Abstract
The place of bevacizumab (BEV) in salvage re-irradiation (Re-RT) settings of malignant glioma is poorly defined. In the current study risk/benefit profiles of two BEV-based Re-RT protocols were analyzed and compared with that of salvage BEV plus irinotecan (BEV/IRI). According to interdisciplinary tumor board recommendations, patients were assigned to one of three BEV-based treatment protocols: (1) BEV/IRI, (2) Re-RT (36 Gy/18 fx) with concomitant BEV (Re-RT/BEV), and (3) Re-RT with concomitant/maintenance BEV (Re-RT/BEV→BEV). Prognostic factors were obtained from proportional hazards models. Adverse events were classified according to the NCI CTCAE, v4.0. 105 consecutive patients were enrolled from 08/2008 to 05/2014. Patients undergoing Re-RT experienced longer time intervals from initial diagnosis to BEV treatment (median: 22.0 months vs. 13.7 months, p = 0.001); those assigned to Re-RT/BEV→BEV rated better on the performance scale (median KPSREC: 90 vs. 70, p = 0.013). Post-recurrence survival after BEV-based treatment (PRS) was longest after Re-RT/BEV→BEV (median: 13.1 months vs. 8 months, p = 0.006). PRS after Re-RT/BEV and BEV/IRI was similar. Multivariately, higher KPSREC and Re-RT/BEV→BEV were associated with longer PRS. Treatment toxicity did not differ among groups. Re-RT/BEV→BEV is safe, feasible and effective and deserves further prospective evaluation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bevacizumab (BEV) has been reported to promote tumor regression [1–4], to reduce vasogenic edema due to normalization of abnormal tumor vessels [5] and to improve performance scores of malignant glioma patients [6, 7]. On the one side, it has been suspected that the improvement of both tumor perfusion and intra-tumoral oxygenation (as a consequence of vessel normalization) might also enhance the efficacy of other chemotherapeutic agents in selected patients [8, 9]. On the other side, it has also been pointed out that anti-angiogenic agents might aggravate tumor hypoxia followed by enhanced angiogenesis, cancer cell invasion, etc [10, 11]. While numerous BEV-based treatment protocols have been evaluated in malignant glioma patients [1–4, 8, 12–16], no conclusion could currently be drawn from these data as to the role of BEV in glioma treatment settings. For example, promising results have been reported from the BELOB-trial: patients with recurrent GBMs did significantly better in terms of survival (OS) and progression-free survival (PFS) after BEV plus lomustine as compared to BEV alone [8]. These results, however, could not be confirmed by the EORTC 26101 trial (median OS in both arms approximately 9 months).
In salvage re-irradiation (Re-RT) settings, BEV has been reported to act as a protective agent [17] and to enhance the efficacy of irradiation by overcoming hypoxia mediated mechanisms of radioresistance [5, 18]. For further clarification the RTOG 1205/NCT01730950 trial has been conducted comparing Re-RT/BEV with BEV alone. The patient accrual for that trial is still ongoing, the study is accruing well but results cannot be awaited in the near future. Thus, treatment decisions in favor of salvage Re-RT still have to rely on retrospective data [19–22]. Different treatment strategies are currently under discussion. Previously, a group of the present study population of patients with Re-RT/BEV was compared with Re-RT alone [19]. However, there was no comparison with patients solely treated by BEV alone or a combined systemic treatment. Moreover, it remained unclear whether concomitant Re-RT/BEV should be supplemented by maintenance BEV treatment. For further clarification, we here present risk/benefit profiles of two Re-RT treatment protocols using either concomitant BEV (Re-RT/BEV) or concomitant and maintenance BEV (Re-RT/BEV→BEV) in patients with multimodal pretreated malignant glioma recurrences; profiles were compared with that of salvage BEV plus irinotecan (BEV/IRI).
Materials and methods
Patient selection
After review by the ethics committee of the University of Munich (# 620-15), the tumor registries of the departments of Neurosurgery and Radiation Oncology were queried (from 04/2007 to 10/2014) for adult patients (aged 18–75 years) with the first to third relapse of supratentorial recurrent malignant glioma undergoing salvage Re-RT with concomitant (Re-RT/BEV) or concomitant and maintenance BEV (Re-RT/BEV→BEV) and for those treated with salvage BEV in combination with irinotecan (BEV/IRI) (Fig. 1). The first patient treated received BEV in 08/2008; the last one received BEV in 05/2014. Date of last follow-up was 10/2015.
Initial histological diagnosis after open tumor resection or molecular stereotactic biopsy was made according to the WHO classification 2007 [23]. MGMT promoter methylation status was determined using methylation-specific PCR (MSP) and bisulfite sequencing; for IDH1/2 mutational status analysis pyrosequencing was used.
All patients had received conventionally fractionated RT (+/− temozolomide) with tumor doses in the range of 60 Gy as part of their initial treatment. In methylated tumors, salvage Re-RT and/or BEV was only considered indicated in those patients who were no longer responsive to alkylating chemotherapeutic agents. Disease progression before the initiation of salvage treatment was documented by follow-up MRI and/or open tumor resection/stereotactic biopsy. All patients had signed informed consent for scientific evaluation of clinical data and tissue information.
Treatment regimens
For all study patients, treatment decisions were made by the interdisciplinary tumor board and assessment of the benefit and the risk of the respective salvage treatment were adjusted for the effects of patient- and treatment-related factors including their molecular genetic biomarker profiles.
Before the initiation of BEV therapy, adequate hematologic, hepatic/renal function and acceptable blood coagulation levels were required. Patients with a recent history of intracranial hemorrhage, arterial thromboembolism, clinically relevant cardiovascular disease, not well controlled hypertension, and/or unhealed wounds were excluded. The following salvage treatment strategies were initiated: (1) BEV in combination with irinotecan (BEV/IRI), (2) Re-RT with concomitant BEV alone (Re-RT/BEV) and (3) Re-RT with concomitant and maintenance BEV (Re-RT/BEV→BEV) (Fig. 1).
Re-RT was principally considered a possible treatment strategy in patients with a KPS score of at least 70 at tumor recurrence (KPSREC) and a minimum time interval of 6 months between the date of projected Re-RT and the end of first radiation therapy procedure [24]. Patients undergoing salvage surgery with complete resection of their recurrence were not considered suitable for Re-RT. In case of multifocal disease, the indication for Re-RT was less frequently given and the primary systemic approach was preferred. The Re-RT protocol implied a total dose of 36 Gy in 18 fractions (2 Gy/fraction) employing 3D conformal radiotherapy or IMRT if adjacent critical structures were present. Gross tumor volume (GTV) included the contrast enhancing lesion of the gadopentetate dimeglumine enhanced (Gd+) T1 weighted MR imaging. Planning target volume (PTV) was defined as GTV plus 10 mm margin at maximum. To ensure reproducibility patients were immobilized with a thermoplastic mask system. Treatment planning was performed using Oncentra MasterPlan® (Nucletron BV, Veenendaal, the Netherlands). During Re-RT, patients received BEV 10 mg/kg per body weight intravenously on days 1 and 15 [25]. In the Re-RT/BEV→BEV group, BEV was continued every 2 weeks accordingly. It was intended to apply at least additional six cycles if no toxicity occurred. In the BEV/IRI group, irinotecan chemotherapy was administered additionally in a dosage of 125 mg/m2 every 2 weeks (up to 340 mg/m2 depending on the use of EIADs).
Generally, it was intended to apply six BEV cycles either in combination with IRI or after Re-RT. Treatment was stopped earlier in case of tumor progression/unacceptable toxicity. Further treatment beyond that intended time point required a new tumor board decision. In case of a clinical response and in patients with excellent clinical score, BEV was continued (patients with >6 cycles, n = 39, 37.1 %).
Treatment schedule and follow-up
Baseline evaluation included MRI, complete physical and neurological examination, and blood tests. MRI control was performed every three months until the end of follow-up with assessment and independently done according to the MacDonald criteria [26, 27] (the RANO criteria were first introduced in 2010 and could not be used in this study). Tumor progression had to be confirmed by clinical and/or neuroradiological follow-up. The standardized MRI (Magnetom Symphony, Siemens; or Signa HDxt, GE Healthcare) protocol comprised 3D T1-weighted sequences (slice thickness: 1 mm) before and after administration of gadopentetate dimeglumine (0.1 mmol per kilogram of body weight, Gd). Volumes of the recurrent tumors before initiation of BEV were determined using the smartbrush iPlan tool (BRAINLAB, Feldkirchen, Germany). Tumor volume calculations referred to measurements of solid enhancing tumor parts including also necrotic/pseudocystic areas (Gd+ volume). In case of suspected pseudoprogression, FET-PET and stereotactic re-biopsy were performed.
Toxicity evaluation
Hematology was performed weekly. Adverse events were defined according to the National Cancer Institute (NCI) Common Toxicity Criteria, version 4.0.
Statistics
Reference point of the study was the date of first BEV treatment. Primary study endpoints were death, treatment toxicity and post-recurrence tumor progression at 6 months. Post-recurrence survival (PRS) was defined as the time interval between the date of first BEV treatment and death. Additionally, post-recurrence progression-free survival at 6 months was analyzed (PR-PFS-6). Secondary endpoint was OS, which was calculated from the date of the initial diagnosis. Survival analyses were made with the Kaplan–Meier method; for comparative analyses the log-rank test was used. Prognostic factors were obtained from proportional hazards models. The distribution of epidemiological data in the respective groups was analyzed and compared using the Kruskal–Wallis/Mann–Whitney U tests (continuously scaled variables) and the χ2 statistics/Fisher’s exact test (categorical variables). A p value of ≤0.05 was considered significant. All calculations were performed using SPSS (version 23.0).
Results
105 patients with recurrent malignant glioma (anaplastic glioma; n = 20, glioblastoma; n = 85) were included. Patient characteristics are summarized in Table 1. Open tumor resection was initially performed in 72.9 %, and molecular stereotactic biopsy in 27.1 %. Information on MGMT promoter methylation status and IDH1/2 mutational status was available for 97 and 96 patients, respectively. The median dose of primary RT was 60 Gy (range: 39–78 Gy) and that of the Re-RT treatment 36 Gy (range: 28–46 Gy). One patient underwent hypofractionated radiotherapy and five patients received conventional lower doses due to inital low-grade histology/tumor extent. One patient received a conventional (dose: 66 Gy) and one a particle boost treatment (dose: 78 Gy). Re-irradiation dose was adapted according to previous dose exposition and organ-at-risk constraints. The number of applied TMZ cycles before BEV treatment was lowest in the Re-RT/BEV group (median: 4 vs. 8, p = 0.031). Tumor progression before initiation of the BEV-based treatment was verified in 43 patients by biopsy/resection and in 62 patients by follow-up MRI imaging including FET-PET.
The median time interval from initial RT to BEV-based treatment was similar in both Re-RT groups (Re-RT/BEV: 19.8 months vs. Re-RT/BEV→BEV: 17.7 months); it was shorter in the BEV/IRI group (12.4 months, p = 0.002) as expected. Patients receiving Re-RT/BEV suffered more often from WHO grade III recurrences (p = 0.043) (Table 1). Gd+ volumes were similar in the Re-RT/BEV→BEV and BEV/IRI groups (median Gd+ volume: 26.0 ml vs. 28.6 ml). Patients of the Re-RT/BEV group exhibited the largest tumor volumes, the difference, however, was not statistically significant (median: 37.8 ml, p = 0.341). Treatment groups did no differ in terms of sex (p = 0.638), age before initiation of BEV-based treatment (p = 0.968), MGMT promoter methylation (p = 0.294) and IDH1/2 mutational status (p = 0.574). The median number of relapses before BEV-based treatment was similar among treatment groups (see Table 1), the same was true for the distribution (one/two/three or four relapses); the patterns of applied modalities did not change over time. Patients of the Re-RT/BEV→BEV group had better performance scores than the BEV/IRI group (median KPSREC: 90 vs. 70, p = 0.013). The median frequency of BEV cycles was similar in the BEV/IRI group and Re-RT/BEV→BEV group (8 cycles vs. 8.5 cycles). Similarly, median treatment time was longer for the BEV/IRI and Re-RT/BEV→BEV group compared to the Re-RT/BEV group (4.4/5.2 months vs. 1.1 months, p = 0.01).
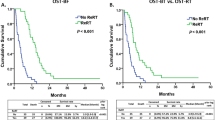
Median follow-up after BEV-based treatment was 25.6 months (95 % CI 11.4–39.9 months). No significant differences were seen in the respective treatment groups. Overall, median OS and PRS were 28.9 months (95 % CI 23.9–33.8 months) and 9.0 months (95 % CI 7.6–10.4 months), respectively. Median OS was significantly shorter after BEV/IRI (BEV/IRI: 23.3vmonths, 95 % CI 19.3–27.2, p = 0.012) and nearly identical among the Re-RT groups (Re-RT/BEV: 30.2 months, 95 % CI 22.5–37.9 months; Re-RT/BEV→BEV: 34 months, 95 % CI 29.7–38.4 months, p = 0.382, Fig. 2a).
a, b OS and PRS for the groups BEV/IRI, Re-RT/BEV and Re-RT/BEV→BEV. Median OS was significantly shorter after BEV/IRI (23.3 months, p = 0.012) and nearly identical among the Re-RT groups (Re-RT/BEV 30.2 months; Re-RT/BEV→BEV 34 months, p = 0.382). Median PRS after Re-RT/BEV→BEV (13.1 months) was longer than after Re-RT/BEV (PRS 8.0 months, p = 0.006). No difference was seen for Re-RT/BEV and BEV/IRI (6.6 months vs. 8.0 months, p = 0.752)
Median PRS after Re-RT/BEV→BEV was longer than after Re-RT/BEV (13.1 months, 95 % CI 10.4–16.1 months vs. 8.0 months, 95 % CI 6.0–10.1 months, p = 0.006). Those of the Re-RT/BEV and BEV/IRI group exhibited similar PRS rates (BEV/IRI: 6.6 months, 95 % CI 4.4–8.8 months, p = 0.752, Fig. 2b).
Grade 3/4 toxicities were seen in seven patients and grade 1/2 toxicities in 25 patients (Table 2). The frequency of adverse events was not influenced by the applied treatment protocols (grade 1–2: p = 0.57, grade 3–4: p = 0.68). There was no radiation-induced symptomatic edema or radiation necrosis in patients with Re-RT/BEV.
Results of one-variable and multivariate prognostic models are summarized in Table 3. Multivariately, a mutated IDH1 mutational status (p = 0.016), WHO grade III (p = 0.002) and Re-RT/BEV→BEV treatment group (p = 0.008) gained favorable influence on length of OS. KPSREC (p = 0.007) and Re-RT/BEV→BEV treatment (p = 0.045) retained prognostic impact on PRS.
Discussion
The place of Re-RT in combination with BEV for salvage treatment of malignant glioma is poorly defined. Here we show that toxicity profiles of Re-RT/BEV, Re-RT/BEV→BEV, and BEV/IRI are similar. We did not observe radiogenic complications such as symptomatic radiation induced edema indicating eventually protective effects of concomitant/maintenance BEV in Re-RT settings. On the basis of the respective toxicity profiles, we suppose that each of the three treatment strategies is applicable for selected patients with malignant glioma recurrences after proven inefficacy of previously applied standard treatment protocols.
The interdisciplinary tumor board selected distinct patient subpopulations for different treatment protocols. For example, a longer time interval (>6 months) after initial radiation predisposes patients for Re-RT protocols whereas those having shorter recurrence free survival after initial radiation were more likely to receive BEV/IRI. This in turn led to a decreased median OS time within the BEV/IRI group as a shorter time interval after initial radiation is per se a clear indicator of decreased OS. Moreover, patients undergoing Re-RT/BEV→BEV rated highest on the performance scale before treatment. Even though the study was not designed to analyze the efficacy of the applied treatment protocols, it was remarkable that Re-RT/BEV→BEV was associated with the longest PRS, independently of the relapse-free survival after initial treatment. An inherent OS superiority cannot be claimed over the BEV/IRI group as the above mentioned characteristics have to be regarded as a certain form of selection bias. Our major result is the influence on PRS on which our report was focused.
The acquired resistance to standard therapies for the tumors of this series is also underlined by loss of prognostic/predictive impact of otherwise powerful molecular biomarkers such as MGMT promoter methylation and IDH1 mutational status [28]; whereas these markers gained powerful influence on OS indicating increased responsibility to alkylating agents and perhaps also radiation in earlier days, no such prognostic effect was seen on PRS.
It was remarkable that the Re-RT/BEV→BEV group contained a higher proportion of grade IV tumors and might have been expected to have inferior outcomes; however, comparable to the loss of the prognostic impact of biomarkers WHO grade did not have a prognostic impact on PRS- and therefore this imbalance should not be overestimated.
The major difference in light of recent evidence on BEV in relapsed GBM is the fact that BEV was combined with Re-RT in this study.
The favorable prognostic impact of Re-RT/BEV→BEV was also confirmed in multivariate models after adjustment for the effects of potentially confounding factors. This is the first retrospective study indicating a potential advantage of concomitant and maintenance BEV application in the framework of Re-RT settings.
BEV/IRI and Re-RT/BEV were associated with similar PRS rates, the salvage treatment time in the Re-RT/BEV group, however, was significantly shorter. Thus, Re-RT/BEV might have the potential to reduce the treatment burden for a considerable number of patients and might be regarded as a valuable treatment option particularly for those not suitable for long-term systemic treatment; this issue warrants further investigation, especially as a substantial part of eligible patients do not present with optimal performance scores.
Representative results could be obtained in the current study compared to previous trials [4, 13, 19–22, 29–33]. However, any comparative analyses should be done cautiously. Reports focusing on Re-RT settings for malignant glioma recurrences did usually not control their results for the effects of the applied selection criteria and other possible prognostic factors. Evaluation of outcome measurements is usually not performed within the framework of comparative adjusted analyses [19–22, 34]. Key variables such as performance scores at the time of tumor progression, volume of the recurrent tumor and the time interval between initial radiation and Re-RT have to be considered, when it comes to analyze the pros and cons of Re-RT settings.
Toxicity after Re-RT compares favorably to other studies dealing with Re-RT/BEV [19–21]. For example, no symptomatic radiation induced edema and/or problems with wound healing were observed. The latter might be explained by the fact that majority of patients received only minimally invasive biopsy procedures before BEV application. To which extent differences in GTVs and PTVs have contributed to the favorable toxicity profile, remains unknown. GTVs and PTVs in the Re-RT/BEV and Re-RT/BEV→BEV groups were comparable; only a few studies have reported on larger target volumes [21, 35].
Our study has the inherent limitations of a retrospective analysis. The relatively small sample size in the respective subgroups might also have contributed to these limitations. The potential superiority of maintenance BEV after Re-RT/BEV deserves further evaluation within the framework of prospective studies. So far, Re-RT strategies in combination with BEV can be offered as a feasible and safe treatment strategy for selected patients with malignant glioma recurrences. Results of this study might provide a basis against which other studies treating similar tumors might be compared.
References
Chamberlain MC, Johnston SK (2010) Salvage therapy with single agent bevacizumab for recurrent glioblastoma. J Neurooncol 96:259–269. doi:10.1007/s11060-009-9957-6
Field KM, Jordan JT, Wen PY, Rosenthal MA, Reardon DA (2014) Bevacizumab and glioblastoma: scientific review, newly reported updates, and ongoing controversies. Cancer. doi:10.1002/cncr.28935
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Wagner M, Bigner DD, Friedman AH, Friedman HS (2007) Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 13:1253–1259. doi:10.1158/1078-0432.CCR-06-2309
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25:4722–4729. doi:10.1200/JCO.2007.12.2440
Gonzalez J, Kumar AJ, Conrad CA, Levin VA (2007) Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys 67:323–326. doi:10.1016/j.ijrobp.2006.10.010
Kaley T, Nolan C, Carver A, Omuro A (2013) Bevacizumab for acute neurologic deterioration in patients with glioblastoma. CNS Oncol 2:413–418. doi:10.2217/cns.13.40
Nagpal S, Harsh G, Recht L (2011) Bevacizumab improves quality of life in patients with recurrent glioblastoma. Chemother Res Pract. doi:10.1155/2011/602812
Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D, de Vos FY, Dinjens WN, Enting RH, Taphoorn MJ, van den Berkmortel FW, Jansen RL, Brandsma D, Bromberg JE, van Heuvel I, Vernhout RM, van der Holt B, van den Bent MJ (2014) Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15:943–953. doi:10.1016/S1470-2045(14)70314-6
Niyazi M, Harter PN, Hattingen E, Rottler M, von Baumgarten L, Proescholdt M, Belka C, Lauber K, Mittelbronn M (2016) Bevacizumab and radiotherapy for the treatment of glioblastoma: brothers in arms or unholy alliance? Oncotarget 7:2313–2328. doi:10.18632/oncotarget.6320
de Groot JF, Fuller G, Kumar AJ, Piao Y, Eterovic K, Ji Y, Conrad CA (2010) Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol 12:233–242. doi:10.1093/neuonc/nop027
Lucio-Eterovic AK, Piao Y, de Groot JF (2009) Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res 15:4589–4599. doi:10.1158/1078-0432.CCR-09-0575
Desjardins A, Reardon DA, Coan A, Marcello J, Herndon JE 2nd, Bailey L, Peters KB, Friedman HS, Vredenburgh JJ (2012) Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer 118:1302–1312. doi:10.1002/cncr.26381
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740. doi:10.1200/JCO.2008.19.8721
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ Jr, Mehta MP (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370:699–708. doi:10.1056/NEJMoa1308573
Soffietti R, Trevisan E, Bertero L, Cassoni P, Morra I, Fabrini MG, Pasqualetti F, Lolli I, Castiglione A, Ciccone G, Ruda R (2014) Bevacizumab and fotemustine for recurrent glioblastoma: a phase II study of AINO (Italian Association of Neuro-Oncology). J Neurooncol 116:533–541. doi:10.1007/s11060-013-1317-x
Vredenburgh JJ, Desjardins A, Reardon DA, Friedman HS (2009) Experience with irinotecan for the treatment of malignant glioma. Neuro Oncol 11:80–91. doi:10.1215/15228517-2008-075
Perez-Torres CJ, Yuan L, Schmidt RE, Rich KM, Drzymala RE, Hallahan DE, Ackerman JJ, Garbow JR (2015) Specificity of vascular endothelial growth factor treatment for radiation necrosis. Radiother Oncol 117:382–385. doi:10.1016/j.radonc.2015.09.004
Anderson JC, Duarte CW, Welaya K, Rohrbach TD, Bredel M, Yang ES, Choradia NV, Thottassery JV, Yancey Gillespie G, Bonner JA, Willey CD (2014) Kinomic exploration of temozolomide and radiation resistance in glioblastoma multiforme xenolines. Radiother Oncol 111:468–474. doi:10.1016/j.radonc.2014.04.010
Flieger M, Ganswindt U, Schwarz SB, Kreth FW, Tonn JC, la Fougere C, Ertl L, Linn J, Herrlinger U, Belka C, Niyazi M (2014) Re-irradiation and bevacizumab in recurrent high-grade glioma: an effective treatment option. J Neurooncol 117:337–345. doi:10.1007/s11060-014-1394-5
Gutin PH, Iwamoto FM, Beal K, Mohile NA, Karimi S, Hou BL, Lymberis S, Yamada Y, Chang J, Abrey LE (2009) Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 75:156–163. doi:10.1016/j.ijrobp.2008.10.043
Hundsberger T, Brugge D, Putora PM, Weder P, Weber J, Plasswilm L (2013) Re-irradiation with and without bevacizumab as salvage therapy for recurrent or progressive high-grade gliomas. J Neurooncol 112:133–139. doi:10.1007/s11060-013-1044-3
Niyazi M, Ganswindt U, Schwarz SB, Kreth FW, Tonn JC, Geisler J, la Fougere C, Ertl L, Linn J, Siefert A, Belka C (2012) Irradiation and bevacizumab in high-grade glioma retreatment settings. Int J Radiat Oncol Biol Phys 82:67–76. doi:10.1016/j.ijrobp.2010.09.002
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. doi:10.1007/s00401-007-0243-4
Niyazi M, Brada M, Chalmers AJ, Combs SE, Erridge SC, Fiorentino A, Grosu AL, Lagerwaard FJ, Minniti G, Mirimanoff RO, Ricardi U, Short SC, Weber DC, Belka C (2016) ESTRO-ACROP guideline “target delineation of glioblastomas”. Radiother Oncol 118:35–42. doi:10.1016/j.radonc.2015.12.003
Niyazi M, Jansen NL, Rottler M, Ganswindt U, Belka C (2014) Recurrence pattern analysis after re-irradiation with bevacizumab in recurrent malignant glioma patients. Radiat Oncol 9:299. doi:10.1186/s13014-014-0299-y
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. doi:10.1200/jco.2009.26.3541
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Kessler J, Guttler A, Wichmann H, Rot S, Kappler M, Bache M, Vordermark D (2015) IDH1(R132H) mutation causes a less aggressive phenotype and radiosensitizes human malignant glioma cells independent of the oxygenation status. Radiother Oncol 116:381–387. doi:10.1016/j.radonc.2015.08.007
Bokstein F, Shpigel S, Blumenthal DT (2008) Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer 112:2267–2273. doi:10.1002/cncr.23401
Demirci U, Tufan G, Aktas B, Balakan O, Alacacioglu A, Dane F, Engin H, Kaplan MA, Gunaydin Y, Ozdemir NY, Tugba Unek I, Karaca H, Akman T, Sonmez OU, Coskun U, Harputluoglu H, Sevinc A, Tonyali O, Buyukberber S, Benekli M (2013) Bevacizumab plus irinotecan in recurrent or progressive malign glioma: a multicenter study of the Anatolian Society of Medical Oncology (ASMO). J Cancer Res Clin Oncol 139:829–835. doi:10.1007/s00432-013-1390-8
Poulsen HS, Grunnet K, Sorensen M, Olsen P, Hasselbalch B, Nelausen K, Kosteljanetz M, Lassen U (2009) Bevacizumab plus irinotecan in the treatment patients with progressive recurrent malignant brain tumours. Acta Oncol 48:52–58. doi:10.1080/02841860802537924
Reardon DA, Desjardins A, Peters KB, Gururangan S, Sampson JH, McLendon RE, Herndon JE 2nd, Bulusu A, Threatt S, Friedman AH, Vredenburgh JJ, Friedman HS (2012) Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naive, recurrent glioblastoma. J Neurooncol 107:155–164. doi:10.1007/s11060-011-0722-2
Minniti G, Agolli L, Falco T, Scaringi C, Lanzetta G, Caporello P, Osti MF, Esposito V, Enrici RM (2015) Hypofractionated stereotactic radiotherapy in combination with bevacizumab or fotemustine for patients with progressive malignant gliomas. J Neurooncol 122:559–566. doi:10.1007/s11060-015-1745-x
Combs SE, Thilmann C, Edler L, Debus J, Schulz-Ertner D (2005) Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol 23:8863–8869. doi:10.1200/JCO.2005.03.4157
Magnuson W, Ian Robins H, Mohindra P, Howard S (2014) Large volume reirradiation as salvage therapy for glioblastoma after progression on bevacizumab. J Neurooncol 117:133–139. doi:10.1007/s11060-014-1363-z
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that conflicts of interest do not exist.
Additional information
Oliver Schnell and Jun Thorsteinsdottir have equally contributed to this study.
All authors except WA are members of the CCC Neuro-Oncology, LMU Munich.
Rights and permissions
About this article
Cite this article
Schnell, O., Thorsteinsdottir, J., Fleischmann, D.F. et al. Re-irradiation strategies in combination with bevacizumab for recurrent malignant glioma. J Neurooncol 130, 591–599 (2016). https://doi.org/10.1007/s11060-016-2267-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2267-x