Abstract
Outcomes after bevacizumab failure for recurrent glioblastoma (GBM) are poor. Our analysis of 16 phase II trials (n = 995) revealed a median overall survival (OS) of 3.8 months (±1.0 month SD) after bevacizumab failure with no discernible activity of salvage chemotherapy. Thus, the optimal treatment for disease progression after bevacizumab has yet to be elucidated. This study evaluated the efficacy of reirradiation for patients with GBM after progression on bevacizumab. An IRB approved retrospective (2/2008–5/2013) analysis was performed of 23 patients with recurrent GBM (after standard radiotherapy/temozolomide) treated with bevacizumab (10 mg/kg) every 2 weeks until progression (median age 53 years; median KPS 80; median progression free survival on bevacizumab 3.7 months). Within 7–14 days of progression on bevacizumab, patients initiated reirradiation to a dose of 54 Gy in 27 fractions using pulsed-reduced dose rate (PRDR) radiotherapy. The median planning target volume was 424 cm3. At the start of reirradiation, bevacizumab (10 mg/kg) was given every 4 weeks for two additional cycles. The median OS and 6 month OS after bevacizumab failure was 6.9 months and 65 %, respectively. Reirradiation was well tolerated with no symptomatic grade 3–4 toxicities. Favorable outcomes of reirradiation after bevacizumab failure in patients with recurrent GBM suggest its role as a treatment option for large volume recurrences not amenable to stereotactic radiosurgery. As PRDR is easily accomplished from a technological standpoint, we are in the process of expanding this approach to a multi-institutional cooperative group trial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The treatment options for recurrent glioblastoma (GBM) remain limited. As vascular endothelial growth factor (VEGF) is highly expressed in GBM cells and the degree of overexpression is predictive of survival [1], VEGF has been a logical therapeutic target. Relative to this, bevacizumab (a humanized monoclonal antibody that targets VEGF) received accelerated US Food and Drug Administration approval in 2009 based on two separate phase II trials: AVF3708g and NCI 06C0064E. AVF3708g randomized patients to bevacizumab alone or in combination with irinotecan and reported a progression free survival (PFS) of 4.2 and 5.6 months and a median overall survival (OS) after bevacizumab progression of 5.0 and 3.1 months, respectively [2]. NCI 06C0064E reported a PFS of 3.7 months and a median OS after bevacizumab progression of 3.5 months [3]. These data, compared to historical controls, suggested the possibility of a modest improvement in survival.
Interestingly, it became clear to the neuro-oncology community that patients who had progressed following bevacizumab therapy tended to be refractory to further therapeutic interventions. In this respect, our review of the literature (Table 1) identified 16 phase II trials (n = 995) that investigated the efficacy of bevacizumab with or without additional chemotherapy after initial bevacizumab failure [2–17]. Our analysis demonstrated a median OS of 3.8 months (±1.0 month SD) with no discernible activity of continued bevacizumab and/or chemotherapy for this patient population. In addition to the poor outcomes observed with the continuation of bevacizumab after progression, patients remain at increased risk for life threatening events such as CNS hemorrhage, thromboembolic events, gastrointestinal perforation and myelosuppression when combined with chemotherapy [2, 3, 5–9, 15]. Thus, the optimal treatment for disease progression after bevacizumab has yet to be elucidated and no chemotherapeutic agent has been shown to salvage patients after bevacizumab failure [4, 6, 7, 9, 10, 12, 16].
Studies presented in the literature have examined reirradiation alone, reirradiation followed by adjuvant systemic therapy or reirradiation concurrent with systemic therapy [18–24]. Most recently, reirradiation for recurrent high-grade glioma with limited volume disease has consisted of hypofractionated treatment regimens employing smaller margins (0.5–1.0 cm) to generate the clinical target volume (CTV) or stereotactic radiosurgery (SRS) [18–24]. For large volume recurrences, our group has elected to utilize reirradiation with pulsed-reduced dose rate (PRDR) technique after progression on bevacizumab, as we believe it to be more effective than continuing systemic therapies.
Our institutional experience utilizing PRDR radiotherapy for the reirradiation of recurrent glioma has been previously described in a series of 103 bevacizumab naïve patients [25]. In brief, an apparent dose rate of 0.0667 Gy/min is achieved by giving 0.2 Gy pulses separated by 3 minute intervals. This technique is hypothesized to improve the therapeutic index by two separate mechanistic pathways. First, the reduction in dose rate may allow the normal brain parenchyma to repair sublethal damage with greater efficacy than adjacent malignant cells [26]. Secondly, dividing each fraction into a number of subfractions may take advantage of low-dose hyper-radiosensitivity; a phenomenon whereby some malignant cells have increased radiosensitivity to doses <0.3–0.5 Gy [27–29]. Using PRDR radiotherapy, it is feasible to safely reirradiate large volumes and include peritumoral cells extending beyond the T2-weighted or fluid attenuation inversion recovery (FLAIR) irregularity to decrease the probability of a marginal miss or recurrence outside the radiation field [25, 30–32]. The report to follow summarizes our experience in a series of 23 patients using reirradiation as a treatment modality for GBM after progression on bevacizumab.
Patients and methods
Study design
The institutional review board at the University of Wisconsin approved this retrospective analysis. Twenty-three patients treated with PRDR reirradiation after bevacizumab failure for recurrent GBM between February 2008 and January 2013 were identified using the departmental database. At initial diagnosis, every patient in the study underwent maximal safe resection, standard external beam radiotherapy (59.4–60 Gy) concurrent with temozolomide and six additional cycles of adjuvant temozolomide [33]. After completion of initial therapy, all patients were followed with serial MRI scans at 2 month intervals (or less if clinical symptoms warranted a shorter interval) until radiographic evidence of disease recurrence as confirmed by a neuroradiologist using the response assessment in neuro-oncology (RANO) criteria [34]. At the time of recurrence, all patients were offered enrollment on a clinical trial or bevacizumab (10 mg/kg). Bevacizumab was continued every 2 weeks until progressive disease was identified on MRI (obtained at 2 month intervals) using the RANO criteria.
Within 7–14 days of progression on bevacizumab, patients initiated reirradiation using PRDR radiotherapy. During reirradiation, bevacizumab (10 mg/kg) was given every 4 weeks for two cycles (i.e. weeks 1 and 5) to minimize radiation effects and necrosis [35]. All patients were evaluated once per week during radiotherapy. Patients returned for follow-up visits 2 weeks after the completion of treatment and approximately every 4 weeks thereafter.
Radiotherapy
A contrast-enhanced MRI brain with 1 mm slices was obtained to document progressive disease on bevacizumab and within 7 days patients underwent treatment planning computed tomography (CT) with 1.25 mm slices. During the planning CT, patients were simulated with a thermoplastic mask system to ensure immobilization and reproducibility. The MRI was co-registered to the treatment planning CT scan and a gross tumor volume (GTV) was outlined by encompassing the contrast enhancing lesion and adjacent T2-weighted or FLAIR irregularity. The CTV was defined as the GTV + 2.0 cm margin. If a clear T2 FLAIR abnormality was not present, the GTV was demarcated by outlining the contrast enhancing abnormality and an expansion of 2.5 cm was applied to generate the CTV. In both situations, the CTVFootnote 1 was trimmed off of anatomic boundaries that would limit spread. The PTV was generated by adding a 3–5 mm margin to the CTV.
Three-dimensional conformal radiotherapy utilizing 6 MV photons was used to deliver a dose of 54 Gy in 27 fractions administered 5 days per week using PRDR radiotherapy. The median duration of treatment was 37 days (range: 37–43 days). In PRDR radiotherapy, each treatment is delivered using 0.2 Gy pulses separated by 3 minute intervals, creating an apparent dose rate of 0.0667 Gy/min. The dose rate of the linear accelerator is decreased from the conventional dose rate of 4–6 to 1 Gy/min during each pulse. Dose constraints for the PRDR plan included limiting the optic chiasm and brainstem to 54 Gy. No constraints were placed on the cumulative dose, including the initial treatment dose. The treatment plans were constructed using Pinnacle planning systems (Fitchburg, WI) and administered at five facilities within our system.
Toxicity evaluation
The toxicity of reirradiation was assessed using the Common Terminology Criteria for Adverse Events, version 4.0. Toxicity was characterized as worsening of previous symptoms or the development of new symptoms during treatment.
Statistical methods
The primary purpose of this analysis was to determine the median OS and 6 month OS of patients receiving PRDR radiotherapy from the point of progression on bevacizumab and compare it to historical controls. The data point for OS was calculated from the date of the MRI brain, which documented progressive disease. Survival estimates were obtained using the Kaplan–Meier method. The other endpoints estimated were OS from the date of initial diagnosis and PFS from the start of bevacizumab. Patients were censored at the time of the most recent follow-up visit. A univariate analysis using log-rank test was performed for factors that may potentially influence prognosis. All statistical analyses were performed using SPSS (Statistical Package for Social Sciences, version 21.0).
The mean of the median overall survivals documented in the literature (Table 1) was calculated via normalization to account for the differing sample sizes between studies. A standard deviation was then calculated to assess the variability among the studies.
Results
Patient characteristics
The patient characteristics are detailed in Table 2. At first recurrence, four patients underwent subtotal resection and chemotherapy, three patients received chemotherapy alone and two patients underwent subtotal resection alone prior to receiving bevacizumab. The median PFS from diagnosis to first recurrence was 6.5 months (range 4.0–20.6 months) and the median PFS on bevacizumab was 3.7 months (range 1.2–14.1 months; see Fig. 1). The median PTV was 424 cm3 (range 74–776 cm3).
Toxicity
Treatment with PRDR radiotherapy was well tolerated with no symptomatic grade 3–4 toxicities related to reirradiation and/or reirradiation concurrent with bevacizumab. At the completion of reirradiation, the median dexamethasone dose per day was 8 mg. Only 40 % of patients required increasing doses of steroids during the course of their treatment. There was no observed clinical or radiographic evidence of rebound edema after cessation of bevacizumab. One patient died within 2 weeks of completion of radiotherapy due to progression of fulminant disease.
Survival
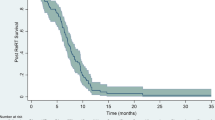
At the time of analysis, 22 of 23 patients had died. The median OS after progression on bevacizumab was 6.9 months (range 2.7–12 months) and the 3, 6 and 12 month OS after bevacizumab failure was 91, 65 and 5 %, respectively (see Fig. 2). The median OS from date of initial diagnosis was 20.5 months and the 12, 18 and 24 month OS was 96, 61 and 30 %, respectively.
On univariate analysis, neither age, KPS, MGMT methylation status, PFS after initial therapy, salvage therapy prior to bevacizumab, PFS on bevacizumab, or PTV was predictive of survival, though statistical power was limited.
Patterns of recurrence
The MRI corresponding to the date of radiographic progression after PRDR radiotherapy was analyzed and compared to the treatment plan in the original treatment planning software. Using the definition used by Shapiro et al. [24], recurrences were defined by the volume of tumor present on T1-weighted post-gadolinium and FLAIR sequences located within the 95 % isodose line: an “infield” recurrence contained >80 % of the tumor recurrence, “marginal” 20–80 % and “out of field” <20 %. Of the 23 patients treated, 20 underwent post-PRDR MRI and the number of patients observed to have an “infield”, “marginal” and “out of field” recurrence was 17 (85 %), 2 (10 %) and 1 (5 %), respectively.
Discussion
Treatment options for recurrent GBM include systemic therapy, reirradiation, maximal safe resection and best supportive care. While these modalities have never been compared in a randomized trial, bevacizumab is often given at first recurrence. The basis for the FDA approval of bevacizumab in the recurrent setting is based on two separate phase II trials, neither of which contained a non-bevacizumab control group [2, 3]. It is notable that outcomes after bevacizumab progression are poor and our analysis of 16 phase II trials (n = 995) revealed a median OS of only 3.8 months (±1.0 month). While our study is limited by its retrospective nature, the median OS of 6.9 months after bevacizumab failure compares favorably with the continuation of systemic therapies (Table 1). The median PFS on bevacizumab of 3.7 months in our cohort is comparable to the literature and thereby supports our use of this historical dataset.
The continuation of bevacizumab during retreatment radiation was predicated on reducing radiation induced edema and necrosis [35]. The advantage of using PRDR radiotherapy lies in the ability to reirradiate large volumes while minimizing toxicity. The reirradiation of large volumes has typically been avoided because of concern for radiation necrosis and surpassing the cumulative dose limits of adjacent critical structures. As a result, reirradiation approaches using hypofractionated schedules or SRS have employed smaller treatment volumes. It is notable that while our series included large treatment volumes, no symptomatic grade 3–4 toxicities were observed.
The RTOG recently opened a study (RTOG-1205) randomizing patients with recurrent GBM to bevacizumab alone or bevacizumab concurrent with hypofractionated radiotherapy to a dose of 35 Gy in 10 fractions. Per the protocol, there is no expansion to the CTV for lesions greater than 3.5 cm and lesions less than 3.5 cm will have a maximum expansion of 5 mm. The PTV expansion is 3–5 mm with daily image guidance. A recent study by Shapiro et al. [24], using similar treatment volumes, examined patterns of failure in the reirradiation setting with concurrent bevacizumab and found that 24 % of recurrences were marginal and 24 % were remote (median PTV 35 cm3, range 3–62 cm3). The largest reirradiation series in the literature reported by Combs [19] employed similar treatment volumes and reported a median PTV of 49 cm3 (range 2.5–636 cm3), but did not report patterns of failure. Conversely, the median PTV in the current study is 424 cm3 (range 74–776 cm3). The marginal recurrence rate of 10 % and remote recurrence rate of 5 % in the current study compares favorably to those employing smaller treatment volumes.
While our median OS after progression on bevacizumab is comparable to reports of the aforementioned hypofractionated regimen [23, 24], both may be inferior to SRS for small volume recurrences. A recent study by Cabrera et al. [18], examining the efficacy of SRS concurrent with bevacizumab after progression on bevacizumab, reported a median OS of 14.4 months. Cuneo et al. [20] evaluated the use of SRS and adjuvant bevacizumab in a group of heavily pretreated patients and demonstrated a median OS of 11.2 months from the time of SRS. While the study by Cabrera did not report treatment volumes, the median PTV in the study by Cuneo was only 4.8 cm3, demonstrating a highly selected group of patients with small volume recurrences. Thus, PRDR radiotherapy may provide an excellent treatment option for large volume recurrences not amenable to SRS.
The concept of PRDR radiotherapy was derived as a means to safely allow for retreatment radiation in the setting of recurrent disease. Our experience with PRDR radiotherapy has shown that reirradiation of large volumes of normal tissue is feasible [25] and likely exploits the ability of normal tissues to repair sublethal damage more effectively than GBM cells when the dose rate is decreased [26]. Moreover, by giving each sub-fraction in a dose of 0.2 Gy, low-dose hyper-radiosensitivity may limit the ability of the GBM cells to effectively repair the damage caused by radiation [27–29]. In a recent preclinical study using an orthotopic mouse model, Dilworth et al. [36] demonstrated that PRDR radiotherapy significantly improved median OS and response rates compared with standard radiotherapy. Furthermore, standard radiotherapy was found to be more toxic, as examination of the adjacent brain parenchyma revealed a significant increase in degenerating neurons and diminished capillary integrity compared to PRDR radiotherapy.
To our knowledge, the only other report of a potentially successful attempt of salvage therapy for large volume recurrences after bevacizumab failure was a subset analysis of patients treated with NovoTTF-100A therapy [4]. The median OS after bevacizumab failure was only 3.1 months in the chemotherapy arm (n = 21), compared with 6.3 months in the NovoTTF-100A arm (n = 23) and this therapeutic lead is being followed up by the RTOG in a prospective study. It is our contention that PRDR radiotherapy is less cumbersome for patients and far more cost effective than NovoTTF-100A therapy.
While our median OS of 6.9 months after bevacizumab failure compares favorably to historical controls, there are limitations to this study. First, the study carries with it all of the limitations inherent in a retrospective analysis. Second, the statistical power is diminished because of the small sample size. Finally, while our results are encouraging, we acknowledge that many of the studies cited as historical controls have significant variability pertaining to the number of therapies patients received prior to bevacizumab.
In conclusion, our series of reirradiation after bevacizumab progression in recurrent GBM suggest a possibility for extended survival compared to the continuation of systemic therapies and may represent an excellent treatment option for recurrences that are not amenable to SRS. Furthermore, PRDR radiotherapy was delivered at five different centers within our system, demonstrating the ability of this technique to be administered in various settings. As PRDR radiotherapy is easily accomplished from a technological standpoint, the RTOG is currently considering a study in which patients with recurrent GBM who have demonstrated progression on bevacizumab would be randomized to one of three arms: bevacizumab plus a chemotherapeutic agent of choice, bevacizumab concurrent with PRDR radiotherapy or bevacizumab concurrent with conventional dose rate radiotherapy using a hypofractionated treatment regimen of 35 Gy in 10 Fx.
Notes
Rationale for CTV: During the first course of radiotherapy, the CTV for the initial field recommended by the radiation therapy oncology group (RTOG) is the contrast enhancing lesion and adjacent T2-weighted or FLAIR irregularity with a 2.0 cm margin. This recommendation is based upon two studies, one of which demonstrated that the isolated tumor cell infiltration extended at least as far as the T2 prolongation on MRI and another which showed that 90 % of recurrences were within 2 cm of the contrast enhancing lesion [30, 31].
References
Zhou YH, Tan F, Hess KR, Yung WK (2003) The expression of PAX6, PTEN, vascular endothelial growth factor, and epidermal growth factor receptor in gliomas: relationship to tumor grade and survival. Clin Cancer Res 9:3369–3375
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740. doi:10.1200/JCO.2008.19.8721
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745. doi:10.1200/JCO.2008.16.3055
Ram Z, Stupp R, et al (2010) Subgroup and quality of life analyses of the phase III clinical trial of NovoTTF-100A versus best standard chemotherapy for recurrent glioblastoma. Neuro Oncol Society for Neuro-Oncology (SNO) Abstract No-55, pages iv 48–49
Bokstein F, Shpigel S, Blumenthal DT (2008) Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer 112:2267–2273. doi:10.1002/cncr.23401
Iwamoto FM, Abrey LE, Beal K, Gutin PH, Rosenblum MK, Reuter VE, DeAngelis LM, Lassman AB (2009) Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology 73:1200–1206. doi:10.1212/WNL.0b013e3181bc0184
Lu-Emerson C, Norden AD, Drappatz J, Quant EC, Beroukhim R, Ciampa AS, Doherty LM, Lafrankie DC, Ruland S, Wen PY (2011) Retrospective study of dasatinib for recurrent glioblastoma after bevacizumab failure. J Neurooncol 104:287–291. doi:10.1007/s11060-010-0489-x
Nghiemphu PL, Liu W, Lee Y, Than T, Graham C, Lai A, Green RM, Pope WB, Liau LM, Mischel PS, Nelson SF, Elashoff R, Cloughesy TF (2009) Bevacizumab and chemotherapy for recurrent glioblastoma: a single-institution experience. Neurology 72:1217–1222. doi:10.1212/01.wnl.0000345668.03039.90
Omuro A, Chan TA, Abrey LE, Khasraw M, Reiner AS, Kaley TJ, DeAngelis LM, Lassman AB, Nolan CP, Gavrilovic IT, Hormigo A, Salvant C, Heguy A, Kaufman A, Huse JT, Panageas KS, Hottinger AF, Mellinghoff I (2013) Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro Oncol 15:242–250. doi:10.1093/Neuonc/Nos295
Quant EC, Norden AD, Drappatz J, Muzikansky A, Doherty L, Lafrankie D, Ciampa A, Kesari S, Wen PY (2009) Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol 11:550–555. doi:10.1215/15228517-2009-006
Raizer JJ, Grimm S, Chamberlain MC, Nicholas MK, Chandler JP, Muro K, Dubner S, Rademaker AW, Renfrow J, Bredel M (2010) A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer 116:5297–5305. doi:10.1002/cncr.25462
Reardon DA, Desjardins A, Peters KB, Vredenburgh JJ, Gururangan S, Sampson JH, McLendon RE, Herndon JE 2nd, Coan A, Threatt S, Friedman AH, Friedman HS (2011) Phase 2 study of carboplatin, irinotecan, and bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. Cancer 117:5351–5358. doi:10.1002/cncr.26188
Sathornsumetee S, Desjardins A, Vredenburgh JJ, McLendon RE, Marcello J, Herndon JE, Mathe A, Hamilton M, Rich JN, Norfleet JA, Gururangan S, Friedman HS, Reardon DA (2010) Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro Oncol 12:1300–1310. doi:10.1093/neuonc/noq099
Selfridge J, Piccioni D, Zurayk M, et al. (2012) Deferred use of bevazicumab for recurrent glioblastoma is not associated with diminished efficacy. Neuro Oncol Society for Neuro-Oncology (SNO) Abstract NO-97, page vi 84
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25:4722–4729. doi:10.1200/JCO.2007.12.2440
Wen PY, Schiff D, Cloughesy TF, Raizer JJ, Laterra J, Smitt M, Wolf M, Oliner KS, Anderson A, Zhu M, Loh E, Reardon DA (2011) A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol 13:437–446. doi:10.1093/neuonc/noq198
Zuniga RM, Torcuator R, Jain R, Anderson J, Doyle T, Ellika S, Schultz L, Mikkelsen T (2009) Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol 91:329–336. doi:10.1007/s11060-008-9718-y
Cabrera AR, Cuneo KC, Desjardins A, Sampson JH, McSherry F, Herndon JE 2nd, Peters KB, Allen K, Hoang JK, Chang Z, Craciunescu O, Vredenburgh JJ, Friedman HS, Kirkpatrick JP (2013) Concurrent stereotactic radiosurgery and bevacizumab in recurrent malignant gliomas: a prospective trial. Int J Radiat Oncol Biol Phys 86:873–879. doi:10.1016/j.ijrobp.2013.04.029
Combs SE, Thilmann C, Edler L, Debus J, Schulz-Ertner D (2005) Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol 23:8863–8869. doi:10.1200/JCO.2005.03.4157
Cuneo KC, Vredenburgh JJ, Sampson JH, Reardon DA, Desjardins A, Peters KB, Friedman HS, Willett CG, Kirkpatrick JP (2012) Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 82:2018–2024. doi:10.1016/j.ijrobp.2010.12.074
Fogh SE, Andrews DW, Glass J, Curran W, Glass C, Champ C, Evans JJ, Hyslop T, Pequignot E, Downes B, Comber E, Maltenfort M, Dicker AP, Werner-Wasik M (2010) Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol 28:3048–3053. doi:10.1200/JCO.2009.25.6941
Gutin PH, Iwamoto FM, Beal K, Mohile NA, Karimi S, Hou BL, Lymberis S, Yamada Y, Chang J, Abrey LE (2009) Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 75:156–163. doi:10.1016/j.ijrobp.2008.10.043
Niyazi M, Ganswindt U, Schwarz SB, Kreth FW, Tonn JC, Geisler J, la Fougere C, Ertl L, Linn J, Siefert A, Belka C (2012) Irradiation and bevacizumab in high-grade glioma retreatment settings. Int J Radiat Oncol Biol Phys 82:67–76. doi:10.1016/j.ijrobp.2010.09.002
Shapiro LQ, Beal K, Goenka A, Karimi S, Iwamoto FM, Yamada Y, Zhang Z, Lassman AB, Abrey LE, Gutin PH (2013) Patterns of failure after concurrent bevacizumab and hypofractionated stereotactic radiation therapy for recurrent high-grade glioma. Int J Radiat Oncol Biol Phys 85:636–642. doi:10.1016/j.ijrobp.2012.05.031
Adkison JB, Tome W, Seo S, Richards GM, Robins HI, Rassmussen K, Welsh JS, Mahler PA, Howard SP (2011) Reirradiation of large-volume recurrent glioma with pulsed reduced-dose-rate radiotherapy. Int J Radiat Oncol Biol Phys 79:835–841. doi:10.1016/j.ijrobp.2009.11.058
Hall EJ, Giaccia AJ (2006) Radiobiology for the radiologist. Lippincott Williams & Wilkins, Philadelphia
Harney J, Short SC, Shah N, Joiner M, Saunders MI (2004) Low dose hyper-radiosensitivity in metastatic tumors. Int J Radiat Oncol Biol Phys 59:1190–1195. doi:10.1016/j.ijrobp.2003.12.029
Joiner MC, Marples B, Lambin P, Short SC, Turesson I (2001) Low-dose hypersensitivity: current status and possible mechanisms. Int J Radiat Oncol Biol Phys 49:379–389
Marples B, Wouters BG, Collis SJ, Chalmers AJ, Joiner MC (2004) Low-dose hyper-radiosensitivity: a consequence of ineffective cell cycle arrest of radiation-damaged G2-phase cells. Radiat Res 161:247–255
Hochberg FH, Pruitt A (1980) Assumptions in the radiotherapy of glioblastoma. Neurology 30:907–911
Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ (1987) Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg 66:865–874. doi:10.3171/jns.1987.66.6.0865
Mohindra P, Robins HI, Tome WA, Hayes L, Howard SP (2013) Wide-field pulsed reduced dose rate radiotherapy (PRDR) for recurrent ependymoma in pediatric and young adult patients. Anticancer Res 33:2611–2618
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. doi:10.1056/NEJMoa043330
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. doi:10.1200/JCO.2009.26.3541
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, Grewal J, Prabhu S, Loghin M, Gilbert MR, Jackson EF (2011) Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 79:1487–1495. doi:10.1016/j.ijrobp.2009.12.061
Dilworth JT, Krueger SA, Dabjan M, Grills IS, Torma J, Wilson GD, Marples B (2013) Pulsed low-dose irradiation of orthotopic glioblastoma multiforme (GBM) in a pre-clinical model: effects on vascularization and tumor control. Radiother Oncol. doi:10.1016/j.radonc.2013.05.022
Acknowledgments
Kathleen Reader Neuro-oncology Fund.
Conflict of interest
HIR has served as a consultant to Novocure and Genentech
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Magnuson, W., Ian Robins, H., Mohindra, P. et al. Large volume reirradiation as salvage therapy for glioblastoma after progression on bevacizumab. J Neurooncol 117, 133–139 (2014). https://doi.org/10.1007/s11060-014-1363-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1363-z