Abstract
Bevacizumab failure is a major clinical problem in the management of high grade gliomas (HGG), with a median overall survival (OS) of < 4 months. This study evaluated the feasibility and efficacy of fractionated stereotactic re-irradiation (FSRT) for patients progressed after Bevacizumab treatment. Retrospective review was conducted of 36 patients treated with FSRT after progression on bevacizumab. FSRT was most commonly delivered in 3.5 Gy fractions to a total dose of 35 Gy. Survival from initial diagnosis, as well as from recurrence and re-irradiation, were utilized as study endpoints. Univariate and multivariate analysis was performed. The median time from initial bevacizumab treatment to FSRT was 8.5 months. The median plan target volume for FSRT was 27.5 cc. The median OS from FSRT was 4.8 months. FSRT treatment was well tolerated with no grade 3 or higher toxicity. Favorable outcomes were observed in patients with recurrent HGG who received salvage FSRT after bevacizumab failure. The treatment was well tolerated. Prospective study is warranted to further evaluate the efficacy of salvage FSRT for selected patients with recurrent HGG amenable to FSRT, who had failed bevacizumab treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant gliomas are the most common maligant brain tumors in adults, with an estimated annual incidence of 3 per 100,000 people in the United States [1]. Despite multi-modality therapy [which includes resection, radiation therapy (RT), and chemotherapy], almost all patients will develop recurrence [2]. Despite recent treatment advances, the long-term outcomes for these patients remain poor [3,4,5,6].
There lacks consensus for management of recurrent high grade gliomas (HGG). A variety of chemotherapies have been evaluated for recurrent glioblastoma (GBM) with modest results. Bevacizumab, an anti-vascular endothelial growth factor monoclonal antibody, was evaluated for recurrent GBM. Phase 2 studies demonstrated favorable 6-month progression-free survival and objective responses with bevacizumab for recurrent GBM, which led to its approval by the US Food and Drug Administration in 2009 for use in recurrent GBM [7,8,9]. Currently, bevacizumab is one of the most commonly utilized treatment options for patients with recurrent GBM in US. However, if patients progressed after bevacizumab treatment, further treatment seldom effective [10]. In addition to disease recurrence itself driving poor outcomes, there is also evidence that these patients who fail bevacizumab also harbor disease that is highly resistant to other systemic therapies [11, 12]. Therefore, treatment options for these recurrent patients remain limited and their prognosis is dismal with a recent review of sixteen studies reporting an overall survival (OS) of under 4 months after bevacizumab failure [11, 13, 14]. It is critical to establish the optimal treatment for disease progression after bevacizumab.
Re-irradiation is another effective treatment option for recurrent HGG. Re-irradiation is frequently administered in the forms of stereotactic radiosurgery (SRS), or as hypofractionated radiotherapy [2, 15,16,17,18,19]. Fractionated stereotactic radiotherapy (FSRT) is a promising treatment modality for the treatment of these refractory HGGs, as it possesses the precise targeting advantages of SRS, as well as the dose-sparing radiobiologic properties of fractionation to allow greater sparing of surrounding critical structures, thus limiting toxicity [20, 21]. Our institution experience on a cohort of patients with recurrent HGGs treated with FSRT demonstrated a favorable median survival time of 11.2 months from re-irradiation [15]. Moreover, it appears that the combination of bevacizumab with SRS or FSRT may provide superior outcomes when compared with either treatment alone [17, 22, 23]. One of the studies showed median OS of 12.5 months for patients treated with FSRT and bevacizumab [17]. A recent report also demonstrated the feasibility and efficacy of using a special re-irradiation technique (pulsed-reduced dose rate) for patients progressed after bevacizumab treatment [14]. However, the pulsed-reduced dose rate radiation is not widely available. The present study sought to evaluate the safety and efficacy of FSRT in patients who failed therapy with bevacizumab.
Materials and methods
Patients
The Thomas Jefferson University institutional review board approved this single-institution, retrospective study. Patients who received FSRT salvage after progression on bevacizumab were included. Patients who received FSRT within 2 months of initiation of bevacizumab were excluded. A total of 36 patients were identified from 2006 to 2013. Patients were followed with MRI scans and clinical assessment, which were obtained 6–8 weeks after FSRT, and at approximately 2-month intervals thereafter. No patient was lost to follow up.
Radiation treatment planning
Treatment decisions were based on consensus recommendations following discussion in our institution’s multidisciplinary brain tumor board consisting of radiologists, neurosurgeons, neuro-oncologists, neuropathologists and radiation oncologists. Prior to 2004, treatment planning was conducted with the X-knife 3-D planning system (Radionics, Burlington, MA, USA). Radiation treatment was which delivered with a dedicated stereotactic 600SR linear accelerator (Varian, Palo Alto, CA, USA). From 2004 to 2013, treatment planning was carried out with Brain Lab iplan (Brainlab, Munich, Germany). Radiation treatment was delivered with Novalis 600N linear accelerator (Varian, Palo Alto, CA) using HD MLC (high definition Multileaf collimator) and exacTRAC (Brainlab, Munich, Germany) on board imaging. All patients undergoing irradiation were fitted with custom-made Brainlab (Munich, Germany) thermal plastic masks for immobilization. Treatment planning MRI and computed tomography (CT) images were obtained and fused. All patients had thin cut (1–1.5 mm) axial post-contrast and T2/FLAIR MRI. The gross tumor volume (GTV) was defined on MRI using the gadolinium enhanced T1 weighted series. Surrounding edema was not purposely included in the treatment volume. The planning target volume was the GTV with minimum margin (0–2 mm per the treating physician). Critical normal structures, such as optic nerves, chiasm, and brainstem were also contoured. The radiation planning used dynamic conformal arcs, IMRT (intensity modulated RT), or hybrid-Arcs (a combination of dynamic arcs with IMRT beams), or VMAT (volume modulated Arc Therapy). The patients were treated with FSRT to a median PTV dose of 35 Gy delivered in 3.5 Gy fractions [15]. The dose was reduced to 30 in 3 Gy fractions for large targets, and/or high critical normal structure dose. The constraints for normal critical structures include: brainstem max dose < 20 Gy; optic nerve max dose < 15 Gy, chiasm max dose < 15 Gy.
Statistical analysis
The primary end point of the study was OS re-irradiation. OS was defined as the time from initiation of FSRT to the time of death. OS from initial diagnosis was also recorded. Date of recurrence after FSRT was defined as the date of radiographic evidence of progression based on RANO criteria [24]. Kaplan–Meier curves were generated for the OS endpoint. Cox proportional hazard modeling was used for multivariate analysis with factors analyzed in a step-wise fashion. Toxicity was graded using Radiation Therapy Oncology Group (RTOG) criteria. All statistical analysis was performed using the STATA data analysis and statistical software version 13.1 (STATA Corporation).
Results
Patient population and treatment parameters
A total of 36 patients with high grade glioma who had tumor progression on bevacizumab and received salvage FSRT between 2006 and 2013 were included in this study. Patients who received FSRT within 2 months of initiation of bevacizumab were excluded. The pathology was either anaplastic astrocytoma (5 patients) or GBM multiforme (GBM) (30 patients), And there was one patient had grade II gemistocytic astrocytoma (Table 1). Patient characteristics are listed in Table 1. There were 17 males and 19 females. All patients received initial surgery and were treated with radiation and temozolomide. The median age at recurrence was 57.1 years (range 37–73). The median Karnofsky Performance Status (KPS) at recurrence was 80%. 33 patients developed local progression, and 3 patients developed local and distant progression after bevacizumab treatment. Following disease progression on bevacizumab, the median target volume treated with FSRT was 27.5 cc (range 1.95–165 cc). The median dose was 35 in 3.5 Gy daily fractions (range 30–37.5 Gy). All patients continued bevacizumab treatment with FSRT.
Survival
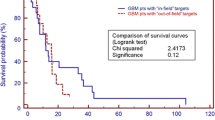
Patients underwent routine surveillance for a median follow up of 20.4 months after initial diagnosis, with an OS from initial diagnosis of 24.9 months. Majority of patients initiated bevacizumab at first recurrence (29), while five patients initiated bevacizumab at second recurrence, and one patient started bevacizumab at third recurrences. The median OS from initiation of bevacizumab was 13.4 months. The median time from initiation of bevacizumab treatment to salvage FSRT was 8.5 months (range 2.4–32 months). The median progression free survival after FSRT was 3.9 months. The median OS from FSRT was 4.8 months (Fig. 1; Table 2).
Multivariate analysis
Multivariate analysis was performed to investigate whether different variables in our study population influenced OS from recurrence or from FSRT therapy (Table 3). These included age at recurrence, KPS score, volume of recurrence, histology (AA vs. GBM) or re-resection status. Importantly, out of all of these variables, only re-resection demonstrated a statistically significant association with OS from recurrence (HR 2.59; p = 0.04). Whether patients underwent re-resection did not impact OS from FSRT (HR 1.87; p = 0.17).
Toxicity
No patients demonstrated clinically significant acute morbidity, with no treatment related grade III or higher toxicity observed. All patients were able to complete the prescribed radiation course without interruption. There were no observed hospitalizations or surgeries for early acute or delayed toxicity in the study population.
Discussion
Treatment failure and disease progression with bevacizumab treatment remain as a clinical challenge for patients with recurrent HGG. The overall prognosis remains quite poor [14]. Despite this clinical need, there is a paucity of literature regarding the management of patients who fail bevacizumab. In that context, the present study investigated FSRT as a potential treatment modality to address this problem.
FSRT had been previously studied in the setting of recurrent HGG, with generally favorable results. Multiple studies have shown FSRT to be efficacious, with OS after recurrence in these studies ranging from 5 to 11 months. Moreover, these studies showed FSRT to be very well tolerated, with a low rate of grade 3 toxicities, radiation necrosis (RN), or reoperation [15, 25, 26]. Of note, one study observed significantly more toxicity and reoperation (11 of 88 patients) than the others [27]. This toxicity outlier can perhaps be explained by the use of a different dosing regimen (24 Gy in four fractions in Lederman et al. vs. 30–35 Gy in six to ten fractions in the other studies [27]). In a head to head trial, Patel et al. compared SRS with FSRT and showed comparable OS and radiographic tumor response between the two modalities, with a trend towards fewer events of RN in the FSRT cohort [28].
The data on FSRT treatment following bevacizumab failure is much more limited. In a retrospective study, Torcuator et al. looked at two cohorts of patients who failed bevacizumab: one that received either FSRT or SRS and one that received no FSRT/SRS. They demonstrated an increased OS in patients receiving FSRT/SRS (7.2 vs. 3.3 months in untreated patients) [29]. Another study that is published only in abstract form by Sarmey et al. similarly looked at RT (including 17 patients who received some form of RT) vs. non-RT regimens following bevacizumab failure and showed statistically significant increased survival in the radiation group (8.8 vs. 5.4 months for untreated) [30].
In the current study, we reported our institutional experience of FSRT with bevacizumab after bevacizumab failure. Indeed, our work builds off of previous work by both our group and others showing comparable benefit and improved safety in FSRT regimens for HGGs. One limitation of our study is the lack of a control cohort for comparison in terms of outcomes to put our OS into context. Historically, patients who fail bevacizumab have been shown in a recent review of sixteen studies to have an OS of 3.8 months [11, 13, 14]. Thus, our observed OS compares favorably to, and indeed exceeds that mark. Taken in the context of the aforementioned studies which show benefit of RT vs. no RT in bevacizumab failure, and also that FSRT and SRS provide similar OS in recurrent gliomas before bevacizumab treatment, our data are consistent with these previous studies and moreover suggest a role for FSRT in the management of patients who fail bevacizumab. Despite our findings, it is worth noting that one limitation of our study is the potential bias of our dataset in that it only includes patients who are amenable to therapy with FSRT. Therefore it is difficult to directly compare our survival data to the existing literature, given that the literature includes all patients, whether or not they are eligible for FSRT. Further head-to-head studies will be needed to evaluate FSRT vs. other modalities to definitively establish a role and identify populations that would most benefit.
Notably, our multivariate analysis yielded only one variable that was associated with OS: re-resection status. Indeed, there is controversy in the literature regarding the prognostic value of re-resection in patients with recurrent HGG [2], but our data suggest that re-resection is actually deleterious in terms of survival outcomes. However, given the retrospective nature of the current study, it is difficult to draw strong conclusions from these data, as re-resection status itself may be confounding by representing underlying patient characteristics that lead to poorer prognosis. Future studies will be needed to identify patient populations who will most benefit from an FSRT regimen.
Other limits to our study include a small patient cohort (36) as well as those shortcomings inherent to all retrospective studies including selection bias and potential treatment differences in a non-randomized study. Despite these potential drawbacks, this study represents, to our knowledge, the largest literature cohort of FSRT patients in the context of bevacizumab failure. Moreover, the dire prognosis of these patients and the paucity of data regarding their management underscores the relevance of the present study, and suggests the need for future prospective randomized trials to improve survival and positively impact the lives of patients with HGG.
Conclusions
Favorable outcomes were observed using FSRT to treat patients with recurrent HGG who progressed after bevacizumab treatment. Thetreatment was well tolerated. Prospective study is warranted to further evaluate the efficacy of salvage FSRT for patients with recurrent HGG after bevacizumab failure.
Change history
08 January 2018
The fourth author’s name was incorrect in the initial online publication. The original article has been corrected.
References
Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G, Group EGW (2014) High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(Suppl 3):iii93–iii101
Palmer JD, Siglin J, Yamoah K, Dan T, Champ CE, Bar-Ad V et al. (2015) Re-resection for recurrent high-grade glioma in the setting of re-irradiation: more is not always better. J Neurooncol 124:215–221
Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA et al (2013) Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 31:4085–4091
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370:699–708
Chinot OL, de La Motte Rouge T, Moore N, Zeaiter A, Das A, Phillips H et al (2011) AVAglio: Phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv Ther 28:334–340
Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA et al (2015) Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA 314:2535–2543
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA et al (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25:4722–4729
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Dowell JM, Reardon DA, Quinn JA et al (2007) Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 13:1253–1259
Iwamoto FM, Abrey LE, Beal K, Gutin PH, Rosenblum MK, Reuter VE et al (2009) Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology 73:1200–1206
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745
Hundsberger T, Reardon DA, Wen PY (2017) Angiogenesis inhibitors in tackling recurrent glioblastoma. Expert Rev Anticancer Ther 17:507–515
Norden AD, Young GS, Setayesh K, Muzikansky A, Klufas R, Ross GL et al (2008) Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 70:779–787
Magnuson W, Ian Robins H, Mohindra P, Howard S (2014) Large volume reirradiation as salvage therapy for glioblastoma after progression on bevacizumab. J Neurooncol 117:133–139
Fogh SE, Andrews DW, Glass J, Curran W, Glass C, Champ C et al (2010) Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol 28:3048–3053
Combs SE, Widmer V, Thilmann C, Hof H, Debus J, Schulz-Ertner D (2005) Stereotactic radiosurgery (SRS): treatment option for recurrent glioblastoma multiforme (GBM). Cancer 104:2168–2173
Gutin PH, Iwamoto FM, Beal K, Mohile NA, Karimi S, Hou BL et al (2009) Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 75:156–163
Park KJ, Kano H, Iyer A, Liu X, Niranjan A, Flickinger JC et al (2012) Salvage gamma knife stereotactic radiosurgery followed by bevacizumab for recurrent glioblastoma multiforme: a case-control study. J Neurooncol 107:323–333
Cuneo KC, Vredenburgh JJ, Sampson JH, Reardon DA, Desjardins A, Peters KB et al (2012) Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 82:2018–2024
Combs SE, Gutwein S, Thilmann C, Debus J, Schulz-Ertner D (2005) Reirradiation of recurrent WHO grade III astrocytomas using fractionated stereotactic radiotherapy (FSRT). Strahlenther Onkol 181:768–773
Combs SE, Gutwein S, Thilmann C, Huber P, Debus J, Schulz-Ertner D (2005) Stereotactically guided fractionated re-irradiation in recurrent glioblastoma multiforme. J Neuro-oncol 74:167–171
Minniti G, Agolli L, Falco T, Scaringi C, Lanzetta G, Caporello P et al (2015) Hypofractionated stereotactic radiotherapy in combination with bevacizumab or fotemustine for patients with progressive malignant gliomas. J Neurooncol 122:559–566
Clark GM, McDonald AM, Nabors LB, Fathalla-Shaykh H, Han X, Willey CD et al (2014) Hypofractionated stereotactic radiosurgery with concurrent bevacizumab for recurrent malignant gliomas: the University of Alabama at Birmingham experience. Neurooncol Pract 1:172–177
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Grosu AL, Weber WA, Franz M, Stark S, Piert M, Thamm R et al (2005) Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys 63:511–519
Fokas E, Wacker U, Gross MW, Henzel M, Encheva E, Engenhart-Cabillic R (2009) Hypofractionated stereotactic reirradiation of recurrent glioblastomas: a beneficial treatment option after high-dose radiotherapy?. Strahlenther Onkol 185:235–240
Lederman G, Wronski M, Arbit E, Odaimi M, Wertheim S, Lombardi E et al (2000) Treatment of recurrent glioblastoma multiforme using fractionated stereotactic radiosurgery and concurrent paclitaxel. Am J Clin Oncol 23:155–159
Patel M, Siddiqui F, Jin JY, Mikkelsen T, Rosenblum M, Movsas B et al (2009) Salvage reirradiation for recurrent glioblastoma with radiosurgery: radiographic response and improved survival. J Neurooncol 92:185–191
Torcuator RG, Thind R, Patel M, Mohan YS, Anderson J, Doyle T et al (2010) The role of salvage reirradiation for malignant gliomas that progress on bevacizumab. J Neurooncol 97:401–407
Sarmey N, Chao ST, murphy ES, Yu JS, Peereboom D, Stevens G et al (2015) The role of salvage radiation in recurrent glioblastoma after bevacizumab failure. ASCO Annual Meeting, Chicago, IL
Acknowledgements
E.S.B. received an F30 Ruth Kirschstein MD-PhD Fellowship Award (CA180500).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No actual or potential conflicts of interest.
Additional information
The original version of this article has been revised: The fourth author's name has been corrected.
A correction to this article is available online at https://doi.org/10.1007/s11060-018-2744-5.
Rights and permissions
About this article
Cite this article
Shi, W., Blomain, E.S., Siglin, J. et al. Salvage fractionated stereotactic re-irradiation (FSRT) for patients with recurrent high grade gliomas progressed after bevacizumab treatment. J Neurooncol 137, 171–177 (2018). https://doi.org/10.1007/s11060-017-2709-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2709-0