patients in 50 reports. The primary endpoints were PFS and OS-rates at 6 and 12 months. The authors reported 6- and 12-month OS-rates of 73 and 36% and corresponding PFS rates of 43 and 17%, respectively. Interestingly, the authors reported significantly higher incidences of grade ≥ 3 toxicities in prospective trials compared with retrospective reports and concluded that re-RT has a reasonable efficacy with an acceptable safety profile [38••]. In order to find an explanation for the heterogeneity in the survival data after re-RT in rGBM, the authors further evaluated the influence of the dosing regimen in re-RT. For this, the EQD2, an equivalence dose for different dosing regimens, was calculated and included in the analysis. There was no advantage seen for any of the regimens, and particularly no dose-response relationship beyond an EQD2 of 36 Gy. However, this finding should be considered with caution, as the EQD2 was calculated with a focus on tumor-control, and the analysis did not include the target volume definition of the re-RT. Furthermore, it is counterintuitive that the dosing regimen did not affect the toxicity rates (9 vs. 8% for < 36 Gy and > 36 Gy).
For rHGG, including anaplastic astrocytomas (AA) and anaplastic oligodendrogliomas (AO), there is currently no meta-analysis available. However, several larger cohorts have been analyzed. Among these, the reports from Kessel, Navarria, and Combs are the largest patient cohorts [35•, 39, 43]. The median OS times after re-RT of HGG were 11.3, 12.2, and 17 months. The simultaneously published results for rGBM were within the range of previously discussed series at 7.9, 8, and 8.5 months, which substantiates the conclusion that HGG remains a better prognosis even after recurrence when compared with rGBM. Noteworthy, none of these articles reported excessive toxicity.
Target volume definition
As previously discussed, both dose and target volume definition potentially affect the efficacy and toxicity of re-RT. In the available literature, a plethora of target volume definitions and dosing concepts have been described [17]; however, none of these regimens has proven superiority over another. This can partially be explained by possible difficulties in the target volume definition after recurrence. Most authors use the area of contrast enhancement on T1-weighted MRI (GdT1w) as a surrogate for the gross tumor volume (GTV) used for re-RT [17]. Unfortunately, radiation necrosis, micro-hemorrhages, and post-ischemic changes can be indistinguishable from “true” rGBM, and some parts of the rGBM go beyond the borders of the contrast enhancement [57]. To overcome this limitation, sophisticated MRI techniques and functional imaging have been included in the planning process, with median survival times of seven to 12.4 months [25, 58,59,60]. In order to quantify the overlap of GTVs based on different imaging modalities, Popp et al. compared the commonly used GdT1w-based (GTV) to GTVs based on diffusion restriction or amino acid positron emission tomography (AA-PET). Interestingly, all of these modalities generated more or less distinct volumes, and especially GTVs based on diffusion-weighted images (DWI) were largely separate from the GdT1w- and PET-based GTVs and were not overlapping with areas of later progression [61]. The same group recently started a trial to compare re-RT based on an AA-PET with re-RT based on GdT1w-GTVs [62].
Another question is whether re-RT is efficient and safe when the entire recurrent glioma has been removed by surgery. An argument in favor of re-RT is that after GTR of rGBM, further recurrences frequently recur in the area of the resection cavity [17]. Another argument is that early re-RT was an independent prognostic factor after surgery in several single- and multi-institutional series [10, 15, 63, 64]. Chun et al. recently presented the first retrospective cohort on re-treating the resection cavity after near-total or GTR. The group re-irradiated the resection cavity with a 5-mm margin, with a median dose of 45 Gy (EQD2). The response was assessed based on the RANO-HGG-criteria and was significantly longer after re-OP followed by re-RT compared with re-OP alone. The OS showed a strong tendency in favor of the re-RT group, but the difference did not reach significance (12 vs. 28 months, p 0.066). A randomized trial is currently open to compare early re-RT with the resection cavity vs. observation [53].
Summary
Re-irradiation has become a cornerstone in the treatment of rHGG and rGBM and should be considered for all recurrences along with all other treatment modalities. Available evidence supports the efficacy and safety of re-RT with or without chemotherapy. Re-RT should not be seen as a single treatment alternative but as a part of a multimodal retreatment approach. This approach should consider the most effective local treatment options, such as surgery and re-RT, which can be complemented by systemic treatments, when feasible. Because of the high complexity of decision-making in this field, a multidisciplinary team approach is highly recommended.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. https://doi.org/10.1016/S1470-2045(06)70665-9.
Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016;2:1460–9. https://doi.org/10.1001/jamaoncol.2016.1373.
Straube C, Schmidt-Graf F, Wiestler B, Zimmer C, Meyer B, Combs SE. The algorithms of adjuvant therapy in gliomas and their effect on survival. J Neurosurg Sci. 2018;63. https://doi.org/10.23736/S0390-5616.18.04610-6.
Shapiro WR, Green SB, Burger PC, Mahaley MS, Selker RG, VanGilder JC, et al. Randomized trial of three chemotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma. J Neurosurg. 1989;71:1–9. https://doi.org/10.3171/jns.1989.71.1.0001.
van den Bent MJ, Baumert B, Erridge SC, Vogelbaum MA, Nowak AK, Sanson M, et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017;6736:1–9. https://doi.org/10.1016/S0140-6736(17)31442-3.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. https://doi.org/10.1056/NEJMoa043330.
Perry JR, Laperriere N, O’Callaghan CJ, Brandes AA, Menten J, Phillips C, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376:1027–37. https://doi.org/10.1056/NEJMoa1611977.
Van Den Bent MJ, Carpentier AF, Brandes AA, Sanson M, Taphoorn MJB, Bernsen HJJA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer p. J Clin Oncol. 2006;24:2715–22. https://doi.org/10.1200/JCO.2005.04.6078.
Park JK, Hodges T, Arko L, Shen M, Dello Iacono D, McNabb A, et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J Clin Oncol. 2010;28:3838–43. https://doi.org/10.1200/JCO.2010.30.0582.
Ringel F, Pape H, Sabel M, Krex D, Bock HC, Misch M, et al. Clinical benefit from resection of recurrent glioblastomas: results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro-Oncology. 2016;18:96–104. https://doi.org/10.1093/neuonc/nov145.
Suchorska B, Weller M, Tabatabai G, Senft C, Hau P, Sabel MC, et al. Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma—results from the DIRECTOR trial. Neuro-Oncology. 2016;18:nov326–556. https://doi.org/10.1093/neuonc/nov326.
Sughrue ME, Sheean T, Bonney PA, Maurer AJ, Teo C. Aggressive repeat surgery for focally recurrent primary glioblastoma: outcomes and theoretical framework. 2015;38:1–7. https://doi.org/10.3171/2014.12.FOCUS14726.DISCLOSURE.
Azoulay M, Santos F, Shenouda G, Petrecca K, Oweida A, Guiot MC, et al. Benefit of re-operation and salvage therapies for recurrent glioblastoma multiforme: results from a single institution. J Neuro-Oncol. 2017;132:419–26. https://doi.org/10.1007/s11060-017-2383-2.
Kim HR, Kim KH, Kong D-S, Seol HJ, Nam D-H, Lim DH, et al. Outcome of salvage treatment for recurrent glioblastoma. J Clin Neurosci. 2015;22:468–73. https://doi.org/10.1016/j.jocn.2014.09.018.
Combs SE, Kessel KA, Hesse J, Straube C, Zimmer C, Schmidt-Graf F, et al. Moving second courses of radiotherapy forward: early re-irradiation after surgical resection for recurrent gliomas improves efficacy with excellent tolerability. Neurosurgery. 2018;0:1–8. https://doi.org/10.1093/neuros/nyx629.
Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:1–17. https://doi.org/10.1016/j.ijrobp.2009.07.1754.
Straube C, Elpula G, Gempt J, Gerhardt J, Bette S, Zimmer C, et al. Re-irradiation after gross total resection of recurrent glioblastoma. Strahlenther Onkol. 2017;193:897–909. https://doi.org/10.1007/s00066-017-1161-6.
Petrecca K, Guiot M-C, Panet-Raymond V, Souhami L. Failure pattern following complete resection plus radiotherapy and temozolomide is at the resection margin in patients with glioblastoma. J Neuro-Oncol. 2013;111:19–23. https://doi.org/10.1007/s11060-012-0983-4.
Ogura K, Mizowaki T, Arakawa Y, Ogura M, Sakanaka K, Miyamoto S, et al. Initial and cumulative recurrence patterns of glioblastoma after temozolomide-based chemoradiotherapy and salvage treatment: a retrospective cohort study in a single institution. Radiat Oncol. 2013;8:97. https://doi.org/10.1186/1748-717X-8-97.
Ang KK, Price RE, Stephens LC, Jiang GL, Feng Y, Schultheiss TE, et al. The tolerance of primate spinal cord to re-irradiation. Int J Radiat Oncol Biol Phys. 1993;25:459–64. https://doi.org/10.1016/0360-3016(93)90067-6.
Lawrence YR, Li XA, el Naqa I, Hahn CA, Marks LB, Merchant TE, et al. Radiation dose–volume effects in the brain. Int J Radiat Oncol. 2010;76:S20–7. https://doi.org/10.1016/j.ijrobp.2009.02.091.
Amichetti M, Amelio D. A review of the role of re-irradiation in recurrent high-grade glioma (HGG). Cancers (Basel). 2011;3:4061–89. https://doi.org/10.3390/cancers3044061.
Magnuson W, Ian Robins H, Mohindra P, Howard S. Large volume reirradiation as salvage therapy for glioblastoma after progression on bevacizumab. J Neuro-Oncol. 2014;117:133–9. https://doi.org/10.1007/s11060-014-1363-z.
Balducci M, Diletto B, Chiesa S, D’Agostino GR, Gambacorta MA, Ferro M, et al. Low-dose fractionated radiotherapy and concomitant chemotherapy for recurrent or progressive glioblastoma: final report of a pilot study. Strahlenther Onkol. 2014;190:370–6. https://doi.org/10.1007/s00066-013-0506-z.
Miwa K, Matsuo M, Ogawa S, Shinoda J, Yokoyama K, Yamada J, et al. Re-irradiation of recurrent glioblastoma multiforme using 11C-methionine PET/CT/MRI image fusion for hypofractionated stereotactic radiotherapy by intensity modulated radiation therapy. Radiat Oncol. 2014;9:181. https://doi.org/10.1186/1748-717X-9-181.
Greenspoon JN, Sharieff W, Hirte H, Overholt A, Devillers R, Gunnarsson T, et al. Fractionated stereotactic radiosurgery with concurrent temozolomide chemotherapy for locally recurrent glioblastoma multiforme: a prospective cohort study. Oncol Targets Ther. 2014;7:485–90. https://doi.org/10.2147/OTT.S60358.
Dincoglan F, Beyzadeoglu M, Sager O, Demiral S, Gamsiz H, Uysal B, et al. Management of patients with recurrent glioblastoma using hypofractionated stereotactic radiotherapy. Tumori. 2015;101:179–84. https://doi.org/10.5301/tj.5000236.
Bir SC, Connor DE, Ambekar S, Wilden JA, Nanda A. Factors predictive of improved overall survival following stereotactic radiosurgery for recurrent glioblastoma. Neurosurg Rev. 2015;38:705–13. https://doi.org/10.1007/s10143-015-0632-4.
Holt D, Bernard M, Quan K, Clump D, Engh J, Burton S, et al. Salvage stereotactic radiosurgery for recurrent glioblastoma multiforme with prior radiation therapy. J Cancer Res Ther. 2016;12:1243. https://doi.org/10.4103/0973-1482.199537.
Schnell O, Thorsteinsdottir J, Fleischmann DF, Lenski M, Abenhardt W, Giese A, et al. Re-irradiation strategies in combination with bevacizumab for recurrent malignant glioma. J Neuro-Oncol. 2016;130:591–9. https://doi.org/10.1007/s11060-016-2267-x.
Moller S, Munck Af Rosenschold P, Costa J, Law I, Poulsen HS, Engelholm SA, et al. Toxicity and efficacy of re-irradiation of high-grade glioma in a phase I dose- and volume escalation trial. Radiother Oncol. 2017;125:223–7. https://doi.org/10.1016/j.radonc.2017.09.039.
van Linde ME, Brahm CG, de Witt Hamer PC, Reijneveld JC, Bruynzeel AMEE, Vandertop WP, et al. Treatment outcome of patients with recurrent glioblastoma multiforme: a retrospective multicenter analysis. J Neuro-Oncol. 2017;135:183–92. https://doi.org/10.1007/s11060-017-2564-z.
Shi W, Scannell Bryan M, Gilbert MR, Mehta MP, Blumenthal DT, Brown PD, et al. Investigating the effect of reirradiation or systemic therapy in patients with glioblastoma after tumor progression: a secondary analysis of NRG Oncology/Radiation tTherapy Oncology Group trial 0525. Int J Radiat Oncol Biol Phys. 2017;100:38–44. https://doi.org/10.1016/j.ijrobp.2017.08.038.
Arvold ND, Shi DD, Aizer AA, Norden AD, Reardon DA, Lee EQ, et al. Salvage re-irradiation for recurrent high-grade glioma and comparison to bevacizumab alone. J Neuro-Oncol. 2017;135:581–91. https://doi.org/10.1007/s11060-017-2611-9.
• Kessel KA, Hesse J, Straube C, Zimmer C, Schmidt-Graf F, Schlegel J, et al. Modification and optimization of an established prognostic score after re-irradiation of recurrent glioma. PLoS One. 2017;12:e0180457. https://doi.org/10.1371/journal.pone.0180457 A simple but powerfull score to predict survival of patients with recurrent malignant glioma after re-RT. The score is based on a multi-institutional database and was internly and externly validated.
Shi W, Blomain ES, Siglin J, Palmer JD, Dan T, Wang Y, et al. Salvage fractionated stereotactic re-irradiation (FSRT) for patients with recurrent high grade gliomas progressed after bevacizumab treatment. J Neuro-Oncol. 2018;137:171–7. https://doi.org/10.1007/s11060-017-2709-0.
Niyazi M, Adeberg S, Kaul D, Boulesteix A-L, Bougatf N, Fleischmann DF, et al. Independent validation of a new reirradiation risk score (RRRS) for glioma patients predicting post-recurrence survival: a multicenter DKTK/ROG analysis. Radiother Oncol. 2018;127:121–7. https://doi.org/10.1016/j.radonc.2018.01.011.
•• Kazmi F, Soon YY, Leong YH, Koh WY, Vellayappan B. Re-irradiation for recurrent glioblastoma (GBM): a systematic review and meta-analysis. J Neuro-Oncol. 2019. https://doi.org/10.1007/s11060-018-03064-0 The first meta-analysis of re-RT articles. The article substantiates the prognosis after re-RT and gives a valid overview about side effects which should be anticipated after re-RT.
Navarria P, Minniti G, Clerici E, Tomatis S, Pinzi V, Ciammella P, et al. Re-irradiation for recurrent glioma: outcome evaluation, toxicity and prognostic factors assessment. A multicenter study of the Radiation Oncology Italian Association (AIRO). J Neuro-Oncol. 2018;142:59–67. https://doi.org/10.1007/s11060-018-03059-x.
Chun SJ, Park SH, Park CK, Kim JW, Kim TM, Choi SH, et al. Survival gain with re-Op/RT for recurred high-grade gliomas depends upon risk groups. Radiother Oncol. 2018;128:254–9. https://doi.org/10.1016/j.radonc.2018.05.024.
Nieder C, Andratschke NH, Grosu AL. Re-irradiation for recurrent primary brain tumors. Anticancer Res. 2016;36:4985–95. https://doi.org/10.21873/anticanres.11067.
Oehlke O, Mix M, Graf E, Schimek-Jasch T, Nestle U, Gotz I, et al. Amino-acid PET versus MRI guided re-irradiation in patients with recurrent glioblastoma multiforme (GLIAA) - protocol of a randomized phase II trial (NOA 10/ARO 2013-1). BMC Cancer. 2016;16:769. https://doi.org/10.1186/s12885-016-2806-z.
Combs SE, Edler L, Rausch R, Welzel T, Wick W, Debus J. Generation and validation of a prognostic score to predict outcome after re-irradiation of recurrent glioma. Acta Oncol. 2013;52:147–52. https://doi.org/10.3109/0284186X.2012.692882.
Kessel KA, Hesse J, Straube C, Zimmer C, Schmidt-Graf F, Schlegel J, et al. Validation of an established prognostic score after re-irradiation of recurrent glioma. Acta Oncol (Madr). 2017;56:422–6. https://doi.org/10.1080/0284186X.2016.1276621.
Straube C, Antoni S, Gempt J, Zimmer C, Meyer B, Schlegel J, et al. Re-irradiation in elderly patients with glioblastoma: a single institution experience. J Neuro-Oncol. 2019;0:0–335. https://doi.org/10.1007/s11060-019-03101-6.
Combs SE, Kessel KA, Hesse J, Straube C, Zimmer C, Schmidt-Graf F, et al. Moving second courses of radiotherapy forward: early re-irradiation after surgical resection for recurrent gliomas improves efficacy with excellent tolerability. Neurosurgery. 2018;83:1241–8. https://doi.org/10.1093/neuros/nyx629.
Schnell O, Thorsteinsdottir J, Fleischmann DF, Lenski M, Abenhardt W, Giese A, et al. Re-irradiation strategies in combination with bevacizumab for recurrent malignant glioma. J Neuro-Oncol. 2016;130:591–9. https://doi.org/10.1007/s11060-016-2267-x.
Niyazi M, Adeberg S, Kaul D, Boulesteix A-L, Bougatf N, Fleischmann DF, et al. Independent validation of a new reirradiation risk score (RRRS) for glioma patients predicting post-recurrence survival: a multicenter DKTK/ROG analysis. Radiother Oncol. 2018;127:121–7. https://doi.org/10.1016/j.radonc.2018.01.011.
•• Chun S-J, Park S-H, Park C-K, Kim JW, Kim TM, Choi SH, et al. Survival gain with re-Op/RT for recurred high-grade gliomas depends upon risk groups. Radiother Oncol. 2018;128:254–9. https://doi.org/10.1016/j.radonc.2018.05.024 The first article which introduces re-RT as an adjuvant treatment after surgery for recurrent malignant glioma.
Combs SE, Debus J, Schulz-Ertner D. Radiotherapeutic alternatives for previously irradiated recurrent gliomas. BMC Cancer. 2007;7:167. https://doi.org/10.1186/1471-2407-7-167.
• Shi W, Scannell Bryan M, Gilbert MR, Mehta MP, Blumenthal DT, Brown PD, et al. Investigating the effect of reirradiation or systemic therapy in patients with glioblastoma after tumor progression: a secondary analysis of NRG Oncology/Radiation Therapy Oncology Group trial 0525. Int J Radiat Oncol Biol Phys. 2018;100:38–44. https://doi.org/10.1016/j.ijrobp.2017.08.038 One of the largest prospectively collected cohorts. The article summarizes and compares salvage strategies for rGBM.
Straube C, Elpula G, Gempt J, Gerhardt J, Bette S, Zimmer C, et al. Re-irradiation after gross total resection of recurrent glioblastoma: spatial pattern of recurrence and a review of the literature as a basis for target volume definition. Strahlenther Onkol. 2017;193:897–909. https://doi.org/10.1007/s00066-017-1161-6.
Straube C, Scherb H, Gempt J, Kirschke J, Zimmer C, Schmidt-Graf F, et al. Adjuvant stereotactic fractionated radiotherapy to the resection cavity in recurrent glioblastoma—the GlioCave study (NOA 17–ARO 2016/3–DKTK ROG trial). BMC Cancer. 2018;18:15. https://doi.org/10.1186/s12885-017-3928-7.
Combs SE, Bischof M, Welzel T, Hof H, Oertel S, Debus J, et al. Radiochemotherapy with temozolomide as re-irradiation using high precision fractionated stereotactic radiotherapy (FSRT) in patients with recurrent gliomas. J Neuro-Oncol. 2008;89:205–10. https://doi.org/10.1007/s11060-008-9607-4.
Wick W, Fricke H, Junge K, Kobyakov G, Martens T, Heese O, et al. A phase II, randomized, study of weekly APG101+reirradiation versus reirradiation in progressive glioblastoma. Clin Cancer Res. 2014;20:6304–13. https://doi.org/10.1158/1078-0432.CCR-14-0951-T.
Huang RY, Rahman R, Ballman KV, Felten SJ, Anderson SK, Ellingson BM, et al. The impact of T2/FLAIR evaluation per RANO criteria on response assessment of recurrent glioblastoma patients treated with bevacizumab. Clin Cancer Res. 2016;22:575–81. https://doi.org/10.1158/1078-0432.CCR-14-3040.
Straube C, Bette S, Pyka T, Einhellig H, Zimmer C, Schwaiger M, et al. Modern imaging in neurooncology. Aktuelle Neurol. 2017;44:160–70. https://doi.org/10.1055/s-0043-111588.
Grosu AL, Weber WA, Franz M, Stärk S, Piert M, Thamm R, et al. Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:511–9. https://doi.org/10.1016/j.ijrobp.2005.01.056.
Minniti G, Scaringi C, De Sanctis V, Lanzetta G, Falco T, Di Stefano D, et al. Hypofractionated stereotactic radiotherapy and continuous low-dose temozolomide in patients with recurrent or progressive malignant gliomas. J Neuro-Oncol. 2013;111:187–94. https://doi.org/10.1007/s11060-012-0999-9.
Conti A, Pontoriero A, Arpa D, Siragusa C, Tomasello C, Romanelli P, et al. Efficacy and toxicity of CyberKnife re-irradiation and “dose dense” temozolomide for recurrent gliomas. Acta Neurochir. 2012;154:203–9. https://doi.org/10.1007/s00701-011-1184-1.
Popp I, Bott S, Mix M, Oehlke O, Schimek-Jasch T, Nieder C, et al. Diffusion-weighted MRI and ADC versus FET-PET and GdT1w-MRI for gross tumor volume (GTV) delineation in re-irradiation of recurrent glioblastoma. Radiother Oncol. 2018;130:121–31. https://doi.org/10.1016/j.radonc.2018.08.019.
Oehlke O, Mix M, Graf E, Schimek-Jasch T, Nestle U, Gotz I, et al. Amino-acid PET versus MRI guided re-irradiation in patients with recurrent glioblastoma multiforme (GLIAA) - protocol of a randomized phase II trial (NOA 10/ARO 2013-1). BMC Cancer. 2016;16:769. https://doi.org/10.1186/s12885-016-2806-z.
Mandl ES, Dirven CMF, Buis DR, Postma TJ, Vandertop WP. Repeated surgery for glioblastoma multiforme: only in combination with other salvage therapy. Surg Neurol. 2008;69:506–9. https://doi.org/10.1016/j.surneu.2007.03.043.
Lee J, Ahn SS, Chang JH, Suh CO. Hypofractionated re-irradiation after maximal surgical resection for recurrent glioblastoma: therapeutic adequacy and its prognosticators of survival. Yonsei Med J. 2018;59:194–201. https://doi.org/10.3349/ymj.2018.59.2.194.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Christoph Straube received a scholarship from Medac GmbH, received a travel grant from NovoCure Ltd., contributed to a brochure for patients about GBM which was partially sponsored by NovoCure Ltd., and received speakers honoria from Teva Pharmaceutical Industries Ltd. and Roche. Kerstin A. Kessel declares that she has no conflict of interest. Claus Zimmer has served on scientific advisory boards for Philips and Bayer Schering, serves as co-editor on the Advisory Board of Clinical Neuroradiology, has received speaker honoraria from Bayer-Schering and Philips, and has received research support and investigator fees for clinical studies from Biogen Idec, Quintiles, MSD Sharp & Dohme, Boehringer Ingelheim, Inventive Health Clinical UK Ltd., Advance Cor, Brainsgate, Pfizer, Bayer-Schering, Novartis, Roche, Servier, Penumbra, WCT GmbH, Syngis, SSS International Clinical Research, PPD Germany GmbH, Worldwide Clinical Trials Ltd., Phenox, Covidien, Actelion, Medivation, Medtronic, Harrison Clinical Research, Concentric, Penumbra, Pharmtrace, Reverse Medical Corp., Premier Research Germany Ltd., Surpass Medical Ltd., and GlaxoSmithKline. Friederike Schmidt-Graf served as an author for Medac GmbH. Jürgen Schlegel declares that he has no conflict of interest. Jens Gempt serves as a consultant for BrainLab. Bernhard Meyer serves as a consultant for BrainLab. Stephanie E. Combs has served on advisory boards of Bristol-Myers Squibb (BMS), Roche, Novocure, Daiichi Synkio, Astra Zeneca, Icotec, and Varian Medical Systems; has served on an advisory board and speaker’s bureau for BrainLab; and has received speaker’s honoraria from BrainLab, Accuray, Dr. Sennewald, BMS, Astra Zeneca, Roche, Varian Medical Systems, Icotec, Elekta, Novocure, and Medac GmbH.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuro-oncology
Rights and permissions
About this article
Cite this article
Straube, C., Kessel, K.A., Zimmer, C. et al. A Second Course of Radiotherapy in Patients with Recurrent Malignant Gliomas: Clinical Data on Re-irradiation, Prognostic Factors, and Usefulness of Digital Biomarkers. Curr. Treat. Options in Oncol. 20, 71 (2019). https://doi.org/10.1007/s11864-019-0673-y
Published:
DOI: https://doi.org/10.1007/s11864-019-0673-y